Significance
Tight and highly controlled transcriptional regulation is pivotal for cell survival under stress conditions. We demonstrate that Gre factor homolog (Gfh) proteins found in the Deinococcus/Thermus lineage of extremophilic bacteria affect multiple steps of transcription and may serve to halt transcription at specific genomic sites. These proteins bind within the secondary channel of RNA polymerase (RNAP) and dramatically increase site-specific transcriptional pausing in the presence of manganese ions, likely by stabilizing inactive enzyme conformation. Both Gfh expression and cellular manganese concentration are increased during stress response, thus providing an efficient way for transcription regulation. Similar mechanisms may be operating with other prokaryotic and eukaryotic factors acting through the secondary channel, which emerges as the central regulatory hub in the control of RNAP activities.
Keywords: RNA polymerase, transcriptional pausing, Gfh factors, Deinococcus radiodurans, stress response
Abstract
Transcriptional pausing has emerged as an essential mechanism of genetic regulation in both bacteria and eukaryotes, where it serves to coordinate transcription with other cellular processes and to activate or halt gene expression rapidly in response to external stimuli. Deinococcus radiodurans, a highly radioresistant and stress-resistant bacterium, encodes three members of the Gre family of transcription factors: GreA and two Gre factor homologs, Gfh1 and Gfh2. Whereas GreA is a universal bacterial factor that stimulates RNA cleavage by RNA polymerase (RNAP), the functions of lineage-specific Gfh proteins remain unknown. Here, we demonstrate that these proteins, which bind within the RNAP secondary channel, strongly enhance site-specific transcriptional pausing and intrinsic termination. Uniquely, the pause-stimulatory activity of Gfh proteins depends on the nature of divalent ions (Mg2+ or Mn2+) present in the reaction and is also modulated by the nascent RNA structure and the trigger loop in the RNAP active site. Our data reveal remarkable plasticity of the RNAP active site in response to various regulatory stimuli and highlight functional diversity of transcription factors that bind inside the secondary channel of RNAP.
Cellular RNA polymerases (RNAPs) are complex molecular machines whose activity during transcription is regulated by DNA- and RNA-encoded signals, protein factors, small molecules, and inhibitors. The catalytic cycle of RNAP can be interrupted by pauses of various natures that play important roles in genetic regulation in all organisms, from the classic systems of transcription attenuation and their variations in bacteria (1) to recently discovered widespread promoter-proximal pausing in eukaryotes (2). The pausing serves to activate or repress transcription rapidly at specific genomic sites in response to regulatory stimuli and to coordinate RNA synthesis with other genetic processes (e.g., DNA replication and repair, RNA translation in bacteria) (1, 3–8).
Recent structural and biochemical studies revealed distinct RNAP conformations corresponding to different functional states of the transcription elongation complex (TEC) during nucleotide addition, RNA proofreading, and pausing (9–11). The control of structural transitions between these states likely underlies the function of multiple regulatory factors acting on RNAP. However, the roles of individual RNAP conformations in transcription and the mechanisms of their switching by transcription factors remain only partially understood. During transcription, RNAP holds the DNA template and the RNA transcript within its main channel, whose opening is controlled by the mobile clamp/shelf module and the flap domain of RNAP (9–11). Nucleotide addition and TEC translocation depend on alternating cycles of the folding of the trigger loop (TL) and kinking of the bridge helix (BH) in the RNAP active site (9–11) (Fig. 1A). Analysis of the structure of a bacterial paused TEC suggested that the first step in pausing, elemental pause formation, is accompanied by partial clamp opening, TL unfolding, and BH kinking (11). During hairpin-dependent pausing, these changes are reinforced by the nascent RNA hairpin formation under the flap domain of RNAP, resulting in stronger TEC inactivation (12–14).
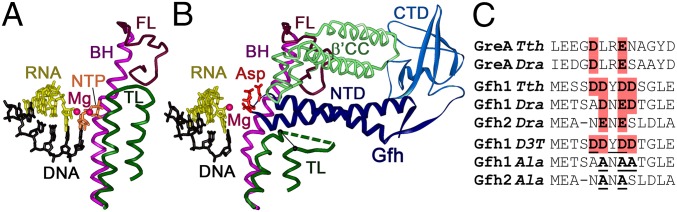
Fig. 1.
Interactions of Gfh factors with the RNAP active site. (A) Structure of the active TEC of Tth RNAP (Protein Data Bank accession number 1IW7) (10). The TL, BH, and F-loop (FL) are shown in green, magenta, and carmine, respectively. (B) Structure of Tth RNAP–Gfh1 complex (3AOI) (22). The acidic residues in the Gfh NTD tip are red; the β′ coiled-coil element (β′CC) interacting with Gfh C-terminal domain (CTD) at the entry of the secondary channel is light green. The position of the analyzed TL deletion (Δ1254–1272) is shown with a black line. (C) Alignment of the active sites of GreA and Gfh factors from Dra and Tth. Acidic residues in the NTD tip are shown in red; residues substituted in the mutant Gfh factors are boldfaced and underlined. The full GreA and Gfh sequences are shown in Fig. S1.
The secondary channel of RNAP serves as the entry gate for NTP substrates during active transcription and accommodates the RNA 3′-end during TEC backtracking. It also serves as the binding site for regulatory factors, including universal bacterial factor GreA and eukaryotic factor TFIIS, which stimulate RNA cleavage by their cognate RNAPs in backtracked TECs. Gre factors were shown to replace the TL and to chelate catalytic divalent metal ions in the RNAP active site during the cleavage reaction (15–17). Several lineage-specific factors also target the secondary channel, including the DksA protein from Proteobacteria and Gre factor homolog (Gfh) factors from the Deinococcus/Thermus phylum of extremophilic bacteria (Fig. 1B and Fig. S1). The Gfh1 protein from Thermus thermophilus (Tth) was shown to inhibit all RNAP activities, including nucleotide addition and RNA cleavage (18–21). Similar to GreA, Gfh1 was proposed to bind metal ions in the RNAP active site by acidic residues from the tip of its N-terminal domain (NTD) (Fig. 1B), but to stabilize them in catalytically nonproductive positions (20, 21). In addition, it induces major changes in RNAP conformation (RNAP “ratcheting”), including opening of the clamp/shelf module and kinking of the BH (9, 22). The activity of Gfh1 is regulated by a pH-dependent rotation of its C-terminal domain, resulting in its activation at low pH values (20).
Fig. S1.
Alignment of GreA and Gfh factors from Tth and Dra. Amino acid residues identical in various Gfh factors are shown in bold. Acidic residues in the NTD loop are shown in red. The alignment is based on Laptenko et al. (20). CTD, C-terminal domain.
Deinococcus radiodurans (Dra) is a highly radioresistant and stress-resistant mesophilic bacterium closely related to Tth. It encodes two Gfh factors, Gfh1 and Gfh2, whose cellular functions remain unknown. In this work, we reveal the effects of Dra Gfh factors on different steps of transcription and demonstrate that they strongly enhance site-specific transcriptional pausing by Dra RNAP. Uniquely, the activity of the Gfh factors is greatly stimulated by manganese ions, which were previously shown to accumulate in Dra cells under stress conditions and to play multiple roles in stress resistance (23–26). We investigate the mechanism of pause stimulation and propose that Gfh factors recognize and stabilize an inactive TEC conformation that is transiently formed at specific pause sites.
Results
Manganese-Dependent Effects of Gfh Factors on Transcription Elongation.
We cloned and purified Gfh factors from Dra and analyzed their effects on Dra RNAP activity at different steps of transcription. All experiments were performed in the presence of either Mg2+ or Mn2+ ions as RNAP cofactors, because manganese ions were previously shown to play essential roles in stress response in Dra (23–26) and to modulate the activity of Dra RNAP (27).
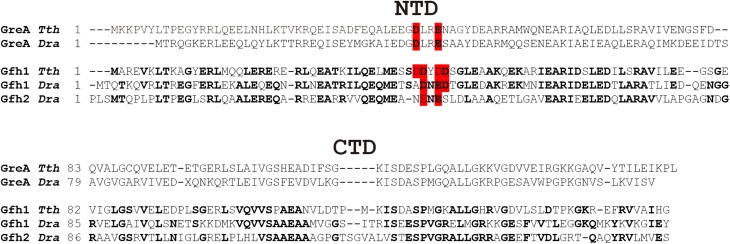
We first tested the effects of the Dra Gfh factors on transcription initiation. Gfh1 and Gfh2 similarly inhibited abortive synthesis by the Dra σA RNAP holoenzyme at pH 6.5 and pH 7.9 (approximately three- to fivefold; Fig. S2). No large differences in the inhibition efficiencies were also observed between reactions containing Mg2+ or Mn2+ ions, except that inhibition by Gfh2 became less efficient in the presence of Mn2+ (Fig. S2B). Thus, in contrast to Tth Gfh1, whose inhibitory activity is greatly stimulated at low pH values (20), Dra Gfh factors have only moderate pH-independent effects on transcription initiation.
Fig. S2.
Effects of Gfh factors on transcription initiation. (A) Sequence of the T7A1cons promoter used in the abortive initiation assay. (B) Inhibition of abortive transcription by Gfh1 and Gfh2 factors from Dra at different pH values in the presence of Mg2+ (Left) or Mn2+ (Right). Transcription was performed with the CpA primer and UTP at 37 °C. RNAP activity is shown as a percentage of the CpApU synthesis measured in the absence of Gfh factors.
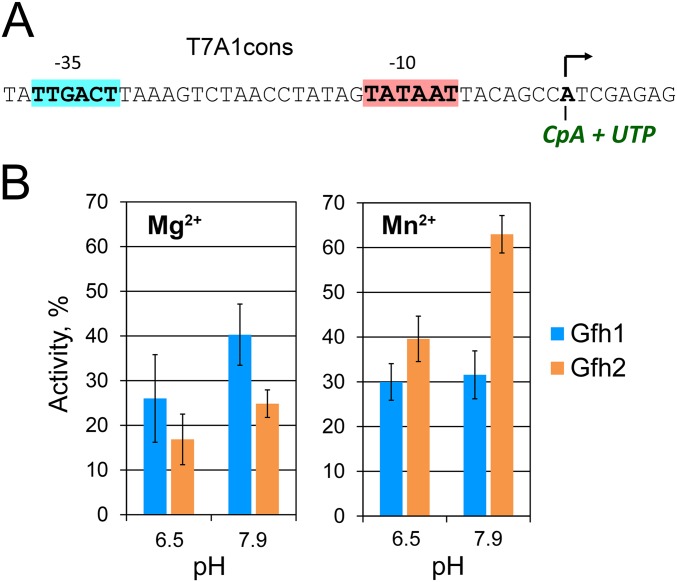
We next analyzed the effects of Dra Gfh factors on the elongation stage of transcription. In the presence of Mg2+, both factors had no effect on the average elongation rate of Dra RNAP measured on a 500-bp-long rpoB-based template (Fig. 2B). In contrast, Tth Gfh1 strongly inhibited transcription elongation by Tth RNAP (Mg2+ reactions; Fig. 2C), in agreement with published data (20). Surprisingly, both Dra Gfh1 and Gfh2 significantly slowed down the average rate of RNA synthesis in the presence of Mn2+ ions, as manifested by the later appearance of full-length transcripts (Fig. 2B). Even stronger inhibition was observed for Tth Gfh1, resulting in almost complete stalling of transcription by Tth RNAP (Mn2+ reactions; Fig. 2C). Therefore, Mn2+ dependence may be a general phenomenon for this family of transcription factors.
Fig. 2.
Effects of Gfh factors on transcription elongation. (A) Scheme of the DNA template and outline of the experiment. The λ phage PR promoter, 26-nt C-less region, and 500-nt run-off RNA (RO) are indicated. The reactions were performed with 10 mM Mg2+ or Mn2+ at pH 7.9 and stopped after 15 and 30 s and 1, 2, 4, and 10 min. (B) Kinetics of full-length RNA synthesis by Dra RNAP; Gfh factors were added to 5 μM. (C) Effects of Tth Gfh1 on RNA synthesis by Tth RNAP.
Although Tth Gfh1 was not functional with Dra RNAP, we analyzed a mutant variant of Dra Gfh1 with replacement of three amino acid residues in the NTD tip with corresponding residues from Tth Gfh1 (Gfh1D3T) (Fig. 1C). The Gfh1D3T mutant and wild-type Dra Gfh1 had comparable effects on transcription elongation by Dra RNAP (Fig. 2B), suggesting that the stronger transcription inhibition by Tth Gfh1 is not explained by its NTD tip structure but may result from specific properties of Tth RNAP and/or differences in RNAP contacts with other Gfh parts (Fig. 1B and Fig. S1).
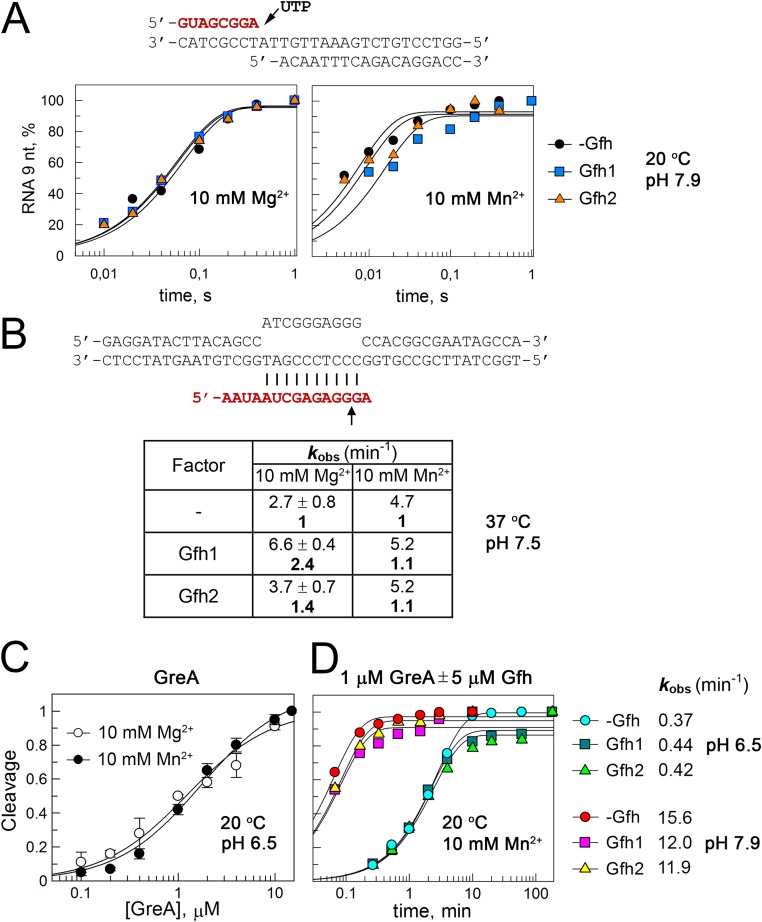
We further analyzed single-nucleotide addition by Dra RNAP. The experiments were performed with the minimal nucleic scaffold template that is bound by RNAP in the posttranslocated state and was previously used extensively to characterize catalysis by Dra RNAP (27–29) (Fig. S3A). The Gfh factors had no effect on the rate of nucleotide incorporation on this template in the presence of Mg2+ and only slightly (twofold or less) inhibited it in the presence of Mn2+ ions (Fig. S3A). Therefore, active posttranslocated TECs are not efficiently targeted by the Dra Gfh factors.
Fig. S3.
Effects of Gfh factors on single-nucleotide addition, intrinsic RNA cleavage, and Gre-induced RNA cleavage by Dra RNAP. RNA oligonucleotides (shown in red) were 5′-labeled before TEC assembly. (A) Reactions with the minimal nucleic acid scaffold containing 8-nt RNA were started by the addition of 1 mM UTP, and the kinetics of 9-nt RNA synthesis were analyzed at 20 °C and pH 7.9 using a rapid-quench flow apparatus in the presence of 10 mM MgCl2 or MnCl2. The data were normalized to the maximal RNA extension in each reaction. (B) Analysis of intrinsic RNA cleavage was performed in the mismatched TEC containing adenine nucleotide at the RNA 3′-end opposite template guanine. The reactions were performed at 37 °C and pH 7.5 in the presence of 10 mM MgCl2 or MnCl2. kobs, observed rate constant. (C) To estimate Kd,app for GreA binding to the rA-dG TEC, analysis of RNA cleavage was performed at different GreA concentrations at 20 °C and pH 6.5 in the presence of 10 mM MgCl2 or MnCl2 (the reaction time was 10 s). (D) No effects of the Gfh factors on GreA-dependent cleavage in the rA-dG TEC. GreA and Gfh1 were added to 1 μM and 5 μM, respectively, and the kinetics of the cleavage reactions were monitored at 20 °C and at pH 6.5 or pH 7.9 after the addition of 10 mM MnCl2.
Dra Gfh Factors Do Not Act as Anticleavage Factors.
Previously, Tth Gfh1 was shown to inhibit intrinsic and GreA-dependent RNA cleavage by Tth RNAP, suggesting that it may function as an “anti-Gre” factor (18–21). In contrast, Dra Gfh factors did not inhibit intrinsic RNA cleavage by Dra RNAP in a synthetic TEC with a mismatched A in the RNA 3′-end opposite template G in the presence of Mn2+, and even slightly stimulated it in the presence of Mg2+ ions (Fig. S3B).
We next tested whether Gfh factors could inhibit GreA-induced RNA cleavage. GreA greatly increased the RNA cleavage rate by Dra RNAP in the same TEC (27) (Fig. S3 C and D); we therefore performed the cleavage reactions not only at pH 7.9 but also at pH 6.5, to decrease the reaction rate and make the measurements more accurate. Titration of GreA, performed at pH 6.5, demonstrated that it binds the TEC with apparent Kds (Kd,apps) of 1.3 ± 0.2 μM and 1.7 ± 0.3 μM in the presence of Mg2+ and Mn2+, respectively (Fig. S3C). When Gfh factors were added in a fivefold molar excess over GreA (5 μM vs. 1 μM) in the presence of Mn2+, they had no effect on GreA-stimulated RNA cleavage, even though the GreA concentration was below its Kd,app for the TEC binding (Fig. S3D). Thus, Gfh factors cannot efficiently compete with GreA during the cleavage reaction.
Gfh Factors Stimulate Transcriptional Pausing in the Presence of Manganese Ions.
The experiments presented above showed that Gfh factors significantly decrease the elongation rate but cannot effectively target transcription complexes involved in RNA synthesis or RNA cleavage. We then proposed that they may recognize a specific state(s) of the TEC formed during transcription elongation. Recent studies suggested that Tth Gfh1 stabilizes a ratcheted TEC conformation probably involved in transcriptional pausing and termination (9, 22). We therefore analyzed Gfh effects on transcriptional pausing by RNAP.
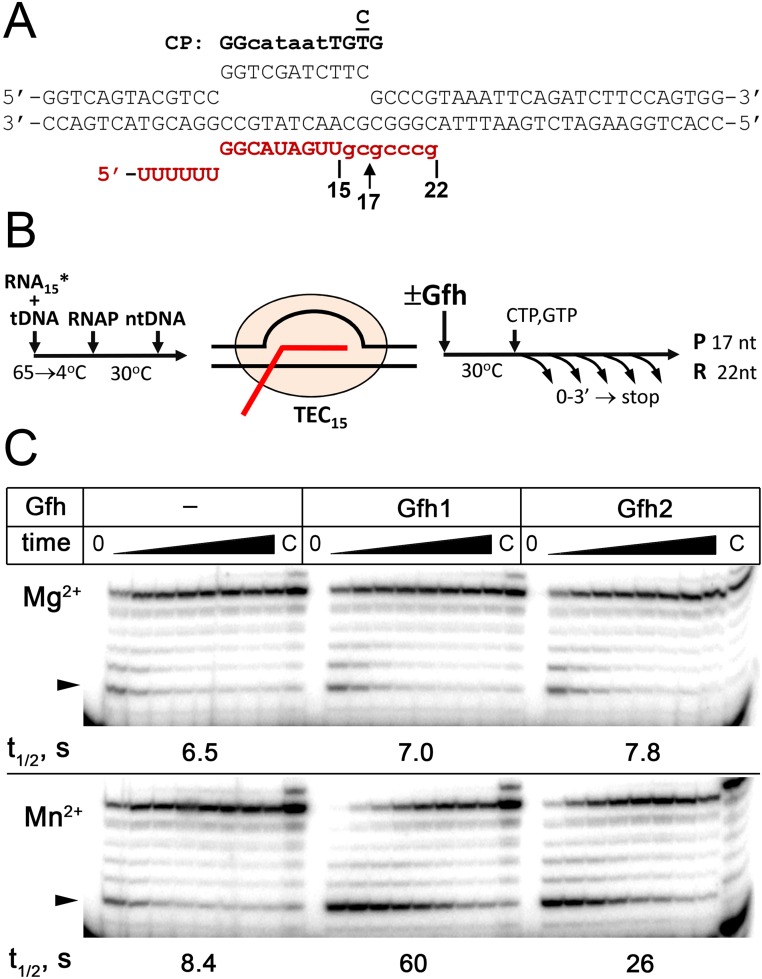
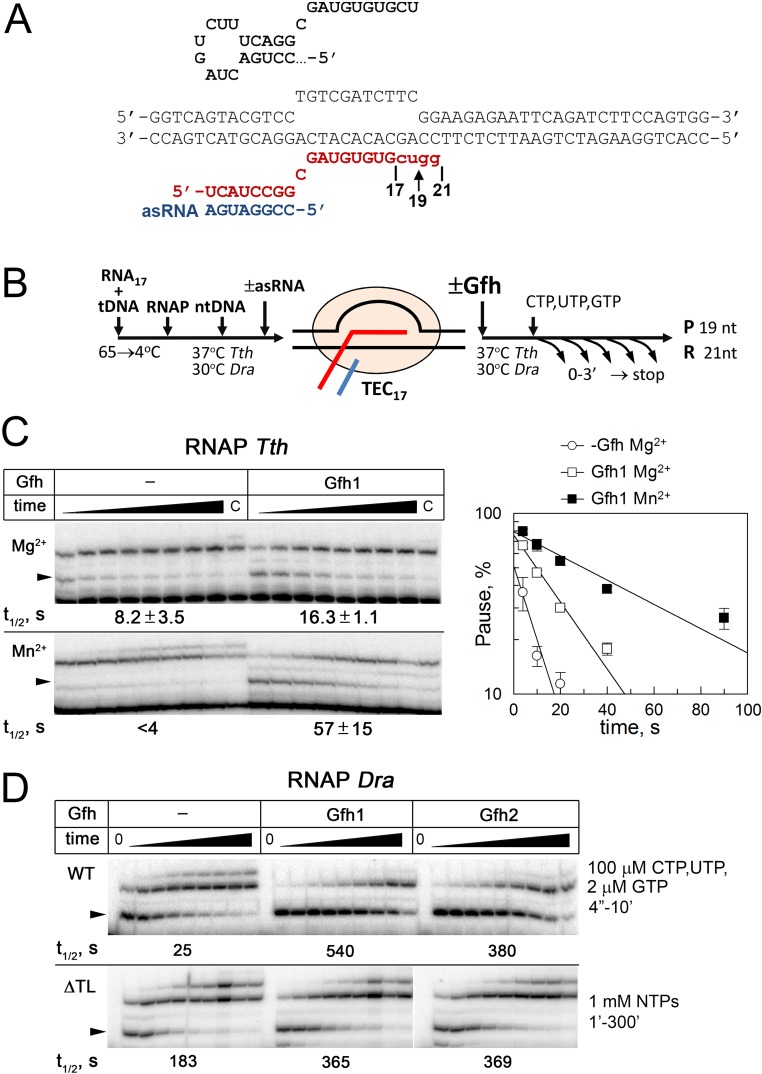
We tested RNAP pausing at a consensus pause site previously identified in genome-wide analyses of transcriptional pausing in Escherichia coli (30, 31) and at a hairpin-dependent hisP pause site. We used a scaffold-based approach to assemble TECs positioned immediately upstream of the pausing sites (Figs. S4 and S5). For the hisP pause site, the RNA hairpin formation was mimicked by the addition of a complementary antisense RNA oligonucleotide (32). Transcription was started by the addition of a limited NTP set, in either the absence or presence of Gfh factors, resulting in the appearance of paused TECs and read-through complexes stalled several nucleotides downstream of the pausing sites (Fig. 3 and Fig. S4C).
Fig. S4.
Effects of Gfh factors on consensus pausing by Dra RNAP. (A) Structure of the nucleic acid scaffold used for pause analysis. The consensus pause (CP) sequence (30) is shown on the top. The starting 15-nt RNA transcript is shown in uppercase letters, and the 3′-terminal nucleotides added during transcription are shown in lowercase letters. Positions of the 17-nt paused and 22-nt readthrough transcripts are indicated. (B) Schematic of the experiment. The 15-mer TEC containing 5′-labeled RNA was assembled from synthetic oligonucleotides, followed by the addition of Gfh (5 μM) and NTP substrates (GTP and CTP, 10 μM of each). (C) Kinetics of RNA synthesis in the presence of 10 mM Mg2+ (Upper) and Mn2+ (Lower) ions. Positions of the paused transcript are indicated with arrowheads. The pause t1/2 times are shown below the gels (also Table S1).
Fig. S5.
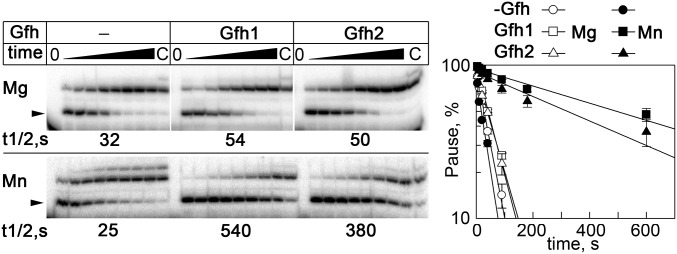
Analysis of hairpin-dependent pausing. (A) Structure of the nucleic acid scaffold used for pause analysis. The wild-type hisP hairpin RNA sequence is shown on the top. The starting 17-nt RNA transcript is shown in uppercase letters, and the 3′-terminal nucleotides added during transcription are shown in lowercase letters. Positions of the paused 19-nt and readthrough 21-nt transcripts are indicated. (B) Schematic of the pausing assay. The reactions were performed at 30 °C for Dra RNAP and 40 °C for Tth RNAP. (C) Effects of Tth Gfh1 on hairpin-dependent pausing by Tth RNAP. Analysis of hisP pausing was performed in the presence of the antisense RNA oligo with 10 mM MgCl2 (Upper) or MnCl2 (Lower). The paused transcripts are indicated with arrowheads. The pause t1/2 times are shown below the gels. (D) Comparison of hisP pausing by wild-type (WT; Upper) and ΔTL Dra (Lower) RNAPs in the presence of Mn2+ (the data for WT RNAP correspond to Fig. 3). Transcription with the ΔTL RNAP was performed in the presence of 1 mM NTPs, and the reaction times were from 1 to 300 min. The pause t1/2 times are shown below the gels.
Fig. 3.
Effects of Gfh factors on hairpin-dependent pausing by Dra RNAP. Analysis of hisP pausing was performed at 30 °C in the absence or presence of Gfh factors (5 μM) with 10 mM Mg2+ (Upper) or 10 mM Mn2+ (Lower). The paused transcripts are indicated with arrowheads. The pause t1/2 times are shown below the gels.
We found that Dra RNAP could recognize both types of the pausing signals in the presence of either Mg2+ or Mn2+ (Table 1 and Table S1). In particular, the t1/2 time of the hisP pause for Dra RNAP measured in the presence of Mg2+ was comparable to E. coli RNAP (t1/2 = 32 vs. 55 s), and was only slightly decreased in the presence of Mn2+ (t1/2 = 25 s). Previously, Tth RNAP was also shown to respond to the hairpin (11) and consensus (30) pause signals. We confirmed that Tth RNAP paused at the hisP site, although the pausing was inefficient in the presence of Mn2+ ions (Fig. S5C). Thus, these signals are universally recognized by RNAPs from different bacterial lineages.
Table 1.
Effects of Gfh factors on hisP pausing
| Reaction* | −Gfh | Gfh1 | Gfh2 | |||
| t1/2, s | Pmax, % | t1/2, s | Pmax, % | t1/2, s | Pmax, % | |
| WT/Mn2+ | 25.4 ± 2.5 | 80.6 ± 1.2 | 542 ± 86 | 94.6 ± 0.9 | 379 ± 90 | 90.4 ± 1.0 |
| 1 | 21.3 | 14.9 | ||||
| → Mg2+ | 31.6 ± 1.5 | 90.5 ± 1.4 | 44.9 ± 2.3 | 97.3 ± 3.0 | 48.0 ± 1.2 | 90.4 ± 3.6 |
| 1 | 1.4 | 1.5 | ||||
| → −asRNA | 14.4 ± 4.0 | 74.5 ± 2.4 | 106 ± 16 | 92.4 ± 1.0 | 79.2 ± 7.2 | 89.2 ± 1.8 |
| 1 | 7.4 | 5.5 | ||||
| → Gfh-Ala | — | — | 88.9 ± 19.1 | 79.9 ± 9.2 | 36.6 ± 5.3 | 79.1 ± 1.2 |
| 3.5 | 1.4 | |||||
| → ΔTL | 183 ± 13 | 77.4 ± 8.4 | 365 ± 20 | 81.2 ± 6.4 | 369 ± 24 | 79.4 ± 6.2 |
| 1 | 2.0 | 2.0 | ||||
Reaction conditions are shown in comparison to standard reactions performed with WT Dra RNAP and Gfh factors in the presence of antisense RNA (asRNA) and 10 mM Mn2+. The arrows indicate components substituted in each reaction. The reactions with the ΔTL RNAP were performed at high NTP concentrations. The numbers in bold indicate the t1/2 values in comparison to Gfh-less reactions. Pmax, projected maximal pausing at zero time point.
Table S1.
Effects of Gfh factors on consensus RNAP pausing
| Reaction* | −Gfh | Gfh1 | Gfh2 | |||
| t1/2, s | Pmax, % | t1/2, s | Pmax, % | t1/2, s | Pmax, % | |
| WT/Mn2+ | 8.4 ± 3.4 | 34.7 ± 14.3 | 60.3 ± 16.7 | 71.7 ± 1.9 | 25.5 ± 0.4 | 67.5 ± 1.4 |
| 1 | 7.2 | 3.0 | ||||
| → Mg2+ | 6.5 ± 2.5 | 37.0 ± 11.8 | 7.0 ± 1.1 | 43.0 ± 12.4 | 7.8 ± 0.5 | 39.2 ± 2.0 |
| 1 | 1.1 | 1.2 | ||||
| → ΔTL | 75.7 | 60.2 | 200 | 61.6 | 165 | 61.7 |
| 1 | 2.6 | 2.2 | ||||
Reaction conditions are shown in comparison to standard reactions (first line), which were performed with wild-type Dra RNAP in the presence of antisense RNA (asRNA) oligonucleotide, 10 mM Mn2+ ions, and 10 μM NTPs (GTP + CTP). The arrows indicate components substituted in each reaction. The reactions with the ΔTL RNAP were performed in the presence of 1 mM NTPs. Averages and SDs from two to four independent experiments are shown. The numbers in bold indicate the t1/2 values in comparison to Gfh-less reactions. Pmax, projected maximal pausing at zero time point.
When the reactions were performed with Mg2+ ions, Dra Gfh factors had essentially no effect on consensus pausing and only weakly stimulated hairpin-dependent pausing by Dra RNAP. In contrast, Gfh1 and Gfh2 dramatically stimulated both types of pausing in the presence of Mn2+ (Fig. 3, Fig. S4C, Table 1, and Table S1). In particular, the pause t1/2 times were increased three- to sevenfold for the consensus pause and 15- to 20-fold for the hairpin-dependent pause. In both cases, Gfh factors also significantly increased the pause efficiencies (Pmax, defined as the maximal pausing at zero time point; details are provided in SI Materials and Methods). Similarly, Tth Gfh1 stimulated hisP pausing by Tth RNAP, and the effect was much stronger in the presence of Mn2+ ions (twofold vs. >10-fold increase in the pause t1/2 in Mg2+ and Mn2+ reactions; Fig. S5C).
Role of the RNA Hairpin in Pause Stimulation by Dra Gfh Factors.
In subsequent experiments, we focused on the analysis of hairpin-dependent pausing by Dra RNAP, which was most strongly affected by Gfh factors. To reveal the role of RNA hairpin in the pause formation, we repeated the experiment without the addition of the antisense RNA oligonucleotide. In this case, the pause t1/2 in the absence of Gfh factors was decreased about twofold (14.4 s vs. 25.4 s in Mn2+ buffer). Furthermore, the pause-stimulatory effect of the Gfh factors was significantly smaller and was comparable to the consensus pause site (five- to sevenfold increase in the pause t1/2; Table 1).
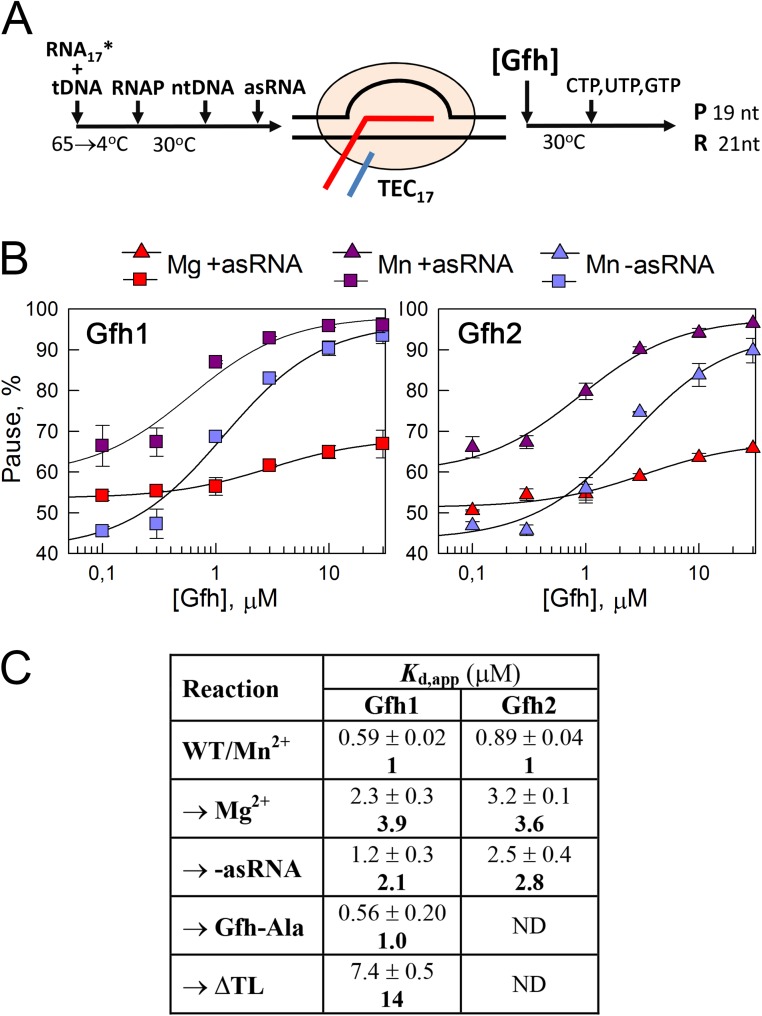
The RNA duplex was shown to affect the conformation of the paused complex significantly, including changes in the clamp domain position and TL folding (12, 13). Tth Gfh1 induces similar conformational changes of RNAP (9, 22). We therefore proposed that the RNA duplex formation may stimulate Gfh binding to the TEC. To test this hypothesis, we determined apparent Gfh affinities (Kd,apps) to the paused TEC by measuring the pausing efficiencies at different Gfh concentrations in the absence or presence of the antisense RNA oligonucleotide (Fig. S6 A and B). The RNA duplex indeed increased Gfh affinities to the TEC approximately two- to threefold (Kd,app = 0.6/0.9 μM and Kd,app = 1.2/2.5 μM for Gfh1/Gfh2 with and without the RNA duplex, respectively; Fig. S6C). The results suggest that Gfh factors preferably recognize a specific paused TEC conformation that is stabilized by the RNA hairpin.
Fig. S6.
Measurement of apparent Gfh affinities to the paused TEC. (A) Schematic of the assay. Gfh was added to the TEC17 at various concentrations in the presence of 10 mM MgCl2 or MnCl2, followed by the addition of nucleotide substrates. (B) Dependence of the pausing efficiencies on Gfh concentrations under different conditions. The pause efficiencies were measured at fixed time points at different Gfh concentrations, and the Kd,app values were calculated from the titration curves (details are provided in SI Materials and Methods). (C) Kd, app values for Gfh binding to the paused TEC. Reaction conditions are shown in comparison to standard reactions (first line), which were performed with wild-type Dra RNAP and Gfh factors in the presence of the antisense RNA (asRNA) oligonucleotide and 10 mM Mn2+ ions. The arrows indicate components substituted in each reaction. The reactions with the ΔTL RNAP were performed for prolonged times at elevated NTP concentrations (1 mM). Averages and SDs from two to four independent experiments are shown. The numbers in bold indicate the Kd,app values in comparison to the WT/Mn2+ reactions.
Gfh Factors Affect the Binding of Metal Ions to the TEC.
To reveal whether the inability of Gfh factors to stimulate pausing with Mg2+ ions might be explained by their inability to interact with the TEC under these conditions, we compared apparent Gfh affinities with the paused TEC in the presence of Mg2+ and Mn2+. Despite the very small effect of Gfh factors on pausing in the presence of magnesium, the Kd,app values could be reliably calculated for both Mg2+- and Mn2+-dependent reactions. We found that the affinities of both Gfh factors to the TEC were indeed three- to fourfold lower in the presence of Mg2+ (Kd,app = 2.3/3.2 μM for Gfh1/Gfh2; Fig. S6 B and C). However, these differences cannot fully explain the great differences in the pause stimulation, because Gfh concentrations (5 μM) in all reactions were higher than the Kd,app values for Gfh binding.
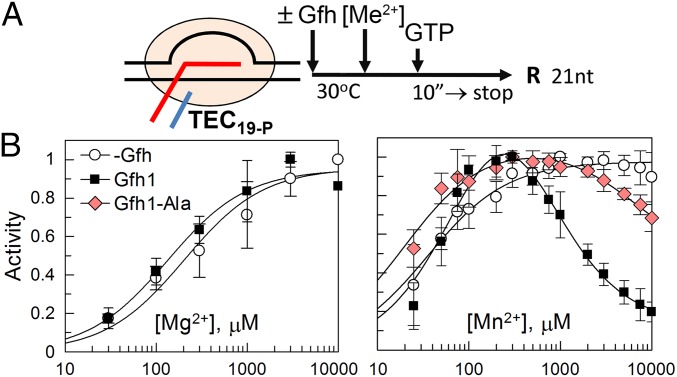
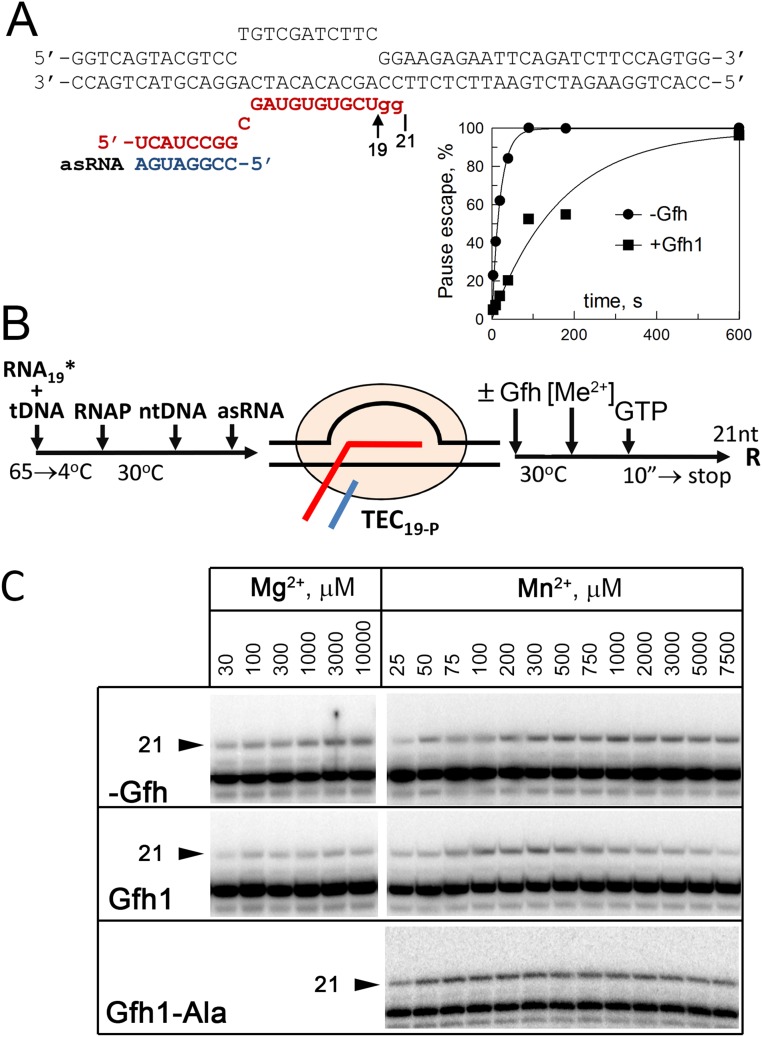
Previously, Gre and Gfh factors were proposed to chelate divalent metal ions in the RNAP active site by acidic residues in their NTD loops, resulting in activation of RNA cleavage (in the case of Gre factors) or inhibition of catalysis (in the case of Tth Gfh1). In particular, both E. coli GreA and Tth Gfh1 were shown to increase the affinity of metal ions to RNAP (15, 17, 20). To reveal possible effects of Dra Gfh factors on the catalytic metal binding, we measured apparent affinities of Mg2+ and Mn2+ ions to RNAP in the reaction of nucleotide addition in paused TEC in the absence and presence of Dra Gfh1. The experiment was performed with the TEC assembled exactly at the hisP site; control experiments confirmed that this complex adopts the paused state and is sensitive to the Gfh action (Fig. 4A and Fig. S7 A and B).
Fig. 4.
Effects of Gfh1 on apparent affinities of Me2+ ions to RNAP. (A) Schematic of the assay. The TEC19-P complex stalled at the pausing site was assembled from core RNAP and synthetic oligonucleotides, Gfh factors (5 μM) and metal ions were added, and RNA extension to 21 nt was measured after the addition of GTP (2 μM). (B) Efficiencies of RNA extension at various Mg2+ or Mn2+ concentrations in the absence or presence of Gfh1 and Gfh1-Ala. RNA extension at each Me2+ concentration was normalized to the maximal extension in the same titration experiment.
Fig. S7.
Analysis of Gfh effects on divalent metal ion binding. (A) Structure of the TEC used in the experiment (compare with Fig. S5B). The starting TEC contained 19-nt RNA corresponding to the site of pausing; the addition of GTP resulted in synthesis of 21-nt RNA transcript. The plot shows efficiencies of pause escape (normalized to the maximal RNA extension) by Dra RNAP in the TEC19-P measured in the absence and presence of Gfh1 (5 μM, 30 °C, 10 mM MnCl2). (B) Schematic of the experiment. The paused TEC19-P was incubated with Gfh (5 μM), and metal ions were added to various concentrations, followed by the addition of GTP (2 μM). The reaction was stopped after 10 s so that ≤25% of total RNA was extended. (C) Analysis of 21-nt RNA synthesis at different Me2+ concentrations in either the absence or presence of the Gfh1 factor and its mutant Gfh1-Ala variant. Note that absolute efficiencies of RNA extension in this experiment do not reflect any differences in the rates of RNA synthesis under corresponding conditions because of fluctuations in the efficiencies of TEC assembly in each particular reaction (because the reactions were performed in solution and not all RNA was bound by RNAP). To calculate the effects of Gfh on the apparent affinities of metal ions, the efficiencies of RNA extension at each Me2+ concentration were normalized to the maximal efficiency in the same titration experiment (Fig. 4B).
In the absence of Gfh factors, titration of Me2+ ions resulted in gradual activation of RNA synthesis, with Kd,app values of 200 ± 35 μM and 30 ± 12 μM for Mg2+ and Mn2+, respectively (Fig. 4B and Fig. S7C), in agreement with previous measurements for E. coli RNAP (33). Because the reaction requires the presence of two catalytic ions in the RNAP active site, these values likely correspond to the binding of the second ion, which is more weakly bound (33). Similar dependence was observed for Mg2+ reactions in the presence of Gfh1, which only slightly increased the metal affinity (Kd,app = 115 ± 50 μM; Fig. 4B, Left). In contrast, titration of Mn2+ in the presence of Gfh1 resulted in initial activation of RNA synthesis, followed by its inhibition at higher Mn2+ concentrations (Fig. 4B, Right and Fig. S7C). We interpret this inhibition as the binding of a third metal ion to the catalytically active TEC, resulting in the decrease in the reaction rate (details are provided in SI Materials and Methods). Based on this model, the Kd,apps of the second and third Mn2+ ions derived from the titration curve were 117 ± 11 μM and 520 ± 105 μM, respectively, which lie within the physiological range of manganese concentrations measured in Dra cells under stress conditions (23, 24).
To reveal the role of acidic residues at the NTD tip in pause stimulation by Gfh factors, we analyzed mutant variants of Gfh1 and Gfh2 with alanine substitutions of these residues (Gfh-Ala; Fig. 1C). The mutant Gfh factors had dramatically impaired ability to stimulate pausing (1.5- to 3.5-fold increase in the hisP t1/2 in comparison to 15- to 20-fold stimulation for wild-type factors; Table 1). At the same time, these substitutions did not change the Gfh affinity to RNAP (Kd,app for Gfh1-Ala of ∼0.6 μM in the presence of Mn2+; Fig. S6C). Titration of Mn2+ ions revealed that the Gfh1-Ala mutant impaired binding of the third Mn2+ ion (Kd,app > 5,000 μM, at least a 10-fold increase in comparison to wild-type Gfh1), without significantly affecting the binding of the second ion (Kd,app = 35 ± 14 μM; Fig. 4B and Fig. S7C). Thus, the Gfh NTD tip is probably involved in the third ion binding in the vicinity of the RNAP active site, and this ion contributes to transcription inhibition (Discussion).
The RNAP TL Is Involved in Stimulation of Transcriptional Pausing by Gfh Factors.
The TL in the RNAP active site has previously been implicated in functional interplay with Gre factors, which should replace it to stimulate the RNA cleavage reaction (16, 29, 34). To reveal whether the TL is important for pause stimulation by Gfh factors, we analyzed mutant Dra RNAP with a deletion in the TL (Fig. 1B). As expected, the deletion significantly increased the hisP pause t1/2 even when transcription was performed at much higher NTP concentrations in the presence of Mn2+, likely because it decreased the rate of RNA synthesis. However, the Gfh factors were almost unable to stimulate pausing by the mutant RNAP (only a twofold increase in the pause t1/2; Fig. S5D and Table 1). Titration of Gfh1 revealed that the TL deletion significantly decreased its affinity to the mutant RNAP (Kd,app was increased ∼14-fold; Fig. S6C). Thus, the TL is likely involved in Gfh binding to RNAP and in transcription inhibition.
Gfh Factors Stimulate Intrinsic Transcription Termination.
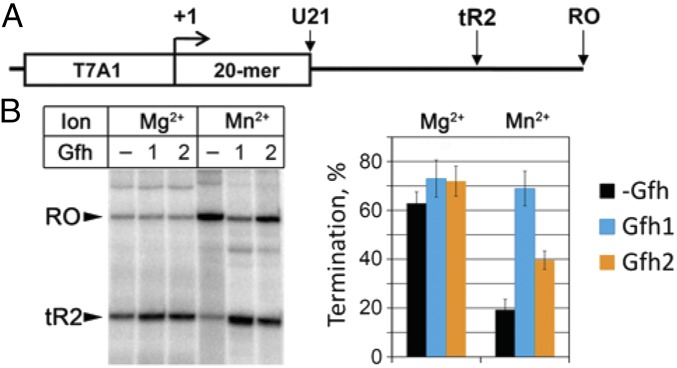
The strong stimulation of hairpin-dependent pausing by Gfh factors prompted us to investigate their effects on intrinsic termination, which also depends on the RNA hairpin formation. Both Gfh1 and Gfh2 only slightly increased termination efficiency on a model λ phage tR2 terminator in the presence of Mg2+ ions (Fig. 5). Interestingly, the termination efficiency by Dra RNAP was significantly decreased when transcription was performed with Mn2+ ions. The addition of Gfh factors restored termination in the presence of Mn2+ (Fig. 5B). Therefore, Gfh1 and Gfh2 can act as termination factors under these conditions.
Fig. 5.
Effects of Gfh factors on intrinsic transcription termination by Dra RNAP. (A) Scheme of the template. Positions of the T7A1 promoter, 20-nt U-less region, λ phage tR2 terminator, and run-off RNA are shown. (B) Analysis of transcription termination was performed in the presence of 10 mM Mg2+ or Mn2+ ions at 37 °C. Termination efficiencies are shown as the percentage of the terminated (tR2) transcripts relative to the sum of tR2 and RO RNAs.
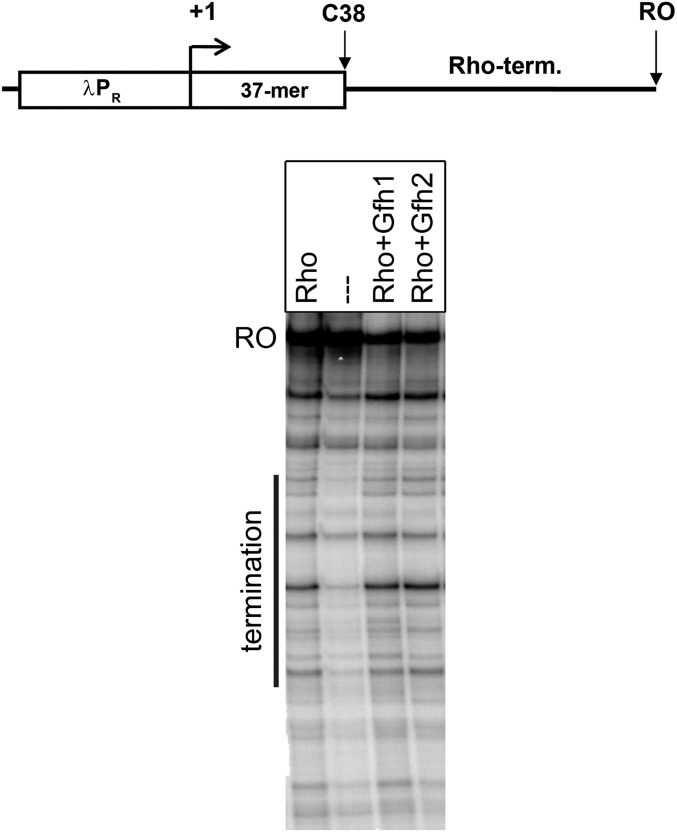
Finally, we tested the effects of Gfh factors on Rho-dependent termination. Because no Rho-dependent terminators from Dra have been described to date, we analyzed termination on a genomic Dra fragment corresponding to the E. coli trpt′ terminator located after the α-synthase gene in the conserved tryptophan operon. The addition of Dra Rho to the transcription reactions resulted in the appearance of terminated RNA products in the expected region (Fig. S8). Gfh1 and Gfh2 did not affect Rho-dependent termination. Thus, the Gfh factors do not generally make RNAP more prone to transcription termination, but act on only a subset of termination signals.
Fig. S8.
Analysis of the effects of Gfh factors on Rho-dependent transcription termination by Dra RNAP. (Upper) Scheme of the template. The λ phage PR promoter, 37-nt C-less region, 400-nt region containing putative trpt′ terminator (term.), and run-off RNA (RO) are indicated. (Lower) Reactions were performed in the presence of 10 mM Mn2+ ions at 20 °C; Gfh factors and Rho were added to 5 μM and 1 μM, respectively. The position of the termination region is shown by a vertical line on the left of the gel.
SI Materials and Methods
Proteins, Plasmids, and DNA Templates.
Wild-type Dra core RNAP was purified either from Dra cells or from Escherichia coli BL21(DE3) cells expressing all RNAP subunits from the plasmid pET28-rpoACBZ-Dra as described previously (27, 62). Recombinant ΔTL Dra RNAP (Δ1254–1272, replaced with three alanines) was purified in the same way. Tth RNAP was purified from strain Tth HB8rpoC::10H kindly provided by L. Minakhin, Waksman Institute of Microbiology, Piscataway, NJ, as described by Sevostyanova et al. (63). The Dra σA subunit, Tth σA subunit, and Dra GreA were expressed in E. coli from pET28-rpoD-Dra, pET28-rpoD-Tth, and pET28-Dra-GreA plasmids, respectively, and purified as described elsewhere (27, 62). The Dra Gfh1 and Gfh2 proteins and Tth Gfh1 containing C-terminal His6-tags were expressed from the pET28-Gfh1-Dra, pET28-Gfh2-Dra, and pET28-Gfh1-Tth vectors, respectively. E. coli BL21(DE3) cells containing these plasmids were grown at 37 °C, and protein expression was induced for 2 h by the addition of 1 mM isopropyl-β-d-thiogalactopyranoside at OD600 = 0.5. The cells were disrupted by sonication, and the Gfh factors were purified similar to the GreA factor (27) by Polymin P precipitation (from the flow-through fraction, to remove RNAP) and Ni2+-affinity chromatography. Mutant variants of Dra Gfh genes were obtained by site-directed PCR mutagenesis, and the proteins were expressed in E. coli and purified in the same way. Dra Rho factor was cloned into the pET28 vector; expressed in E. coli; and purified in the same way as Gfh factors, including Polymin P precipitation and Ni2+-affinity chromatography.
In Vitro Transcription.
Analysis of abortive initiation was performed in transcription buffers containing 40 mM KCl, 10 mM MgCl2 or MnCl2, and 40 mM Tris⋅HCl (for pH 7.9) or Pipes⋅NaOH (for pH 6.5). Dra RNAP holoenzyme (50 nM core RNAP plus 250 nM σA subunit) was incubated with a PCR DNA fragment (25 nM) containing the T7A1cons promoter (Fig. S1) for 10 min at 37 °C. Gfh factors were added to 5 μM, and the samples were incubated for another 5 min, followed by the addition of CpA (25 μM) and UTP (1 μM, with addition of α-[32P]-UTP). The reactions were stopped after 5 min by the addition of an equal volume of stop-solution (8 M urea, 20 mM EDTA), and RNA products were separated by 23% (wt/vol) PAGE and quantified with Typhoon 9500 scanner (GE Healthcare).
Measurements of the elongation rates and transcription termination by Dra RNAP were performed at 20 °C and 37 °C, respectively, at pH 7.9 as previously described (27, 35). TECs containing labeled RNA transcripts and stalled at positions +26 and +20 of the λ phage PR-rpoB (Fig. 2A) and T7A1-λtR2 (Fig. 5A) templates were obtained by transcription with limited substrate sets (C-less and U-less NTP mixtures, respectively); Gfh factors were added to 5 μM and incubated for 3 min, followed by the addition of all four NTPs (200 μM each) and heparin (10 μg/mL) to prevent reinitiation. Analysis of transcription elongation by Tth RNAP was performed in the same way at 40 °C. Rho-dependent termination by Dra RNAP was analyzed at 37 °C on a DNA template containing a putative Rho-dependent trpt′ terminator (positions 958,560–959,070 in the Dra genome) fused to the λ phage PR promoter containing a 37-nt C-less initially transcribed region (Fig. S7). TECs containing labeled 37-nt RNA were obtained by transcription with C-less NTP mix (with addition of α-[32P]-UTP). Rho (1 μM) and Gfh factors (5 μM) were added to the stalled TECs, and transcription was restarted by the addition of all four NTPs (2 mM ATP and 250 μM CTP, GTP, and UTP). The reactions were terminated after 10 min by the addition of the stop-solution, the samples were treated with chloroform, and RNA was ethanol-precipitated and analyzed by 10% (wt/vol) PAGE.
Analysis of single-nucleotide addition and RNA cleavage was performed on synthetic nucleic acid scaffold templates (Fig. S3) as previously described (27, 29). The reaction conditions are indicated in the legend for Fig. S3.
Analysis of RNAP Pausing.
Analysis of the consensus and hisP pausing was performed using synthetic templates shown in Figs. S4 and S5, respectively, in transcription buffers containing 40 mM Tris⋅HCl (pH 7.9), 40 mM KCl, and 10 mM MgCl2 or MnCl2, unless otherwise indicated. The TECs were assembled from DNA, RNA oligonucleotides, and core RNAP as previously described (32, 35). The RNA oligonucleotides were 5′-labeled with γ-[32P]-ATP and T4 polynucleotide kinase before TEC assembly. During analysis of hisP pausing, antisense RNA was added to 2 μM when required and the samples were incubated for 3 min at 30 °C. Gfh factors (5 μM) were added, and the samples were incubated for an additional 3 min. NTP substrates (2 μM GTP, 100 μM UTP and CTP for hisP pausing; 10 μM GTP and CTP for consensus pausing) were added, and the reactions were stopped after increasing time intervals (30 s to 10 min) by addition of the stop-solution. Control chase reactions were performed for 5 min with 500 μM NTPs to reveal the background fraction of inactive TECs. In the case of the ΔTL RNAP, transcription was performed for 1–300 min with 1 mM NTPs, without chase reactions. In the case of Tth RNAP and Tth Gfh1, transcription was performed at 37 °C in the presence of 100 μM CTP and UTP and 10 μM GTP.
The pause efficiencies at each time point were calculated as the ratio of the paused RNA product (minus the background value) to the sum of the paused and read-through products. Observed rate constants for the pause decay and corresponding pause t1/2 times were calculated by fitting the data to the single-exponential equation:
where P is the pausing efficiency, Pmax is the projected maximal pausing at zero time point, and kobs is the observed rate constant for the pause decay.
To measure apparent Gfh affinities to the paused TECs, the reactions with the hisP scaffold were performed in the same way, but the Gfh factors were added at various concentrations (from 0.1 to 30 μM). The samples were incubated for 3 min at 30 °C, and nucleotides were added [2 μM GTP, 100 μM UTP and CTP for wild-type RNAP, and 1 mM NTPS (all three) for ΔTL RNAPs]. The reactions were stopped after 10 s for wild-type RNAP in the presence MnCl2, after 30 s for wild-type RNAP in the presence of MgCl2, and after 2 min for the ΔTL RNAP. The pausing efficiencies were fit to the hyperbolic equation:
where P is the pausing efficiency at a given Gfh concentration, P0 is pausing in the absence of Gfh, A is the amplitude of changes in the pausing efficiency, and Kd,app is the apparent dissociation constant for Gfh binding to the TEC.
Calculation of Apparent Me2+ Affinities to RNAP.
Analysis of Gfh effects on apparent affinities of metal ions to RNAP was performed in the transcription complex stalled at the hisP site. TEC19-P was assembled from synthetic DNA oligonucleotides and 5′-labeled 19-nt RNA shown in Fig. S7A as described above. To confirm that this complex is in the paused state and is sensitive to Gfh1, the rate of RNA extension was measured after the addition of 2 μM GTP in both the absence and presence of Gfh1 (5 μM). The efficiencies of pause escape were normalized to the maximal RNA extension and fit to the single-exponential equation:
where R is the efficiency of RNA extension, Rmax is the maximal RNA extension, and kobs is the observed rate constant for the pause escape.
To calculate the apparent affinities of metal ions, TEC19-P was incubated with wild-type Gfh1 or the Gfh1-Ala mutant (5 μM) for 3 min at 37 °C, followed by the addition of 2 μM GTP. After 15 s, transcription was started by the addition of MgCl2 or MnCl2 at different concentrations (from 2 μM to 10 mM). The reactions were stopped after 10 s to ensure that the reaction did not go to completion; under these conditions, no more than 25% of the starting RNA was extended. For monophasic curves, the Kd,app values were calculated from the hyperbolic equation:
where R is the amount of extended RNA, Rmax is the maximal activity, and Kd-2 is the apparent dissociation constant for binding of the second catalytic metal ion to RNAP.
In the case of the biphasic curves observed in the presence of Gfh for the Mn2+ reactions, we assumed that inhibition results from the binding of a third Mn2+ ion to the active TECs that already contained both catalytic ions. Furthermore, this inhibition was not complete, and the TECs retained some activity even at saturating Mn2+ concentrations (Fig. 4B) because of pause escape and/or as a result of incomplete saturation of complexes with Gfh. Accordingly, the data were fit to the following equation:
where R is the amount of extended RNA, Rmax is the projected maximal RNA extension that would be observed in the case of no inhibition by Gfh, Imax is the maximal efficiency of TEC inhibition at saturating Mn2+ concentration, and Kd-2 and Kd-3 are apparent dissociation constants for the second and third metal ions. It should be noted that although other models (e.g., independent binding of the second and third ions) can also be used for data fitting, yielding slightly different Kd values, this difference would not affect the principal conclusion on the binding of an additional ion(s) in the presence of Gfh1. Additional experiments are required to distinguish between these models.
All data on the observed rate constants and Kd,apps presented in this paper are averages from two to four independent experiments with SDs.
Discussion
Gfh factors found in extremophilic bacteria from the Deinococcus/Thermus phylum belong to the notable family of transcription factors that bind within the secondary channel of RNAP and directly modulate its catalytic activity, but their functional role in transcription remains largely unknown. We demonstrate that the Gfh1 and Gfh2 proteins from the radioresistant bacterium Dra preferentially target transcription complexes involved in pausing and termination, and are able to halt RNAP at specific genomic sites. In particular, both Gfh1 and Gfh2 strongly enhance recognition of two common types of the pausing signals found in bacteria, consensus and hairpin-dependent pauses, by Dra RNAP. They also stimulate intrinsic termination, which likely proceeds through the same structural intermediate(s) as hairpin-dependent pausing (9, 35, 36). Previously, the pause RNA hairpin was shown to induce long-range conformational changes of the TEC, including opening of the clamp domain and inhibition of the TL folding (12, 13). These changes likely stabilize the initial elemental paused state, which is common for various pause types (11), and improve Gfh binding in the secondary RNAP channel, thus enhancing their effects on pausing. Because the Gfh factors inefficiently inhibit ΔTL Dra RNAP (whereas the TL deletion by itself stimulates pausing, likely by decreasing the rate of nucleotide incorporation), the unfolded TL likely contributes to Gfh binding, either directly or by changing the conformation of the secondary RNAP channel, and may be required for pause-associated TEC rearrangements. In the paused TECs, the secondary channel and the active center of RNAP are partially occluded by Gfh, the TL is unfolded, and the binding site of the incoming nucleotide is occupied by the kinked BH, thus inhibiting nucleotide addition (11, 22).
Notably, both consensus and hisP pauses that are stimulated by Gfh were shown to be resistant to the action of Gre factors (30, 37). GreA is also unable to target active TECs involved in RNA synthesis (9, 16). Conversely, the Dra Gfh proteins have only minor or no effects on intrinsic and GreA-stimulated RNA cleavage in mismatched TECs, although both types of factors have comparable affinities to their target TECs. Thus, the two classes of factors likely recognize distinct structural states of the TEC. Recent structural studies revealed different degrees of RNAP ratcheting (clamp opening and BH kinking) in GreA- and Gfh1-bound Tth TECs (9, 11, 22), probably explaining the different TEC specificities that we observed for the Dra GreA and Gfh factors. Although Tth Gfh1 can induce conversion of active TECs into the inactive conformation (9, 20), the Dra Gfh factors have likely evolved specifically to target paused TECs that are transiently formed during transcription, resulting in weaker average effects on transcription elongation in comparison to Tth Gfh1.
E. coli Gre factors and DksA were also shown to target different types of the TEC, thus avoiding direct competition between these factors during transcription elongation (38). Although the exact TEC state targeted by DksA remains to be identified, both DksA and Gre ultimately suppress formation of backtracked complexes by E. coli RNAP and decrease the transcription/replication conflicts (5, 39). In addition, DksA was proposed to decrease nucleotide misincorporation and associated transcriptional pausing (40, 41). The Gfh factors might also prevent TEC backtracking at the sites of pausing, by stabilizing inactive TECs that could be further disassembled by cellular machineries involved in DNA replication and repair (3).
The Dra Gfh factors depend on manganese ions for their effects on transcriptional pausing and intrinsic termination, but not on transcription initiation, suggesting that the mechanisms of inhibition might be different for different steps of transcription. Our experiments revealed that the Gfh factors likely promote binding of an additional (third) Mn2+ ion to RNAP, which may help to stabilize the paused TEC conformation. Alanine substitutions of acidic residues in the Gfh1 NTD interfered with its action and impaired the third ion affinity, suggesting that the NTD tip contributes to the Mn2+ binding at or near the RNAP active site, either directly or through interactions with other elements in the active site (20, 22) (Fig. 1B). Mn2+ ions, by themselves, may also affect the active site structure and the TL conformation (27, 42, 43), thus making RNAP more sensitive to the Gfh action and increasing its affinity to Gfh.
Manganese ions were shown to reach millimolar concentrations in Dra under stress conditions (23, 24). Importantly, manganese accumulation was also observed during stress response in other bacterial phyla as well as in eukaryotic organisms (44). Although the data on intracellular distribution of Mn2+ ions in Dra are controversial (26, 45–47), several reports suggest that a significant fraction of Mn2+ ions is bound to proteins (46, 48). Accordingly, Mn2+ ions were shown to activate several stress-induced enzymes in Dra, including DNA polymerases (49, 50), Mn2+-dependent superoxide dismutase (45, 48), and the PprI protease responsible for activation of dozens of stress-related genes through the cleavage of DdrO repressor (51). Our data suggest that RNAP is another likely target for manganese-dependent regulation in Dra. In support of this hypothesis, the expression of Gfh1 (DR1970) and GreA (DR1162) in Dra is strongly induced after irradiation, although Gfh2 (DR2375) is expressed constitutively (52, 53). Notably, the effects of Gfh1 on transcriptional pausing are stronger than the effects of Gfh2, likely resulting in large overall changes in transcription. Gfh-dependent and Mn-dependent stimulation of transcriptional pausing and termination may therefore serve as a specific mechanism of genetic regulation during stress response and may help to coordinate transcription with other genetic processes.
Although little is known about the role of manganese in stress resistance in Thermus, there is evidence that cellular Mn2+ concentrations are sufficiently high in Tth, and several Mn2+-dependent enzymes have been implicated in the oxidative stress response in this bacterium (54–56). We found that although Tth Gfh1 is functional in the presence of magnesium ions, its pause-stimulatory activity is also greatly enhanced by manganese. In line with this finding, Tth Gfh1 was shown to strongly inhibit exonucleolytic RNA cleavage by Tth RNAP in the presence of Mn2+ ions (21), and to have different effects on transcription initiation depending on divalent metal ions included in the reaction (20). We therefore propose that the activity of Gfh factors from various bacteria may be similarly modulated by the type of metal ions present in the transcription reaction.
Recently, a DksA2 paralog of DksA in Pseudomonas aeruginosa, which lacks the Zn-finger motif required for the function of the canonical factor, was shown to be induced under Zn-limiting conditions and to regulate transcription of virulence genes through binding to RNAP (57). Altogether, these observations extend the concept of factor-dependent exchange of RNAP activities in response to various regulatory stimuli (16, 21, 34), and suggest that various secondary channel regulators may play a general role in metal homeostasis and, more broadly, in adaptation to complex ecological niches.
At least some details of the pausing and termination pathways appear to be similar in bacterial and eukaryotic RNAPs. In particular, mammalian RNAP II was shown to respond to the bacterial consensus pause signal (30) and yeast RNAP III was suggested to recognize RNA hairpins during termination (58). Furthermore, an open, ratcheted RNAP conformation was observed in eukaryotic RNAP I (59, 60) and in archaeal RNAP (61). Thus, similar mechanisms of transcriptional regulation by secondary channel factors that affect RNAP conformation may function in other domains of life.
Materials and Methods
The detailed experimental procedures are described in SI Materials and Methods. RNAP pausing was analyzed using synthetic nucleic scaffold templates at 30 °C in in the presence of 10 mM MgCl2 or MnCl2 and 5 μM Gfh factors, unless otherwise indicated.
Acknowledgments
We thank S. Borukhov and two anonymous reviewers of the manuscript for helpful suggestions and discussions. This work was supported by the Russian Science Foundation (Grant 14-14-01074).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1603531113/-/DCSupplemental.
References
- 1.Zhang J, Landick R. A two-way street: Regulatory interplay between RNA polymerase and nascent RNA structure. Trends Biochem Sci. 2016;41(4):293–310. doi: 10.1016/j.tibs.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jonkers I, Lis JT. Getting up to speed with transcription elongation by RNA polymerase II. Nat Rev Mol Cell Biol. 2015;16(3):167–177. doi: 10.1038/nrm3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haines NM, Kim YI, Smith AJ, Savery NJ. Stalled transcription complexes promote DNA repair at a distance. Proc Natl Acad Sci USA. 2014;111(11):4037–4042. doi: 10.1073/pnas.1322350111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Proshkin S, Rahmouni AR, Mironov A, Nudler E. Cooperation between translating ribosomes and RNA polymerase in transcription elongation. Science. 2010;328(5977):504–508. doi: 10.1126/science.1184939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dutta D, Shatalin K, Epshtein V, Gottesman ME, Nudler E. Linking RNA polymerase backtracking to genome instability in E. coli. Cell. 2011;146(4):533–543. doi: 10.1016/j.cell.2011.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Epshtein V, et al. UvrD facilitates DNA repair by pulling RNA polymerase backwards. Nature. 2014;505(7483):372–377. doi: 10.1038/nature12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belogurov GA, Artsimovitch I. Regulation of transcript elongation. Annu Rev Microbiol. 2015;69:49–69. doi: 10.1146/annurev-micro-091014-104047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts JW, Shankar S, Filter JJ. RNA polymerase elongation factors. Annu Rev Microbiol. 2008;62:211–233. doi: 10.1146/annurev.micro.61.080706.093422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sekine S, Murayama Y, Svetlov V, Nudler E, Yokoyama S. The ratcheted and ratchetable structural states of RNA polymerase underlie multiple transcriptional functions. Mol Cell. 2015;57(3):408–421. doi: 10.1016/j.molcel.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 10.Vassylyev DG, et al. Structural basis for substrate loading in bacterial RNA polymerase. Nature. 2007;448(7150):163–168. doi: 10.1038/nature05931. [DOI] [PubMed] [Google Scholar]
- 11.Weixlbaumer A, Leon K, Landick R, Darst SA. Structural basis of transcriptional pausing in bacteria. Cell. 2013;152(3):431–441. doi: 10.1016/j.cell.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hein PP, et al. RNA polymerase pausing and nascent-RNA structure formation are linked through clamp-domain movement. Nat Struct Mol Biol. 2014;21(9):794–802. doi: 10.1038/nsmb.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nayak D, Voss M, Windgassen T, Mooney RA, Landick R. Cys-pair reporters detect a constrained trigger loop in a paused RNA polymerase. Mol Cell. 2013;50(6):882–893. doi: 10.1016/j.molcel.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toulokhonov I, Zhang J, Palangat M, Landick R. A central role of the RNA polymerase trigger loop in active-site rearrangement during transcriptional pausing. Mol Cell. 2007;27(3):406–419. doi: 10.1016/j.molcel.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Laptenko O, Lee J, Lomakin I, Borukhov S. Transcript cleavage factors GreA and GreB act as transient catalytic components of RNA polymerase. EMBO J. 2003;22(23):6322–6334. doi: 10.1093/emboj/cdg610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roghanian M, Yuzenkova Y, Zenkin N. Controlled interplay between trigger loop and Gre factor in the RNA polymerase active centre. Nucleic Acids Res. 2011;39(10):4352–4359. doi: 10.1093/nar/gkq1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sosunova E, et al. Donation of catalytic residues to RNA polymerase active center by transcription factor Gre. Proc Natl Acad Sci USA. 2003;100(26):15469–15474. doi: 10.1073/pnas.2536698100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laptenko O, Borukhov S. Biochemical assays of Gre factors of Thermus thermophilus. Methods Enzymol. 2003;371:219–232. doi: 10.1016/S0076-6879(03)71016-7. [DOI] [PubMed] [Google Scholar]
- 19.Hogan BP, Hartsch T, Erie DA. Transcript cleavage by Thermus thermophilus RNA polymerase. Effects of GreA and anti-GreA factors. J Biol Chem. 2002;277(2):967–975. doi: 10.1074/jbc.M108737200. [DOI] [PubMed] [Google Scholar]
- 20.Laptenko O, et al. pH-dependent conformational switch activates the inhibitor of transcription elongation. EMBO J. 2006;25(10):2131–2141. doi: 10.1038/sj.emboj.7601094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Symersky J, et al. Regulation through the RNA polymerase secondary channel. Structural and functional variability of the coiled-coil transcription factors. J Biol Chem. 2006;281(3):1309–1312. doi: 10.1074/jbc.C500405200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tagami S, et al. Crystal structure of bacterial RNA polymerase bound with a transcription inhibitor protein. Nature. 2010;468(7326):978–982. doi: 10.1038/nature09573. [DOI] [PubMed] [Google Scholar]
- 23.Daly MJ, et al. Accumulation of Mn(II) in Deinococcus radiodurans facilitates gamma-radiation resistance. Science. 2004;306(5698):1025–1028. doi: 10.1126/science.1103185. [DOI] [PubMed] [Google Scholar]
- 24.Daly MJ, et al. Small-molecule antioxidant proteome-shields in Deinococcus radiodurans. PLoS One. 2010;5(9):e12570. doi: 10.1371/journal.pone.0012570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agapov AA, Kulbachinskiy AV. Mechanisms of stress resistance and gene regulation in the radioresistant bacterium Deinococcus radiodurans. Biochemistry (Mosc) 2015;80(10):1201–1216. doi: 10.1134/S0006297915100016. [DOI] [PubMed] [Google Scholar]
- 26.Slade D, Radman M. Oxidative stress resistance in Deinococcus radiodurans. Microbiol Mol Biol Rev. 2011;75(1):133–191. doi: 10.1128/MMBR.00015-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Esyunina D, et al. Lineage-specific variations in the trigger loop modulate RNA proofreading by bacterial RNA polymerases. Nucleic Acids Res. 2016;44(3):1298–1308. doi: 10.1093/nar/gkv1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miropolskaya N, Artsimovitch I, Klimasauskas S, Nikiforov V, Kulbachinskiy A. Allosteric control of catalysis by the F loop of RNA polymerase. Proc Natl Acad Sci USA. 2009;106(45):18942–18947. doi: 10.1073/pnas.0905402106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miropolskaya N, et al. Interplay between the trigger loop and the F loop during RNA polymerase catalysis. Nucleic Acids Res. 2014;42(1):544–552. doi: 10.1093/nar/gkt877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larson MH, et al. A pause sequence enriched at translation start sites drives transcription dynamics in vivo. Science. 2014;344(6187):1042–1047. doi: 10.1126/science.1251871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vvedenskaya IO, et al. Interactions between RNA polymerase and the “core recognition element” counteract pausing. Science. 2014;344(6189):1285–1289. doi: 10.1126/science.1253458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolb KE, Hein PP, Landick R. Antisense oligonucleotide-stimulated transcriptional pausing reveals RNA exit channel specificity of RNA polymerase and mechanistic contributions of NusA and RfaH. J Biol Chem. 2014;289(2):1151–1163. doi: 10.1074/jbc.M113.521393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sosunov V, et al. The involvement of the aspartate triad of the active center in all catalytic activities of multisubunit RNA polymerase. Nucleic Acids Res. 2005;33(13):4202–4211. doi: 10.1093/nar/gki688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zenkin N. Multiple personalities of the RNA polymerase active centre. Microbiology. 2014;160(Pt 7):1316–1320. doi: 10.1099/mic.0.079020-0. [DOI] [PubMed] [Google Scholar]
- 35.Esyunina D, Klimuk E, Severinov K, Kulbachinskiy A. Distinct pathways of RNA polymerase regulation by a phage-encoded factor. Proc Natl Acad Sci USA. 2015;112(7):2017–2022. doi: 10.1073/pnas.1416330112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peters JM, Vangeloff AD, Landick R. Bacterial transcription terminators: The RNA 3′-end chronicles. J Mol Biol. 2011;412(5):793–813. doi: 10.1016/j.jmb.2011.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feng GH, Lee DN, Wang D, Chan CL, Landick R. GreA-induced transcript cleavage in transcription complexes containing Escherichia coli RNA polymerase is controlled by multiple factors, including nascent transcript location and structure. J Biol Chem. 1994;269(35):22282–22294. [PubMed] [Google Scholar]
- 38.Furman R, Sevostyanova A, Artsimovitch I. Transcription initiation factor DksA has diverse effects on RNA chain elongation. Nucleic Acids Res. 2012;40(8):3392–3402. doi: 10.1093/nar/gkr1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y, et al. DksA guards elongating RNA polymerase against ribosome-stalling-induced arrest. Mol Cell. 2014;53(5):766–778. doi: 10.1016/j.molcel.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roghanian M, Zenkin N, Yuzenkova Y. Bacterial global regulators DksA/ppGpp increase fidelity of transcription. Nucleic Acids Res. 2015;43(3):1529–1536. doi: 10.1093/nar/gkv003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Satory D, et al. DksA involvement in transcription fidelity buffers stochastic epigenetic change. Nucleic Acids Res. 2015;43(21):10190–10199. doi: 10.1093/nar/gkv839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Čabart P, Jin H, Li L, Kaplan CD. Activation and reactivation of the RNA polymerase II trigger loop for intrinsic RNA cleavage and catalysis. Transcription. 2014;5(3):e28869. doi: 10.4161/trns.28869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walmacq C, et al. Rpb9 subunit controls transcription fidelity by delaying NTP sequestration in RNA polymerase II. J Biol Chem. 2009;284(29):19601–19612. doi: 10.1074/jbc.M109.006908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Culotta VC, Daly MJ. Manganese complexes: Diverse metabolic routes to oxidative stress resistance in prokaryotes and yeast. Antioxid Redox Signal. 2013;19(9):933–944. doi: 10.1089/ars.2012.5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bruch EM, de Groot A, Un S, Tabares LC. The effect of gamma-ray irradiation on the Mn(II) speciation in Deinococcus radiodurans and the potential role of Mn(II)-orthophosphates. Metallomics. 2015;7(5):908–916. doi: 10.1039/c5mt00009b. [DOI] [PubMed] [Google Scholar]
- 46.Bruch EM, Thomine S, Tabares LC, Un S. Variations in Mn(II) speciation among organisms: what makes D. radiodurans different. Metallomics. 2015;7(1):136–144. doi: 10.1039/c4mt00265b. [DOI] [PubMed] [Google Scholar]
- 47.Sharma A, et al. Responses of Mn2+ speciation in Deinococcus radiodurans and Escherichia coli to γ-radiation by advanced paramagnetic resonance methods. Proc Natl Acad Sci USA. 2013;110(15):5945–5950. doi: 10.1073/pnas.1303376110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tabares LC, Un S. In situ determination of manganese(II) speciation in Deinococcus radiodurans by high magnetic field EPR: Detection of high levels of Mn(II) bound to proteins. J Biol Chem. 2013;288(7):5050–5055. doi: 10.1074/jbc.C112.444992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heinz K, Marx A. Lesion bypass activity of DNA polymerase A from the extremely radioresistant organism Deinococcus radiodurans. J Biol Chem. 2007;282(15):10908–10914. doi: 10.1074/jbc.M611404200. [DOI] [PubMed] [Google Scholar]
- 50.Lecointe F, Shevelev IV, Bailone A, Sommer S, Hübscher U. Involvement of an X family DNA polymerase in double-stranded break repair in the radioresistant organism Deinococcus radiodurans. Mol Microbiol. 2004;53(6):1721–1730. doi: 10.1111/j.1365-2958.2004.04233.x. [DOI] [PubMed] [Google Scholar]
- 51.Wang Y, et al. Protease activity of PprI facilitates DNA damage response: Mn2+-dependence and substrate sequence-specificity of the proteolytic reaction. PLoS One. 2015;10(3):e0122071. doi: 10.1371/journal.pone.0122071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang C, et al. Proteomic analysis of Deinococcus radiodurans recovering from gamma-irradiation. Proteomics. 2005;5(1):138–143. doi: 10.1002/pmic.200300875. [DOI] [PubMed] [Google Scholar]
- 53.Liu Y, et al. Transcriptome dynamics of Deinococcus radiodurans recovering from ionizing radiation. Proc Natl Acad Sci USA. 2003;100(7):4191–4196. doi: 10.1073/pnas.0630387100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kondo N, et al. Insights into different dependence of dNTP triphosphohydrolase on metal ion species from intracellular ion concentrations in Thermus thermophilus. Extremophiles. 2008;12(2):217–223. doi: 10.1007/s00792-007-0118-6. [DOI] [PubMed] [Google Scholar]
- 55.Agari Y, Kuramitsu S, Shinkai A. Identification of novel genes regulated by the oxidative stress-responsive transcriptional activator SdrP in Thermus thermophilus HB8. FEMS Microbiol Lett. 2010;313(2):127–134. doi: 10.1111/j.1574-6968.2010.02133.x. [DOI] [PubMed] [Google Scholar]
- 56.Ebihara A, et al. Roles of Mn-catalase and a possible heme peroxidase homologue in protection from oxidative stress in Thermus thermophilus. Extremophiles. 2015;19(4):775–785. doi: 10.1007/s00792-015-0753-2. [DOI] [PubMed] [Google Scholar]
- 57.Blaby-Haas CE, Furman R, Rodionov DA, Artsimovitch I, de Crécy-Lagard V. Role of a Zn-independent DksA in Zn homeostasis and stringent response. Mol Microbiol. 2011;79(3):700–715. doi: 10.1111/j.1365-2958.2010.07475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nielsen S, Yuzenkova Y, Zenkin N. Mechanism of eukaryotic RNA polymerase III transcription termination. Science. 2013;340(6140):1577–1580. doi: 10.1126/science.1237934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Engel C, Sainsbury S, Cheung AC, Kostrewa D, Cramer P. RNA polymerase I structure and transcription regulation. Nature. 2013;502(7473):650–655. doi: 10.1038/nature12712. [DOI] [PubMed] [Google Scholar]
- 60.Fernández-Tornero C, et al. Crystal structure of the 14-subunit RNA polymerase I. Nature. 2013;502(7473):644–649. doi: 10.1038/nature12636. [DOI] [PubMed] [Google Scholar]
- 61.Jun SH, et al. The X-ray crystal structure of the euryarchaeal RNA polymerase in an open-clamp configuration. Nat Commun. 2014;5:5132. doi: 10.1038/ncomms6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Esyunina DM, Kulbachinskiy AV. Purification and characterization of recombinant Deinococcus radiodurans RNA polymerase. Biochemistry (Mosc) 2015;80(10):1271–1278. doi: 10.1134/S0006297915100077. [DOI] [PubMed] [Google Scholar]
- 63.Sevostyanova A, et al. Temporal regulation of viral transcription during development of Thermus thermophilus bacteriophage phiYS40. J Mol Biol. 2007;366(2):420–435. doi: 10.1016/j.jmb.2006.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]