Significance
Bacterial viruses often express functions that affect host RNA synthesis. Phage HK022 Nun protein interacts with the Escherichia coli ternary elongation complex to arrest transcription elongation. This paper probes the structural basis of this interaction and shows that Nun approaches both ends of the RNA:DNA hybrid within the ternary elongation complex, making it unique among known transcription factors.
Keywords: Nun protein, Gre factors, transcription arrest, phage HK022, ternary elongation complex
Abstract
The coliphage HK022 protein Nun transcription elongation arrest factor inhibits RNA polymerase translocation. In vivo, Nun acts specifically to block transcription of the coliphage λ chromosome. Using in vitro assays, we demonstrate that Nun cross-links RNA in an RNA:DNA hybrid within a ternary elongation complex (TEC). Both the 5′ and the 3′ ends of the RNA cross-link Nun, implying that Nun contacts RNA polymerase both at the upstream edge of the RNA:DNA hybrid and in the vicinity of the catalytic center. This finding suggests that Nun may inhibit translocation by more than one mechanism. Transcription elongation factor GreA efficiently blocked Nun cross-linking to the 3′ end of the transcript, whereas the highly homologous GreB factor did not. Surprisingly, both factors strongly suppressed Nun cross-linking to the 5′ end of the RNA, suggesting that GreA and GreB can enter the RNA exit channel as well as the secondary channel, where they are known to bind. These findings extend the known action mechanism for these ubiquitous cellular factors.
Transcription elongation by RNA polymerase (RNAP) is regulated by pausing and termination signals encoded in the transcribed DNA sequence, as well as by numerous protein factors and free RNA molecules. In transcribing ternary elongation complexes (TEC) (Fig. 1A), the RNA product remains transiently bound to a template DNA strand, forming a RNA:DNA hybrid 9- to 10-bp long (1). Transcription, although processive, is frequently interrupted by RNAP pausing. Mechanisms of such pausing include intrinsic pausing (2, 3) and backtracking (1, 4, 5), the process by which RNAP reverse-translocates. During backtracking, the RNA 3′ terminus moves from the catalytic center into the secondary channel, thus rendering TEC catalytically inactive. Bacterial transcription factors (TFs) GreA and GreB (6–10), as well as the analogous eukaryotic TFIIS factor (11, 12), rescue backtracked TEC by promoting degradation of this disengaged RNA, thus restoring the 3′ end of the transcript within the RNAP active center and allowing transcription to proceed.
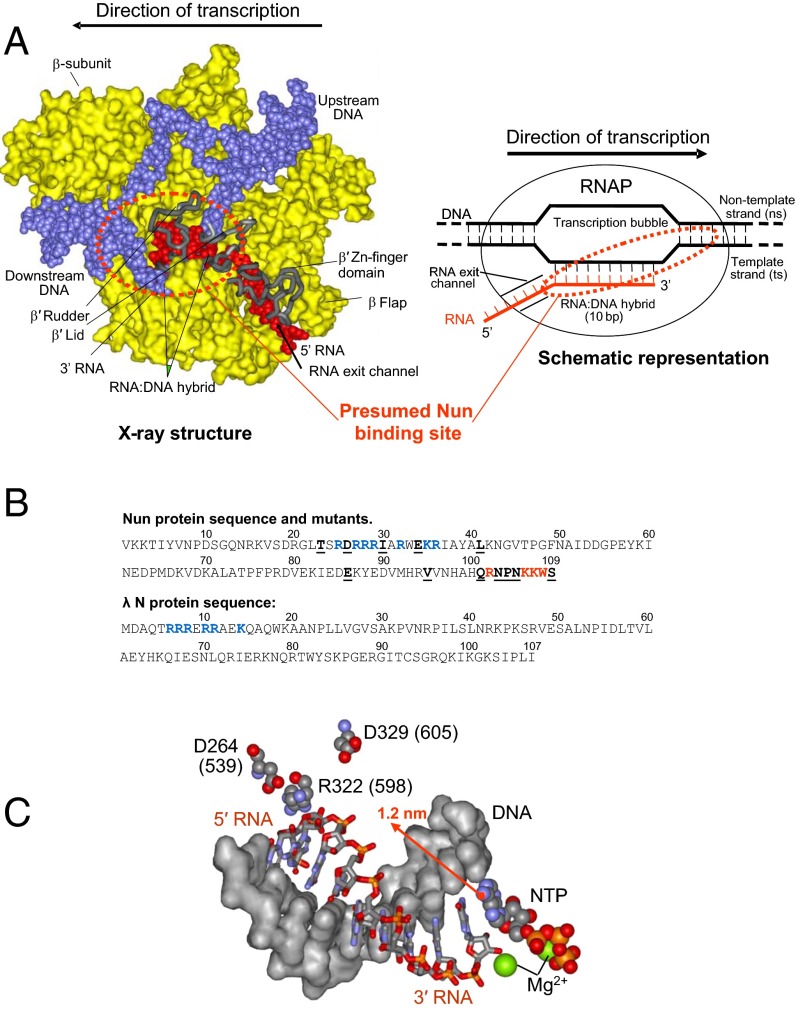
Fig. 1.
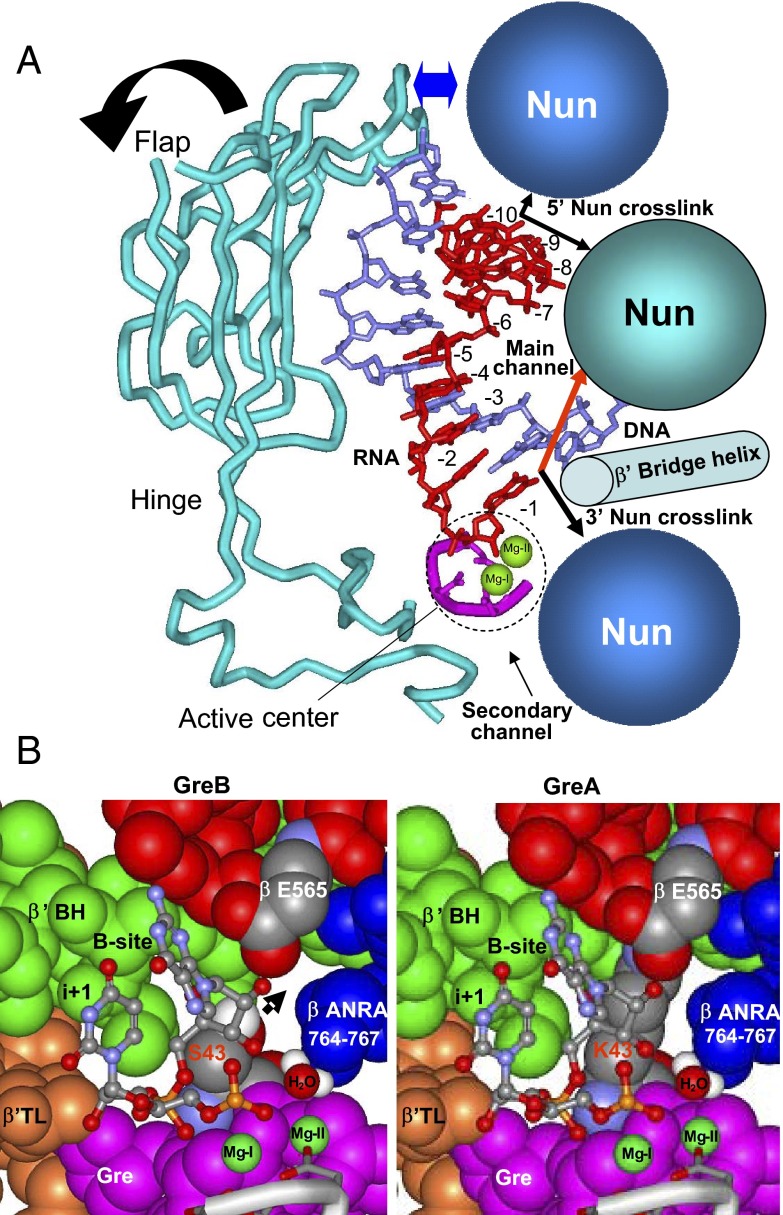
Nun interactions in transcription elongation complex. (A, Left) X-ray model of TEC. DNA is shown in blue, RNA in red, RNAP proteins in yellow (surface representation). Most of the β′ subunit is omitted for clarity. Main features of TEC are marked. The dotted circle shows the Nun binding sites inferred from our cross-linking data. A schematic representation of TEC featuring its nucleic acid scaffold is shown on the right. (B) Sequence of Nun and λ N proteins. The residues constituting the ARM motif are rendered in blue. Residues that when mutated display strong phenotypes are highlighted in red. Other mapped positions of Nun mutations are underlined. (C) Spatial arrangement of RNA:DNA hybrid of TEC and mutations in β′ subunit affecting Nun function. Residue numbering is as in Tth RNAP and in E. coli (in parentheses). The arrow indicates a possible position of the 3′ RNA-attached cross-linking group extruding into RNAP main channel.
The 12-kDa Nun protein of coliphage HK022 excludes phage λ superinfection by specifically blocking TEC translocation on λ DNA (13, 14). Nun competes with the λ N protein, which, conversely, enhances elongation and suppresses transcription termination. The amino-terminal RNA-binding domain (arginine-rich motif, or ARM) (Fig. 1B) is similar and plays the same role for both proteins: that of binding to the λ nascent transcript NUT sequence. The carboxyl-terminal domain (CTD) of Nun or λ N interacts with RNAP to block or stimulate transcription elongation, respectively (13). At high Nun concentrations, however, transcription arrest occurs in vitro or in vivo on templates lacking the λ NUT sequence (14, 15). Interaction between the Nun CTD and RNAP was probed using several different approaches. The CTD conjugated to a photoreactive group cross-links with a DNA template ∼9-bp downstream to the catalytic center in a paused TEC (16). Nun may also interact with RNAP near the 5′ edge of the RNA:DNA hybrid (Fig. 1C). This finding is supported by the fact that in a nuclease toe-printing assay, Nun protects an additional 1–2 nt upstream to the pretranlocated rear-end boundary of TEC. This toe-print is only found at sites of Nun-mediated arrest (14). Furthermore, mutations in the RNAP β′ subunit near the upstream end of the transcription bubble (Fig. 1C) suppress Nun binding and arrest (14, 17).
Despite the numerous biochemical studies of the effect of Nun on transcription, little is known about the structural mechanism of Nun action. In the present study we used a cross-linking approach to probe Nun interactions with the RNA transcript and TEC.
Results
Nun Contacts TEC in the RNA Exit Channel and Near the Active Center.
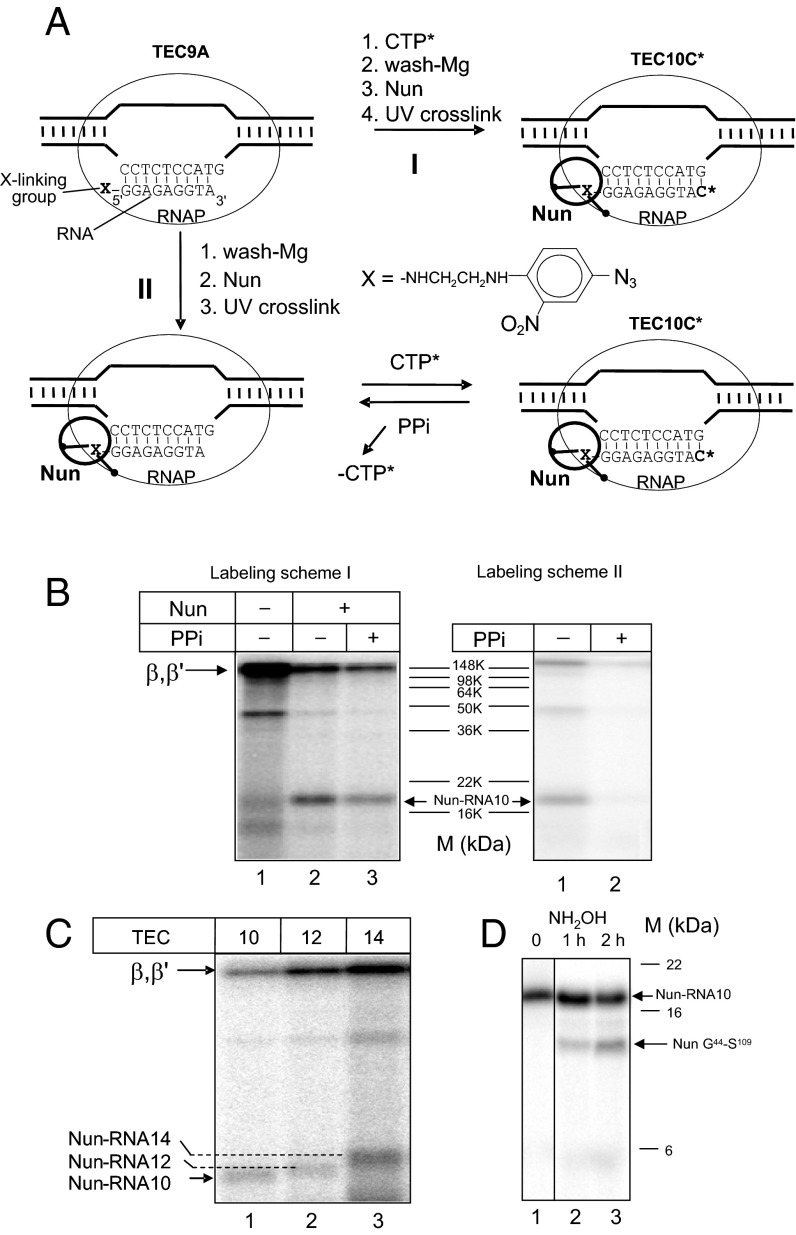
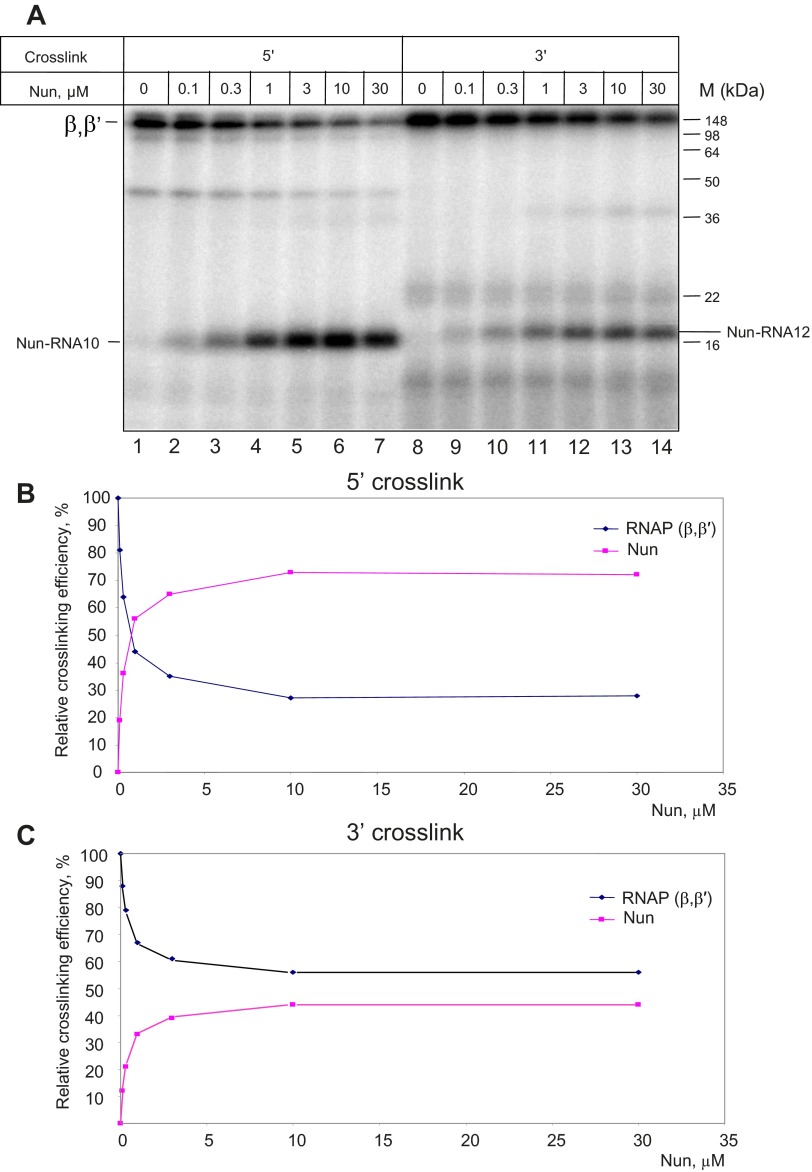
A previous study (14) suggested that Nun acts by binding to TEC at the upstream edge of the RNA:DNA hybrid. We tested this prediction using a cross-linking assay. In one version of this assay, TEC was assembled with a 9-nt RNA (TEC9A) bearing a photoreactive group attached to the 5′ phosphate. This assembly was followed by elongation with the next cognate [α-32P] CTP substrate, addition of Nun, and induction of the cross-link by UV irradiation (Fig. 2A, scheme I). In the second approach (Fig. 2A, scheme II), Nun was cross-linked to TEC9A followed by elongation with the [α-32P] CTP substrate. The latter protocol (18, 19) specifically detects TEC that retain catalytic competence after cross-link formation. Nonspecific cross-links resulting from occasional dissociation of RNA from TEC, as well as cross-links in intact but catalytically inactive complexes, are not detected. As seen in Fig. 2B, Left, lane 2, one additional radioactive product appeared upon TEC10C irradiation in the presence of Nun. The size of this product (∼18 kDa) correlates with the predicted mobility of Nun cross-linked to the RNA primer. The slow migrating cross-linked products represent the labeled RNAP large subunits, β and β′ (20). The 18-kDa product disappeared upon treatment with protease (Fig. S1, lane 9), indicating that it consists of a protein–RNA complex. Essentially the same results were seen when the second cross-linking scheme was applied (Fig. 2B, Right, lanes 1 and 2), except that pyrophosphate treatment almost entirely removed radioactivity from the labeled cross-linking products. Because pyrophosphorolysis of nascent RNA occurs exclusively in TEC active centers, this confirms that this labeling protocol detects only functional complexes. These results indicate that Nun interacts with transcribing TEC at the upstream edge of the RNA:DNA hybrid.
Fig. 2.
The 5′ Nun cross-linking. (A) Cross-linking strategy. Scheme I: Photoreactive TEC9 was first elongated by a radioactive substrate followed by Nun addition and UV irradiation. Scheme II: Nun was added to TEC9 assembled with RNA bearing a 5′ photoreactive group, followed by UV irradiation and incubation with radioactive substrate. (B) Phosphoimage of the reaction mixtures treated as in A after SDS denaturing and PAGE separation. Positions of RNAP cross-linked subunits and Nun are indicated along with protein size markers. (C) Nun cross-linking in TEC10, -11, and -12 following scheme I (A). (D) Partial hydroxylamine cleavage of the purified Nun cross-linked product. Arrow indicated the position of the cleavage site in Nun sequence.
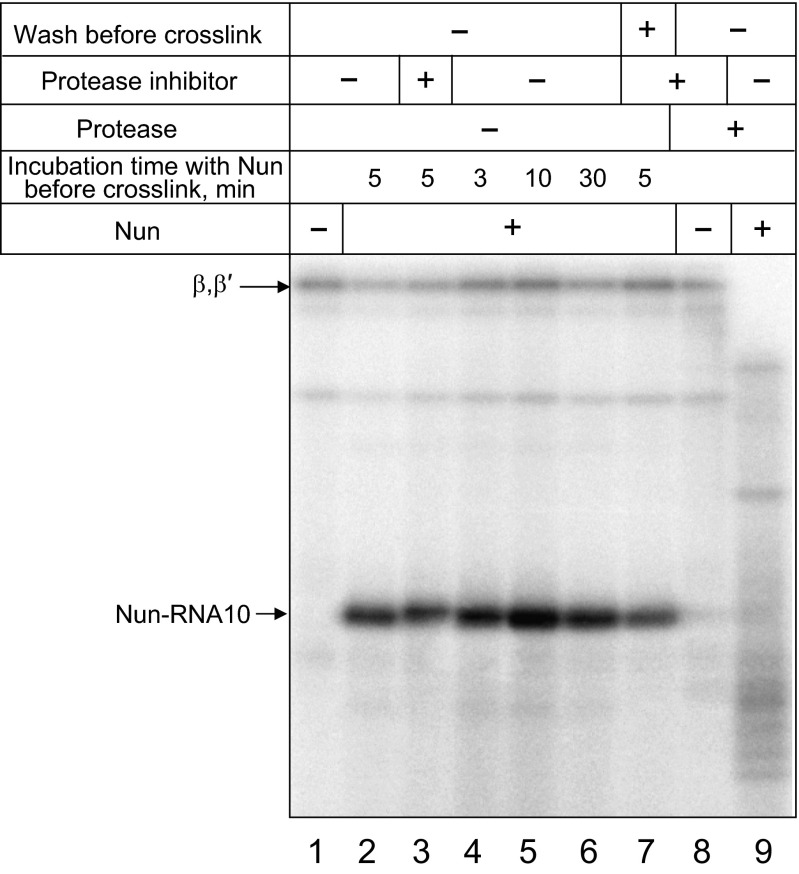
Fig. S1.
Nun cross-linking under incubation conditions that affect protein stability.
We next performed Nun cross-linking assays in TECs with longer RNA products (12 nt and 14 nt RNA) using scheme I. As seen in Fig. 2C, Nun cross-linked to all these labeled RNAs. Notably, the size of the cross-linked product increased as a function of RNA size, reflecting the difference in the length of the cross-linked RNA transcript. Thus, Nun can bind to TEC with various RNA lengths. We excluded the possibility that the 5′ cross-linked band was a proteolytic fragment of labeled RNAP subunits that might be generated by traces of protease in Nun preparation. As seen in Fig. S1, no difference in labeled 18-kDa product intensity was observed in the presence of protease inhibitor added to the labeling mixture (compare lanes 2 and 3 in Fig. S1) when the incubation time of TEC with Nun was increased (compare lanes 4–6 in Fig. S1), or when the beads containing Nun-bound TEC were washed before the cross-link was induced. In the positive control, added protease efficiently degraded labeled TEC (lane 9 in Fig. S1), and protease inhibitor prevented TEC degradation (compare lanes 1 and 8 in Fig. S1). Finally, we treated the purified 18-kDa radioactive product with hydroxylamine, which cleaves peptides between asparagine and glycine residues. We recovered a single radioactive product, whose electrophoretic mobility was consistent with cleavage at unique site (N43-G44) in the Nun sequence (Fig. 2D). The size of the product (∼14 kDa) suggests that the cross-linking site resides in the C-terminal Nun fragment, between G44 and S109.
These results strongly suggest that the observed Nun-dependent radioactive cross-linking product represents a true Nun cross-link, rather than a proteolytic degradation product.
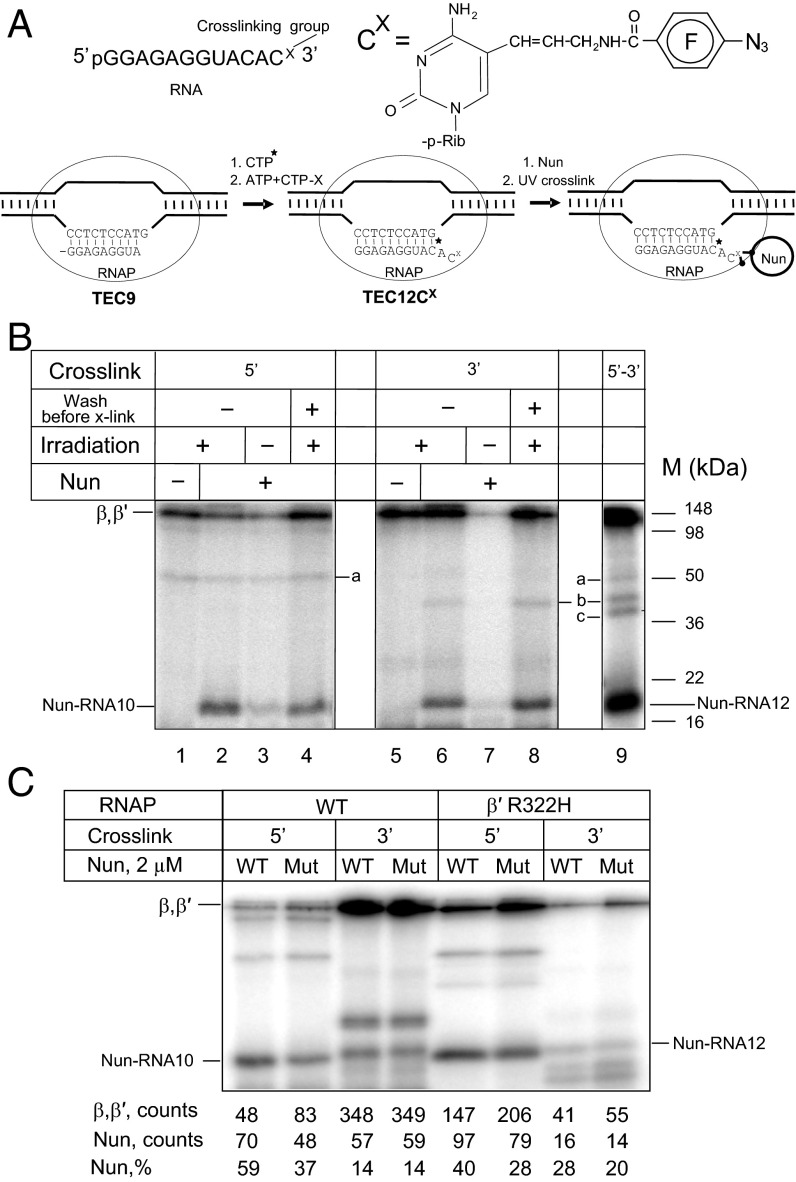
We then introduced a photo–cross-linking group into the RNA 3′ terminus of TEC12C using the scheme of Fig. 3A. Nun addition to the photoreactive TEC was followed by irradiation with UV light. To our surprise, we observed a Nun-dependent radioactive band with the same mobility (18 kDa) as the 5′ cross-link product (Fig. 3B, lane 6). This 18-kDa product was also protease-sensitive, and its intensity was the same when the beads containing Nun-bound TEC were washed before the cross-link was induced (compare lanes 6 and 8 in Fig. 3B). We conclude that the 18-kDA radioactive products represent RNA cross-linked to Nun and that Nun contacts TEC at both upstream and downstream edges of the RNA:DNA hybrid. This finding suggests two possibilities: (i) Nun enters TEC through the RNA exit channel or the secondary channel, contacting either the 5′ or 3′ end of the RNA:DNA hybrid, respectively; or (ii) Nun enters through either the exit channel or the secondary channel, but can contact both ends of the RNA:DNA hybrid simultaneously.
Fig. 3.
Nun cross-linking with photoreactive probes introduced at 5′, 3′, and both 3′ and 5′ termini. (A) The scheme for Nun 3′ cross-linking as in TEC12. The structure of the photo–cross-linkable 3′ RNA residue is presented. The double 3′, 5′ cross-linking was performed similarly, but using an RNA 5′ photoreactive derivative (Fig. 2) in the above scheme. (B) Cross-linking results. Cross-linked radioactive products are indicated. “a,” “b,” and “c” represent minor radioactive products detected under various cross-linking conditions. (C) Effect of amino acid substitutions in Nun (K106/107A) and RNAP (β′R322H) on RNA–Nun cross-linking. The cross-linking reaction was performed at 20 °C in transcription buffer in the absence of Mg2+ with 2 µM Nun. Quantification of RNAP and Nun cross-links is presented at the bottom of the panel.
How Many Nun Molecules Bind to TEC?
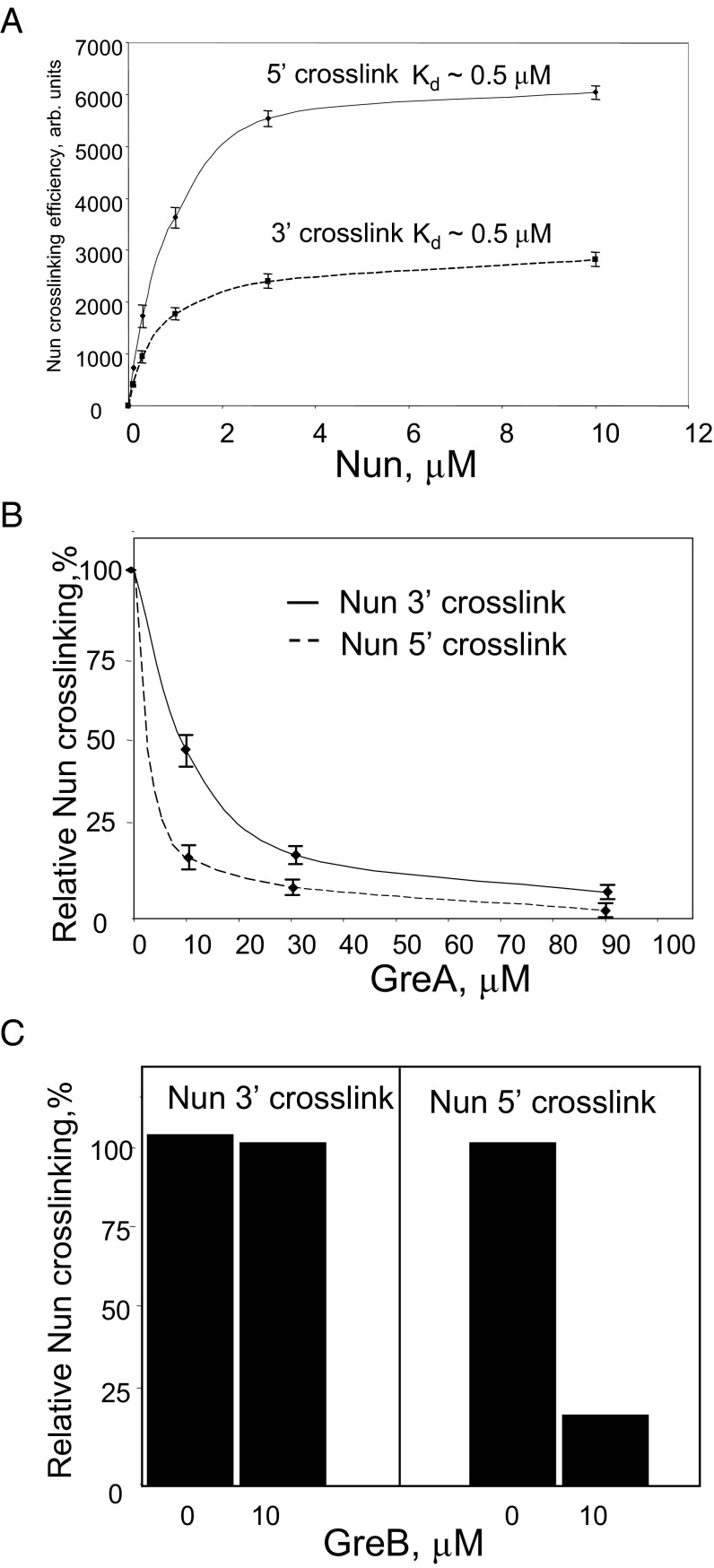
The 5′ and 3′ RNA ends reside at the opposite sides of TEC and are deeply buried in the protein. A single 12-kDa Nun protein might be too small to contact both ends, in which case two Nun molecules would interact with TEC. To test this notion, we determined the apparent binding constants for Nun to the RNA 3′ and 5′ proximal sites by measuring cross-linking efficiency as a function of Nun concentration. As seen in Fig. 4A and Fig. S2, the calculated dissociation constants were essentially the same for both sites (about 0.5 µM), consistent with the proposal that both cross-links originate from single binding event. Importantly the concentration-dependent increase in Nun cross-linking was accompanied by a simultaneous decrease in the labeling of RNAP subunits in both cases (Fig. S2). This finding indicates the competitive nature of the cross-links and provides additional evidence that Nun interacts with both ends of the RNA within TEC. In another approach, we introduced the cross-linking group to both 3′ and 5′ RNA termini and irradiated this TEC in the presence of Nun. One additional product (Fig. 3B, lane 9, product “c”) appeared along with the previously observed cross-linked Nun (note that products “a” and “b” appeared in the 5′ and 3′ cross-links, respectively). The size of this band was close to that of two Nun molecules connected by RNA, and it was not seen with RNA carrying single 5′ or 3′ cross-linking groups. The intensity of the putative Nun–5′-RNA-3′–Nun was close to that expected for the product of the probabilities of individual cross-links from the 5′ and 3′ termini, which suggests that the product represents an RNA cross-linked with two Nun molecules. However, a single Nun molecule with two cross-links to RNA might exhibit an abnormal gel mobility consistent with this band. Thus, (i) there may be two TEC binding sites with the same affinity for Nun, or (ii) two Nun molecules bind to a single site and then each reacts with a different end of the nascent RNA, or (iii) one Nun binds to a single site and then can react with the 5′, the 3′, or both ends of the RNA. These experiments thus leave open the question of Nun/TEC stoichiometry.
Fig. 4.
Determination of Nun/TEC Kd and GreA,B/TEC Kd for 3′ and 5′ RNA cross-links. (A) Quantitative analysis of Nun 5′ and 3′ cross-linking products with various Nun concentrations. (B and C) The same analysis for Nun cross-linking products in the presence of various concentrations of GreA (B) or GreB (C). Concentration of GreA/B proteins is indicated.
Fig. S2.
(A) Efficiency of Nun/TEC cross-linking with RNA 5′ (lanes 1–7) or 3′ (lanes 8–14) end in the presence of various concentrations of Nun. (B and C) Quantification of the results of A.
Effect of Substitutions Disrupting Nun Action on Nun Cross-Linking in TEC.
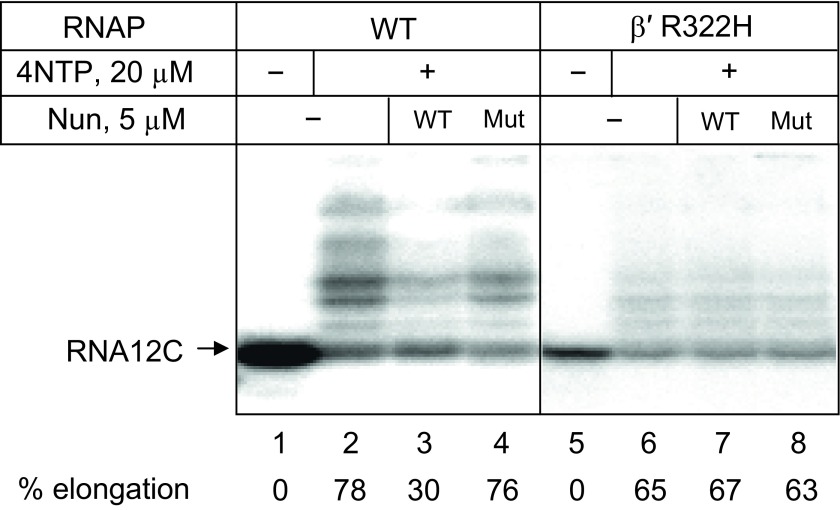
A double substitution in the Nun C terminus (K106/107A, indicated in Fig. 1B) abrogates Nun arrest activity, as do β′ rudder mutations in RNAP near the 5′ end of the RNA:DNA hybrid (14, 16, 17) (Fig. 1C). As previously observed, the K106/107A mutant, unlike wild-type Nun, failed to arrest transcription (Fig. S3, compare lanes 3 and 4). We next tested the Nun-resistant RNAP β′R322H mutant. Except for a slight (∼twofold) defect in elongation (Fig. S3, compare lanes 2 and 6), this mutant is essentially wild-type for all catalytic activities of RNAP. As expected (Fig. S3, lanes 6–8), neither wild-type nor mutant Nun inhibited elongation of TEC12 assembled with mutant RNAP.
Fig. S3.
Effect of amino acid substitutions in Nun (K106/107A) and RNAP (β′R322H) on Nun arrest activity.
To determine whether mutant Nun is unable to arrest transcription because of a lower binding affinity for TEC, we repeated the cross-linking assay in TEC12C with the 5′ and 3′ photoreactive RNA derivatives described above (Fig. 3C). Both wild-type and mutant Nun formed 3′ RNA cross-links with equivalent efficiency (14% relative to total cross-linking products). Mutant Nun, however, yielded fewer 5′ cross-links than wild-type (37% vs. 59%). The β′R322H mutation decreased both wild-type and mutant Nun binding to 5′-tagged RNA (40% and 28%, respectively). Formation of 3′ cross-links was increased for both wild-type and mutant Nun, but the latter reacted less efficiently than the former (28% and 20%, respectively). Thus, the Nun and RNAP mutations reduce, but do not eliminate, Nun cross-linking to RNA. These results support our previous in vivo findings that both the mutant Nun and the mutant RNAP interact to form TEC that do not terminate at the λ tR1 terminator, yet fail in some subsequent step and cannot abrogate transcription elongation (21).
Effect of Gre Factors on Nun Cross-Linking in TEC.
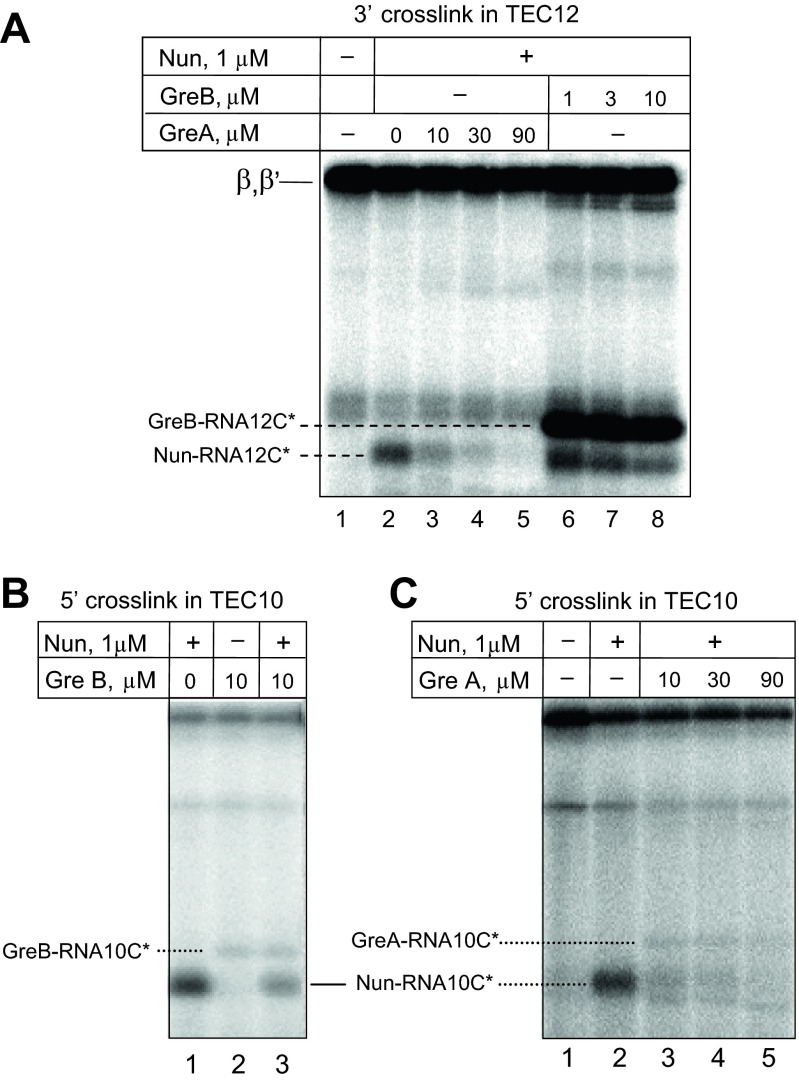
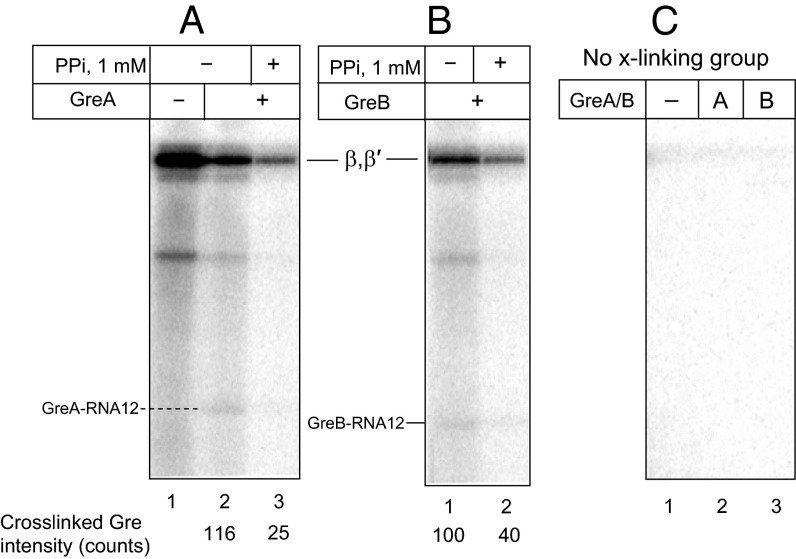
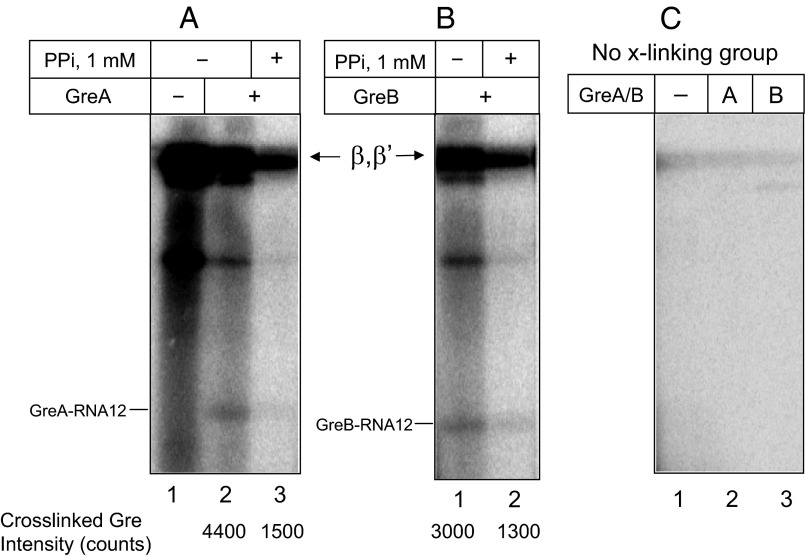
Efficient cross-linking with the 3′ RNA terminus suggested that Nun binds in the vicinity of the RNAP active center, which is thought to be approachable only through the secondary channel. We therefore reasoned that GreA and GreB factors, which enter TEC through the secondary channel, might compete with Nun for cross-linking. Indeed, GreA efficiently blocked Nun–3′ RNA cross-link formation (Fig. 4B; see also Fig. S4A, lanes 2–5). Interestingly, the highly homologous GreB factor had little if any effect on Nun cross-linking (Fig. 4C, Left, and Fig. S4A, lanes 6–8). Although we did not see an RNA–GreA cross-link product, we did detect a radioactive band that corresponds in size to RNA–cross-linked GreB factor. Notably, the efficiency of GreB cross-linking was the same at factor concentrations 1 µM and 10 µM, indicating saturation of the GreB binding center (Fig. S4A, lanes 6 and 8). Therefore, the inability of GreB factor to abolish Nun cross-linking was not a result of insufficient GreB concentration. Note that Mg2+ was depleted in these reaction mixtures to avoid RNA cleavage by Gre factors.
Fig. S4.
Nun 3′ and 5′ RNA cross-linking in the presence of Gre factors. (A–C) 3′ and 5′ cross-links respectively. The mixtures were irradiated at 0° for 2 min at the indicated incubation conditions.
In what were to have been control tests, we monitored 5′ RNA-Nun cross-linking in the presence of Gre factors. To our surprise, we observed significant inhibition of Nun cross-linking by both GreA and GreB factors (Fig. 4 B and C, Right; see also Fig. S4B, compare lanes 1 and 3, and Fig. S4B, lanes 2–5). These results imply that Gre factors might prevent 5′ RNA–Nun cross-link by binding in the vicinity of upstream edge of RNA:DNA hybrid, a mode of binding that has never been previously reported. Remarkably, faint radioactive bands (indicated in Fig. 5 and Figs. S4 B and C and S5) are seen (about 21 kDa with GreB and 22 kDa with GreA) with Gre in the presence or absence of Nun. The size of these bands is consistent with GreA–RNA and GreB–RNA cross-linking products. We confirmed that the Gre cross-links occurred in catalytically competent TEC. The 5′ RNA cross-links in TEC9 were induced in the presence of GreA or GreB factors, followed by elongation of the cross-linked RNA with [α-32P] CTP. As seen in Fig. 5, this protocol yielded the same radioactive bands detected in the previous experiment (Fig. S4 B and C). Notably, pyrophosphate treatment resulted in significant loss of radioactivity from the cross-linking products (about 4-fold for GreA and 2.5-fold for GreB), indicating that cross-linking occurred in a functional transcription complex. No cross-linking was observed in the absence of the 5′ arylazido group (Fig. 5C, lanes 1–3; see also more exposed image of the same gel in Fig. S5), indicating that the Gre-RNA cross-link indeed originated from the 5′ RNA terminus, rather than from possible photoreactivity of RNA nitrous bases at the 3′ end of the RNA:DNA hybrid.
Fig. 5.
GreA and GreB cross-linking to the 5′ RNA terminus in TEC10. (A and B) Analysis of the cross-linking products with GreA and GreB correspondingly under various incubation conditions. (C) Control experiment demonstrating the dependence of the RNA–protein cross-linking on the presence of the photoreactive group at RNA 5′ terminus.
Fig. S5.
(A–C) Overexposed image of Fig. 5.
Discussion
Our studies demonstrate that Nun cross-links to both the 5′ and 3′ RNA ends in TEC. This is an unexpected and striking finding that sheds light on the topography of the Nun-arrested transcription complex and the Nun mechanism of action. We have excluded the possibility that Nun interacts with free RNA: (i) the TEC structures are very stable; (ii) the Nun–cross-linked RNA is in the active center and it can add nucleotides or undergo pyrophosphorolysis; (iii) Nun competes with β and β′ for both 5′ and 3′ RNA cross-linking; and (iv) initial experiments show that the Nun-depended cross-linking product can be cleaved by hydroxylamine to produce a degradation product of expected size, suggesting the cross-link between G44 and the C terminus.
X-ray structures of TEC (Fig. 1A) indicate that the RNA:DNA hybrid and nascent transcript are buried deep within the enzyme. Indeed, the photoreactive group on the RNA residue at the upstream (5′) edge of RNA:DNA hybrid is not surface-accessible, but is instead secluded in the RNA exit channel. Structural mobility of the β′ rudder and β Flap loops that form the channel has recently been described (22, 23). We propose that Nun uses this inherent flexibility of the exit channel components to rearrange the β and β′ RNAP subunits in the channel, exposing the RNA for cross-linking.
Efficient cross-linking to the RNA 3′ terminus suggests that Nun also interacts with the TEC active center. As seen from the crystallographic model of TEC, this is possible if Nun enters through the secondary channel (Fig. 6A), as do prokaryotic transcript cleavage factors GreA and GreB. Nun-induced active center rearrangement might affect the ability of the enzyme to synthesize RNA. Because the linker length between the 3′ RNA attachment site and the photoreactive group is 1.2 nm, it is possible that Nun cross-links as the phenylazido group protrudes into the main, rather than the secondary channel (Figs. 1C and 6A, red arrow).
Fig. 6.
Models for Nun and Gre binding in TEC. (A) The relative arrangement of the RNA:DNA hybrid, active center, β′ Flap domain, and Nun in TEC. The basic features on the DNA:DNA hybrid surrounding the hybrid are indicated. Active center Mg2+ ions are shown as green circles. The blue arrow indicates the proposed displacement of the Flap loop by Nun, which opens the RNA exit channel. In the first model, two TEC-bound Nun molecules (blue spheres) cross-link to the 3′ and 5′ RNA ends. In the second model, a single Nun molecule (cyan sphere) cross-links to both RNA ends. The red arrow indicates a possible 3′ cross-link to Nun through the main channel. (B) High resolution model for GreA (Right) and GreB (Left) binding in the vicinity of the active center. The Black arrow indicates the possible exit for the 3′ backtracked RNA segment in the GreB complex, which is blocked in the GreA complex.
The ability of Nun to cross-link to both 3′ and 5′ RNA termini is consistent with the possibility that two Nun molecules can bind TEC. Indeed, we detect a unique cross-linking product when cross-linking groups were introduced at both 3′ and 5′ RNA termini. The size of this product is equal to two Nun molecules linked to the RNA transcript. However, the Nun binding constant, as deduced from the efficiency of Nun cross-linking at various Nun concentrations, was the same for both 5′ and 3′ cross-links. Both cross-links might involve the binding of a single Nun dimer, or two Nun molecules bound with the same affinity to both upstream and downstream sites, as shown in Fig. 6A. We have not excluded the possibility that a single Nun molecule bound near the 5′ or 3′ end of the hybrid could extend through the TEC to contact the opposite end (Fig. 6A). Finally, the apparent Nun–RNA–Nun band might be a single Nun molecule cross-linked to both 5′ and 3′ ends of the RNA whose migration does not reflect its true molecular weight.
We have also shown that Nun arrest activity and Nun binding to TEC are not completely correlated. For example, the Nun K106/107A mutant is able to bind to TEC, albeit with reduced efficiency, but cannot arrest transcription. Notably, the above Nun C-terminal residues are involved in cross-linking to the DNA template (16). Therefore, the anchoring of these residues to DNA may be required for arrest, even though the contribution of this interaction to Nun binding to TEC might be small. Similarly, the RNAP β′R322H mutation prevents Nun transcription arrest, without entirely abrogating the ability of Nun to bind TEC. We note that both the Nun and RNAP mutations reduce RNA cross-linking efficiency.
Nun blocks the antitermination activity of the essential phage λ N protein in vivo (24). The two proteins share a highly homologous N-terminal ARM (Fig. 1B), but diverge significantly at their C termini (17). Both Nun and λ N compete for binding to the nut sequence of λ nascent transcript and thus access to TEC (25). Although λ N, like Nun, binds at the 5′ edge of the RNA:DNA hybrid, the two proteins have opposite effects on transcription elongation (26). λ N accelerates transcription and prevents termination and backtracking, whereas Nun arrests transcription (14, 17). It has recently been shown that λ N stabilizes the 5′ edge of RNA:DNA hybrid to prevent transcription slippage (26). By stabilizing the RNA:DNA hybrid, or interacting with the RNA exit channel, λ N could suppress formation of the stem-loop structures found in intrinsic termination sites. To allow rapid transcription elongation, λ N stabilization of the RNA:DNA hybrid must be transitory. In contrast, Nun stabilization of the RNA:DNA hybrid is irreversible, thus blocking TEC translocation.
Another unexpected finding of this study is the effect of Gre factors on Nun/RNA cross-linking. GreA—but not GreB—protected Nun from 3′ cross-linking. Although both factors are highly homologous, they differ in substrate specificity. Thus, GreA factor acts on slightly backtracked TECs, cleaving RNA transcripts in short increments (2-to 3-nt segments). Deeply backtracked or arrested TECs are resistant to GreA. As seen in our high-resolution model (Fig. 6B), this difference can be explained by GreA blocking the secondary channel so that backtracked 3′ RNA segments remain secluded in the cavity of the active center. However, with GreB, the disengaged RNA segment freely exits into the secondary channel. Our model attributes this difference to the bulky GreA Lys43 residue. GreA Lys43, which is stabilized by a salt bridge with βE565, blocks RNA exit. The smaller Ser residue in GreB at the equivalent position allows RNA access to the secondary channel. Moreover, RNA exit is promoted by the GreB “basic patch,” a cluster of positively charged residues which pull RNA out of the active center by establishing salt bridges with RNA phosphate residues. The protective effect of GreA can thus be explained by assuming that the 3′ RNA–Nun cross-link occurs outside the active center in the secondary channel, so that GreA blocks the cross-link by preventing RNA entering the secondary channel. In contrast, GreB does not block Nun cross-linking, because it allows RNA to exit into the secondary channel. This explanation requires that Nun and GreB can simultaneously occupy the secondary channel, because bound GreB does not prevent 3′ Nun cross-linking.
Inhibition of 5′ Nun cross-linking by GreA/B was not predicted, because Gre factors bind in the secondary channel, and thus approach the opposite (3′) edge of the RNA:DNA hybrid. This edge is about 3.4 nm away from the 5′ edge of the hybrid. However, the 5′ RNA cross-linking of both GreA and GreB in TEC9 and TEC10 suggests that these factors can enter the RNA exit channel to block Nun 5′ cross-linking. We cannot exclude the possibility that GreA/B binding in the secondary channel allosterically alters TEC such that the 5′ end of the RNA becomes unaccessible. We note that the binding constants for GreA, which were calculated from the concentration dependence on inhibiting Nun 3′ and 5′ RNA cross-linking (∼10 µM and 2.5 µM, respectively), differ significantly. This finding suggests that GreA binds at different sites to block Nun 5′ and 3′ cross-linking. Furthermore, we find both 5′ RNA cross-links to both GreA and GreB. This finding extends the known action mechanism for these ubiquitous cellular factors.
Materials and Methods
All chemicals were from Sigma-Aldrich. His6-tagged RNA polymerase was purified from the RL721 E. coli strain. Mutant RNAPs were obtained as previously described (26). Ultrapure ribonucleoside-5′-triphosphates were from Pharmacia. The [α-32P] CTP was from MP Biomed. Oligonucleotides were from Oligos. Synthesis of the cross-linking RNA derivative was performed as previously described (27). Radioactive products were resolved by electrophoresis, and quantified by phosphoimagery using a Molecular Dynamics device (GE Healthcare) and using a Typhoon FLA7000 device (GE Healthcare). Molecular modeling was performed using WebLab ViewerLight 4.0 (Molecular Simulations).
Elongation Complexes.
TEC9A and TEC 11A were assembled as previously described (12). Briefly, modified or nonmodified RNA9 or RNA11 was mixed with equivalent amounts of DNA template strand to a final concentration of 1 µM in transcription buffer, TB (20 mM Tris⋅HCl, pH 8.0, 0.1 M NaCl, 10 mM MgCl2) at 40 °C and left to cool in a water bath to room temperature for ∼30 min. This mixture was added to an equivalent amount of His6-tagged RNAP immobilized on NTA agarose, and kept at room temperature in a shaker for 10 min followed by addition of the equivalent amount of DNA nontemplate strand. The mixture was agitated for another 10 min at room temperature and supplemented with TB containing 1 M NaCl. After 10 min the pellet was separated and washed with regular TB. Labeled TEC10C, and TEC12C, were obtained by elongation of TEC9A or TEC11A by an equivalent amount of [α-32P] CTP (3000 Ci/mmol, 10 mCi/mL; MP Biomedicals) followed by washing with TB (4 × 1 mL). TEC11A was obtained from TEC10C by elongation with 10 µM ATP for 1 h at room temperature in TB containing 1 mM MgCl2. TEC12CR with a photoreactive group at the 3′ terminal cytidine residue was obtained from labeled TEC10C by elongation with 10 µM ATP and the photoreactive CTP analog (30 µM), as described in ref. 10. Elongation complex TEC12CR with the photoreactive group at the 5′ terminus was obtained as TEC12CR, but using 5′ modified photoreactive RNA oligonucleotide. TEC14G was obtained from TEC10C using a “walking” protocol (10). Labeled TEC12C assembled on the T7 A1 promoter was obtained as described in ref. 10.
Cross-Linking in TEC.
For cross-linking in TEC9A, the TEC was assembled with photoreactive nanonucleotide RNA and irradiated with a UV monitor (254 nm) for 2 min in the presence or absence of Nun, or Gre factors at concentrations indicated in the figures and their legends. This process was followed by elongation with 0.1 µM [α-32P] CTP for 2 min at room temperature. Alternatively, elongation of TEC9A with radioactive CTP was followed by UV irradiation in the presence or absence of Nun or Gre factors. In all of these experiments, Mg2+ was removed before cross-link formation by washing the NTA beads with transcription buffer lacking Mg2+. For cross-links in TEC12A, TEC14G, and TEC12CR, the TECs obtained as above were mixed with Nun (5 µM) and UV-irradiated. The cross-linked samples were supplemented with 5 µL of denaturing mixture (3% (wt/vol) SDS, 3% (wt/vol) DTT, 30% (vol/vol) glycerol, 0.1% Bromophenol blue), kept for 10 min at 56 °C, and analyzed by 12–15% (wt/vol) SDS PAGE.
Elongation of TEC12C in the Presence of Nun.
Reaction mixtures containing 20–100 nM TEC12C were preincubated with 5 µM Nun for 5 min at room temperature and supplemented with a complete set of NTPs (final concentration is indicated in Fig. S3). After incubation for 5 min at room temperature, the reaction was stopped by addition of an equal volume of hot 12 M urea and 50 mM Na-EDTA, pH 8.0. The samples were then analyzed by electrophoresis (15% (wt/vol) PAAG, 7 M urea).
Pyrophosphorolysis in TEC.
The reaction was performed in transcription buffer containing 10 mM sodium pyrophosphate for 10 min at ambient temperature.
Hydroxylamine Cleavage.
After SDS/PAGE separation, the products corresponding to cross-linked Nun were visualized by autoradiography and excised. The gel pieces were washed with water (10 min) while shaking, crushed, and the radioactive material then eluted with 0.3% SDS. The eluted material was concentrated by freeze-drying and redissolved in water to achieve 1% SDS concentration in the sample. A sample aliquot was supplemented with equal volume of 2 M hydroxylamine/0.2 M Na2CO3, pH 10, and incubated at 37 °C. At 1- and 2-h time intervals, aliquots were withdrawn, supplemented with 1/5 (V/V) of 0.5 M Tris⋅HCl, pH 8.0, 50% (vol/vol) glycerol, and 0.1% Bromophenol blue, and stored at −20° before analysis by 15% (wt/vol) SDS/PAGE.
Acknowledgments
This work was supported by Grant 2R01GM037219 (to M.E.G. and C.L.V.), and by a Public Health Research Institute Research Support grant (to A.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1601056113/-/DCSupplemental.
References
- 1.Nudler E, Mustaev A, Lukhtanov E, Goldfarb A. The RNA-DNA hybrid maintains the register of transcription by preventing backtracking of RNA polymerase. Cell. 1997;89(1):33–41. doi: 10.1016/s0092-8674(00)80180-4. [DOI] [PubMed] [Google Scholar]
- 2.Weixlbaumer A, Leon K, Landick R, Darst SA. Structural basis of transcriptional pausing in bacteria. Cell. 2013;152(3):431–441. doi: 10.1016/j.cell.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kireeva ML, Kashlev M. Mechanism of sequence-specific pausing of bacterial RNA polymerase. Proc Natl Acad Sci USA. 2009;106(22):8900–8905. doi: 10.1073/pnas.0900407106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reeder TC, Hawley DK. Promoter proximal sequences modulate RNA polymerase II elongation by a novel mechanism. Cell. 1996;87(4):767–777. doi: 10.1016/s0092-8674(00)81395-1. [DOI] [PubMed] [Google Scholar]
- 5.Komissarova N, Kashlev M. RNA polymerase switches between inactivated and activated states By translocating back and forth along the DNA and the RNA. J Biol Chem. 1997;272(24):15329–15338. doi: 10.1074/jbc.272.24.15329. [DOI] [PubMed] [Google Scholar]
- 6.Borukhov S, Sagitov V, Goldfarb A. Transcript cleavage factors from E. coli. Cell. 1993;72(3):459–466. doi: 10.1016/0092-8674(93)90121-6. [DOI] [PubMed] [Google Scholar]
- 7.Stebbins CE, et al. Crystal structure of the GreA transcript cleavage factor from Escherichia coli. Nature. 1995;373(6515):636–640. doi: 10.1038/373636a0. [DOI] [PubMed] [Google Scholar]
- 8.Opalka N, et al. Structure and function of the transcription elongation factor GreB bound to bacterial RNA polymerase. Cell. 2003;114(3):335–345. doi: 10.1016/s0092-8674(03)00600-7. [DOI] [PubMed] [Google Scholar]
- 9.Laptenko O, Lee J, Lomakin I, Borukhov S. Transcript cleavage factors GreA and GreB act as transient catalytic components of RNA polymerase. EMBO J. 2003;22(23):6322–6334. doi: 10.1093/emboj/cdg610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sosunova E, et al. Donation of catalytic residues to RNA polymerase active center by transcription factor Gre. Proc Natl Acad Sci USA. 2003;100(26):15469–15474. doi: 10.1073/pnas.2536698100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reines D, Ghanouni P, Li QQ, Mote J., Jr The RNA polymerase II elongation complex. Factor-dependent transcription elongation involves nascent RNA cleavage. J Biol Chem. 1992;267(22):15516–15522. [PMC free article] [PubMed] [Google Scholar]
- 12.Kettenberger H, Armache KJ, Cramer P. Architecture of the RNA polymerase II-TFIIS complex and implications for mRNA cleavage. Cell. 2003;114(3):347–357. doi: 10.1016/s0092-8674(03)00598-1. [DOI] [PubMed] [Google Scholar]
- 13.Hung SC, Gottesman ME. The Nun protein of bacteriophage HK022 inhibits translocation of Escherichia coli RNA polymerase without abolishing its catalytic activities. Genes Dev. 1997;11(20):2670–2678. doi: 10.1101/gad.11.20.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vitiello CL, Kireeva ML, Lubkowska L, Kashlev M, Gottesman M. Coliphage HK022 Nun protein inhibits RNA polymerase translocation. Proc Natl Acad Sci USA. 2014;111(23):E2368–E2375. doi: 10.1073/pnas.1319740111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uc-Mass A, Khodursky A, Brown L, Gottesman ME. Overexpression of phage HK022 Nun protein is toxic for Escherichia coli. J Mol Biol. 2008;380(5):812–819. doi: 10.1016/j.jmb.2008.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watnick RS, Gottesman ME. Binding of transcription termination protein nun to nascent RNA and template DNA. Science. 1999;286(5448):2337–2339. doi: 10.1126/science.286.5448.2337. [DOI] [PubMed] [Google Scholar]
- 17.Robledo R, Atkinson BL, Gottesman ME. Escherichia coli mutations that block transcription termination by phage HK022 Nun protein. J Mol Biol. 1991;220(3):613–619. doi: 10.1016/0022-2836(91)90104-e. [DOI] [PubMed] [Google Scholar]
- 18.Grachev MA, Kolocheva TI, Lukhtanov EA, Mustaev AA. Studies on the functional topography of Escherichia coli RNA polymerase. Highly selective affinity labelling by analogues of initiating substrates. Eur J Biochem. 1987;163(1):113–121. doi: 10.1111/j.1432-1033.1987.tb10743.x. [DOI] [PubMed] [Google Scholar]
- 19.Tsarev IG, Alikina T, Grachev MA, Zaychikov EF, Mustaev AA. Highly selective labeling of E. coli RNA polymerase–promoter complex with the reactive derivatives of oligonucleotide primers of different specificity. Bioorganicheskaya Khimia (Rus) 1990;16:765–774. [PubMed] [Google Scholar]
- 20.Korzheva N, et al. A structural model of transcription elongation. Science. 2000;289(5479):619–625. doi: 10.1126/science.289.5479.619. [DOI] [PubMed] [Google Scholar]
- 21.Kim HC, Gottesman ME. Transcription termination by phage HK022 Nun is facilitated by COOH-terminal lysine residues. J Biol Chem. 2004;279(14):13412–13417. doi: 10.1074/jbc.M313206200. [DOI] [PubMed] [Google Scholar]
- 22.Tagami S, et al. Crystal structure of bacterial RNA polymerase bound with a transcription inhibitor protein. Nature. 2010;468(7326):978–982. doi: 10.1038/nature09573. [DOI] [PubMed] [Google Scholar]
- 23.Toulokhonov I, Landick R. The Flap domain is required for pause RNA hairpin inhibition of catalysis by RNA polymerase and can modulate intrinsic termination. Mol Cell. 2003;12(5):1125–1136. doi: 10.1016/s1097-2765(03)00439-8. [DOI] [PubMed] [Google Scholar]
- 24.Hung SC, Gottesman ME. Phage HK022 Nun protein arrests transcription on phage λ DNA in vitro and competes with the phage λ N antitermination protein. J Mol Biol. 1995;247(3):428–442. doi: 10.1006/jmbi.1994.0151. [DOI] [PubMed] [Google Scholar]
- 25.Chattopadhyay S, et al. Interaction between the phage HK022 Nun protein and the nut RNA of phage lambda. Proc Natl Acad Sci USA. 1995;92(26):12131–12135. doi: 10.1073/pnas.92.26.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parks AR, et al. Bacteriophage λ N protein inhibits transcription slippage by Escherichia coli RNA polymerase. Nucleic Acids Res. 2014;42(9):5823–5829. doi: 10.1093/nar/gku203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sosunova E, Sosunov V, Epshtein V, Nikiforov V, Mustaev A. Control of transcriptional fidelity by active center tuning as derived from RNA polymerase endonuclease reaction. J Biol Chem. 2013;288(9):6688–6703. doi: 10.1074/jbc.M112.424002. [DOI] [PMC free article] [PubMed] [Google Scholar]