Abstract
Previous studies suggest that insulin-sensitizing agents could play a significant role in the treatment of major depression, particularly depression in patients with documented insulin resistance or those who are resistant to standard psychopharmacological approaches. This study aimed to assess the effects on depressive symptoms with adjuvant treatment with the PPARγ-agonist pioglitazone. Patients (N=37) with non-psychotic, non-remitting depression receiving standard psychiatric regimens for depression were randomized across an insulin sensitivity spectrum in a 12-week double blind, randomized controlled trial of pioglitazone or placebo. Improvement in depression was associated with improvement in glucose metabolism but only in patients with insulin resistance. An age effect was also shown in that response to pioglitazone was more beneficial in younger aged patients. Study findings suggest differential improvement in depression severity according to both glucose metabolic status and level of depression at baseline. A greater understanding of the reciprocal links between depression and IR may lead to a dramatic shift in the way in which depression is conceptualized and treated, with a greater focus on treating and/or preventing metabolic dysfunction.
Keywords: depressive disorders, insulin resistance, PPAR-γ agonist, antidepressant response, treatment resistant, clinical trial
1. Introduction
While the association between insulin resistance (IR) and depressive symptoms is well documented (Gold et al., 2005), causal aspects of the relationship are incompletely documented and likely bidirectional. As the current prevalence rates of DM2 and related diseases grow worldwide and its associated metabolic consequences become more salient, it is increasingly critical to understand the role of IR in depressive disorders.
We previously proposed that treatment of depressive disorders results in reversal of IR (Rasgon et al., 2010) and recently reported pilot work demonstrating significant improvement in depression severity upon open-label add-on of a PPAR-gamma-agonist to treatment as usual (TAU) (Rasgon et al., 2010). There are two main potential mechanisms by which PPAR-γ agonists may improve depressive symptoms: insulin-sensitization (Rasgon et al., 2010) and anti-inflammation (Kemp et al., 2014).
A greater understanding of the reciprocal links between depression and IR may lead to a dramatic shift in the way in which depression is conceptualized and treated, with a greater focus on treating and/or preventing metabolic dysfunction. To date, studies evaluating IR in patients with depressive disorders before and after treatment have reported a decrease in IR upon improvement in mood (Heninger, et al., 1975). These studies reported that IR normalizes in patients who respond to antidepressant treatment compared to non-responders who show no significant improvement in IR (Nathan et al., 1981; Boyd et al., 1985; Okamura et al., 2000). Specifically, selective serotonin reuptake inhibitors (SSRIs) appear to improve insulin sensitivity (Goodnick, 2001), with improvement in glucose tolerance generally observed in treatment responders compared with non-responders.
While only a few studies explored the potential mood-modifying effects of insulin-sensitizing agents, all showed improvement in depressive symptoms (Rasgon et al., 2010a; Rasgon et al., 2010b; Sepanjnia et al., 2012). This suggests that insulin-sensitizing agents could play a significant role in the treatment of major depression, particularly depression in patients with documented IR or those who are resistant to standard psychopharmacological approaches.
Insulin has been shown to alter central nervous system (CNS) concentrations of neurotransmitters such as dopamine (Lozovsky et al., 1981) and norepinephrine (Boyd et al., 1985), by a variety of mechanisms, as well as to have direct electrophysiological effects on neuronal activity (Boyd et al., 1985). Additionally, induced IR in the CNS has been shown to result in cognitive and behavioral alterations in animal models (Kovacs and Hajnal, 2009).
Accordingly, when depression manifests as a sequela of metabolic disorders such as obesity and DM2, it is hypothesized to be associated with resistance of CNS structures to the effects of insulin, which may derive from genetic polymorphisms, as well as from long-term exposure to excess amounts of circulating insulin due to peripheral IR (Okamura et al., 2000). Thus, “overcoming” central IR," for example by pharmacological interventions, could be an attractive strategy in the treatment and prevention of these disorders.
This study aimed to assess mood effects in a 12-week double-blind, randomized-controlled trial of adjuvant treatment with the PPARγ-agonist pioglitazone, an insulin-sensitizing agent, compared with treatment with placebo, in participants with non-psychotic, non-remitting depression receiving standard psychiatric regimens for unipolar or bipolar depression. Pioglitazone is an FDA-approved, insulin-sensitizing treatment for DM2 and has particularly beneficial effects on lipid profile (Goldberg et al., 2005) and rate of cardiovascular events (Lincoff et al., 2007) in this population. Furthermore, in assessing the utility of an additive insulin-sensitizing agent on mood outcomes in both insulin sensitive and insulin resistant patients, this study attempted to disentangle the role of insulin- sensitizing and anti-inflammatory mechanisms.
In an a prior manner, this study’s aims were twofold: 1) To assess whether treatment with pioglitazone would result in significantly greater mood improvement compared to treatment with placebo among patients with unremitted depression despite treatment as usual (TAU) and 2) To examine mechanisms of proposed effects of a PPARγ-agonist on mood outcomes by comparing treatment outcomes based on surrogate markers of glucose metabolic status (hereafter referred to IR and IS) between IR and insulin sensitive (IS) participants.
2. Methods and materials
The Stanford University Institutional Review Board approved the current study in its entirety. All participants provided informed consent prior to study enrollment after they received detailed information regarding all study procedures, potential side effects of study medication, risks and benefits of participation, and contact personnel in case of questions or concerns. Study were participants were recruited on an rolling basis from November 2011 to April 2014 through collaboration with established investigators in the area of adult depression within the Department of Psychiatry & Behavioral Sciences at Stanford University, as well as community health providers. In addition, participants were recruited through advertisements in local newspapers and clinical trial recruitment websites.
Inclusion criteria included being between 21 and 75 years old, having a body mass index (BMI) of 18.5 to 40 kg/m2, having at least 12 years of education, having a history of depression with at least 8 weeks of stable TAU for depression. We did not exclude subjects on a basis of a type of medication(s) used in treatment of depression, as our primary goal was to evaluate antidepressant qualities of adjunctive Pioglitazone.
Exclusion criteria included a history of liver dysfunction, electroconvulsive therapy (ECT) within the previous 6 months, diagnosis of possible or probable any dementia or evidence of cognitive decline, history of Type I or Type II diabetes, history of significant CVD or myocardial infarction, cerebrovascular, pulmonary disease, or cancer, untreated hypothyroidism, unstable or untreated hypertension, known osteoporosis or prior history of non-traumatic fracture, history of a neurological disorder or evidence of neurologic or other physical illness that could produce cognitive deterioration.
Forty-two medically stable men and women ages 23–71 with non-psychotic, non-remitted depression were enrolled. Thirty-seven participants completed the study. Among enrolled participants, glucose metabolism ranged across the insulin sensitivity spectrum, including IS, IR, and/or pre- diabetes
2.1 RCT design and randomization
This randomized controlled trail consisted of a parallel design in which 50% of participants were allocated to 12 weeks of treatment with 30 mg/day of Pioglitazone and 50% of participants were randomized to 12 weeks treatment with placebo pill. Random allocation was generated by use of a random number generator that assigned half of subjects’ identification numbers to each study condition. Randomization and maintenance of the unblinded study list was performed by a staff member of the Stanford University Department of Psychiatry who did not participate in the implementation of the study. Active and placebo medication were bottled and labeled with participant identification numbers by this unblinded staff person according to randomized assignment. Study investigators, coordinators, raters and clinical laboratory staff remained blinded to subject treatment assignments throughout their participation in the study.
2.2 Clinical assessment
Clinical assessment consisted of a physical examination and laboratory tests, including measures of height and weight, an oral glucose tolerance test (OGTT), fasting plasma glucose (FPG), and fasting plasma insulin (FPI). Other data collected included current medications and family medical history. All clinical and laboratory tests were repeated at the end-of-treatment (Week 12) with the exception of the genetic sample.
During baseline examination, insulin resistant status was determined if participants met one of the following criteria. Otherwise they were considered to be insulin sensitive:
Fasting plasma glucose > 100 mg/dL;
Fasting plasma insulin > 15ulU/ml;
Oral Glucose Tolerance Test at 120 minutes > 140 mg/dL.
2.2.1. Oral glucose tolerance test
The OGTT measures individual ability to metabolize glucose and can distinguish between normal patterns and the patterns of diabetes and IR (Mueller et al., 1969). We chose this test over other direct and surrogate measures of IR, such as the insulin suppression test or the hyperinsulinemic euglycemic clamp, because of its relatively easy administration and participant tolerance. Furthermore, it does not involve administration of pharmacological agents, as do other assessments of glucose or insulin tolerance. Participants undergoing OGTT began in a fasting state (no food or drink except water for >10–16 hours). Blood samples were obtained to measure baseline glucose and insulin concentrations, followed by administration of 75mg oral glucose, after which additional samples were obtained at +30, +60, +90, and +120 minutes. A meal was given to each participant immediately following completion of the procedure.
Surrogate markers of IR were categorized according to having one or more of the following: 1) FPG greater than or equal to 100 mg/dL, 2) An OGTT at 120 minutes greater than or equal to 140 mg/dL, 3) FPI greater than or equal to 15 mIU/L, or 4) HOMA-IR greater than 3.8 mmol/L.
2.3 Psychiatric assessment
The psychiatric examination at screening included the Structured Clinical Interview for DSM-IV (SCID) (First et al., 1997), the 17-item Hamilton Depression Rating Scale (HDRS-21) (Hamilton, 1960), and the MMSE (Folstein et al., 1975). The HDRS-21 was used to screen for unremitted depression, as characterized by a score of >7. Administration of the HDRS-21 was repeated at each interim visit (Weeks 2, 4, 6, and 8) and at the end-of-treatment (Week 12). They were also questioned at each interval regarding medication compliance, side effects, and or whether any major medical events had occurred. The MMSE, a brief measure of global cognitive functioning was used to screen out current cognitive impairment. Information regarding chronicity and duration of depressive disorder was not an aim of the study, but was collected as part of the recruitment criteria. Participant characteristics were as follows: 15 identified as having 3 or fewer lifetime episodes of depression, 15 identified as having 4 or more episodes, and 12 participants were unsure of number of lifetime episodes. The mean duration of the current depressive episode was 397.21 days (SD= 827.59). Twelve subjects were unable to estimate the duration of the current episode, or identified it as duration greater than 10 years. There were four bipolar patients, 3 in the active and 1 in the placebo group. The rest of the participants had MDD. Sixteen had of diabetes, 23 did not have a family history of diabetes, 3 did not know.
2.4 Statistical analysis
Statistical analyses were conducted using Statistical Analysis System 9.4 (SAS Institute, Cary NC). Study was powered based on previous studies of patients with mood disorders assessed with the HDRS-21 rating scale (Sepanjnia et al., 2012). Missing metabolic data, including FPG, FPI and OGTT were imputed using fully conditional specification multiple imputation. T-tests were conducted in order to assess baseline differences between groups and to assess for differences in the degree of change in HDRS-21 and metabolic measures. Generalized linear models (GLM) were utilized to assess treatment response both unadjusted and adjusted for differences in baseline age, and baseline metabolic outcomes. Due to limited sample size and multiple comparisons, model significance was set as p=0.006 and p=0.05 with and without adjustment for multiple comparisons, respectively. Correlational analyses were conducted between predictors such as age, baseline metabolic characteristics and change in metabolic measures relative to change in HDRS-21 to test for associations with treatment response.
We tested the following general linear models for Aim 1:
Model 1: Change in HDRS-21 as predicted by treatment group.
Models 2–6: Change in HDRS-21 as predicted by treatment group, and significantly associated predictors including: age, age by treatment group interaction, OGTT at baseline, change in OGTT and FPG at baseline, change in FPG.
We tested the following general linear models for Aim 2:
Models 1–2: Change in HDRS-21 among IR and IS participants, respectively as predicted by treatment group.
Models 3–8: Change in HDRS-21 among IR and IS participants, respectively as predicted by treatment group, and significantly associated predictors including: age, age by treatment group interaction, change in OGTT and change in FPG.
3. Results
Forty-two subjects were enrolled and 37 participants (8 males and 29 females) completed the study. Five participants withdrew from the study; 2 moved, 1 withdrew due to a side effect (edema) of treatment with Pioglitazone, and 2 did not specify a reason for withdrawal.
Significant baseline differences between treatment groups were found in HDRS-21 scores and OGTT (Table 1). No significant baseline differences were found in BMI, HOMA, FPG or FPI between treatment and placebo groups. No significant changes were found in FPI, FPG, OGTT or HOMA for either treatment group from baseline to final assessment.
Table 1.
Baseline clinical characteristics of the active and placebo groups.
| Active Group (N=19) | Placebo Group (N=18) | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Mean | SD | Mean | SD | t | df | p |
| Age | 49.42 | 15.11 | 43.28 | 11.80 | 1.38 | 34 | 0.18 |
| BMI | 30.83 | 6.24 | 31.30 | 7.76 | −0.20 | 33 | 0.84 |
| Fasting Plasma Glucose | 97.05 | 13.16 | 98.20 | 8.81 | −0.31 | 32 | 0.76 |
| Fasting Plasma Insulin | 12.20 | 4.87 | 11.17 | 4.60 | 0.58 | 25 | 0.57 |
| HOMA | 2.79 | 1.63 | 3.41 | 1.93 | 1.06 | 33 | 0.30 |
| Oral Glucose Tolerance Test | 100.9 | 20.11 | 136.8 | 58.69 | −2.47 | 35 | 0.02 |
| Baseline HDRS-21 | 17.26 | 5.52 | 13.94 | 4.03 | 2.08 | 35 | 0.05 |
a. Mean clinical characteristics are compared via t-tests. Bold p-values indicate a significant baseline difference between treatment groups.
b. BMI: Body Mass Index; HOMA: Homeostasis Model Assessment; HDRS-21: 21 Item Hamilton Depression Rating Scale
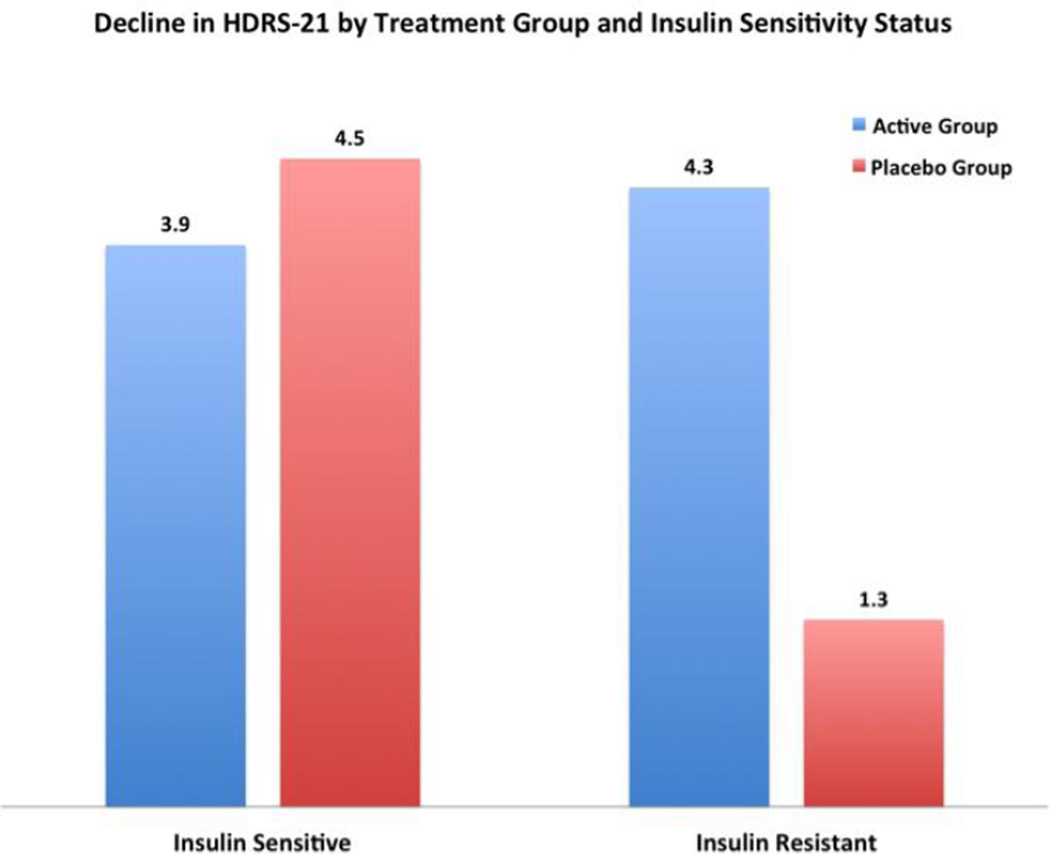
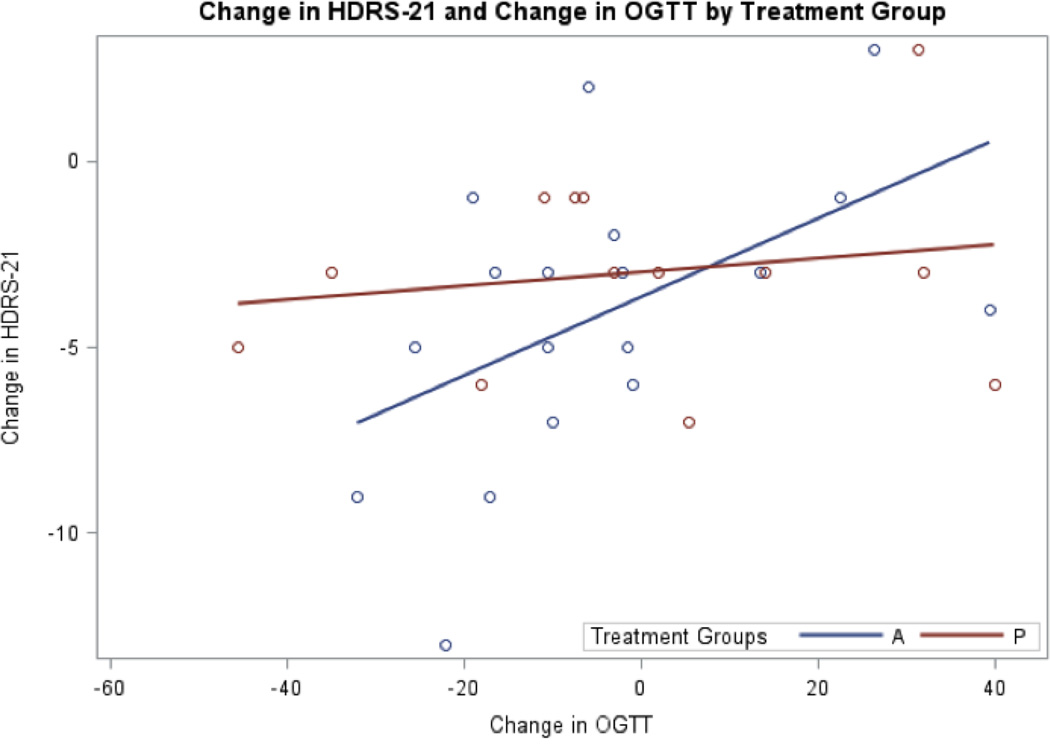
There was no significant difference in mean decline of HDRS-21 scores between treatment groups (t(29)=−1.22, p=0.23). A significant decline in HDRS-21 scores was found within each treatment group from baseline to final assessment (placebo group: t(13)=3.41, p<0.01; active group: t(17)=4.48, p<0.01) (Figure 1). The active and placebo groups had a 24% and 23% decline in HDRS-21 scores, respectively from baseline to final assessment. Within the active group, change in HDRS-21 was positively correlated to change in OGTT, r(18)=0.51, p=0.03. No such correlation was found in the placebo group, r(14)=−0.32, p=0.25.
Figure 1.
Decline in Hamilton Depression Rating Scale (HDRS-21) by Treatment Group and Insulin Sensitivity Status. Hamilton Mean reduction in HDRS-21 is noted that top of each bar. All groups significantly reduced HDRS-21 from week 0 to week 12 of treatment with the exception of the insulin resistant placebo group.
3.1 Baseline insulin sensitivity status and treatment response
There was a significant decline of HDRS-21 scores among the active IS group, t(8) = 5.94, p < 0.001 active IR group, t(8) = 2.45, p < 0.05, and the placebo IS group, t(5) = 6.26, p < 0.005. However, HDRS did not significantly decline in the placebo IR group, t(5) = 1.23, p =0.26
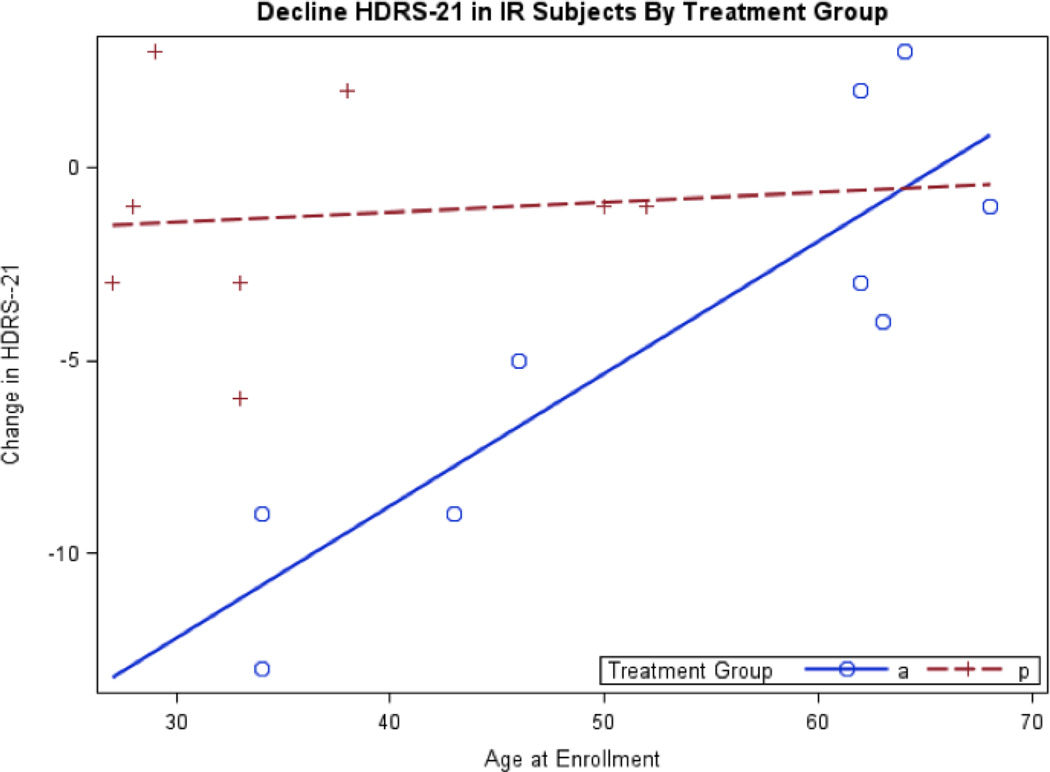
There continued to be a significant treatment effect on change in HDRS-21 scores within the IR group when adjusting for baseline differences in age (Table 3). The active IR group declined in HDRS-21 scores an average of 7.15 points more than that of the placebo IR group, where lower age was associated with greater decline (Figure 2). Again, there was no difference in decline in HDRS-21 scores between the active and placebo IS groups.
Table 3.
Generalized Linear Models of treatment response within IR and IS subgroups.
| Model 1 |
Model 2 | Model 3 | Model 4 | |||||||||
| Variable | B | SE B |
p | B | SE B | p | B | SE B | p | B | SE B | |
| Insulin Sensitive |
||||||||||||
| Treatment Group |
0.61 | 1.00 | 0.55 | −0.65 | 0.73 | 0.39 | −0.68 | .70 | 0.62 | −0.58 | 0.82 | 0.93 |
| Age | - | - | - | − 0.09 | 0.20 | 0.005 | − 0.97 | 0.71 | 0.36 | −0.08 | 0.03 | 0.01 |
| Change in OGTT |
- | - | - | - | - | - | −0.02 | 0.03 | 0.003 | - | - | - |
| Change in FPG |
- | - | - | - | - | - | - | - | - | 0.2 | 0.10 | 0.81 |
| Age x Treatment |
- | − 0.03 |
NS | NS | NS 0.52* |
NS | NS | NS 0.58* |
- | - | − 0.52* |
|
| R2 | ||||||||||||
| Model 1 |
Model 2 | Model 3 | Model 4 | |||||||||
| Variable | B | SE B |
p | B | SE B | p | B | SEB | p | B | SEB | p |
| Insulin Resistant Sample |
||||||||||||
| Treatment Group |
3.08 | 2.11 | 0.17 | 20.29 | 4.09 | 0.0001 | 5.64 | 2.01 | 0.002 | 6.94 | 3.69 | 0.003 |
| Age | - | - | - | 0.34 | 0.07 | .004 | 0.21 | 0.07 | 0.001 | 0.23 | 0.07 | 0.005 |
| Change in OGTT |
- | - | - | - | - | - | 0.04 | 0.07 | 0.10 | - | - | - |
| Change in FPG |
- | - | - | - | - | - | - | - | - | −0.31 | 0.16 | 0.08 |
| Age x Treatment |
- | - | - | −0.32 | 0.14 | 0.04 | NS | NS | NS | NS | NS | NS |
a. Model 1 predicts change in Hamilton Depression Rating Scale (HDRS-21) score via treatment group alone;
b. Model 2 predicts change in HDRS-21 with treatment group and adjusting for age and age by treatment group interaction (where significant)
c. Model 3 predicts change in HDRS-21 with treatment group adjusting for age and change in fasting plasma glucose (FPG). We elected to adjust for proportional change in FPG due to baseline metabolic and age differences between groups.
d, Model 4: predicts change in HDRS-21 with treatment group adjusting for age and change in Oral Glucose Tolerance Test at 120 minutes (OGTT) from baseline to final assessment.. We elected to adjust for proportional change in OGTT due to baseline metabolic and age differences between groups.
e. Bolded p-values indicate models that are significant at the criterion p<0.006.
f. IR: Insulin resistant; IS: Insulin Sensitive; NS: Non-significant interaction; OGTT: Oral Glucose Tolerance Test at 120 minutes; FPG: Fasting Plasma Glucose.
Figure 2.
Decline in Hamilton Depression Rating Scale (HDRS-21) in Insulin Resistant (IR) Subjects by Treatment Group. a. Blue circles and the corresponding solid blue regression line represent the active treatment group. Red crosses and the red patterned regression line represent the placebo group. b. This figure corresponds to Model 2 for the insulin resistant sample in Table 3. c. Age at in enrollment significantly differs between treatment groups therefore age is adjusted for in this generalized linear model. d. There is a significant age by treatment group interaction where the active group shows a significant decline in HDRS among younger subjects relative to older subjects.
In addition to an age and treatment effect, change in OGTT and change in FPG predicted the degree of decline of HDRS-21 but only among IR subjects (Table 3); the greater the decline in OGTT and FPG, the greater the decline in HDRS-21. No such findings were found among the IS subjects.
4. Discussion
Our main findings showed that improvement in glucose metabolism was associated with improvement in depression. Additionally, treatment with pioglitazone was effective in improving mood but only when IR was present, particularly with younger aged individuals.
Insulin resistance (IR) occurs in approximately one third of otherwise apparently healthy individuals to such an extent that it significantly increases risk for cardiovascular disease (CVD) (Yip et al., 1998) and type 2 diabetes (DM2) and is often accompanied by depression (Kenna and Rasgon, 2008). In turn, depression has a lifetime prevalence of over 23% (Okamura et al., 2000), and patients with a history of depressive disorders have high prevalence of IR and glucose dysregulation (Heninger et al., 1975; Okamura et al., 2000; Rasgon et al., 2002; Stemmle et al., 2009) and DM2 (Okamura et al., 2000).
The purpose of this study was to follow up on previous findings from an open label trial that showed an antidepressant effect of the PPARγ-agonist, rosiglitazone, in individuals with depression (Rasgon et al., 2010). The current study evaluated the effects of another PPARγ-agonist, pioglitazone, in a sample of patients with unremitted depression despite TAU across a spectrum of insulin sensitivity. Additionally, predictors of mood response were examined.
No significant treatment effect was seen for mood ratings or glucose metabolic measures between active and placebo groups as a whole. When baseline insulin sensitivity status was considered, mood and glucose metabolic improvements were found to differ between the pioglitazone and placebo groups but only in patients with IR. Patients who were IS improved regardless of treatment group
A surprising aspect of our findings was that the IS group showed equivalent levels of improvement on both adjunctive pioglitazone and placebo, and to an approximately equivalent extent as IR subjects on pioglitazone. One possible explanation of this finding is that participants benefited across the board from their concomitant individualized treatment for depression, but that IR represents a biological gating mechanism that prevents depressed individuals from benefiting from other interventions, thus generating a treatment-resistant depression. Our results would be consistent with this hypothesis in that pioglitazone treatment reduced the treatment resistance associated with baseline IR, but had no effect on mood for the IS subjects, who would not have had IR-associated treatment resistance in the first place.
Several animal (Eissa et al., 2009; Shahsavarian et al., 2014) and human (Rasgon et al., 2010b; Kemp et al., 2012; Sepanjnia et al., 2012; Kashani et al., 2013; Kemp et al., 2014; Zeinoddini et al., 2015) studies support antidepressant effects of PPARγ-agonists. However, these studies exclusively used either IS or IR participants. Our study was the first to our knowledge to examine factors that mediate a PPARγ-agonist, insulin- sensitizing agent on antidepressant response in patients with depression. We found that IS status at baseline as well as age predicted metabolic as well as mood response to treatment with pioglitazone.
In a similar study, early improvement and overall response to pioglitazone treatment was shown in a sample of moderately to severely depressed patients with normal glucose metabolism (Sepanjnia et al., 2012). Although remission rate was higher with pioglitazone treatment compared to placebo in the Sepanjnia et al study (45% versus 15%) (Sepanjnia et al., 2012), remission was not achieved in the majority of that IS sample. That study also utilized citalopram in addition to pioglitazone or placebo in a sample that was more depressed than our sample.
Treatment with pioglitazone has also been shown to be successful in bipolar patients with unremitting depression and metabolic syndrome (Rasgon et al., 2010; Kemp et al., 2012). These studies combined with our findings suggest that successful treatment of unremitting depression with pioglitazone is more likely when there is corresponding glucose metabolic dysfunction.
Among our important and thought provoking results, is an age-specific effect. We showed that younger age was also a factor in predicting enhanced mood response with pioglitazone treatment. Studies that found depression treatment response of pioglitazone in patients either with or without impaired glucose metabolism used younger samples (Sepanjnia et al., 2012; Kashani et al., 2013; Kemp et al., 2014) or did not specifically examine age effects. In general, younger adults respond better to antidepressant therapies than older adults (Zanardi et al., 2003; Thase et al., 2005). Although evidence is limited to DM2 studies, there does not appear to be age-based effects on glucose metabolism with glucose lowering medications (Pandya et al., 2013). Older adults also show slower reductions in depressive symptoms with antidepressant treatments when compared to younger adults (Zanardi et al., 2003) hence, a longer treatment trial with pioglitazone may be necessary for older patients with unremitting depression to achieve optimal mood response. To our knowledge no other studies have shown age effects in depression treatment response with pioglitazone.
4.1 Mechanisms of action
The proposed insulin sensitization mechanism stems from neurobiological evidence that suggests that circulating insulin exerts pivotal functions in central nervous networks (Kerr et al., 1993; Davis et al., 1995). While the origin of CNS insulin is still under debate, it is clear that insulin has multiple functions within the CNS. Insulin has been shown to affect the release and reuptake of catecholamines and influence the regulation of regional cerebral blood flow, the autonomic nervous system, and the HPA axis (Kerr et al., 1993; Davis et al., 1995).
An increase in pro-inflammatory cytokines is common in depression, obesity, metabolic syndrome, and DM2, due to activation of the glutamatergic system, resulting in a reduction of glutamate re-uptake (Raison et al., 2006). IR in itself is a proinflammatory state, hence improvement in treatment outcome of depressed patients may be mediated by an anti-inflammatory effect (Lanquillon et al., 2000; Kemp et al., 2012), rather than insulin-sensitizing effect of thiazolidinediones (TZD). Depression and inflammation have also been linked although it appears that depression increases inflammatory factors more so than inflammatory factors increase depression (Stewart et al., 2009).
The anti-inflammatory hypothesis arises from evidence that, when compared with non-depressed individuals, both medically ill and medically healthy patients with major depression have been found to exhibit all of the cardinal features of inflammation, including elevations in relevant inflammatory cytokines and their soluble receptors in peripheral blood and cerebrospinal fluid, as well as elevations in peripheral blood concentrations of acute phase proteins and chemokines (Zorrilla et al., 2001; Raison et al., 2006). Further, depressed patients with evidence of increased inflammatory activity prior to treatment have been reported to be less responsive to antidepressants, or lithium (Sluzewska et al., 1997; Lanquillon et al., 2000), suggesting that reducing inflammation may result in enhanced antidepressant response. Nonsteroidal anti-inflammatory treatments for major depression have also been explored, and while these have had mixed results, multiple promising alternative anti-inflammatory targets are currently under study (Maes et al., 2012).
Our finding that treatment-resistant depression is associated with IR and relieved by a PPARγ-agonist could be explained either by an inflammatory mechanism (i.e., that IR is associated with systemic inflammation which in turn enhances treatment-resistant depression) or by a direct insulin-mediated mechanism (i.e., that CNS-based IR alters neural responses in a way that contributes to treatment-resistant depression and is mitigated by reduction in IR). Since our study did not collect data on inflammatory responses, it is difficult to speculate what role any changes in inflammatory markers might have played in mediating the observed responses to pioglitazone. Future studies that would collect adjunctive data on inflammatory markers would help to differentiate between these possible explanations.
4.2 Limitations
Study limitations include small sample size, particularly when treatment groups were dichotomized into IS and IR status. Small sample sizes also precluded investigation into gender effects of adjuvant pioglitazone treatment. Study patients were on a variety of antidepressant medications prescribed by their personal physicians thus interactions with specific antidepressant use was not possible. Treatment protocol was brief and long-term effects of pioglitazone treatment could not be assessed. It is possible that in older patients treatment response may be slower and require a longer medication trial. However, mood effects were seen over the trial period consistent with other studies that show mood response to pioglitazone within several weeks.
4.3 Conclusion
Consistent with other studies, the current study showed that pioglitazone might be an effective adjuvant treatment for unremitting depression, particularly for individuals who have IR and who are younger. Our findings help to differentiate between populations of patients with depression who may or may not benefit from adjuvant treatment with PPARγ-agonists. Future studies with larger sample sizes are warranted.
Figure 3.
Change in Hamilton Depression Rating Scale (HDRS-21) and Change in Oral Glucose Tolerance Test at 120 minutes (OGTT) by Treatment Group. a. Blue circles and the corresponding blue regression line represent the active treatment group. Red circles and the red regression line represent the placebo group. b. Change in OGTT is from week 0 to week 2 is significantly positively correlated to change in HDRS-21 in the active but not placebo treatment.
Table 2.
Baseline clinical characteristics of active and placebo groups by insulin sensitivity status.
| Active IS (N=9) | Placebo IS (N=8) | Active IR (N=10) | Placebo IR (N=10) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Mean | SD | Mean | SD | p | Mean | SD | Mean | SD | P |
| Age | 48.00 | 16.45 | 47.50 | 13.75 | 0.95 | 52.89 | 13.60 | 36.25 | 9.77 | 0.01 |
| BMI | 26.64 | 3.92 | 29.20 | 7.02 | 0.64 | 33.37 | 6.76 | 33.43 | 8.82 | 0.81 |
| Fasting Plasma | 88.00 | 13.19 | 92.25 | 5.64 | 0.41 | 106.2 | 5.47 | 103.1 | 10.02 | 0.46 |
| Glucose Fasting | 10.61 | 2.49 | 9.18 | 3.98 | 0.46 | 13.98 | 6.35 | 14.12 | 4.42 | 0.96 |
| Plasma Insulin HOMA | 2.29 | 0.40 | 2.12 | 0.60 | 0.70 | 3.14 | 2.23 | 3.70 | 1.10 | 0.53 |
| Oral Glucose Tolerance Test | 90.44 | 6.89 | 113.40 | 7.03 | 0.04 | 111.20 | 15.50 | 162.5 | 29.00 | 0.09 |
| Baseline HDRS-21 | 15.22 | 2.64 | 13.67 | 1.51 | 0.17 | 19.22 | 7.26 | 13.25 | 4.91 | 0.06 |
a. Mean clinical characteristics compared by treatment group within insulin sensitive (IS) and insulin resistant groups (IR) via t-test. Bold p-values indicate a significant baseline difference between treatment groups.
b. BMI: Body Mass Index; HOMA: Homeostasis Model Assessment; HDRS-21: 21 Item Hamilton Depression Rating Scale.
Highlights.
We aimed to assess the effects on depressive symptoms with adjuvant treatment with the PPARγ-agonist pioglitazone.
Patients (N=37) with non-remitting depression receiving standard psychiatric regimens for depression.
Improvement in depression was associated with improvement in glucose metabolism but only in patients with insulin resistance.
An age effect was also shown in that response to pioglitazone was more beneficial in younger aged patients.
Acknowledgments
Dr. Rasgon has been a consultant for the following companies: Shire Pharmaceuticals, and Sunovion Pharmaceuticals. She has received research support from the following companies: Magceutics, Inc., ADA (American Diabetes Association), and Corcept Pharmaceuticals.
Funding was provided by the National Institutes of Health grant number R21 MH093948-01A1. The authors thank Norma Costello and Abigail Faisal for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical registry name: Pioglitazone in patients with mood disorders.
URL: https://clinicaltrials.gov/ct2/show/NCT01559857
Registration number: NCT01559857
Disclosures
Ms. Watson Lin, Dr. Wroolie and Dr. Robakis have no biomedical financial interests, potential conflicts of interest or funding to report.
References
- Boyd FT, Jr, Clarke DW, Muther TF, Raizada MK. Insulin receptors and insulin modulation of norepinephrine uptake in neuronal cultures from rat brain. J. Biol. Chem. 1985;260:15880–15884. [PubMed] [Google Scholar]
- Davis S, Colburn C, Dobbins R. Evidence that the brain of the conscious dog is insulin sensitive. Journal of Clinical Investigation. 1995;95:593–602. doi: 10.1172/JCI117703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissa AA, Al-Rasheed NM. Antidepressant-like effects of rosiglitazone, a PPARgamma agonist, in the rat forced swim and mouse tail suspension tests. Behavioural Pharmacology. 2009;20:635–642. doi: 10.1097/FBP.0b013e328331b9bf. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon MWJB, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders-Patient Edition. Washington, DC: American Psychiatric Press, Inc; 1997. (SCID-I/P, 2/2001 revision) [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gold PW, Martinez P, Haim A, Eskandari F, Cizza G, Alesci S, Kling M, Quon M. Decreased insulin sensitivity and increased plasma insulin, glucose, and triglyceride concentrations and their interactions in remitted patients with major depression: evidence for an incipient metabolic syndrome. Society of Biological Psychiatry 60th Annual Scientific Convention and Meeting; Atlanta, GA. 2005. [Google Scholar]
- Goldberg RB, Kendall DM, Deeg MA, Buse JB, Zagar AJ, Pinaire JA, Jacober SJ. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care. 2005;28:1547–1554. doi: 10.2337/diacare.28.7.1547. [DOI] [PubMed] [Google Scholar]
- Goodnick P. Use of antidepressants in treatment of comorbid diabetes mellitus and depression as well as in diabetic neuropathy. Annal of clinical psychiatry. 2001;13(1):31–41. doi: 10.1023/a:1009012815127. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heninger G, Mueller P, Davis L. Depressive symptoms and the glucose tolerance test and insulin tolerance test. The Journal of Nervous and Mental Disease. 1975;161:421–432. [PubMed] [Google Scholar]
- Kashani L, Omidvar T, Farazmand B, Modabbernia A, Ramzanzadeh F, Tehraninejad ES, Akhondzadeh S. Does pioglitazone improve depression through insulin-sensitization? Results of a randomized double-blind metformin-controlled trial in patients with polycystic ovarian syndrome and comorbid depression. Psychoneuroendocrinology. 2013;38:767–776. doi: 10.1016/j.psyneuen.2012.08.010. [DOI] [PubMed] [Google Scholar]
- Kemp DE, Ismail-Beigi F, Ganocy SJ, Conroy C, Gao K, Obral S, Calabrese JR. Use of insulin sensitizers for the treatment of major depressive disorder: a pilot study of pioglitazone for major depression accompanied by abdominal obesity. Journal of Affective Disorders. 2012;136:1164–1173. doi: 10.1016/j.jad.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp DE, Schinagle M, Gao K, Conroy C, Ganocy SJ, Ismail-Beigi F, Calabrese JR. PPAR- gamma agonism as a modulator of mood: proof-of-concept for pioglitazone in bipolar depression. CNS Drugs. 2014;28:571–581. doi: 10.1007/s40263-014-0158-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenna H, Rasgon N. Insulin resistance link between depressive disorders and cognitive dysfunction. In: Stubenrauch J, editor. Metabolic Syndrome and Neuropsychiatric Disorders. New York: Informa; 2008. [Google Scholar]
- Kerr D, Stanley JC, Barron M, Thomas R, Leatherdale BA, Pickard J. Symmetry of cerebral blood flow and cognitive responses to hypoglycemia in humans. Diabetologia. 1993;36:73–78.33. doi: 10.1007/BF00399097. [DOI] [PubMed] [Google Scholar]
- Kovacs P, Hajnal A. In vivo electrophysiological effects of insulin in the rat brain. Neuropeptides. 2009;43:283–293. doi: 10.1016/j.npep.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanquillon S, Krieg JC, Bening-Abu-Shach U, Vedder H. Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology. 2000;22(4):370–379. doi: 10.1016/S0893-133X(99)00134-7. [DOI] [PubMed] [Google Scholar]
- Lincoff AM, Wolski K, Nicholls SJ, Nissen SE. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA. 2007;298:1180–1188. doi: 10.1001/jama.298.10.1180. [DOI] [PubMed] [Google Scholar]
- Lozovsky D, Saller CF, Kopin IJ. Dopamine receptor binding is increased in diabetic rats. Science. 1981;214:1031–1033. doi: 10.1126/science.6458088. [DOI] [PubMed] [Google Scholar]
- Maes M, Fisar Z, Medina M, Scapagnini G, Nowak G, Berk M. New drug targets in depression: inflammatory, cell-mediated immune, oxidative and nitrosative stress, mitochondrial, antioxidant, and neuroprogressive pathways. And new drug candidates--Nrf2 activators and GSK-3 inhibitors. Inflammopharmacology. 2012;20:127–150. doi: 10.1007/s10787-011-0111-7. [DOI] [PubMed] [Google Scholar]
- Mueller PS, Heninger GR, McDonald RK. Intravenous glucose tolerance test in depression. Archives of General Psychiatry. 1969;21:470–477. doi: 10.1001/archpsyc.1969.01740220086010. [DOI] [PubMed] [Google Scholar]
- Nathan RS, Sachar EJ, Asnis GM, Halbreich U, Halpern FS. Relative insulin insensitivity and cortisol secretion in depressed patients. Psychiatry Research. 1981;4(3):291–300. doi: 10.1016/0165-1781(81)90031-7. [DOI] [PubMed] [Google Scholar]
- Okamura F, Tashiro A, Utumi A, Imai T, Suchi T, Tamura D, Sato Y, Suzuki S, Hongo M. Insulin resistance in patients with depression and its changes during the clinical course of depression: minimal model analysis. Metabolism. 2000;49(10):1255–1260. doi: 10.1053/meta.2000.9515. Psychiatry Research. 132, 141–147. [DOI] [PubMed] [Google Scholar]
- Okamura F, Tashiro A, Utumi A, Imai T, Suchi T, Tamura D, Hongo M. Insulin resistance in patients with depression and its changes during the clinical course of depression: minimal model analysis. Metabolism: Clinical and Experimental. 2000;49:1255–1260. doi: 10.1053/meta.2000.9515. [DOI] [PubMed] [Google Scholar]
- Pandya N, DiGenio A, Gao L, Patel M. Efficacy and safety of insulin glargine compared to other interventions in younger and older adults: a pooled analysis of nine open-label, randomized controlled trials in patients with type 2 diabetes. Drugs & Aging. 2013;30:429–438. doi: 10.1007/s40266-013-0069-9. [DOI] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends in Immunology. 2006;27(1):24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasgon NL, Kenna HA, Williams KE, Powers B, Wroolie T, Schatzberg AF. Rosiglitazone add-on in treatment of depressed patients with insulin resistance: a pilot study. Scientific World Journal. 2010;10:321–328. doi: 10.1100/tsw.2010.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasgon NL, Kenna HA, Reynolds-May MF, Stemmle PG, Vemuri M, Marsh W, Ketter TA. Metabolic dysfunction in women with bipolar disorder: the potential influence of family history of type 2 diabetes mellitus. Bipolar Disorders. 2010;12(5):504–513. doi: 10.1111/j.1399-5618.2010.00839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasgon NL, Altshuler LL, Elman S, Frye MA, Gitlin M. Increased insulin resistance in women with bipolar disorder, The One Hundred and Fifty-Fifth Annual Meeting of the American Psychiatric Association. Philadelphia, PA: American Psychiatric Association; 2002. [Google Scholar]
- Sepanjnia K, Modabbernia A, Ashrafi M, Modabbernia MJ, Akhondzadeh S. Pioglitazone adjunctive therapy for moderate-to-severe major depressive disorder: randomized double- blind placebo-controlled trial. Neuropsychopharmacology. 2012;37:2093–2100. doi: 10.1038/npp.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahsavarian A, Javadi S, Jahanabadi S, Khoshnoodi M, Shamsaee J, Shafaroodi H, Dehpour A. Antidepressant-like effect of atorvastatin in the forced swimming test in mice: the role of PPAR- gamma receptor and nitric oxide pathway. European Journal of Pharmacology. 2014;745:52–58. doi: 10.1016/j.ejphar.2014.10.004. [DOI] [PubMed] [Google Scholar]
- Sluzewska A, Sobieska M, Rybakowski JK, Rybakowski JK. Changes in acute-phase proteins during lithium potentiation of antidepressants in refractory depression. Neuropsychobiology. 1997;35:123–127. doi: 10.1159/000119332. [DOI] [PubMed] [Google Scholar]
- Stemmle PG, Kenna HA, Wang PW, Hill SJ, Ketter TA, Rasgon NL. Insulin resistance and hyperlipidemia in women with bipolar disorder. Journal of Psychiatric Research. 2009;43(3):341–343. doi: 10.1016/j.jpsychires.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Stewart JC, Rand KL, Muldoon MF, Kamarck TW. A prospective evaluation of the directionality of the depression-inflammation relationship. Brain, Behavior, and Immunity. 2009;23:936–944. doi: 10.1016/j.bbi.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thase ME, Entsuah R, Cantillon M, Kornstein SG. Relative antidepressant efficacy of venlafaxine and SSRIs: sex-age interactions. Journal of Women's Health. 2005;14:609–616. doi: 10.1089/jwh.2005.14.609. [DOI] [PubMed] [Google Scholar]
- Yip J, Facchini FS, Reaven GM. Resistance to insulin-mediated glucose disposal as a predictor of cardiovascular disease. The Journal of Clinical Endocrinology and Metabolism. 1998;83:2773–2776. doi: 10.1210/jcem.83.8.5005. [DOI] [PubMed] [Google Scholar]
- Zanardi R, Cusin C, Rossini D, De Ronchi D, Serretti A. Comparison of response to fluvoxamine in nondemented elderly compared to younger patients affected by major depression. Journal of Clinical Psychopharmacology. 2003;23:535–539. doi: 10.1097/01.jcp.0000095344.32154.d3. [DOI] [PubMed] [Google Scholar]
- Zeinoddini A, Sorayani M, Hassanzadeh E, Arbabi M, Farokhnia M, Salimi S, Akhondzadeh S. Pioglitazone adjunctive therapy for depressive episode of bipolar disorder: a randomized, double-blind, placebo-controlled trial. Depression and Anxiety. 2015;32:167–173. doi: 10.1002/da.22340. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A, McCorkle R, Seligman DA, Schmidt K. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain, Behavior and Immunity. 2001;15(3):199–226. doi: 10.1006/brbi.2000.0597. [DOI] [PubMed] [Google Scholar]