Abstract
Glucosylceramide (GL-1) level in human has been considered as a surrogate biomarker for enzyme replacement and substrate reduction therapies (ERT and SRT) for Gaucher and Fabry patients. Due to the high endogenous level of GL-1 in human plasma, it is difficult to achieve the analytical sensitivity of plasma GL-1 below the normal endogenous level (1.7 μg/mL to 6.6 μg/mL) when using the standard addition method and regular plasma matrix for standard curve. A high sensitivity plasma GL-1 assay with LLOQ at 0.1 μg/mL was developed and validated using delipidized plasma so that patient plasma concentrations that are below normal reference range can be measured accurately. The normal reference range was established from 120 healthy donors using this developed new method. Twenty-three Fabry patient plasma samples including baseline and post-investigation drug treatment samples were measured. All post-treatment samples showed GL-1 concentration below 2.0 μg/mL, indicating the utility of the reported high sensitivity assay using delipidized plasma for monitoring the plasma GL-1 biomarker level in patients.
Keywords: Glucosylceramide, Globotriaosylceramide, Liquid chromatography–tandem mass spectrometry (LC/MS/MS), Delipidized plasma, Sensitivity, Standard addition method
1. Introduction
Gaucher disease and Fabry disease are lysosomal storage disorders. The two diseases are caused by deficiency of the catabolic enzyme β-glucocerebrosidase and α-galactosidase A (EC 3.2.1.22) [1], respectively. Both enzyme deficiency leads to a systematic accumulation of glucocerebroside (GL-1, also known as glucosylceramide) and globotriasylceramide (GL-3, also known as ceramide trihexoside, CTH, or Gb3) in blood and various organ tissues. These metabolic defect causes a painful neuropathy, angiokertomas, cardiac and renal failure, and cerebrovascular injury [2], [3], [4], [5], [6].
In the Fabry SRT clinical trial, very low plasma GL-1 levels were observed post treatment in patients, suggesting a 20-time more sensitive plasma GL-1 analytical method than the original plasma method with LLOQ of 2.0 μg/mL is needed. In the article, we reported a high sensitivity GL-1 assay using delipidized human plasma as the surrogate matrix of standards. Normal human plasma GL-1 reference range (n = 120) was established using this high sensitivity GL-1 assay. Twenty-three Fabry patient samples were analyzed and results showed the assay suitable for monitoring plasma GL-1 levels at low concentration.
2. Materials and methods
Glucosylceramide (GL-1) and Internal Standard (IS), N-C18:0 galactosylceramide-D35, (perdeuterated, C18:0 fatty acid), were purchased from Matreya (Pleasant Gap, PA). Delipidized plasma/serum was purchased from Golden West Biologicals, Inc. (Temecula, CA). Human plasma is delipidized using silica to remove the lipids. The product is then depth filtered, diafiltered, and 0.2 um filtered and filled. The sample extraction method was modified from the previous GL-1 method published in our laboratory but used delipidized human plasma as standard curve matrix [7]. Briefly, 50 μL plasma in a 96-wel plate was extracted with chloroform: methanol solution (2:1, v/v) containing internal standard. The bottom organic phase was transferred, evaporated, and reconstituted with chloroform. The extracted GL-1 in chloroform was then loaded onto a C18 based solid phase extraction cartridge (LiChrolut RP-18, 500 mg, Merck, VWR #: 48219-244), washed with chloroform, and eluted with acetone:methanol solvent (9:1, v/v). The final eluent was then dried and reconstituted with the mobile phase for LC/MS/MS analysis. The LC/MS/MS condition was the same as the GL-1 method reported [7]. Chromatographic separation was achieved using a hydrophilic interaction liquid chromatography (HILIC) column at 40 °C (XBridge™ HILIC column, 3.5 μm, 2.1 × 50 mm, Waters Corporation, Milford, MA) with an isocratic mobile phase of 95% Methanol with 2 mM Ammonium Acetate and 0.1% Formic Acid on a Waters Acquity UPLC system. The triple quadrupole Sciex API-4000 mass spectrometer was operated at positive electrospray ionization mode in multiple reaction monitoring (MRM) mode. The 7 isoforms and IS were monitored at below MS/MS transitions: 700.8 → 520.7 for C16:0 GL-1; 729.3 → 549.8 for C18:0 GL-1; 756.9 → 576.9 for C20:0 GL-1; 785.1 → 604.9 for C22:0 GL-1; 798.9 → 618.9 for C23:0 GL-1; 811.0 → 631.1 for C24:1 GL-1; 812.9 → 632.8 for C24:0 GL-1; 764.0 → 583.9 for IS N-C18:0 galactosylceramide-d35. The assay linear range was from 0.1 to 20 μg/mL. The ratio of the total peak area of each GL-1 isoform to peak area of internal standard was plotted against GL-1 concentration by MultiQuant™ software. Twenty three K2EDTA human plasma samples were obtained from 4 Fabry patients who received an investigational substrate reduction therapy drug treatment from Week 0 to Week 18. Patient informed consent for biomarker plasma GL-1 was provided.
3. Results and discussions
This high sensitivity GL-1 assay using delipidized plasma was validated. The results demonstrated that the method was precise and accurate, and met bioanalytical analysis industrial acceptance criteria. The linear range of the method was 0.1 to 20 μg/mL and extraction recovery was 68.4% for GL-1 and 73.2% for IS, respectively. In original normal plasma method, the recovery for GL-1 and IS was 84.1% and 69.1%, respectively. The slope comparison of standard curve in regular human plasma and delipidized human plasma showed a very similar slope, which demonstrated the delipdized plasma can replace human plasma and eliminated the interference of the endogenous GL-1 level in the blank matrix. In a side by side comparison experiment, the slope is 0.79893 for normal plasma and 0.80936 for delipidized plasma. The difference is 1.3%. Also, all 7 lots of delipidized plasma have consistent slopes, with %CV of 1.9%. The relative matrix effect was evaluated using one lot of delipidized plasma and human plasma from six individual healthy donors. The matrix effect precision (% CV) and accuracy (% Bias) were acceptable for both individual normal plasma and delipidized plasma pool.
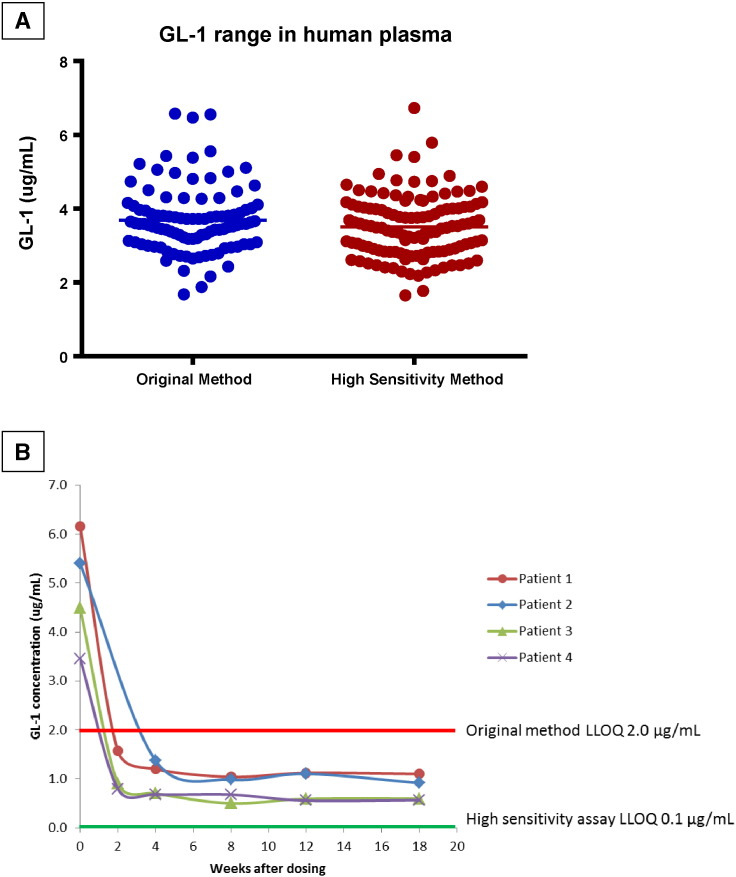
The normal reference range of plasma GL-1 was evaluated and varied from 1.7 to 6.7 μg/mL (n = 120) using the high sensitivity method, and from 1.7 μg/mL to 6.6 μg/mL (n = 100) using regular plasma GL-1 method (Fig. 1A). These data showed two methods gave very similar reference ranges and were in agreement with literature [2], in which GL-1 levels were determined in plasma samples obtained from 28 human healthy subjects through liquid-liquid extraction followed by LC/MS/MS analysis. For the 4 patients in the Fabry SRT trial week 0 to week 18, 19 out of 23 patient samples showed GL-1 concentrations below 2.0 μg/mL. As shown in Fig. 1B, all four patients had GL-1 concentration dropped below 2 μg/mL at 4 weeks post treatment in SRT trial.
Fig. 1.
(A) Comparison of high sensitivity GL-1 assay (n = 120) vs. current human plasma assay (n = 100) using different normal plasma samples (B) Patient GL-1 Concentration after Dosing in SRT Trial.
Standard addition approach is commonly used to quantify biomarkers that contain high endogenous level in the human samples [8], [9], [10] in order to minimize matrix effect difference between standard curve and patient samples. However, it is technically challenging to develop a method with an LLOQ less than the normal reference range of the endogenous analyte present in the blank plasma (standard zero). Alternatively, isotope labeled analyte can be used for standard curve preparation [11]. However, obtaining stable labeled total GL-1 could be costly. Delipidized plasma without endogenous GL-1 level is a better choice for standard curve matrix to enhance the sensitivity. The surrogate matrices should mimic regular plasma characteristics during extraction. The surrogate matrices like human serum albumin in phosphate buffer saline, as well as artificial plasma were tried and showed significant difference in slopes comparing to regular human plasma. The delipidized plasma mimicked regular human plasma in the current reported method, which produced a similar slope as measured in regular human plasma for GL-1 analysis.
In summary, the high sensitivity GL-1 assay was successfully developed and validated for its intended use to measure GL-1 in human plasma samples. Use of delipidized plasma as the blank matrix provided a much more sensitive LLOQ for monitoring GL-1 decrease in Fabry patients during an investigational substrate reduction therapy clinical trial.
Acknowledgements
We thank Lynn Callison for participation in the assay validation, Brian Weir for the samples testing, and William Chen, who developed and validated the original plasma GL-1 method in 2005.
References
- 1.Ferraz M., Kallemeijn W., Mirzaian M., Moro D., Marques A., Wisse P., Boot R., Willems L., Overkleeft H., Aerts J. Gaucher disease and Fabry disease: new markers and insights in pathophysiology for two distinct glycoshphingolipdoses. Biochim. Biophys. Acta. 2014;1841:811–825. doi: 10.1016/j.bbalip.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Dekker N., van Dussen L., Hollak C.E.M., Overkleeft H., Scheij S., Ghauharali K., van Breemen M.J., Ferraz M.J., Groener J.E.M., Maas M., Wijburg F.A., Speijer D., Tylki-Szymanska A., Mistry P.K., Boot R.G., Aerts J.M. Elevated plasma glucosylsphingosine in Gaucher disease: relation to phenotype, storage cell markers, and therapeutic response. Blood. 2011;118:118–127. doi: 10.1182/blood-2011-05-352971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marshall J., McEachern K., Chuang W., Hutto E., Siegel C., Shayman J., Graboweski G., Scheule R., Copeland D., Cheng S. Improved management of lysosomal glucosylceramide levels in a mouse model of type 1 Gaucher disease using enzyme and substrate reduction therapy. J. Inherit. Metab. Dis. 2010;33:281–289. doi: 10.1007/s10545-010-9072-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muller M., Petry A., Vianna L., Breier A., Michelin-Tirelli K., Pires R., Trindade V., Cohelho J. Quantification of glucosylceramide in plasma of Gaucher disease patients. Brazilian J. Pharmaceutical Sci. 2010;46:644–649. [Google Scholar]
- 5.Roddy T.P., Nelson B.C., Sung C.C., Araghi S., Wilkens D., Zhang X.K., Thomas J.J., Richards S.M. Liquid Chromatograhy-tandem mass spectrometry quantification of globotriaosylceramide in plasma for long-term monitoring of Fabry patients treated with enzyme replacement therapy. Clin. Chem. 2005;51:237–240. doi: 10.1373/clinchem.2004.038323. [DOI] [PubMed] [Google Scholar]
- 6.Auray-Blais C., Cyr D., Ntwari A., West M.L., Cox-Brinkman J., Bichet D.G., Germain D.P., Laframboise R., Melancon S.B., Stockley T., Clarke J.T.R., Drouin R. Urinary globotriaosylceramide excretion correlates with the genotype in children and adults with Fabry disease. Mol. Genet. Metab. 2008;93:331–340. doi: 10.1016/j.ymgme.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Ji A., Wang H., Ziso-Qejvanaj E., Zheng K., Chung L.L., Foley T., Chuang W., Richards S., Sung C. A novel approach for quantitation of glucosylceramide in human dried blood spot using LC-MS/MS. Bioanalysis. 2015;7(12):1483–1496. doi: 10.4155/bio.15.77. [DOI] [PubMed] [Google Scholar]
- 8.Groener J.E.M., Poorthuis B.J.H.M., Kuiper S., Helmond M.T.J., Hollak C.E.M., Aerts J.M.F.G. HPLC for simultaneous quantification of total ceramide, glucosylceramide, and ceramide, trihexoside concentrations in plasma. Clin. Chem. 2007;53:742–747. doi: 10.1373/clinchem.2006.079012. [DOI] [PubMed] [Google Scholar]
- 9.Domínguez A., Jarne C., Cebolla V.L., Galbán J., Savirón M., Orduna J., Membrado L., Lapieza M.-P., Romero E., Vicente I.S., de Marcos S., Garriga R. A hyphenated technique based on high-performance thin layer chromatography for determining neutral sphingolipids: a proof of concept. Chromatographia. 2015;2:167–187. [Google Scholar]
- 10.Scherer M., Leuthäuser-Jaschinski K., Ecker J., Schmitz G., Liebisch G. A rapid and quantitative LC-MS/MS method to profile sphingolipids. J. Lipid Res. 2010;51:2001–2011. doi: 10.1194/jlr.D005322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mirzaian M., Wisse P., Ferraz M., Gold H., Donker-Koopman W., Verhoek M., Overkleeft H., Boot R., Kramer G., Dekker N., Averts J. Mass spectrometric quantification of glucolsylsphingosine in plasma and urine of type 1 Gaucher patients using an isotope standard. Blood Cell Mol. Dis. 2015;54:307–314. doi: 10.1016/j.bcmd.2015.01.006. [DOI] [PubMed] [Google Scholar]