Abstract
Background
The dorsal mesenchymal protrusion (DMP) is a second heart field (SHF) derived tissue involved in cardiac septation. Molecular mechanisms controlling SHF/DMP development include the Bone Morphogenetic Protein and Wnt/β-catenin signaling pathways. Reduced expression of components in these pathways leads to inhibition of proliferation of the SHF/DMP precursor population and failure of the DMP to develop. While the Sonic Hedgehog (Shh) pathway has also been demonstrated to be critically important for SHF/DMP development and atrioventricular septation, its role in the regulation of SHF proliferation is contentious.
Results
Tissue-specific deletion of the Shh receptor Smoothened from the SHF resulted in compromised DMP formation and atrioventricular septal defects (AVSDs). Immunohistochemical analysis at critical stages of DMP development showed significant proliferation defect as well as reduction in levels of the Wnt/β-catenin pathway-intermediates β-catenin, Lef1, and Axin2. To determine whether the defects seen in the conditional Smoothened knock-out mouse could be attributed to reduced Wnt/β-catenin signaling, LiCl, a pharmacological activator of this Wnt/β-catenin pathway, was administered. This resulted in restoration of proliferation and partial rescue of the AVSD phenotype.
Conclusions
The data presented suggest that the Wnt/β-catenin pathway interact with the Shh pathway in the regulation of SHF/DMP-precursor proliferation and, hence, the development of the DMP.
Keywords: atrioventricular, septal defect, heart, mouse, proliferation
Introduction
Atrioventricular septal defects (AVSDs) are congenital heart malformations found in approximately 7% of all individuals suffering from congenital heart disease (CHD) (Pierpont et al., 2000) and 3.5 of 10,000 live births (Ferencz et al., 1997). Approximately 2/3 of isolated AVSDs occur in the context of Down syndrome (Delisle et al., 1999). Furthermore, up to 1/3 of AVSDs diagnosed prenatally occur in the context of heterotaxy syndrome (Huggon et al., 2000). While all AVSDs are characterized by the presence of a common AV junction, two major subtypes can be distinguished based on the potential for shunting at the atrial and ventricular level (Anderson et al., 2010). In partial (or incomplete) AVSDs, shunting of blood is restricted to the atrial level by means of an ostium primum defect (or primum/primary atrial septal defect, pASD). In this defect, the lower part of the atrial septum, the muscularized (antero) inferior rim, is missing (Briggs et al., 2012). Complete AVSDs are characterized by having an inlet type ventricular septal defect (VSD) in addition to the pASD. In complete AVSDs, shunting of blood can occur at the ventricular as well as at the atrial level (Anderson et al., 2010).
For many years, it was believed that perturbation of development of the AV endocardial cushions was the only mechanism leading to AVSDs, which has led to the use of the term “endocardial cushion defect” as a synonym for AVSD (Hiltgen et al., 1996; Dor et al., 2001; Gaussin et al., 2002). Studies in recent years have revealed, however, that abnormal development of tissues derived from the posterior second heart field (pSHF), specifically the dorsal mesenchymal protrusion (DMP) and the primary atrial septum (pAS), play a critical role in the pathogenesis of AVSDs as well (Webb et al., 1999; Snarr et al., 2007a, 2008; Wirrig et al., 2007; Goddeeris et al., 2008; Hoffmann et al., 2009; Tian et al., 2010; Cole-Jeffrey et al., 2012; Xie et al., 2012; Briggs et al., 2013).
Insight into how the development of the pSHF and pSHF-derived structures at the venous pole is regulated is slowly emerging. In the past few years, several pathways and mechanisms have been identified as being involved in this process. These include, the Hedgehog (Hh), the Wnt(2)/β-catenin, and the bone morphogenetic protein (BMP) signaling pathway, as well as events regulated by the transcription factors Tbx1 and Tbx5 (Goddeeris et al., 2008; Tian et al., 2010; Xie et al., 2012; Briggs et al., 2013; Rana et al., 2014).
Hedgehog signaling is mediated through ligand binding to a receptor complex that includes patched (Ptch) and Smoothened (Smo). In the absence of a Hedgehog ligand, Ptch catalytically inhibits the activity of Smo (Taipale et al., 2002). Binding of a ligand to Ptch results in decreased activity of Ptch, enabling Smo to transduce Hh signal to the cytoplasm (Stone et al., 1996; Taipale et al., 2002). Therefore, deletion of Smo effectively blocks all Hh signaling. A requirement for Shh signaling in SHF-dependent AV septation was first demonstrated by Goddeeris and colleagues (Goddeeris et al., 2008). They used a Mef2c-AHF-cre mouse in combination with a floxed Smo mouse (Smofl/fl) to conditionally delete Smo from the SHF in haploinsufficient Smo knockout mice (Smo+/−). The resulting SHF-Smofl/− cko mice were characterized by having an AVSD, which was attributed to the abnormal development of the DMP. Based on their analysis of SHF-Smofl/− cko mice, the authors concluded that loss of DMP tissue in Mef2C-AHF-Cre;Smofl/− embryos was likely not the result of decreased proliferation or increased cell death of the pSHF cell population. Instead, it was suggested that it was the consequence of premature myocardialization and/or loss of the mesenchymal phenotype of the pSHF, preventing the addition of this cell population to the developing AV septal complex (Goddeeris et al., 2008).
The importance of the Wnt/beta-catenin signaling pathway for atrioventricular development is well-documented (Tian et al., 2010). Tian and colleagues demonstrated that mice deficient for Wnt2 are characterized by AVSDs resulting from decreased proliferation of the pSHF cells and compromised DMP development. The proliferation defects and the AVSD phenotype found in this mouse model could be rescued through administration of LiCl, a pharmacological activator of the Wnt/β-catenin signaling pathway (Tian et al., 2010).
The Moskowitz laboratory recently reported that mice haploinsufficient for Tbx5 have pASDs resulting from a significant reduction of proliferating cells in the pSHF (Xie et al., 2012). Realizing that the decrease in proliferation was taking place in pSHF cells that also receive Hh-signaling, they stimulated Hh signaling by constitutively overexpressing Smo in this cell population using a SmoM2 mutant mouse. This resulted in normalization of cell proliferation and in significantly reduced penetrance of the pASD phenotype. This result led the authors to conclude that Tbx5 may act upstream, or parallel to, Hh signaling in the pSHF progenitor cells that are involved in atrial septation (Xie et al., 2012).
Finally, BMP signaling has also been identified as a critical player in atrioventricular septation. When canonical BMP signaling is perturbed in the SHF by conditionally deleting Alk3 from the SHF, a decrease in proliferation of the pSHF cells results in hypoplasia of the DMP and AVSDs (Briggs et al., 2013). Importantly, AVSDs are also observed when Bmp4, normally abundantly expressed in the myocardial dorsal mesocardial reflections flanking the developing DMP (Briggs et al., 2013), is conditionally deleted from the myocardium (Jiao et al., 2003).
Although the aforementioned studies have identified multiple signaling pathways to regulate the process of DMP development and AV septation, little is known about whether the respective pathways act independently of each other or whether they control pSHF development and atrial/atrioventricular septation in a synergistic manner.
Unlike earlier studies in which Smo was deleted from specific cell populations in heterozygous Smo knockout mice (Goddeeris et al., 2007, 2008), in this study a strategy was followed that removed Smo only from the SHF-derived cell populations. In this method, other subpopulations in the heart, as well as non-SHF populations in other parts of the embryo, express Smo at wildtype levels. This approach led to inhibition of the development of pSHF-derived AV septal structures, and resulted in AVSDs. Analysis of the pSHF in the SHF-Smofl/fl cko mice showed significant reduction of proliferation and decreased Wnt activity. Activation of the Wnt/β-catenin pathway in these SHF-Smofl/fl cko mice restored pSHF proliferation and led to a significant reduction of the penetrance of the AVSD phenotype. Combined, these observations further emphasize the importance of cell cycle regulation to SHF development and AV septation, and suggest that the Wnt/β-catenin pathway acts downstream of the Shh pathway in controlling this aspect of AV septation.
Results
Conditional Deletion of Smo From the SHF Results in a Spectrum of Congenital Cardiac Malformations
Shh signaling is involved in the embryonic patterning of multiple organ systems. In the heart, Shh regulates developmental events at both the arterial and the venous poles. Inhibition of Shh signaling leads to a spectrum of severe cardiac malformations (Washington Smoak et al., 2005; Dyer and Kirby, 2009).
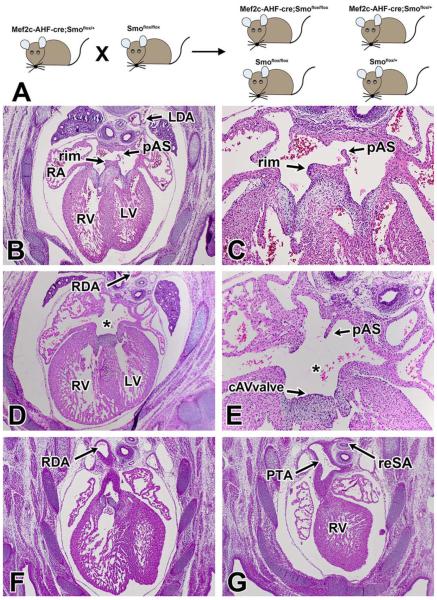
Expression of Smo is required for transduction of Shh signaling to the cytoplasm. To determine how SHF-specific deletion of Smo affects cardiovascular development, we crossed male Mef2c-AHF-cre;Smofl/+ with female Smofl/fl mice (Fig. 1A) to obtain conditional Mef2c-AHF-cre;Smofl/fl knockout mice (SHF-Smofl/fl cko). Offspring was collected from embryonic day (ED) 10.5–ED17.5. A total of 23 SHF-Smofl/fl cko specimens at ED14.5 and beyond were histologically examined for cardiovascular abnormalities (see also Fig. 5G). All control specimens displayed a properly developed atrial septal complex with a primary atrial septum and a well-developed muscular inferior rim (Fig. 1B,C,). In 65% (15/23) of the SHF-Smofl/fl cko specimens we found a pASD (asterisk in Fig. 1D,E), a hallmark feature of virtually all types of AVSDs. In these hearts the muscular inferior rim was missing and a common AV valve was observed (Fig. 1D,E). While several hearts possibly had small slit-like inlet type VSDs (usually only seen in a few serial sections), this observation did not warrant classifying them as complete AVSDs. In 87% (20/23) of the conditional knockout mice, we also found a single artery originating from the right ventricle, a condition known as persistent truncus arteriosus (PTA; Fig. 1G). In approximately 61% (14/23) of the SHF-Smofl/fl cko mice, the position of the descending aorta was abnormal, being either midline or situated on the right side of the embryo (Fig. 1D,F). Other malformations included aberrant origin of the arteries supplying the lungs and the presence of a retro-esophageal subclavian artery (Fig. 1G).
Fig. 1.
Deletion of Smoothened from the SHF results in severe cardiac abnormalities. A: To specifically delete Smoothened from the second heart field, male Mef2c-AHF-cre;Smoflox/+ mice were mated to female Smoflox/flox mice to generate Mef2c-AHF-cre;Smoflox/flox (SHF-Smofl/fl) mice. D–G: Histological analysis of SHF-Smofl/fl ckos at ED14.5 and beyond revealed a wide spectrum of congenital heart defects. B,C: While control embryos showed proper atrioventricular septation with a well-developed DMP-derived inferior rim (arrows), the majority of SHF-Smofl/fl ckos were found to have an ostium primum atrial septal defects, (asterisk in D,E) with a common AV valve orifice. F,G: The embryos also displayed severe outflow abnormalities, including persistent truncus arteriosus, right descending aorta, and retroesophageal subclavian arteries. cAVvalve, common AV valve; DMP, dorsal mesenchymal protrusion; LDA, left descending aorta; RDA, right descending aorta; PTA, persistent truncus arteriosus; RV, right ventricle; LV, left ventricle; reSA, retroesophageal subclavian artery; RA, right atrium; LA, left atrium; PuV, pulmonary vein; pAS, primary atrial septum; AVSD, atrioventricular septal defect
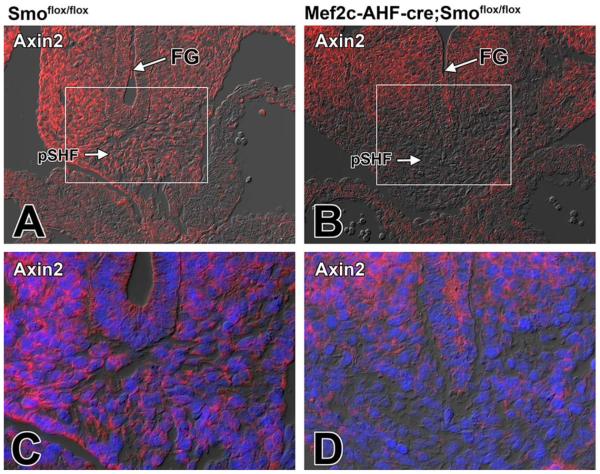
Fig. 5.
Deleting Smoothened from the SHF results in a reduction of Axin2 in the pSHF. A–D: Sections from the venous pole of Smofl/fl control (A,C) and SHF-Smofl/fl (B,D) littermates at ED10.5 were stained for Axin 2 (red). C: Higher magnification of boxed area in A. D: Higher magnification of boxed are in B. The immunofluorescently labeling showed reduced expression of Axin 2 in the pSHF of SHF-Smofl/fl cko embryos (B,D) when compared with control littermates (A,C). Blue staining in C and D is DAPI nuclear stain. FG, foregut; pSHF, posterior second heart field
Conditional Deletion of Smo From the SHF Leads to Perturbation of DMP Development and Reduced Proliferation in the pSHF
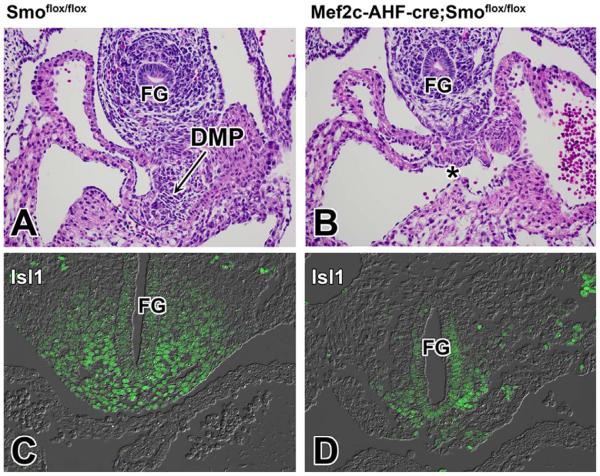
During normal cardiac development, the DMP develops between stages ED9.5–ED10.5. To establish how conditional deletion of Smo from the SHF affects the development of the DMP, we analyzed SHF-Smofl/fl cko specimens and control littermates at ED10.5. This histological examination revealed inhibited DMP development (Fig. 2A,B). To determine whether this DMP defect could be attributed to a reduction of the pSHF/DMP precursor population as previously reported for the SHF-Alk3fl/fl model for AVSD (Briggs et al., 2013) and Wnt2 knockout mouse (Tian et al., 2010), histological and immunohistochemical analyses were performed at 9.5ED which showed significant reduction in the population of Isl1 expressing pSHF cells (Fig. 2C,D).
Fig. 2.
Deletion of Smoothened from the SHF leads to reduction of the size of the SHF and failure of the DMP to form. A–D: This figure shows H/E (A,B) and Isl1 (C,D) staining of the venous pole of Smofl/fl control specimens (A,C) and SHF-Smofl/fl ckos (B,D) at ED10.5. When compared with control littermates, the venous pole of SHF-Smofl/fl ckos is characterized by reduced numbers of Isl1-expressing SHF cells (D) and a severely underdeveloped/missing DMP (asterisk in B). FG, foregut; DMP, dorsal mesenchymal protrusion
The observed abnormality in DMP development was reminiscent of what we observed in the SHF-Alk3fl/fl mouse (Briggs et al., 2013). In this model for AVSD, we established that the failure of the DMP to develop properly was associated with a reduction in the number of cells in the pSHF caused by compromised pSHF proliferation (Briggs et al., 2013). Furthermore, reduced proliferation of the pSHF was also described in the Wnt2 knockout mouse (Tian et al., 2010).
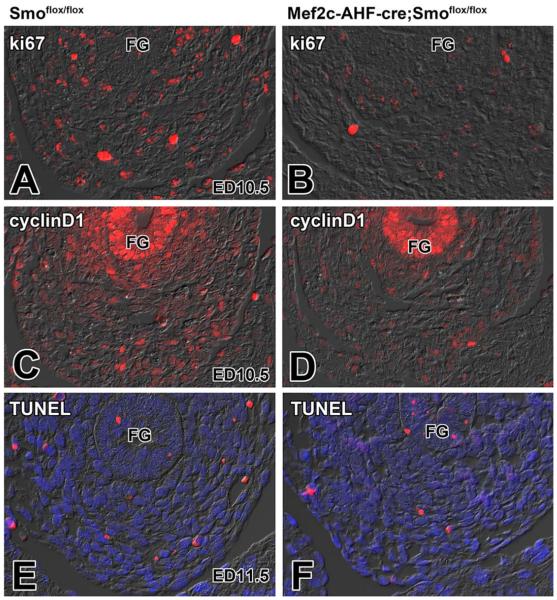
The Shh signaling pathway plays an important role in the regulation of cell proliferation and differentiation in multiple cell lineages, including the SHF (Dyer and Kirby, 2009). To determine whether a proliferation defect was causing the observed phenotype in the SHF-Smofl/fl model, we assessed the level of proliferation with the proliferation marker Ki67 in the SHF. The Ki67 staining revealed, when compared with littermate controls (Fig. 3A,A′), reduced numbers of proliferating SHF cells in SHF-Smofl/fl ckos (Fig. 3B,B′). In addition, we found reduced expression of cyclinD1, important for cell cycle progression in the pSHF of SHF-Smofl/fl ckos (Fig. 3C,C′,D,D′). To establish whether increased apoptosis was involved in the reduction of the pSHF/DMP population, we investigated the level of apoptosis in the SHF-Smofl/fl mice using TUNEL staining. This analysis showed that, when compared with control littermates (Fig. 3E), the number of TUNEL positive cells at the venous pole in the SHF-Smofl/fl ckos was not increased (Fig. 3F).
Fig. 3.
Loss of Smoothened from the SHF does not affect apoptosis but leads to decreased proliferation of SHF cells at the venous pole. This figure shows that, compared with Smofl/fl control embryos (A,C), the level of proliferation in SHF-Smofl/fl ckos (B,D) is greatly reduced as determined by the expression of proliferation marker Ki67 (red in A and B) and the cell-cycle regulator cyclin D1 (red in C and D). The level of apoptosis in SHF-Smofl/fl ckos (F) is not changed as compared to Smofl/fl control specimens (E). FG, foregut; DMP, dorsal mesenchymal protrusion; SHF, second heart field.
Conditional Deletion of Smo From the SHF Negatively Affects the Wnt/β-Catenin Signaling Pathway
As demonstrated above, Shh signaling through Smo is crucially important for the regulation of proliferation of the pSHF, the expression of cyclinD1 as a downstream target, and the development of the DMP. Earlier it was reported that Wnt2 expression at the venous pole is critically important for the development of the pSHF and DMP, as Wnt2 knockout mice are also characterized by a reduction in SHF proliferation and perturbation of DMP development (Tian et al., 2010). These observations support the notion that both the Shh and Wnt/β-catenin pathways are important in the regulation of the cell cycle.
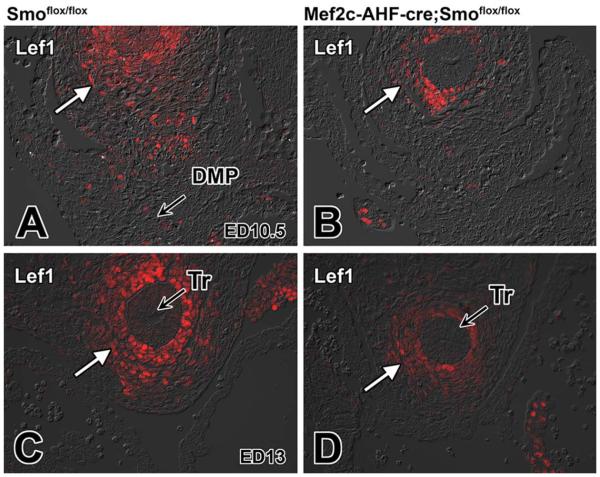
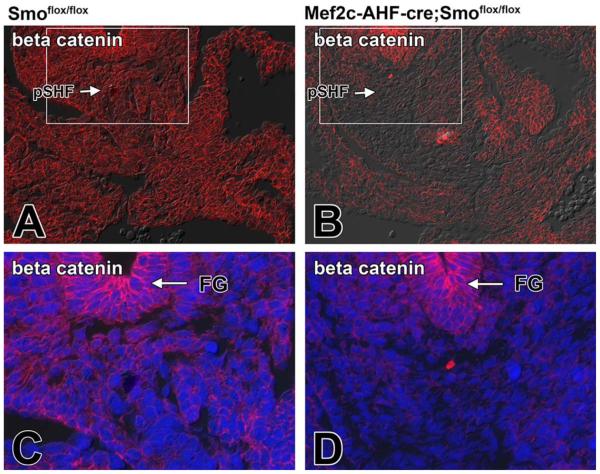
It is well established that the Shh and Wnt/β-catenin pathways interact in tissue development. How they do this differs, however, from tissue to tissue (Lei et al., 2006; Iwatsuki et al., 2007; Alvarez-Medina et al., 2009). In neural progenitor proliferation, Shh activation has been demonstrated to act upstream of Wnt signaling (Alvarez-Medina et al., 2009). To determine whether perturbation of the Shh pathway compromises Wnt/β-catenin signaling in the pSHF, we assessed the expression level of two downstream targets of this pathway, Lef1 and Axin2, as well as the expression of β-catenin itself, in SHF-Smofl/fl cko embryos. Immunofluorescent labeling showed lower levels of expression of Lef1 and Axin2 in SHF-Smofl/fl cko embryos when compared with those found in control Smofl/fl littermates (Figs. 4A–D, 5A–D). Furthermore, immunofluorescent staining for β-catenin also showed reduction of expression in SHF-Smofl/fl cko embryos (Fig. 6A–D).
Fig. 4.
Loss of Smoothened from the SHF leads to reduction of Lef1 expression in the pSHF. Sections from the venous pole of Smofl/fl control and SHF-Smofl/fl littermates at ED10.5 and ED13 were labeled for the expression of the Wnt/β-catenin target gene Lef1 (red). A,B: This analysis revealed reduction of Lef1 in SHF cells of SHF-Smofl/fl ckos (B) when compared with control littermates (A). C,D: Decreased Lef1 expression was also noted around the trachea of SHF-Smofl/fl ckos at later developmental stages. DMP, dorsal mesenchymal protrusion; SHF, second heart field; Tr, trachea
Fig. 6.
Loss of Smoothened from the SHF results in a reduction of β-catenin expression in the pSHF. A,C: The normal expression of β-catenin in the venous pole of a ED10.5 Smofl/fl control embryo (C is an enlargement of the boxed area in A). B,D: The reduced expression of β-catenin expression in a SHF-Smofl/fl littermate. Note that the β-catenin staining in the foregut endoderm is not affected by conditional deletion of Smo from the SHF. Blue staining in C and D is DAPI nuclear stain. FG, foregut; pSHF, posterior second heart field
Activation of the Wnt/β-Catenin Signaling Pathway Partly Rescues the AVSD Phenotype and Restores pSHF Proliferation in SHF-Smofl/fl ckos
Given the overlapping expression domain of Wnt and Shh at the venous pole, the similarity in phenotype of Wnt2 mutants and our SHF-Smofl/fl ckos, the reduction in proliferation, and the reduced expression of Lef1, Axin2, β-catenin, and cyclinD1 observed in our SHF-Smofl/fl ckos, we hypothesized that the Wnt pathway might act downstream of the Shh pathway in the regulation of DMP development and the formation of the AV septal complex. To test this hypothesis, we administered LiCl, a pharmacologic inhibitor of GSK3β and activator of Wnt/β-catenin signaling (Tian et al., 2010), to female mice carrying SHF-Smofl/fl embryos in the expectation that this would reactivate the Wnt/β-catenin signaling, restore proliferation, and reduce the penetrance of the AVSD phenotype.
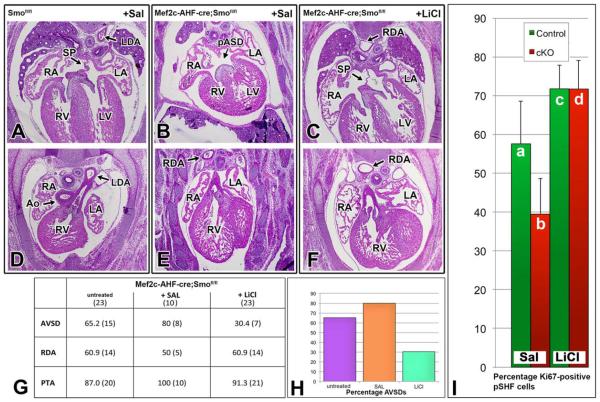
After mating Smofl/fl females with male Mef2c-AHF-cre:Smofl/+, 24 pregnant Smofl/fl females were injected (i.p.) daily with either 200 mg/kg LiCl in saline or saline-only from ED7.5 to ED11.5 to cover the timeline of SHF expansion and addition to the developing venous pole. A total of 33 SHF-Smofl/fl (23 from dams treated with LiCl, 10 from dams injected with saline) at ED14.5 were processed and histologically examined. This analysis revealed a significant reduction in the penetrance of AVSDs in the LiCltreated SHF-Smofl/fl ckos (30% - 7/23) compared with 80% (8/10) in the saline-treated SHF-Smofl/fl ckos (Fig. 7G). Of interest, the LiCl treatment did not significantly affect the penetrance of the abnormalities at the arterial pole (Fig. 7G). The abnormal position of the descending aorta was also not effected by the LiCl treatment as the aorta was positioned either midline or on the right in 14/23 of SHF-Smofl/fl exposed to LiCl and in 5/10 in the salinetreated SHF-Smofl/fl (Fig. 7).
Fig. 7.
Administration of LiCl, an activator of Wnt/β-catenin signaling, lowers penetrance of AVSD phenotype and rescues defective SHF proliferation in SHF-Smofl/fl ckos. A–F: H/E stainings of ED14.5 hearts of Smofl/fl controls (A,D) and Smofl/fl ckos (B,E) of saline treated pregnant mice and sections of a ED14.5 SHF-Smofl/fl cko of a mouse treated with LiCl (C,F). B,E: Note the intact septum primum (SP) and left descending aorta (LDA) in the control heart (A,D) and presence of a primary atrial septum defect (pASD) and right descending aorta (RDA) in the “saline treated” SHF-Smofl/fl cko. C,F: A “LiCl treated” SHF-Smofl/fl cko with an intact atrial septum and with a right descending aorta. G: Summary of the histological analysis of 23 untreated ED14.5 control specimen, 10 SHF-Smofl/fl ckos from saline treated pregnant mice, and 23 SHF-Smofl/fl ckos from pregnant mice treated with LiCl. H: This analysis demonstrates how treatment with LiCl reduces penetrance of the AVSD phenotype in SHF-Smofl/fl ckos (Mef2c-AHF-cre;S-mofl/fl) by more than 50%, but that, regardless of treatment, the vast majority of SHF-Smofl/fl ckos are still characterized by outflow tract defects, including persistent truncus arteriosus and right descending aorta (RDA). I: The percentage of proliferating, Ki67-positive, cells within the posterior SHF at ED10 (as determined by Isl1 staining). When compared with saline-treated control littermates (a), saline-treated ED10 SHF-Smofl/fl ckos (b) show a pronounced decrease in the Ki67:Isl1 ratio (approximately 30%, P<0.05). Administration of LiCl significantly increased the proliferation index in the SHF (P<0.0005) thereby normalizing the proliferation defect (c,d). Ao, aorta; RDA, right descending aorta; RA, right atrium; RV, right ventricle; LA, left atrium; SP, septum primum; AVSD, atrioventricular septal defect; PTA, persistent truncus arteriosus; LiCl, lithium chloride
To test the hypothesis that the LiCl-mediated rescue of the AVSD phenotype in the SHF-Smofl/fl ckos results from restoration of proliferation, the level of proliferation was assessed in the pSHF of saline and LiCl-treated SHF-Smofl/fl ckos and in the pSHF of corresponding saline and LiCl-treated controls at ED10 using the proliferation marker Ki67 in combination with the SHF-marker Isl1. Analysis of saline treated SHF-Smofl/fl ckos (Fig.7Ib) and controls (Fig. 6Ia) revealed a significant reduction (approximately 30%, P<0.05) in the number of proliferating SHF cells. Comparison of LiCl treated SHF-Smofl/fl ckos (Fig. 7d) with saline treated SHF-Smofl/fl ckos (Fig. 7Ib) showed that the proliferation index of the SHF was increased by almost two-fold in LiCl treated embryos (P<0.0005). Furthermore, LiCl administration normalized differences in proliferation between SHF-Smofl/fl cko and control groups.
Discussion
In recent years, the SHF-derived DMP has emerged as a critical component of AV septation. The fusion of the DMP with the endocardially derived mesenchymal cap of the pAS and the endocardially derived AV cushions leads to the formation of the AV mesenchymal complex (Snarr et al., 2007b; Wirrig et al., 2007). In murine models, failure of the DMP to properly develop is associated with pASD (or ostium primum defect), a hallmark feature of nearly all AVSDs (Snarr et al., 2007a; Wirrig et al., 2007; Goddeeris et al., 2008; Tian et al., 2010; Xie et al., 2012). As the role of the DMP in AV septation becomes more clearly defined, so do the molecular mechanisms governing its formation.
Multiple studies have demonstrated that the Shh pathway is critical for SHF-dependent events in cardiovascular development. Shh ligand is secreted by the pharyngeal endoderm adjacent to the SHF (Goddeeris et al., 2007; Dyer and Kirby, 2009) and Patched 2 (Ptc2), a Shh receptor, is expressed within the SHF, with the highest expression found in the posterior SHF (Dyer and Kirby, 2009). In ovo inhibition of Hh signaling, through use of cyclopamine, results in loss of SHF proliferation in only those regions where Ptc2 expression is most abundant while treatment of cultured SHF explants with the Hh agonist SAG results in increased SHF proliferation (Dyer et al., 2010). Murine Shh mutants demonstrate reduced SHF deployment and survival (Washington Smoak et al., 2005). Shh is positioned upstream of genes (e.g., cyclins D and E1, n-myc, and CDC25B) that regulate cell cycle progression by controlling G1-S and G2-M transitions (Kenney and Rowitch, 2000; Oliver et al., 2003; Benazeraf et al., 2006).
Consistent with published studies demonstrating the role of Shh in SHF proliferation, we found a significant reduction of pSHF (approximately 40%) in our SHF-Smofl/fl ckos and a decreased cyclin D1 expression. In an earlier study on the role of Shh signaling in atrioventricular septation, the SHF-Smofl/− model system was used (Goddeeris et al., 2008). While this approach is very similar to the one we followed, it is not identical as it removes Smo from the SHF in a mouse haploinsufficient for Smo in all other tissues. In this earlier study, no decreased levels of proliferation were found using the proliferation marker phosphorylated histone H3 (pHH3) and it is possible that the methods used for assessing proliferation may have contributed to the discrepancy. The antigen recognized by Ki67 is present in all stages of the cell cycle (G1, S, G2, and mitosis) (Cattoretti et al., 1992) while pHH3 is considered a marker of mitosis and is only detectable in a very short window (M-phase) of the cell cycle (Ribalta et al., 2004).
In the study using the SHF-Smofl/− model, some data were presented that suggested premature myocardialization as a mechanism responsible for the compromised development of the DMP (Goddeeris et al., 2008). In our SHF-Smofl/fl ckos, however, we did, not observe ectopic expression of genes associated with myocardial differentiation in the pSHF (data not shown). This apparent difference could also result from the difference in mating strategy. In this context it is important to note that many haploinsufficient mutant mouse models have (cardiac) defects in and of themselves, such as for instance observed in the haploinsufficient Hdf mouse (Wirrig et al., 2007) and the haploinsufficient Tbx5 mouse (Xie et al., 2012). It is therefore to be expected that, in certain situations, conditionally deleting a gene of interest from a specific cell population in a systemic haploinsufficient mouse might lead to different results than conditionally deleting the same gene in that specific subpopulation only.
Intact canonical Wnt signaling has been demonstrated to be of critical importance for SHF proliferation. Loss of canonical Wnt signaling from the SHF results in hypoplasia of SHF-derived structures due to decreased proliferation of SHF cells (Kwon et al., 2007; Lin et al., 2007). Gain-of-function studies have demonstrated that stabilization of Wnt/β-catenin leads to increased proliferation of SHF cells as well as up-regulation of cyclin D2 (Kwon et al., 2007). Both in vivo and in vitro activation of canonical Wnt signaling through inhibition of glycogen synthase kinase-3 (GSK-3) leads to expansion of Isl1-positive cardiovascular progenitors by positively regulating proliferation (Cohen et al., 2007; Qyang et al., 2007). With respect to the developing venous pole, loss of Wnt2 prevents proper SHF expansion, resulting in malformation of the DMP, and AVSD (Tian et al., 2010). Inhibition of GSK-3β in Wnt2 mutants rescued DMP proliferation and resulted in a significant reversal of the AVSD phenotype (Tian et al., 2010).
Similar to the cardiac phenotype observed in Wnt2 mutants, we observed AVSDs secondary to impaired SHF proliferation and subsequent malformation of the DMP in our SHF-Smofl/fl ckos. We also observed a reduction of β-catenin expression as well as the expression of the Wnt/β-catenin signaling downstream target Lef1 and Axin2. Thus, we hypothesized that the AVSD phenotype could be rescued if SHF proliferation defects were corrected. To drive SHF proliferation, we administered LiCl, which inhibits GSK3b and therefore acts as an activator of Wnt/β-catenin signaling, to SHF-Smo ckos at embryonic stages corresponding with SHF expansion at the venous pole. Consistent with our hypothesis, activation of Wnt/β-catenin rescued decreased SHF proliferation at the venous pole as well as expression of cyclin D1. Furthermore, penetrance of AVSD, a defect associated with malformation of the DMP, was reduced in LiCl treated SHF-Smo ckos.
These results not only illustrate the crucial importance of cell cycle regulation in SHF development at the developing venous pole, they also suggest cross-talk between Shh and Wnt signaling pathways within this population of cells. Throughout cardiogenesis, Shh and Wnt ligands and receptors exhibit similar spatiotemporal expression patterns. Furthermore, mice deficient for Wnt or Shh signaling demonstrate similar cardiac malformations including outflow tract and AV septal defects. Empirical evidence suggests that Shh and Wnt pathways may interact to regulate developmental events, including cyclin D1 expression and cell cycle progression (Borycki et al., 2000; Lin et al., 2007; Alvarez-Medina et al., 2009). However, canonical Wnt signaling has also been demonstrated to independently activate transcription of other genes associated with proliferation, including cyclin D2 (Baek et al., 2003; Ai et al., 2007) and cyclin D1 (Shtutman et al., 1999).
Malformations of the outflow tract, including single outflow artery, have been described in Wnt and Shh mutants. Although activation of Wnt signaling rescued penetrance of AV septal defects in SHF-Smofl/fl ckos, arterial pole malformations were found at nearly identical frequencies regardless of treatment. Within SHF cells at the venous pole of the heart, Shh and Wnt signaling are required for SHF expansion, proper formation of the DMP and its subsequent contribution to AV septation. At the developing arterial pole, however, both SHF and cardiac neural crest cells contribute to elongation and septation of the outflow tract. Within these populations, both Shh and canonical Wnt signaling have been implicated in cell proliferation, deployment and survival. The noncanonical Wnt (planar cell polarity) pathway has also been implicated in outflow tract development (Henderson et al., 2006). Thus, the fact that outflow tract defects were not corrected in SHF-Smo ckos through activation of canonical Wnt signaling may reflect the requirement for both canonical and noncanonical Wnt signaling pathways in outflow tract development.
In summary, we demonstrate that loss of Shh signaling from SHF results in decreased SHF proliferation at the developing venous pole, potentially through loss of downstream Wnt signaling. Activation of canonical Wnt signaling restores SHF proliferation and expansion, thereby rescuing malformation of the DMP as well as penetrance of the AVSD phenotype. The work presented here reinforces a requirement for SHF proliferation in proper formation of the DMP and its subsequent contribution to AV septation and suggests interaction between two pathways known to play a role in SHF development at the cardiac venous pole. In combination with other work showing that Tbx5 might act upstream to the Hedgehog pathway in regulation of the SHF in the context of atrioventricular sepation (Xie et al., 2012), this study contributes to increasing our insight into how different regulatory mechanisms and pathways synergistically act in the regulation of the development of the pSHF and atrial/atrioventricular septation.
Experimental Procedures
Mice
The Mef2c-AHF-cre mouse model was kindly provided by Dr. Brian Black. The STOCK Smotm2Amc/J (floxed Smo) mouse was obtained from JAX mice (Jackson Laboratory; stock # 004526). Generation and use of both animal models have been described previously (Zhang et al., 2001; Verzi et al., 2005). To trace the fate of cells derived from the SHF, the Mef2c-AHF-cre mouse was used in combination with the B6.129(Cg)-Gt(ROSA)26-Sortm4(ACTB-tdTomato,dTomatLuo/J (R26mT/mG reporter mouse; Jackson Laboratory; stock no 007576). All experiments using animals were approved by the MUSC Institutional Animal Care and Use Committee and complied with federal and institutional guidelines.
Histology
Male Mef2c-AHF-cre;Smofl/+ mice were mated with female Smof/f mice to generate Mef2c-AHF-cre;Smof/f conditional knockout (SHF-Smofl/fl cko) mice as well as control littermates at stages ranging from ED9 to ED17. Presence of a vaginal plug was defined as ED0.5. Following killing of time-pregnant dams, embryos were isolated in phosphate-buffered saline (PBS) and inspected using a dissecting microscope to establish developmental stage. Embryos were then fixed overnight at 4°C in freshly dissolved paraformaldehyde (4% w/v in PBS), processed through a graded series of ethanol, cleared in toluene, embedded in Paraplast Plus (Fisher Brand, catalogue#: 23-021-400), serially sectioned (5 μm), mounted on Superfrost/Plus microscope slides (Fisherbrand catalogue#: 12-550-15) and stored at room temperature. Hematoxylin/eosin staining was performed as previously described (Waller and Wessels, 2000; Snarr et al., 2007a, 2007b).
Immunofluorescent Antigen Detection
Immunofluorescence was performed as previously described (Briggs et al., 2013) with antibodies recognizing the following antigens: Smo (Abcam AB72130); Lef1 (Cell Signaling 22305); cyclin D1 (Cell Signaling 29785); Islet1 (Isl1; DSHB 39.4D5), myosin heavy chain (MF20; DSHB), pSMAD1,5,8 (pSMAD1/5/8; Millipore; catalogue#: AB3848), α-sarcomeric actin (sAct; Sigma; catalogue#: A2172), FilaminA (FLNA; Epitomics; catalogue#: 2242-1), pSMAD3 (pSMAD3; Epitomics; catalogue#: 1880-1), Crtl1 (DSHB; 9/30/8-A-4); HABP (Seikagaku; catnr 400763); Ki67 (Dako; catnr M7248), beta-catenin (Cell Signaling; 95815), Axin2 (Abcam; ab 32197), and EGFP (Abcam; ab 13970). Secondary antibodies (Jackson Immunoresearch) included anti-rabbit TRITC (711-025-152), anti-rabbit FITC (711-095-152), anti-rabbit Cy5 (711-175-152), anti-mouse TRITC, anti-mouse FITC (715-095-151), anti-mouse Cy5 (715-175-150), anti-rat TRITC (715-025-150), anti-rat FITC (715-095-150), anti-rat Cy5 (711-175-150), and anti-chicken Cy5 (703-495-155). The ApopTag fluorescein direct in situ kit (Millipore; catalogue#: S7160) was used to label apoptotic cells. Nuclei were visualized using DAPI (4′,6-diamidine-2-phenylidole-dihydrochloride, Invitrogen; Slowfade Gold Antifade Reagent with DAPI; catalogue#: S36938) and fluorescence visualized using a Zeiss AxioImager II microscope.
Proliferation
The proportion of actively dividing SHF cells within the splanchnic mesoderm, situated between the pulmonary pit and foregut, was assessed using eight evenly spaced 5-mm sections from five SHF-Smofl/fl cko mice and five control littermates at ED10. In addition, five ED10 SHF-Smo cKOs and five control littermates harvested from mothers administered LiCl were analyzed. Each section was co-labeled with Ki67 and Isl1. Average SHF proliferation was calculated by dividing the number of Isl1-positive;Ki67-positive cells by the total number of Isl1-positive cells. Overall proliferation was determined by dividing the number of Ki67-positive cells by the total number of cells. Proportion of Isl1-positive cells was determined by dividing the number of Isl1-positive cells by the total number of cells in a predetermined elliptical area. Significance was determined using a two-tailed student’s t-test.
Administration of LiCl
Pregnant Smofl/fl dams, crossed with Mef2c-AHF-cre;Smofl/+ male mice, were injected intraperitoneally daily with either saline (vehicle) or 200 mg/kg LiCl starting at embryonic stages ED7.5 and continuing through ED11.5. Embryos were collected at ED10.5, ED11.5, and ED14.5.
Acknowledgments
The authors acknowledge the financial support by the following grants: NIH NCRR, the “South Carolina COBRE for Developmentally Based Cardiovascular Diseases,” (A.W.), NIH-NHLBI (A.W.), NIH-NHLBI (A.W), NIH (L.E.B.), American Heart Association Grant-in-Aid (A.W.), American Heart Association Predoctoral grants (L.E.B.) and (M.L.), European Community’s Sixth Framework Program Grant (MJBvdH), Netherlands Heart Foundation Grant (MJBvdH). This publication was supported by Project 1233 from the South Carolina Clinical & Translational Research (SCTR) Institute, with an academic home at the Medical University of South Carolina CTSA, NIH. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH and AHA.
Grant sponsor: National Institutes of Health; Grant number: P30 GM103342; Grant number: NIH-NHLBI 1R56HL122906; Grant number: NIH-NHLBI R01HL033756; Grant number: NIH 5T32-GM008716; Grant number: NIH 5T32HL007260; Grant number: UL1RR029882; Grant number: UL1TR000062; Grant sponsor: American Heart Association; Grant number: 09GRNT2060075; Grant number: 11PRE7310036; Grant number: 12PRE11340000; Grant sponsor: European Community’s Sixth Framework; Grant number: LSHM-CT-2005-018630; Grant sponsor: Netherlands Heart Foundation; Grant number: 1996M002.
References
- Ai D, Fu X, Wang J, Lu MF, Chen L, Baldini A, Klein WH, Martin JF. Canonical Wnt signaling functions in second heart field to promote right ventricular growth. Proc Natl Acad Sci U S A. 2007;104:9319–9324. doi: 10.1073/pnas.0701212104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Medina R, Le Dreau G, Ros M, Marti E. Hedgehog activation is required upstream of Wnt signalling to control neural progenitor proliferation. Development. 2009;136:3301–3309. doi: 10.1242/dev.041772. [DOI] [PubMed] [Google Scholar]
- Anderson RH, Wessels A, Vettukattil JJ. Morphology and morphogenesis of atrioventricular septal defect with common atrioventricular junction. World J Pediatr Congenit Heart Surg. 2010;1:59–67. doi: 10.1177/2150135109360813. [DOI] [PubMed] [Google Scholar]
- Baek SH, Kioussi C, Briata P, Wang D, Nguyen HD, Ohgi KA, Glass CK, Wynshaw-Boris A, Rose DW, Rosenfeld MG. Regulated subset of G1 growth-control genes in response to derepression by the Wnt pathway. Proc Natl Acad Sci U S A. 2003;100:3245–3250. doi: 10.1073/pnas.0330217100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benazeraf B, Chen Q, Peco E, Lobjois V, Medevielle F, Ducommun B, Pituello F. Identification of an unexpected link between the Shh pathway and a G2/M regulator, the phosphatase CDC25B. Dev Biol. 2006;294:133–147. doi: 10.1016/j.ydbio.2006.02.035. [DOI] [PubMed] [Google Scholar]
- Borycki A, Brown AM, Emerson CP., Jr. Shh and Wnt signaling pathways converge to control Gli gene activation in avian somites. Development. 2000;127:2075–2087. doi: 10.1242/dev.127.10.2075. [DOI] [PubMed] [Google Scholar]
- Briggs LE, Kakarla J, Wessels A. The pathogenesis of atrial and atrioventricular septal defects with special emphasis on the role of the dorsal mesenchymal protrusion. Differentiation. 2012;84:117–130. doi: 10.1016/j.diff.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs LE, Phelps AL, Brown E, Kakarla J, Anderson RH, van den Hoff MJ, Wessels A. Expression of the BMP receptor Alk3 in the second heart field is essential for development of the dorsal mesenchymal protrusion and atrioventricular septation. Circ Res. 2013;112:1420–1432. doi: 10.1161/CIRCRESAHA.112.300821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattoretti G, Becker MH, Key G, Duchrow M, Schluter C, Galle J, Gerdes J. Monoclonal antibodies against recombinant parts of the Ki-67 antigen (MIB 1 and MIB 3) detect proliferating cells in microwave-processed formalin-fixed paraffin sections. J Pathol. 1992;168:357–363. doi: 10.1002/path.1711680404. [DOI] [PubMed] [Google Scholar]
- Cohen ED, Wang Z, Lepore JJ, Lu MM, Taketo MM, Epstein DJ, Morrisey EE. Wnt/beta-catenin signaling promotes expansion of Isl-1-positive cardiac progenitor cells through regulation of FGF signaling. J Clin Invest. 2007;117:1794–1804. doi: 10.1172/JCI31731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole-Jeffrey CT, Terada R, Neth MR, Wessels A, Kasahara H. Progressive anatomical closure of foramen ovale in normal neonatal mouse hearts. Anat Rec (Hoboken) 2012;295:764–768. doi: 10.1002/ar.22432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delisle MF, Sandor GG, Tessier F, Farquharson DF. Outcome of fetuses diagnosed with atrioventricular septal defect. Obstet Gynecol. 1999;94:763–767. doi: 10.1016/s0029-7844(99)00389-0. [DOI] [PubMed] [Google Scholar]
- Dor Y, Camenisch TD, Itin A, Fishman GI, McDonald JA, Carmeliet P, Keshet E. A novel role for VEGF in endocardial cushion formation and its potential contribution to congenital heart defects. Development. 2001;128:1531–1538. doi: 10.1242/dev.128.9.1531. [DOI] [PubMed] [Google Scholar]
- Dyer LA, Kirby ML. Sonic hedgehog maintains proliferation in secondary heart field progenitors and is required for normal arterial pole formation. Dev Biol. 2009;330:305–317. doi: 10.1016/j.ydbio.2009.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer LA, Makadia FA, Scott A, Pegram K, Hutson MR, Kirby ML. BMP signaling modulates hedgehog-induced secondary heart field proliferation. Dev Biol. 2010;348:167–176. doi: 10.1016/j.ydbio.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferencz C, Correa-Villasenor A, Loffredo C. Genetic and environmental risk factors of major cardiovascular malformations: the Baltimore-Washington Infant Study - 1981-1989. Futura Publishing Co. Inc.; Armonk, NY: 1997. p. 463. [Google Scholar]
- Gaussin V, Van de Putte T, Mishina Y, Hanks MC, Zwijsen A, Huylebroeck D, Behringer RR, Schneider MD. Endocardial cushion and myocardial defects after cardiac myocyte-specific conditional deletion of the bone morphogenetic protein receptor ALK3. Proc Natl Acad Sci U S A. 2002;99:2878–2883. doi: 10.1073/pnas.042390499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddeeris MM, Rho S, Petiet A, Davenport CL, Johnson GA, Meyers EN, Klingensmith J. Intracardiac septation requires hedgehog-dependent cellular contributions from outside the heart. Development. 2008;135:1887–1895. doi: 10.1242/dev.016147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddeeris MM, Schwartz R, Klingensmith J, Meyers EN. Independent requirements for Hedgehog signaling by both the anterior heart field and neural crest cells for outflow tract development. Development. 2007;134:1593–1604. doi: 10.1242/dev.02824. [DOI] [PubMed] [Google Scholar]
- Henderson DJ, Phillips HM, Chaudhry B. Vang-like 2 and noncanonical Wnt signaling in outflow tract development. Trends Cardiovasc Med. 2006;16:38–45. doi: 10.1016/j.tcm.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Hiltgen GG, Markwald RR, Litke LL. Morphogenetic alterations during endocardial cushion development in the trisomy 16 (Down syndrome) mouse. Pediatr Cardiol. 1996;17:21–30. doi: 10.1007/BF02505807. [DOI] [PubMed] [Google Scholar]
- Hoffmann AD, Peterson MA, Friedland-Little JM, Anderson SA, Moskowitz IP. sonic hedgehog is required in pulmonary endoderm for atrial septation. Development. 2009;136:1761–1770. doi: 10.1242/dev.034157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggon IC, Cook AC, Smeeton NC, Magee AG, Sharland GK. Atrioventricular septal defects diagnosed in fetal life: associated cardiac and extra-cardiac abnormalities and outcome. J Am Coll Cardiol. 2000;36:593–601. doi: 10.1016/s0735-1097(00)00757-9. [DOI] [PubMed] [Google Scholar]
- Iwatsuki K, Liu HX, Gronder A, Singer MA, Lane TF, Grosschedl R, Mistretta CM, Margolskee RF. Wnt signaling interacts with Shh to regulate taste papilla development. Proc Natl Acad Sci U S A. 2007;104:2253–2258. doi: 10.1073/pnas.0607399104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao K, Kulessa H, Tompkins K, Zhou Y, Batts L, Baldwin HS, Hogan BL. An essential role of Bmp4 in the atrioventricular septation of the mouse heart. Genes Dev. 2003;17:2362–2367. doi: 10.1101/gad.1124803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney AM, Rowitch DH. Sonic hedgehog promotes G(1) cyclin expression and sustained cell cycle progression in mammalian neuronal precursors. Mol Cell Biol. 2000;20:9055–9067. doi: 10.1128/mcb.20.23.9055-9067.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon C, Arnold J, Hsiao EC, Taketo MM, Conklin BR, Srivastava D. Canonical Wnt signaling is a positive regulator of mammalian cardiac progenitors. Proc Natl Acad Sci U S A. 2007;104:10894–10899. doi: 10.1073/pnas.0704044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Q, Jeong Y, Misra K, Li S, Zelman AK, Epstein DJ, Matise MP. Wnt signaling inhibitors regulate the transcriptional response to morphogenetic Shh-Gli signaling in the neural tube. Dev Cell. 2006;11:325–337. doi: 10.1016/j.devcel.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Lin L, Cui L, Zhou W, Dufort D, Zhang X, Cai CL, Bu L, Yang L, Martin J, Kemler R, Rosenfeld MG, Chen J, Evans SM. Beta-catenin directly regulates Islet1 expression in cardiovascular progenitors and is required for multiple aspects of cardiogenesis. Proc Natl Acad Sci U S A. 2007;104:9313–9318. doi: 10.1073/pnas.0700923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver TG, Grasfeder LL, Carroll AL, Kaiser C, Gillingham CL, Lin SM, Wickramasinghe R, Scott MP, Wechsler-Reya RJ. Transcriptional profiling of the Sonic hedgehog response: a critical role for N-myc in proliferation of neuronal precursors. Proc Natl Acad Sci U S A. 2003;100:7331–7336. doi: 10.1073/pnas.0832317100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpont ME, Markwald RR, Lin AE. Genetic aspects of atrioventricular septal defects. Am J Med Genet. 2000;97:289–296. [PubMed] [Google Scholar]
- Qyang Y, Martin-Puig S, Chiravuri M, Chen S, Xu H, Bu L, Jiang X, Lin L, Granger A, Moretti A, Caron L, Wu X, Clarke J, Taketo MM, Laugwitz KL, Moon RT, Gruber P, Evans SM, Ding S, Chien KR. The renewal and differentiation of Isl1+ cardiovascular progenitors are controlled by a Wnt/beta-catenin pathway. Cell Stem Cell. 2007;1:165–179. doi: 10.1016/j.stem.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Rana MS, Theveniau-Ruissy M, De Bono C, Mesbah K, Francou A, Rammah M, Dominguez JN, Roux M, Laforest B, Anderson RH, Mohun T, Zaffran S, Christoffels VM, Kelly RG. Tbx1 coordinates addition of posterior second heart field progenitor cells to the arterial and venous poles of the heart. Circ Res. 2014;115:790–799. doi: 10.1161/CIRCRESAHA.115.305020. [DOI] [PubMed] [Google Scholar]
- Ribalta T, McCutcheon IE, Aldape KD, Bruner JM, Fuller GN. The mitosis-specific antibody anti-phosphohistone-H3 (PHH3) facilitates rapid reliable grading of meningiomas according to WHO 2000 criteria. Am J Surg Pathol. 2004;28:1532–1536. doi: 10.1097/01.pas.0000141389.06925.d5. [DOI] [PubMed] [Google Scholar]
- Shtutman M, Zhurinsky J, Simcha I, Albanese C, D’Amico M, Pestell R, Ben-Ze’ev A. The cyclin D1 gene is a target of the ?-catenin/LEF-1 pathway. Proc Natl Acad Sci U S A. 1999;96:5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snarr BS, Kern CB, Wessels A. Origin and fate of cardiac mesenchyme. Dev Dyn. 2008;237:2804–2819. doi: 10.1002/dvdy.21725. [DOI] [PubMed] [Google Scholar]
- Snarr BS, O’Neal JL, Chintalapudi MR, Wirrig EE, Phelps AL, Kubalak SW, Wessels A. Isl1 expression at the venous pole identifies a novel role for the second heart field in cardiac development. Circ Res. 2007a;101:971–974. doi: 10.1161/CIRCRESAHA.107.162206. [DOI] [PubMed] [Google Scholar]
- Snarr BS, Wirrig EE, Phelps AL, Trusk TC, Wessels A. A spatiotemporal evaluation of the contribution of the dorsal mesenchymal protrusion to cardiac development. Dev Dyn. 2007b;236:1287–1294. doi: 10.1002/dvdy.21074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone DM, Hynes M, Armanini M, Swanson TA, Gu Q, Johnson RL, Scott MP, Pennica D, Goddard A, Phillips H, Noll M, Hooper JE, de Sauvage F, Rosenthal A. The tumour-suppressor gene patched encodes a candidate receptor for Sonic hedgehog. Nature. 1996;384:129–134. doi: 10.1038/384129a0. [DOI] [PubMed] [Google Scholar]
- Taipale J, Cooper MK, Maiti T, Beachy PA. Patched acts catalytically to suppress the activity of Smoothened. Nature. 2002;418:892–897. doi: 10.1038/nature00989. [DOI] [PubMed] [Google Scholar]
- Tian Y, Yuan L, Goss AM, Wang T, Yang J, Lepore JJ, Zhou D, Schwartz RJ, Patel V, Cohen ED, Morrisey EE. Characterization and in vivo pharmacological rescue of a Wnt2-Gata6 pathway required for cardiac inflow tract development. Dev Cell. 2010;18:275–287. doi: 10.1016/j.devcel.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verzi MP, McCulley DJ, De Val S, Dodou E, Black BL. The right ventricle, outflow tract, and ventricular septum comprise a restricted expression domain within the secondary/anterior heart field. Dev Biol. 2005;287:134–145. doi: 10.1016/j.ydbio.2005.08.041. [DOI] [PubMed] [Google Scholar]
- Waller BR, 3rd, Wessels A. Cardiac morphogenesis and dysmorphogenesis. An immunohistochemical approach. Methods Mol Biol. 2000;135:151–161. doi: 10.1385/1-59259-685-1:151. [DOI] [PubMed] [Google Scholar]
- Washington Smoak I, Byrd NA, Abu-Issa R, Goddeeris MM, Anderson R, Morris J, Yamamura K, Klingensmith J, Meyers EN. Sonic hedgehog is required for cardiac outflow tract and neural crest cell development. Dev Biol. 2005;283:357–372. doi: 10.1016/j.ydbio.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Webb S, Anderson RH, Lamers WH, Brown NA. Mechanisms of deficient cardiac septation in the mouse with trisomy 16. Circ Res. 1999;84:897–905. doi: 10.1161/01.res.84.8.897. [DOI] [PubMed] [Google Scholar]
- Wirrig EE, Snarr BS, Chintalapudi MR, O’Neal JL, Phelps AL, Barth JL, Fresco VM, Kern CB, Mjaatvedt CH, Toole BP, Hoffman S, Trusk TC, Argraves WS, Wessels A. Cartilage link protein 1 (Crtl1), an extracellular matrix component playing an important role in heart development. Dev Biol. 2007;310:291–303. doi: 10.1016/j.ydbio.2007.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Hoffmann AD, Burnicka-Turek O, Friedland-Little JM, Zhang K, Moskowitz IP. Tbx5-hedgehog molecular networks are essential in the second heart field for atrial septation. Dev Cell. 2012;23:280–291. doi: 10.1016/j.devcel.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XM, Ramalho-Santos M, McMahon AP. Smoothened mutants reveal redundant roles for Shh and Ihh signaling including regulation of L/R asymmetry by the mouse node. Cell. 2001;105:781–792. [PubMed] [Google Scholar]