Abstract
Purpose
Trans-signaling of interleukin (IL)-6 through its soluble receptor (sIL-6R) is critically involved in the promotion of chronic inflammatory diseases. The aim of the present study was to estimate IL-6, sIL-6R, and soluble gp130 (sgp130, a natural antagonist of IL-6 trans-signaling) concentrations in the serum and aqueous humor (AqH) of patients with diabetic retinopathy (DR).
Methods
Paired AqH and serum samples were collected from 152 consecutive diabetic patients (105 with DR and 47 without DR, NDR) and 51 healthy controls. The IL-6, sIL-6R, and sgp130 concentrations were measured with multiplex bead immunoassay.
Results
The sgp130 concentrations in the serum and AqH were statistically significantly elevated in patients with DR compared with the NDR patients and the healthy controls (p<0.001). The sgp130 concentrations in the serum and AqH increased as the DR severity increased (p = 0.008, p<0.001, respectively). Higher serum and AqH concentrations of IL-6 and sIL-6R were also observed in patients with DR when compared with the NDR patients and the healthy controls (p<0.001). The AqH concentration of sgp130 was found to be statistically significantly correlated with sIL-6R and IL-6. Similarly, the IL-6 concentration in the AqH was statistically significantly correlated with sIL-6R (p<0.001). Elevated sgp130, sIL-6R, and IL-6 concentrations in the AqH were associated with longer disease duration and higher body mass index, plasma glucose, and glycosylated hemoglobin (HbA1c).
Conclusions
The sgp130, IL-6, and sIL-6R concentrations were statistically significantly elevated in patients with DR, suggesting a probable contributing role of the IL-6 trans-signaling pathway to the pathophysiology of DR.
Introduction
Diabetic retinopathy (DR) is a severe microvascular complication of diabetes mellitus and a common vision-threatening disease, characterized by retinal microvascular damage leading to vascular leakage and ischemia-induced retinal neovascularization [1-3]. However, the pathogenesis of DR is not well understood. Currently, it is known that DR is associated with low-grade subclinical inflammatory disease, manifested as the influx of macrophages and leukocytes and the increased concentrations of inflammatory cytokines, such as interleukin-1β (IL-1β), tumor necrosis factor α, and IL-6 [4-8].
IL-6 is a pleiotropic cytokine that regulates various physiologic processes, such as inflammation and immune responses, by acting on a wide variety of cells, including leukocytes, endothelial cells (ECs), and fibroblasts [9,10]. The bioactivity of IL-6 is controlled by a membrane-bound receptor that is composed of two subunits: a cognate receptor subunit (IL-6R) that specifically recognizes IL-6 and a signal-transducing molecule glycoprotein 130 (gp130) [11-14]. In addition to the membrane-bound receptors, soluble forms of both receptors have been found in biologic fluids, the soluble IL-6R (sIL-6R) and the soluble gp130 (sgp130). sIL-6R, together with IL-6, can bind surface gp130 and elicit a biologic response in cells that do not express surface IL-6R. This type of activation has been named “trans-signaling,” and it not only renders a large new spectrum of cells responsive to IL-6 activation but also can elicit changes in physiologic processes ranging from control of the immune response to the maintenance of pathological states [15]. The trans-signaling mechanism is regulated by sgp130, which is a natural inhibitor and forms a complex with IL-6/sIL-6R, preventing the binding of IL-6/sIL-6R to membrane-bound gp130 [16].
The IL-6/sIL-6R/sgp130 complex has been shown to be involved in several inflammatory conditions, such as inflammatory bowel disease and arthritis [17,18]. It has been suggested that during an inflammatory process, the trans-signaling mechanism is critical in the switch from the initial recruitment of neutrophils during acute inflammation to the recruitment of lymphocytes in the chronic phase [11]. Previous findings of chronic low-grade inflammation associated with DR in a patient with type 2 diabetes mellitus (T2DM) suggest the possible involvement of the IL-6 trans-signaling mechanism in these individuals [19,20]. In animal models of uveitis, an elevation in IL-6 and sIL-6R is found in the AqH [18,21]. Unlike IL-6, sIL-6R and sgp130 concentrations fluctuate much less and give more precise information about the activation of the IL-6 pathway. Given this evidence, it is likely that the IL-6 pathway is also involved in the pathogenesis of DR. Therefore, to test this hypothesis, we investigated the serum and AqH concentrations of IL-6, sIL-6R, and sgp130 in patients with DR. The relationship of these parameters to disease severity was also determined.
Methods
Study population
Consecutive patients with T2DM and non-diabetic controls who were undergoing cataract surgery between January 2015 and April 2016 were recruited for this study. Diagnosis of T2DM was confirmed using the 2016 American Diabetes Association standard [22]. All patients underwent a complete ophthalmic examination and a general physical examination. This included visual acuity, slit-lamp-assisted biomicroscopy of the anterior segment, a fundus examination, and fluorescence fundus angiography, which was used for the clinical diagnosis of DR. According to the Diabetic Retinopathy Disease Severity Scale, the diabetic patients were classified into three groups: no apparent retinopathy (NDR), non-proliferative diabetic retinopathy (NPDR), and proliferative diabetic retinopathy (PDR). Diabetic macular edema (DME) was defined as being present if clinically significant macular edema was detected [23].
All patients with T2DM were on an appropriate diet and receiving glucose-lowering medication at the time of recruitment. Detailed demographic information was collected from every subject, such as family history, duration of disease, current medication, height, weight, body mass index (BMI = weight/height2), blood pressure, fasting serum glucose, and glycosylated hemoglobin (HbA1c). Exclusion criteria included the following: acute myocardial infarction, organ failure, liver disease, stroke, recent systemic infection, previous intraocular surgery, earlier intravitreal therapies, photocoagulation during the preceding 3 months, uveitis, trauma, vitreous hemorrhage, and retinal detachment. Patients who had taken immunosuppressive drugs were also excluded. The project followed the tenets of the Declaration of Helsinki and adhered to the ARVO statement on human subjects. All experimental protocols were approved by the ethics committee of our institution. Patients gave their written informed consent to participate in the study.
Sex and age breakdown
We obtained age- and sex-matched samples from 152 T2DM patients and 51 healthy subjects. The mean age of the T2DM patients (75 men and 77 women) was 61.0±7.4 years. The mean age of the healthy control subjects (31 men and 20 women) was 61.4±8.2 years. Of the 152 T2DM patients, there were 47 with NDR, 52 with NPDR, and 53 with PDR. The male/female ratio and mean age were 25/22 and 60.2±8.7 years in the NDR group, 20/32 and 62.3±7.9 years in the NPDR group, and 30/23 and 60.6±5.1 years in the PDR group.
Serum collection
Five milliliters of whole blood was collected from the patients and healthy controls at their first visit, using a standard venipuncture technique between 9:00 and 11:00 AM. Serum samples were obtained after centrifugation at 800 ×g for 10 min, aliquoted, and stored at −80 °C until assayed.
Aqueous humor collection
A limbal paracentesis was performed with a sterile tuberculin syringe at the beginning of cataract surgery before the initial incision was made. Undiluted AqH samples (0.1–0.2 ml) were collected from the paracentesis site. The samples were immediately frozen and stored at −80 °C until analysis.
Cytokine measurement
The simultaneous measurement of IL-6, sIL-6R, and sgp130 in serum and AqH was performed using multiplex bead analysis assays (Millipore UK Ltd., Watford, UK). The AqH samples were diluted 1:5 with PBS (1X; 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.4), 1% bovine serum albumin (BSA), and 0.05% Tween-20 and incubated with monoclonal antibody-coated capture beads for 2 h at 20 °C. The washed beads were further incubated with a biotin-labeled anti-human cytokine antibody for 2 h, followed by streptavidin/phycoerythrin for 30 min. Samples were analyzed with a Luminex® 100TM bioassay analysis system (Luminex, Austin, TX) and STarStation 2.0 commercial software (Applied Cytometry Systems, Sheffield, UK). Standard curves of known concentrations of recombinant human cytokines were used to convert fluorescence units to cytokine concentration. The sensitivities of each assay were <1, <2, and <10 pg/ml for IL-6, sIL-6R, and sgp130, respectively. The value for the buffer alone (PBS, 1% BSA) was subtracted for each sample.
Statistical analysis
The Kruskal–Wallis test was used to compare the concentrations of each cytokine measured between multiple groups. When statistically significant differences were found, the Dunn multiple comparison post-test was employed. The Student t test or the Mann–Whitney (two-tailed) test was used when only two groups were compared (depending on the normality assumptions and the homogeneity of variances). Spearman (two-tailed, nonparametric) correlations were used to assess the significance of the correlations between each cytokine. Data were analyzed using SPSS Version 19.0 (IBM, New York, NY). Graphs were prepared with Prism version 5 (GraphPad Software Inc., La Jolla, CA). A p value of less than 0.05 was considered statistically significant.
Results
Characteristics of controls and patients with diabetes mellitus
The principal characteristics of the enrolled patients are described in Table 1. With regard to age and sex, no statistically significant difference was observed between the study groups (p = 0.50 and p = 0.12, respectively). BMI distribution was statistically significantly higher in patients with T2DM compared with the non-diabetic subjects (p<0.001). The mean duration of diabetes was statistically significantly longer in the PDR group than in the NPDR and NDR groups (p<0.001). HbA1c values between 4.27% and 6.07% were considered normal in our laboratory. HbA1c and fasting glucose levels were also found to differ statistically significantly between the NDR and PDR groups (p<0.001 and p<0.001, respectively).
Table 1. Clinical and biochemical characteristics of type 2 diabetic patients and healthy control subjects.
| Variable | Control |
NDR |
NPDR |
PDR |
p |
|---|---|---|---|---|---|
| (n=51) | (n=47) | (n=52) | (n=53) | ||
| Sex (m/f) |
31/20 |
25/22 |
20/32 |
30/23 |
0.118 |
| Age (years) |
61.4±8.2 |
60.2±8.7 |
62.3±7.9 |
60.6±5.1 |
0.496 |
| BMI (kg/m2) |
22.6±2.3 |
22.9±2.1 |
24.2±4.3 |
25.4±4.5 |
<0.001* |
| Diabetes Duration (years) |
- |
8.7±3.3 |
12.8±3.1 |
15.6±3.9 |
<0.001* |
| FPG (mmol/l) |
- |
7.7±1.3 |
9.2±2.0 |
11.0±2.0 |
<0.001* |
| HbAlc (%) | - | 8.9±1.9 | 12.7±3.1 | 14.6±2.4 | <0.001* |
NDR: type 2 diabetic patients without diabetic retinopathy; NPDR: non-proliferative diabetic retinopathy; PDR: proliferative diabetic retinopathy; BMI, Body mass index; FPG: fasting plasma glucose; HbA1c, glycated hemoglobin Data are expressed as mean ± SD
The IL-6, sIL-6R, and sgp130 concentrations in patients with and without DR
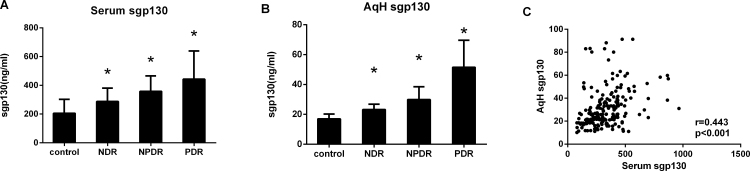
The serum and AqH concentrations of sgp130 according to DR status are reported in Figure 1. In the serum and AqH concentrations, the median sgp130 concentration was statistically significantly higher in the DR and NDR groups compared with the non-diabetic controls (p<0.001). In the subjects with DR, the median sgp130 concentrations in serum and AqH were higher in the PDR group than in the NPDR group (p<0.001). In general, the sgp130 levels were statistically significantly lower in the AqH than in the serum (p<0.001; Table 2), and there was a statistically significant correlation between the sgp130 concentration in the AqH and serum (r = 0.443, p<0.001; Figure 1C).
Figure 1.
Concentrations of sgp130 in serum and AqH from patients with T2DM and non-diabetic controls. Soluble gp130 (sgp130) was measured with multiplex bead immunoassay in serum (A) and aqueous humor (AqH; B) from controls (n = 51), non-diabetic retinopathy (NDR, n = 47), nonproliferative diabetic retinopathy (NPDR, n = 52), and proliferative diabetic retinopathy (PDR, n = 53). All groups were compared using the Kruskal–Wallis test followed by the Dunn multiple comparison test. The relationship between sgp130 in AqH and serum was tested, when matched samples were available, with the Spearman correlation (C). *p<0.001.
Table 2. Cytokine concentrations in patients with or without diabetic macular edema.
| Sample | Cytokine | DME (n=58) |
No-DME (n=47) |
p |
|---|---|---|---|---|
| Median (range) | Median (range) | |||
| Aq (pg/ml) |
IL-6 |
46.77 (15.99–67.80) |
43.11 (17.98–63.80) |
0.038* |
| Serum |
IL-6 |
1.69 (1.25–1.94) |
1.66 (1.08–1.93) |
0.616 |
| Aq (ng/ml) |
sIL-6R |
1.55 (1.01–2.47) |
1.26 (0.96–1.96) |
0.022 |
| Serum |
sIL-6R |
130.90 (74.67–166.34) |
121.56 (85.76–165.34) |
0.038 |
| Aq (ng/ml) |
sgp130 |
40.62 (13.32–91.28) |
31.71 (11.03–83.32)) |
0.002* |
| Serum | sgp130 | 429.48 (140.10–965.46) | 310.61 (118.16–622.61) | <0.001* |
DME: diabetic macular edema; IL: interleukin
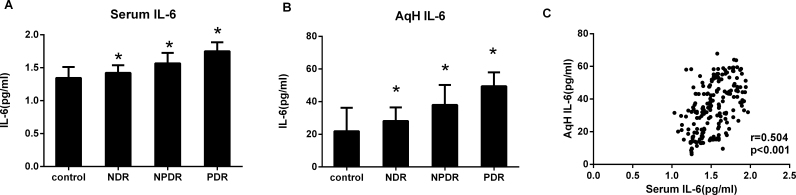
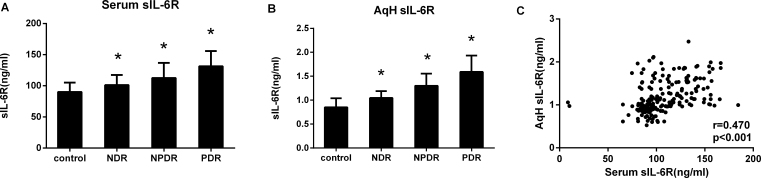
Using multiplex bead immunoassays, we simultaneously measured the IL-6 and sIL-6R concentrations in the serum and AqH (Figure 2, Figure 3). The patients with DR had markedly higher sIL-6R and IL-6 concentrations compared with the non-diabetic subjects (p<0.001). The sIL-6R and IL-6 concentrations in patients with PDR were also found to be higher than in the NPDR group (p<0.001). For all patients, the IL-6 levels were statistically significantly higher in the AqH than in the serum (p<0.001), whereas the sIL-6R level was statistically significantly lower in the AqH than in the serum (p<0.001; Table 2). There was a statistically significant correlation between the IL-6 and sIL-6R concentrations in the AqH and serum (r = 0.504, p<0.001; r = 0.470, p<0.001, respectively; Figure 2C, Figure 3C).
Figure 2.
IL-6 concentrations in serum and AqH from patients with T2DM and non-diabetic controls. Interleukin-6 (IL-6) was measured with multiplex bead immunoassay in serum (A) and aqueous humor (AqH; B) from controls (n = 51), non-diabetic retinopathy (NDR, n = 47), nonproliferative diabetic retinopathy (NPDR, n = 52), and proliferative diabetic retinopathy (PDR, n = 53). All groups were compared using the Kruskal–Wallis test followed by the Dunn multiple comparison test. C: The relationship between IL-6 in AqH and serum was tested, when matched samples were available, with the Spearman correlation. *p<0.001.
Figure 3.
sIL-6R concentrations in serum and AqH from patients with T2DM and non-diabetic controls. Soluble IL-6R (sIL-6R) was measured with multiplex bead immunoassay in serum (A) and aqueous humor (AqH; B) from controls (n = 51), non-diabetic retinopathy (NDR, n = 47), nonproliferative diabetic retinopathy (NPDR, n = 52), and proliferative diabetic retinopathy (PDR, n = 53). All groups were compared using the Kruskal–Wallis test followed by the Dunn multiple comparison test. The relationship between sIL-6R in the AqH and serum was tested, when matched samples were available, with the Spearman correlation (C). *p<0.001.
The IL-6, sIL-6R, and sgp130 concentrations in patients with DR with and without diabetic macular edema
The sgp130 and sIL-6R concentrations were statistically significantly higher in the serum (p<0.001 and p = 0.038, respectively) and AqH (p = 0.002 and p = 0.022, respectively) of patients with DME compared with patients without DME. The IL-6 concentration in the AqH was statistically significantly higher in the DME group when compared with the non-DME group (p = 0.038). In contrast, the serum IL-6 levels were comparable between the two groups (p = 0.616; Table 3).
Table 3. Cytokine levels in the aqueous humor and serum.
| Cytokine | Aqueous | Serum level | P |
|---|---|---|---|
| IL-6 (pg/ml) |
34.19 (6.25–67.80) |
1.51 (1.03–1.97) |
<0.001* |
| sIL-6R (ng/ml) |
1.11 (0.53–2.47) |
103.99 (85.76–184.41) |
<0.001* |
| sgp130 (ng/ml) | 25.8 (10.12–91.28) | 304.60 (80.10–965.46) | <0.001* |
Data are expressed as median (range).
Correlation analysis
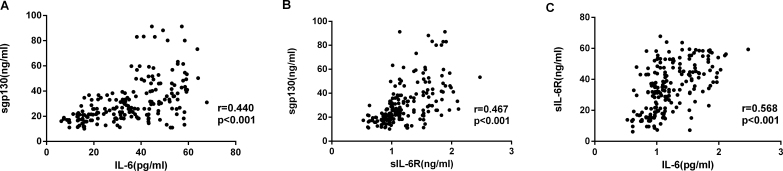
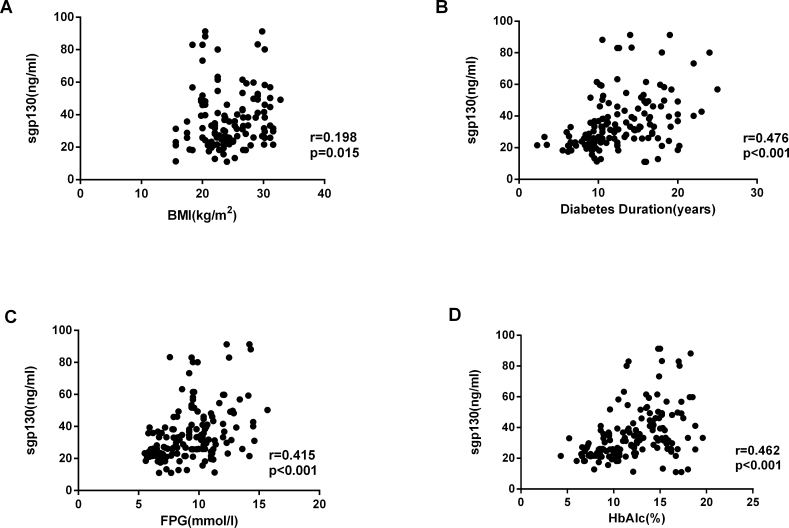
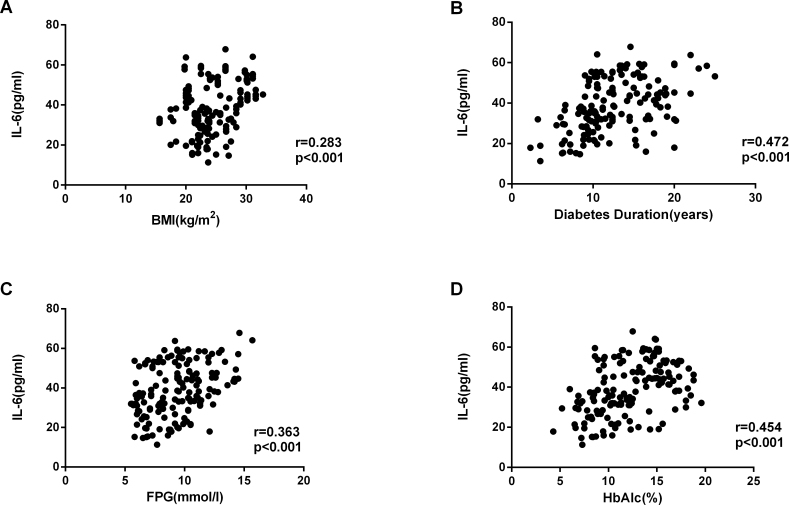
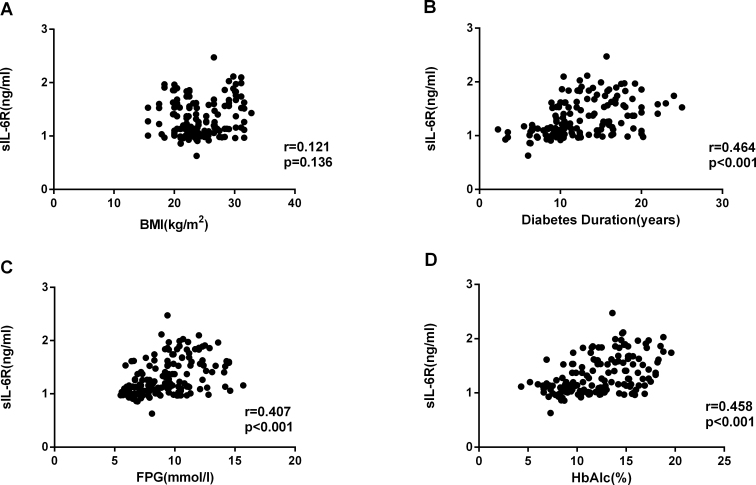
There were statistically significant correlations between the cytokine concentrations (Figure 4), with the strongest association between sIL-6R and sgp130 (Figure 4C: IL-6 versus sIL-6R, r = 0.568, p<0.001; IL-6 versus sgp130, r = 0.440, p<0.001, sIL-6R versus sgp130, r = 0.467, p<0.001). Correlation analysis showed that the diabetes duration, HbA1c level, and fasting blood glucose at the time of surgery were statistically significantly associated with the sgp130, IL-6, and sIL-6R concentrations in the serum and AqH from patients with T2DM (p<0.001; Figure 5, Figure 6, Figure 7).
Figure 4.
IL-6, sIL-6R, and sgp130 concentrations in patients with DR. Aqueous humor (AqH) concentrations were determined by using a multiplex bead-based immunoassay. A: Relationships between interleukin (IL)-6 and soluble gp130 (sgp130) are shown. B: Relationships between IL-6 and soluble IL-6R (sIL-6R) are shown. C: Relationships between sIL-6R and sgp130 are shown. Data were analyzed by using a Spearman’s rank correlation test.
Figure 5.
Correlation analysis showed that sgp130 concentrations in AqH were positively correlated with the BMI (r = 0.198, p = 0.015), duration of diabetes (r = 0.476, p<0.001), FPG (r = 0.415, p<0.001), and HbA1c (r = 0.462, p<0.001) in patients with T2DM. Data were analyzed by using a Spearman’s rank correlation test.
Figure 6.
Correlation analysis showed that IL-6 concentrations in AqH were positively correlated with the BMI (r = 0.283, p<0.001), duration of diabetes (r = 0.472, p<0.001), FPG (r = 0.363, p<0.001), and HbA1c (r = 0.454, p<0.001) in patients with T2DM. Data were analyzed by using a Spearman’s rank correlation test.
Figure 7.
Correlation analysis showed that sIL-6R concentrations in AqH were positively correlated with the BMI (r = 0.121, p = 0.136), duration of diabetes (r = 0.464, p<0.001), FPG (r= 0.407, p<0.001), and HbA1c (r = 0.458, p<0.001) in patients with T2DM. Data were analyzed by using a Spearman’s rank correlation test.
Discussion
In the present study, patients with DR had higher sgp130 concentrations in the serum and AqH compared with the healthy non-diabetic subjects. Higher sIL-6R and IL-6 concentrations were also observed in subjects with DR compared to those in the NDR and control groups. The association between DR and the IL-6, sIL-6R, and sgp130 levels was independent of age and sex. To our knowledge, this is the first study reporting an increase in sIL-6R and sgp130 in subjects with DR.
Elevated sIL-6R can increase the activity of IL-6, lead to greater IL-6/sIL-6R/sgp130 complex formation, and stimulate a greater diversity of cells involved in many inflammatory processes [12,24,25]. The elevated sgp130, sIL-6R, and IL-6 concentrations in DR suggest that IL-6, acting via sIL-6R and sgp130, enhances the trans-signaling as part of the inflammatory response and consequently, may enhance disease activity. It has been suggested that IL-6 trans-signaling is critically involved in the pathophysiology of metabolic disorders, such as T2DM and diabetic nephropathy, by promoting chronic inflammation [26]. A statistically significantly higher plasma level of the IL-6/sIL-6R complex has been shown in patients with T2DM [19], and in addition, consistent with our research, sIL-6R levels were found to correlate with BMI among patients with end-stage renal disease [27]. Elevation of sIL-6R and sgp130 is also associated with endothelial cell (EC) inflammation [28]. Our results suggest the increased IL-6 concentration in patients with DR and that the IL-6 concentration correlated statistically significantly with HbA1c in diabetic patients. Therefore, it can be hypothesized that hyperglycemia stimulates IL-6/sIL-6R/sgp130 complex production in hepatocytes, monocytes, or ECs in patients with DR.
In ophthalmology, the important role of IL-6 and sIL-6R in mediating choroidal neovascularization, experimental uveitis, atopic keratoconjunctivitis, and PDR has been reported [29-32]. In addition, gp130 mRNA expression on the ocular surface was also found to be statistically significantly higher in patients with allergic conjunctivitis [33]. It has been shown that in specific pathophysiological conditions such as inflammation, T cells, ECs, skin fibroblasts, smooth muscle cells, and epithelial cells can be targeted by IL-6 trans-signaling [15,17,34-36].
We observed expression of IL-6, sIL-6R, and sgp130 in the AqH of patients with DR. Retinal cells and the serum component might be sources of IL-6, sIL-6R, and sgp130 in the AqH. In the present study, the AqH level of sIL-6R and sgp130 was found to be related to the presence of DME. Breakdown of the blood–retina barrier and influx of serum proteins are characteristic of the vascular dysfunction observed in the pathophysiology of DR and contributes to vasogenic macular edema. We observed that the sgp130 and sIL-6R concentrations were statistically significantly correlated and both cytokines were found at higher levels in the serum than in the AqH. Therefore, we hypothesize that sIL-6R and sgp130 originate mainly from the serum. These results suggest that the serum is a major component of the sIL-6R and sgp130 observed in the AqH and binding of IL-6 on the surface of proliferating cells may diminish sIL-6R and sgp130 molecules within the AqH. In contrast, the AqH levels of IL-6 were statistically significantly higher than in the serum. It is possible that IL-6 is synthesized locally by resident macrophages, fibroblasts, and infiltrating leukocytes [29,32,37].
These findings indicate a potential link between IL-6 trans-signaling and the development of DR. The IL-6/sIL-6R complexes accumulating in the eye are likely to interact with the gp130-expressing ECs lining the blood vessels. IL-6 trans-signaling not only directly increases vascular permeability but also induces endothelium-derived vascular endothelial growth factor production [38]. Alternatively, it has been demonstrated that sIL-6R may be released from activated leukocytes [10,14]. The sIL-6R released by infiltrating leukocytes would promote further leukocyte recruitment, which is commonly associated with several inflammatory disorders, suggesting that the IL-6/sIL-6R complex may play a positive role in the proinflammatory activation of ECs during the DR process.
Furthermore, it has been reported that a high level of sIL-6R is associated with T-cell abnormalities, such as acquired immunodeficiency syndrome and uveitis [29]. Regarding T2DM, T helper (Th17) lymphocytes play a key role in the pathogenesis of this disease, and studies have demonstrated that production of the Th17 cytokine, IL-17, is elevated in patients with DR [39]. The IL-6/sIL-6R complex may affect Th17 lymphocytes via gp130 and regulate the production of IL-17. Therefore, an increased level of the IL-6/sIL-6R complex in the AqH may induce inflammatory CD4+ Th17 polarization and enhance the inflammatory response. These processes are thought to be related to the exacerbation of the clinical activity of DR.
Finally, the patients with DR showed statistically significant increases not only in IL-6 and sIL-6R but also in sgp130, which acts as a negative regulator of IL-6 trans-signaling. A correlation was observed between the circulating concentrations of sgp130 and sIL-6R, suggesting that these two types of receptors are regulated by the same mechanism in DR. In addition, there was a statistically significant correlation between the sgp130 and IL-6 concentrations in patients with DR. These findings suggest that sgp130 might be involved in regulation of the systemic inflammatory processes associated with classical receptor signaling and the trans-signaling pathway. This is the first study to report elevated sgp130 in patients with DR. Elevated sgp130 concentrations have also been observed in various diseases connected with insulin resistance, including metabolic syndrome [40], polycystic ovary syndrome [41], and preeclampsia [42]. Based on these findings, it can be hypothesized that in the presence of IL-6-mediated chronic low-grade inflammation, increased levels of sgp130 in patients with DR might represent a compensatory mechanism that controls intracellular IL-6 signaling and prevents the activation of the IL-6/IL-6R pathway [43,44].
Data from this study demonstrate that during the course of DR, serum and AqH sgp130 and sIL-6R concentrations increase concurrently with IL-6. As sgp130 might prevent activation of the IL-6/sIL-6R pathway, sgp130 may be of benefit as a therapeutic agent for the treatment of DR. However, there are some limitations. As this is a cross-sectional study, we cannot define cause and effect relationships. In addition, the sample size in this study was small, and further studies with larger sample sizes are warranted. Further research is also needed to determine the sources of upregulation in the AqH concentrations of IL-6, sIL-6R, and sgp130 and to underscore the mechanism of the IL-6/IL-6R/sgp130 complex in the processes of DR.
Acknowledgments
This study was supported by the Fund for National Natural Science Foundation (81300784). The sponsor of the study had no role in the design of the original study protocol, data collection, data analysis, data interpretation, writing of the report, or decision to submit the manuscript for publication. No conflicting relationship exists for any author.
References
- 1.Tang J, Kern TS. Inflammation in diabetic retinopathy. Prog Retin Eye Res. 2011;30:343–58. doi: 10.1016/j.preteyeres.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Durham JT, Herman IM. Microvascular modifications in diabetic retinopathy. Curr Diab Rep. 2011;11:253–64. doi: 10.1007/s11892-011-0204-0. [DOI] [PubMed] [Google Scholar]
- 3.Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund J, Haffner S, Hamman RF, Ikram MK, Kayama T, Klein BE, Klein R, Krishnaiah S, Mayurasakorn K, O'Hare JP, Orchard TJ, Porta M, Rema M, Roy MS, Sharma T, Shaw J, Taylor H, Tielsch JM, Varma R, Wang JJ, Wang N, West S, Xu L, Yasuda M, Zhang X, Mitchell P, Wong TY. Meta-Analysis for Eye Disease Study G. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–64. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Semeraro F, Cancarini A, dell'Omo R, Rezzola S, Romano MR, Costagliola C. Diabetic Retinopathy. 2015;xxx:582060. doi: 10.1155/2015/582060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adamis AP, Berman AJ. Immunological mechanisms in the pathogenesis of diabetic retinopathy. Semin Immunopathol. 2008;30:65–84. doi: 10.1007/s00281-008-0111-x. [DOI] [PubMed] [Google Scholar]
- 6.Gustavsson C, Agardh CD, Agardh E. Profile of intraocular tumour necrosis factor-alpha and interleukin-6 in diabetic subjects with different degrees of diabetic retinopathy. Acta Ophthalmol (Copenh) 2013;91:445–52. doi: 10.1111/j.1755-3768.2012.02430.x. [DOI] [PubMed] [Google Scholar]
- 7.Kowluru RA, Odenbach S. Role of interleukin-1beta in the pathogenesis of diabetic retinopathy. Br J Ophthalmol. 2004;88:1343–7. doi: 10.1136/bjo.2003.038133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong N, Xu B, Wang B, Chu L. Study of 27 aqueous humor cytokines in patients with type 2 diabetes with or without retinopathy. Mol Vis. 2013;19:1734–46. [PMC free article] [PubMed] [Google Scholar]
- 9.Hirano T, Akira S, Taga T, Kishimoto T. Biological and clinical aspects of interleukin 6. Immunol Today. 1990;11:443–9. doi: 10.1016/0167-5699(90)90173-7. [DOI] [PubMed] [Google Scholar]
- 10.Rose-John S, Scheller J, Elson G, Jones SA. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. J Leukoc Biol. 2006;80:227–36. doi: 10.1189/jlb.1105674. [DOI] [PubMed] [Google Scholar]
- 11.Maggio M, Guralnik JM, Longo DL, Ferrucci L. Interleukin-6 in aging and chronic disease: a magnificent pathway. J Gerontol A Biol Sci Med Sci. 2006;61:575–84. doi: 10.1093/gerona/61.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones SA, Horiuchi S, Topley N, Yamamoto N, Fuller GM. The soluble interleukin 6 receptor: mechanisms of production and implications in disease. FASEB J. 2001;15:43–58. doi: 10.1096/fj.99-1003rev. [DOI] [PubMed] [Google Scholar]
- 13.Jostock T, Mullberg J, Ozbek S, Atreya R, Blinn G, Voltz N, Fischer M, Neurath MF, Rose-John S. Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. Eur J Biochem. 2001;268:160–7. doi: 10.1046/j.1432-1327.2001.01867.x. [DOI] [PubMed] [Google Scholar]
- 14.Hurst SM, Wilkinson TS, McLoughlin RM, Jones S, Horiuchi S, Yamamoto N, Rose-John S, Fuller GM, Topley N, Jones SA. Il-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity. 2001;14:705–14. doi: 10.1016/s1074-7613(01)00151-0. [DOI] [PubMed] [Google Scholar]
- 15.Scheller J, Ohnesorge N, Rose-John S. Interleukin-6 trans-signalling in chronic inflammation and cancer. Scand J Immunol. 2006;63:321–9. doi: 10.1111/j.1365-3083.2006.01750.x. [DOI] [PubMed] [Google Scholar]
- 16.Taga T, Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol. 1997;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]
- 17.Atreya R, Mudter J, Finotto S, Mullberg J, Jostock T, Wirtz S, Schutz M, Bartsch B, Holtmann M, Becker C, Strand D, Czaja J, Schlaak JF, Lehr HA, Autschbach F, Schurmann G, Nishimoto N, Yoshizaki K, Ito H, Kishimoto T, Galle PR, Rose-John S, Neurath MF. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nat Med. 2000;6:583–8. doi: 10.1038/75068. [DOI] [PubMed] [Google Scholar]
- 18.Klimiuk PA, Sierakowski S, Latosiewicz R, Cylwik JP, Cylwik B, Skowronski J, Chwiecko J. Interleukin-6, soluble interleukin-2 receptor and soluble interleukin-6 receptor in the sera of patients with different histological patterns of rheumatoid synovitis. Clin Exp Rheumatol. 2003;21:63–9. [PubMed] [Google Scholar]
- 19.Kado S, Nagase T, Nagata N. Circulating levels of interleukin-6, its soluble receptor and interleukin-6/interleukin-6 receptor complexes in patients with type 2 diabetes mellitus. Acta Diabetol. 1999;36:67–72. doi: 10.1007/s005920050147. [DOI] [PubMed] [Google Scholar]
- 20.Papanicolaou DA, Wilder RL, Manolagas SC, Chrousos GP. The pathophysiologic roles of interleukin-6 in human disease. Ann Intern Med. 1998;128:127–37. doi: 10.7326/0003-4819-128-2-199801150-00009. [DOI] [PubMed] [Google Scholar]
- 21.Curnow SJ, Scheel-Toellner D, Jenkinson W, Raza K, Durrani OM, Faint JM, Rauz S, Wloka K, Pilling D, Rose-John S, Buckley CD, Murray PI, Salmon M. Inhibition of T cell apoptosis in the aqueous humor of patients with uveitis by IL-6/soluble IL-6 receptor trans-signaling. J Immunol. 2004;173:5290–7. doi: 10.4049/jimmunol.173.8.5290. [DOI] [PubMed] [Google Scholar]
- 22.Chamberlain JJ, Rhinehart AS, Shaefer CF, Jr, Neuman A. Diagnosis and Management of Diabetes: Synopsis of the 2016 American Diabetes Association Standards of Medical Care in Diabetes. Ann Intern Med. 2016;164:542–52. doi: 10.7326/M15-3016. [DOI] [PubMed] [Google Scholar]
- 23.Expert Committee on the D Classification of Diabetes M. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26(Suppl 1):S5–20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 24.Schaper F, Rose-John S. Interleukin-6: Biology, signaling and strategies of blockade. Cytokine Growth Factor Rev. 2015;26:475–87. doi: 10.1016/j.cytogfr.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Rotter V, Nagaev I, Smith U. Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J Biol Chem. 2003;278:45777–84. doi: 10.1074/jbc.M301977200. [DOI] [PubMed] [Google Scholar]
- 26.Wang H, Zhang Z, Chu W, Hale T, Cooper JJ, Elbein SC. Molecular screening and association analyses of the interleukin 6 receptor gene variants with type 2 diabetes, diabetic nephropathy, and insulin sensitivity. J Clin Endocrinol Metab. 2005;90:1123–9. doi: 10.1210/jc.2004-1606. [DOI] [PubMed] [Google Scholar]
- 27.Pecoits-Filho R, Barany P, Lindholm B, Heimburger O, Stenvinkel P. Interleukin-6 is an independent predictor of mortality in patients starting dialysis treatment. Nephrol Dial Transplant. 2002;17:1684–8. doi: 10.1093/ndt/17.9.1684. [DOI] [PubMed] [Google Scholar]
- 28.Weiss TW, Arnesen H, Seljeflot I. Components of the interleukin-6 transsignalling system are associated with the metabolic syndrome, endothelial dysfunction and arterial stiffness. Metabolism. 2013;62:1008–13. doi: 10.1016/j.metabol.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 29.Simon D, Denniston AK, Tomlins PJ, Wallace GR, Rauz S, Salmon M, Murray PI, Curnow SJ. Soluble gp130, an antagonist of IL-6 transsignaling, is elevated in uveitis aqueous humor. Invest Ophthalmol Vis Sci. 2008;49:3988–91. doi: 10.1167/iovs.08-1953. [DOI] [PubMed] [Google Scholar]
- 30.Izumi-Nagai K, Nagai N, Ozawa Y, Mihara M, Ohsugi Y, Kurihara T, Koto T, Satofuka S, Inoue M, Tsubota K, Okano H, Oike Y, Ishida S. Interleukin-6 receptor-mediated activation of signal transducer and activator of transcription-3 (STAT3) promotes choroidal neovascularization. Am J Pathol. 2007;170:2149–58. doi: 10.2353/ajpath.2007.061018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sugaya S, Sakimoto T, Shoji J, Sawa M. Regulation of soluble interleukin-6 (IL-6) receptor release from corneal epithelial cells and its role in the ocular surface. Jpn J Ophthalmol. 2011;55:277–82. doi: 10.1007/s10384-011-0002-x. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto H, Hayashi H, Uchida H, Kato H, Oshima K. Increased soluble interleukin-6 receptor in vitreous fluid of proliferative vitreoretinopathy. Curr Eye Res. 2003;26:9–14. doi: 10.1076/ceyr.26.1.9.14251. [DOI] [PubMed] [Google Scholar]
- 33.Shoji J, Kawaguchi A, Gotoh A, Inada N, Sawa M. Concentration of soluble interleukin-6 receptors in tears of allergic conjunctival disease patients. Jpn J Ophthalmol. 2007;51:332–7. doi: 10.1007/s10384-007-0461-2. [DOI] [PubMed] [Google Scholar]
- 34.Hashizume M, Hayakawa N, Suzuki M, Mihara M. IL-6/sIL-6R trans-signalling, but not TNF-alpha induced angiogenesis in a HUVEC and synovial cell co-culture system. Rheumatol Int. 2009;29:1449–54. doi: 10.1007/s00296-009-0885-8. [DOI] [PubMed] [Google Scholar]
- 35.Wang XP, Schunck M, Kallen KJ, Neumann C, Trautwein C, Rose-John S, Proksch E. The interleukin-6 cytokine system regulates epidermal permeability barrier homeostasis. J Invest Dermatol. 2004;123:124–31. doi: 10.1111/j.0022-202X.2004.22736.x. [DOI] [PubMed] [Google Scholar]
- 36.Matsumoto S, Hara T, Mitsuyama K, Yamamoto M, Tsuruta O, Sata M, Scheller J, Rose-John S, Kado S, Takada T. Essential roles of IL-6 trans-signaling in colonic epithelial cells, induced by the IL-6/soluble-IL-6 receptor derived from lamina propria macrophages, on the development of colitis-associated premalignant cancer in a murine model. J Immunol. 2010;184:1543–51. doi: 10.4049/jimmunol.0801217. [DOI] [PubMed] [Google Scholar]
- 37.Qu D, Liu J, Lau CW, Huang Y. IL-6 in diabetes and cardiovascular complications. Br J Pharmacol. 2014;171:3595–603. doi: 10.1111/bph.12713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakahara H, Song J, Sugimoto M, Hagihara K, Kishimoto T, Yoshizaki K, Nishimoto N. Anti-interleukin-6 receptor antibody therapy reduces vascular endothelial growth factor production in rheumatoid arthritis. Arthritis Rheum. 2003;48:1521–9. doi: 10.1002/art.11143. [DOI] [PubMed] [Google Scholar]
- 39.Xu H, Cai M, Zhang X. Effect of the blockade of the IL-23-Th17-IL-17A pathway on streptozotocin-induced diabetic retinopathy in rats. Graefe's archive for clinical and experimental ophthalmology = Albrecht Von Graefes Arch Klin Exp Ophthalmol. 2015;253:1485–92. doi: 10.1007/s00417-014-2842-9. [DOI] [PubMed] [Google Scholar]
- 40.Zuliani G, Galvani M, Maggio M, Volpato S, Bandinelli S, Corsi AM, Lauretani F, Cherubini A, Guralnik JM, Fellin R, Ferrucci L. Plasma soluble gp130 levels are increased in older subjects with metabolic syndrome. The role of insulin resistance. Atherosclerosis. 2010;213:319–24. doi: 10.1016/j.atherosclerosis.2010.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nikolajuk A, Kowalska I, Karczewska-Kupczewska M, Adamska A, Otziomek E, Wolczynski S, Kinalska I, Gorska M, Straczkowski M. Serum soluble glycoprotein 130 concentration is inversely related to insulin sensitivity in women with polycystic ovary syndrome. Diabetes. 2010;59:1026–9. doi: 10.2337/db09-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Z, Gao Y, Zhang L, Jia L, Wang P, Zhang L, Li H. Alterations of IL-6, IL-6R and gp130 in early and late onset severe preeclampsia. Hypertens Pregnancy. 2013;32:270–80. doi: 10.3109/10641955.2013.798332. [DOI] [PubMed] [Google Scholar]
- 43.Rebouissou C, Wijdenes J, Autissier P, Tarte K, Costes V, Liautard J, Rossi JF, Brochier J, Klein B. A gp130 interleukin-6 transducer-dependent SCID model of human multiple myeloma. Blood. 1998;91:4727–37. [PubMed] [Google Scholar]
- 44.Nishimoto N, Kishimoto T, Yoshizaki K. Anti-interleukin 6 receptor antibody treatment in rheumatic disease. Ann Rheum Dis. 2000;59(Suppl 1):i21–7. doi: 10.1136/ard.59.suppl_1.i21. [DOI] [PMC free article] [PubMed] [Google Scholar]