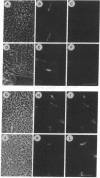
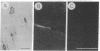
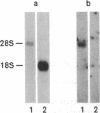
Abstract
The ligand binding site of the nicotinic acetylcholine receptor (AcChoR) is within a short peptide from the alpha subunit that includes the tandem cysteine residues at positions 192 and 193. To elucidate the molecular basis of the binding properties of the AcChoR, we chose to study nonclassical muscle AcChoRs from animals that are resistant to alpha-neurotoxins. We have previously reported that the resistance of snake AcChoR to alpha-bungarotoxin (alpha-BTX) may be accounted for by several major substitutions in the ligand binding site of the receptor. In the present study, we have analyzed the binding site of AcChoR from the mongoose, which is also resistant to alpha-neurotoxins. It was shown that mongoose AcChoR does not bind alpha-BTX in vivo or in vitro. cDNA fragments of the alpha subunit of mongoose AcChoR corresponding to codons 122-205 and including the presumed ligand binding site were cloned, sequenced, and expressed in Escherichia coli. The expressed protein fragments of the mongoose, as well as of snake receptors, do not bind alpha-BTX. The mongoose fragment is highly homologous (greater than 90%) to the respective mouse fragment. Out of the seven amino acid differences between the mongoose and mouse in this region, five cluster in the presumed ligand binding site, close to cysteines 192 and 193. These changes are at positions 187 (Trp----Asn), 189 (Phe----Thr), 191 (Ser----Ala), 194 (Pro----Leu), and 197 (Pro----His). The mongoose like the snake AcChoR has a potential glycosylation site in the binding site domain. Sequence comparison between species suggests that substitutions at positions 187, 189, and 194 are important in determining the resistance of mongoose and snake AcChoR to alpha-BTX. In addition, it was shown that amino acid residues that had been reported to be necessary for acetylcholine binding are conserved in the toxin-resistant animals as well.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson S. N., Ellisman M. H., Deerinck T. J., Maulet Y., Gentry M. K., Doctor B. P., Taylor P. Differences in structure and distribution of the molecular forms of acetylcholinesterase. J Cell Biol. 1989 Jun;108(6):2301–2311. doi: 10.1083/jcb.108.6.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramson S. N., Li Y., Culver P., Taylor P. An analog of lophotoxin reacts covalently with Tyr190 in the alpha-subunit of the nicotinic acetylcholine receptor. J Biol Chem. 1989 Jul 25;264(21):12666–12672. [PubMed] [Google Scholar]
- Asher O., Neumann D., Fuchs S. Increased levels of acetylcholine receptor alpha-subunit mRNA in experimental autoimmune myasthenia gravis. FEBS Lett. 1988 Jun 20;233(2):277–281. doi: 10.1016/0014-5793(88)80442-3. [DOI] [PubMed] [Google Scholar]
- Barkas T., Mauron A., Roth B., Alliod C., Tzartos S. J., Ballivet M. Mapping the main immunogenic region and toxin-binding site of the nicotinic acetylcholine receptor. Science. 1987 Jan 2;235(4784):77–80. doi: 10.1126/science.2432658. [DOI] [PubMed] [Google Scholar]
- Bartfeld D., Fuchs S. Immunological characterization of an irreversibly denatured acetylcholine receptor. FEBS Lett. 1977 May 15;77(2):214–218. doi: 10.1016/0014-5793(77)80237-8. [DOI] [PubMed] [Google Scholar]
- Boulter J., Evans K., Goldman D., Martin G., Treco D., Heinemann S., Patrick J. Isolation of a cDNA clone coding for a possible neural nicotinic acetylcholine receptor alpha-subunit. 1986 Jan 30-Feb 5Nature. 319(6052):368–374. doi: 10.1038/319368a0. [DOI] [PubMed] [Google Scholar]
- Burden S. J., Hartzell H. C., Yoshikami D. Acetylcholine receptors at neuromuscular synapses: phylogenetic differences detected by snake alpha-neurotoxins. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3245–3249. doi: 10.1073/pnas.72.8.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J. P., Devillers-Thiéry A., Chemouilli P. Acetylcholine receptor: an allosteric protein. Science. 1984 Sep 21;225(4668):1335–1345. doi: 10.1126/science.6382611. [DOI] [PubMed] [Google Scholar]
- Cohen J. B., Sharp S. D., Liu W. S. Structure of the agonist-binding site of the nicotinic acetylcholine receptor. [3H]acetylcholine mustard identifies residues in the cation-binding subsite. J Biol Chem. 1991 Dec 5;266(34):23354–23364. [PubMed] [Google Scholar]
- Dennis M., Giraudat J., Kotzyba-Hibert F., Goeldner M., Hirth C., Chang J. Y., Lazure C., Chrétien M., Changeux J. P. Amino acids of the Torpedo marmorata acetylcholine receptor alpha subunit labeled by a photoaffinity ligand for the acetylcholine binding site. Biochemistry. 1988 Apr 5;27(7):2346–2357. doi: 10.1021/bi00407a016. [DOI] [PubMed] [Google Scholar]
- Fuchs S., Neumann D., Safran A., Pizzighella S., Mantegazza R., Daniels M. P., Vogel Z. Species specificity of anti-acetylcholine receptor antibodies elicited by synthetic peptides. Biochemistry. 1987 Jul 28;26(15):4611–4616. doi: 10.1021/bi00389a003. [DOI] [PubMed] [Google Scholar]
- Galzi J. L., Revah F., Bessis A., Changeux J. P. Functional architecture of the nicotinic acetylcholine receptor: from electric organ to brain. Annu Rev Pharmacol Toxicol. 1991;31:37–72. doi: 10.1146/annurev.pa.31.040191.000345. [DOI] [PubMed] [Google Scholar]
- Galzi J. L., Revah F., Black D., Goeldner M., Hirth C., Changeux J. P. Identification of a novel amino acid alpha-tyrosine 93 within the cholinergic ligands-binding sites of the acetylcholine receptor by photoaffinity labeling. Additional evidence for a three-loop model of the cholinergic ligands-binding sites. J Biol Chem. 1990 Jun 25;265(18):10430–10437. [PubMed] [Google Scholar]
- Gershoni J. M. Expression of the alpha-bungarotoxin binding site of the nicotinic acetylcholine receptor by Escherichia coli transformants. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4318–4321. doi: 10.1073/pnas.84.12.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershoni J. M., Hawrot E., Lentz T. L. Binding of alpha-bungarotoxin to isolated alpha subunit of the acetylcholine receptor of Torpedo californica: quantitative analysis with protein blots. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4973–4977. doi: 10.1073/pnas.80.16.4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggerty J. G., Froehner S. C. Restoration of 125I-alpha-bungarotoxin binding activity to the alpha subunit of Torpedo acetylcholine receptor isolated by gel electrophoresis in sodium dodecyl sulfate. J Biol Chem. 1981 Aug 25;256(16):8294–8297. [PubMed] [Google Scholar]
- KARNOVSKY M. J., ROOTS L. A "DIRECT-COLORING" THIOCHOLINE METHOD FOR CHOLINESTERASES. J Histochem Cytochem. 1964 Mar;12:219–221. doi: 10.1177/12.3.219. [DOI] [PubMed] [Google Scholar]
- Kao P. N., Dwork A. J., Kaldany R. R., Silver M. L., Wideman J., Stein S., Karlin A. Identification of the alpha subunit half-cystine specifically labeled by an affinity reagent for the acetylcholine receptor binding site. J Biol Chem. 1984 Oct 10;259(19):11662–11665. [PubMed] [Google Scholar]
- Low B. W., Corfield P. W. Erabutoxin b. Structure/function relationships following initial protein refinement at 0.140-nm resolution. Eur J Biochem. 1986 Dec 15;161(3):579–587. doi: 10.1111/j.1432-1033.1986.tb10481.x. [DOI] [PubMed] [Google Scholar]
- Mishina M., Tobimatsu T., Imoto K., Tanaka K., Fujita Y., Fukuda K., Kurasaki M., Takahashi H., Morimoto Y., Hirose T. Location of functional regions of acetylcholine receptor alpha-subunit by site-directed mutagenesis. 1985 Jan 31-Feb 6Nature. 313(6001):364–369. doi: 10.1038/313364a0. [DOI] [PubMed] [Google Scholar]
- Neumann D., Barchan D., Fridkin M., Fuchs S. Analysis of ligand binding to the synthetic dodecapeptide 185-196 of the acetylcholine receptor alpha subunit. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9250–9253. doi: 10.1073/pnas.83.23.9250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann D., Barchan D., Horowitz M., Kochva E., Fuchs S. Snake acetylcholine receptor: cloning of the domain containing the four extracellular cysteines of the alpha subunit. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7255–7259. doi: 10.1073/pnas.86.18.7255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann D., Barchan D., Safran A., Gershoni J. M., Fuchs S. Mapping of the alpha-bungarotoxin binding site within the alpha subunit of the acetylcholine receptor. Proc Natl Acad Sci U S A. 1986 May;83(9):3008–3011. doi: 10.1073/pnas.83.9.3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann D., Gershoni J. M., Fridkin M., Fuchs S. Antibodies to synthetic peptides as probes for the binding site on the alpha subunit of the acetylcholine receptor. Proc Natl Acad Sci U S A. 1985 May;82(10):3490–3493. doi: 10.1073/pnas.82.10.3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovadia M., Kochva E. Neutralization of Viperidae and Elapidae snake venoms by sera of different animals. Toxicon. 1977;15(6):541–547. doi: 10.1016/0041-0101(77)90105-2. [DOI] [PubMed] [Google Scholar]
- Ravdin P., Axelrod D. Fluorescent tetramethyl rhodamine derivatives of alpha-bungarotoxin: preparation, separation, and characterization. Anal Biochem. 1977 Jun;80(2):585–592. doi: 10.1016/0003-2697(77)90682-0. [DOI] [PubMed] [Google Scholar]
- Schoepfer R., Conroy W. G., Whiting P., Gore M., Lindstrom J. Brain alpha-bungarotoxin binding protein cDNAs and MAbs reveal subtypes of this branch of the ligand-gated ion channel gene superfamily. Neuron. 1990 Jul;5(1):35–48. doi: 10.1016/0896-6273(90)90031-a. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Sussman J. L., Harel M., Frolow F., Oefner C., Goldman A., Toker L., Silman I. Atomic structure of acetylcholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein. Science. 1991 Aug 23;253(5022):872–879. doi: 10.1126/science.1678899. [DOI] [PubMed] [Google Scholar]
- Tzartos S. J., Remoundos M. S. Fine localization of the major alpha-bungarotoxin binding site to residues alpha 189-195 of the Torpedo acetylcholine receptor. Residues 189, 190, and 195 are indispensable for binding. J Biol Chem. 1990 Dec 15;265(35):21462–21467. [PubMed] [Google Scholar]
- Wilson P. T., Lentz T. L. Binding of alpha-bungarotoxin to synthetic peptides corresponding to residues 173-204 of the alpha subunit of Torpedo, calf, and human acetylcholine receptor and restoration of high-affinity binding by sodium dodecyl sulfate. Biochemistry. 1988 Sep 6;27(18):6667–6674. doi: 10.1021/bi00418a004. [DOI] [PubMed] [Google Scholar]
- Wilson P. T., Lentz T. L., Hawrot E. Determination of the primary amino acid sequence specifying the alpha-bungarotoxin binding site on the alpha subunit of the acetylcholine receptor from Torpedo californica. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8790–8794. doi: 10.1073/pnas.82.24.8790. [DOI] [PMC free article] [PubMed] [Google Scholar]