Significance
We live in a time of increasing terror, stress, and trauma, and yet humans show a remarkable ability to cope under high stress states. How the brain supports such active resilient coping is not well-understood. Findings showed high stress levels are accompanied by dynamic brain signals in circuits representing the stress reaction, adaptation, and behavioral control responses. In addition, a ventromedial prefrontal cortical region showed initial decreases in brain activation, but then mobilized with increased activation, and this dynamic change was correlated with active coping. Conversely, individuals who failed to show such “neuroflexibility” in this specific ventromedial prefrontal region reported higher maladaptive coping behaviors. Findings suggest that strategies to promote such neuroflexibility under stress may increase stress resilience in humans.
Keywords: functional neuroimaging, stress, resilience coping, binge alcohol intake, emotional eating
Abstract
Active coping underlies a healthy stress response, but neural processes supporting such resilient coping are not well-known. Using a brief, sustained exposure paradigm contrasting highly stressful, threatening, and violent stimuli versus nonaversive neutral visual stimuli in a functional magnetic resonance imaging (fMRI) study, we show significant subjective, physiologic, and endocrine increases and temporally related dynamically distinct patterns of neural activation in brain circuits underlying the stress response. First, stress-specific sustained increases in the amygdala, striatum, hypothalamus, midbrain, right insula, and right dorsolateral prefrontal cortex (DLPFC) regions supported the stress processing and reactivity circuit. Second, dynamic neural activation during stress versus neutral runs, showing early increases followed by later reduced activation in the ventrolateral prefrontal cortex (VLPFC), dorsal anterior cingulate cortex (dACC), left DLPFC, hippocampus, and left insula, suggested a stress adaptation response network. Finally, dynamic stress-specific mobilization of the ventromedial prefrontal cortex (VmPFC), marked by initial hypoactivity followed by increased VmPFC activation, pointed to the VmPFC as a key locus of the emotional and behavioral control network. Consistent with this finding, greater neural flexibility signals in the VmPFC during stress correlated with active coping ratings whereas lower dynamic activity in the VmPFC also predicted a higher level of maladaptive coping behaviors in real life, including binge alcohol intake, emotional eating, and frequency of arguments and fights. These findings demonstrate acute functional neuroplasticity during stress, with distinct and separable brain networks that underlie critical components of the stress response, and a specific role for VmPFC neuroflexibility in stress-resilient coping.
Unpredictable and uncontrollable events are highly common in daily life, and our cognitive and behavioral coping responses are central to determining the long-term negative or positive effects of stress on health. Consider the following example. You are in the underground on your way to give a critical presentation at a large meeting and the train stops. There is no information on what has happened and the phone signal is down. We almost instantaneously begin to consider options of what to do: Perhaps seek out someone in authority or, alternatively, quickly without much thought push alarm buttons to get out. Active coping (involving strategies such as altering perception via appraisal strategies, reframing or reasoning, exercising cognitive and behavioral control, and problem solving during stress) signals resilience and regulates stress to promote adaptive behaviors and positive health outcomes (1–3). On the other hand, extensive research documents that poor emotional and behavioral coping (using avoidance, suppression, rumination, and habitual motivation during stress) is associated with a number of maladaptive health behaviors and poor health outcomes (3–7). Despite this evidence, the specific neural components of the stress response that may underlie acute stress reactivity, adaptation, and active coping that support stress resilience mechanisms in humans are not well-understood.
Growing basic science research suggests that there is an evolutionary bias toward reduced prefrontal executive control during high stress to promote short-term stress-related habitual behavioral responses for survival (8–11). A host of human functional neuroimaging and behavioral laboratory studies document decreased prefrontal activity and reduced executive function during stress (9, 12, 13) and increased activation in the limbic-striatal network associated with high emotional and behavioral reactivity in healthy and patient samples (13–17). Nonetheless, humans often face high levels of stress, trauma, and aggression in daily life, but they also show a remarkable ability to adapt and reduce stress levels and actively cope and persist in the face of such unpredictable and uncontrollable events, which has led to an increasing focus on identifying brain responses to stress and stress resilience mechanisms that may drive individual differences in stress coping and stress-related negative sequelae (11, 18, 19).
Although previous neuroimaging research has documented increased brain limbic-striatal activation during stress, with evidence of reduced ventromedial prefrontal cortex (VmPFC) or dorsolateral prefrontal cortex (DLPFC) response, these functional responses may represent an initial and early neural stress response as shown by research using event-related presentation of aversive images or very brief block presentation of movie clips or trauma/stress imagery to assess neural stress responses (14, 17, 20). Thus, few studies have focused on the acute temporal dynamics of the limbic-striatal responses and the prefrontal cortical responses to stress or how the brain may dynamically adapt to reduce and respond to acute stress in the moment. For example, research in laboratory animals has shown plasticity in the VmPFC, encompassing the orbitofrontal cortex (OFC) and rostral anterior cingulate cortex (rACC) and subgenual cingulate regions, as a key locus of the emotional and behavioral control network for regaining behavioral control during uncontrollable stress (18, 19). Human neuroimaging evidence suggests that the VmPFC is a key region of the adaptive behavioral coping circuit that plays a role in increased persistence responses in the face of uncontrollable setbacks (21) and in regulation of anxious emotion (22). Disrupted VmPFC signaling during stress also predicts alcohol relapse and failed recovery from alcoholism (23). Childhood trauma, cumulative adversity, and a history of mood disorders or posttraumatic stress disorder (PTSD) are each associated with blunted VmPFC activation during emotion or stress exposure, and disrupted VmPFC connectivity with amygdala is suggestive of poor adaptive coping (13, 14, 16, 24, 25). On the other hand, it is plausible that brief, sustained stress exposure may provide an approach to assess more automatic neural processes that underlie stress adaptive and resilient coping responses. Based on this evidence, we hypothesized that the VmPFC is one of the critical loci of neuroplasticity in a resilience-coping network that signals increased emotional and behavioral control and active coping even in the face of continued stress exposure. We developed a paradigm involving sustained unpredictable exposure to novel highly stressful stimuli and also hypothesized sustained increases in the stress reactivity and distress-signaling circuit involving the amygdala, hippocampus, and hypothalamic responses during stress exposure. Furthermore, because the VmPFC is anatomically connected to other executive and attentional control regions, such as the DLPFC and the inferior parietal lobule (IPL) that are part of the resilience-coping network, we also predicted that the VmPFC response will increase functional connectivity between these regions during stress relative to neutral (S-N) conditions. In addition, because the medial prefrontal cortex (PFC) is modulated by glucocorticoids that in turn impact motivated behavioral responses (26, 27), we further hypothesized that stress-induced cortisol release will be associated not only with functional changes in stress reactivity network regions, such as the hypothalamus, amygdala. and hippocampus, but also with the reduced medial prefrontal activity previously documented during stress and emotion exposure. Finally, because humans vary significantly in how they cope under stress and their associated behavioral responses, we used an individual difference approach to assess whether dynamic changes in VmPFC response during stress will be associated with a subject’s reports of emotional and behavioral coping.
Results
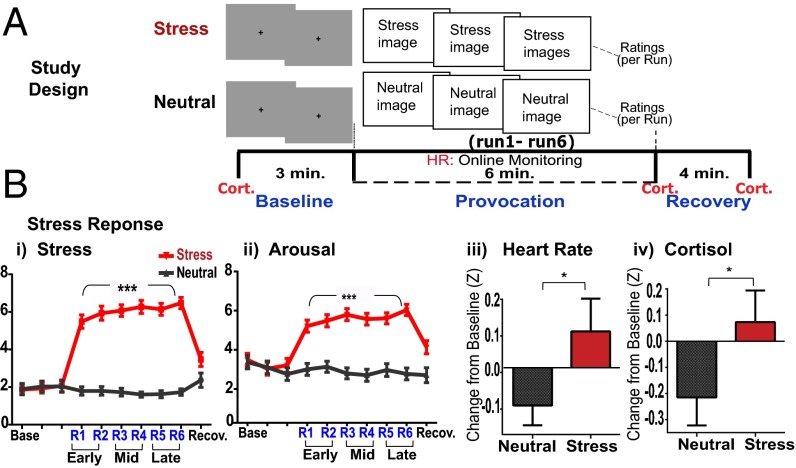
Thirty community young adult participants (mean age 25.7 (8.61) y with no history of physical or mental health disorders participated in a multimethod functional magnetic resonance imaging (fMRI) scan involving a brief and passive sustained provocation procedure to assess separable and distinct temporally related neural processes involved in the stress response and in active coping. The fMRI paradigm involved brief successive exposure to a block of highly aversive images of terror, violence, mutilation, fear, disgust, and desperation that were compared with a no-stress, neutral control block involving nonaversive neutral images [all pictures from the International Affective Picture System (28)]; each image of the stress/neutral block was presented for 5 s per image with a 1-s interstimulus interval (ISI), over six successive runs of 60 s each (10 images per run) making up each of the stress and neutral blocks to provoke sustained unpredictable emotional stress versus a brief sustained no-stress control state. Each stress and neutral block was preceded by three 60-s runs of gray fixation baseline blocks (29) for comparison with the respective stress or neutral runs. The order of stress and neutral blocks was randomly assigned and counterbalanced across subjects. Participants made subjective ratings of stressfulness and arousal after each 60-s run; heart rate was assessed continuously using a pulse oximeter; and plasma cortisol levels were assessed via a previously inserted i.v. line for repeated assessment at a baseline time point : prior to baseline runs, immediately after the 6-min run block, and at 4 min after each of the stress/neutral blocks (see Fig. 1A and SI Appendix for detailed task description).
Fig. 1.
Study design and subjective, physiologic, and neuroendocrine stress response. (A) A sample experimental successive run block made up of three baseline gray fixation runs, followed by six stress/neutral provocation runs and a 4-min recovery period to constitute each of the stress and neutral run blocks. (B) Significantly increased subjective stress and arousal ratings (visual analog 1–9 scale; P < 0.0001 each) and z-transformed scores for average heart rate (*P < 0.03) and plasma cortisol (*P = 0.05). ***P < 0.0001.
Self-Report and Physiologic, Endocrine, and Neural Response to Stress.
Significant main effects of condition (stress versus neutral) indicated sustained increases in subjective stress [condition main effect, F(1,29) = 894.91, P < 0.0001] and arousal [condition main effect, F(1,29) = 198.12, P < 0.0001] ratings, increased average heart rate [condition main effect, F(1,28) = 4.51, P < 0.03] and plasma cortisol response [condition main effect, F(1,26) = 3.73, P = 0.05] during stress vs. no-stress blocks (Fig. 1 B, i–iv), thereby validating that a brief and sustained stress state was provoked relative to the neutral no-stress condition. There were no significant condition × time point interactions for heart rate and cortisol, and thus averaged responses across time points in each condition are shown in Fig. 1 B, iii and iv. Whole brain random effects analysis of variance (ANOVA) analyses using AFNI software (30) assessed condition main effects that contrasted the stress (stress runs − stress baseline runs) versus neutral condition (neutral runs − neutral baseline runs) and condition × time period (early, mid, and late runs, each relative to their respective baseline) interaction effects in all brain analyses comparing stress versus neutral run blocks. Findings indicated a main effect of condition, with significant increases in neural activity during stress vs. no-stress conditions in cortico-limbic striatal regions, including the hypothalamus, amygdala, hippocampus, thalamus, ventral and dorsal striatum, insula and midtemporal regions, dorsal anterior cingulate cortex, DLPFC, and the midbrain regions [P < 0.05 whole brain corrected (WBC); large effect sizes over 1.0]. In addition, during only stress relative to baseline, significant VmPFC deactivation and increased activity in the ventrolateral prefrontal regions (VLPFC) were observed (P < 0.05, WBC) (SI Appendix, Fig. S1A). Sustained stress neural responses in specific representative limbic regions of interest (ROIs) of the amygdala and hypothalamus relative to activation in the neutral condition across runs are presented in SI Appendix, Fig. S1B.
Whole Brain Association with Stress-Related Increases in Cortisol.
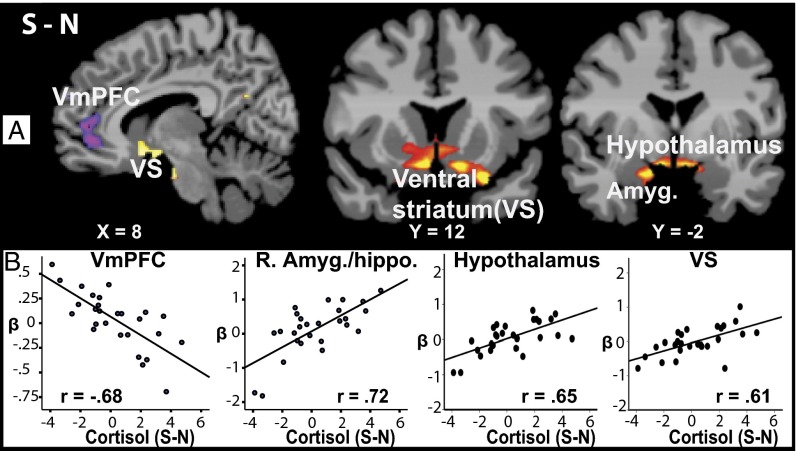
To further examine the coordinated neural and neuroendocrine response, whole brain regression analysis of the plasma cortisol response during the S vs. N condition was conducted. Cortisol increases (S-N) were associated with decreased VmPFC response (r = −0.68; R2 = 0.47) and increased hypothalamus (r = 0.65; R2 = 0.43), right (R) amygdala/hippocampus (r = 0.72; R2 = 0.52) and ventral striatum (r = 0.61; R2 = 0.38) S-N whole brain response (P < 0.05 WBC) [Fig. 2 and see SI Appendix, Table S2 for Montreal Neurological Institute (MNI) coordinates]. Secondary analyses showed that sex did not influence these significant effects.
Fig. 2.
Correlation images from whole brain regression analysis showing association between stress-neutral (S-N) brain activity and cortisol response (S-N) are shown in A (P < 0.05, whole brain corrected) (see SI Appendix, Table S2 for MNI coordinates from the whole brain regression analyses). Corresponding correlation scatterplots from extracted beta weights of ROIs indicating areas of association from the S-N regression map and the S-N cortisol responses are shown in B. Red/yellow, positive correlation; blue-purple, negative correlation.
Dynamic Temporal Changes in Stress/Neutral Brain Activation.
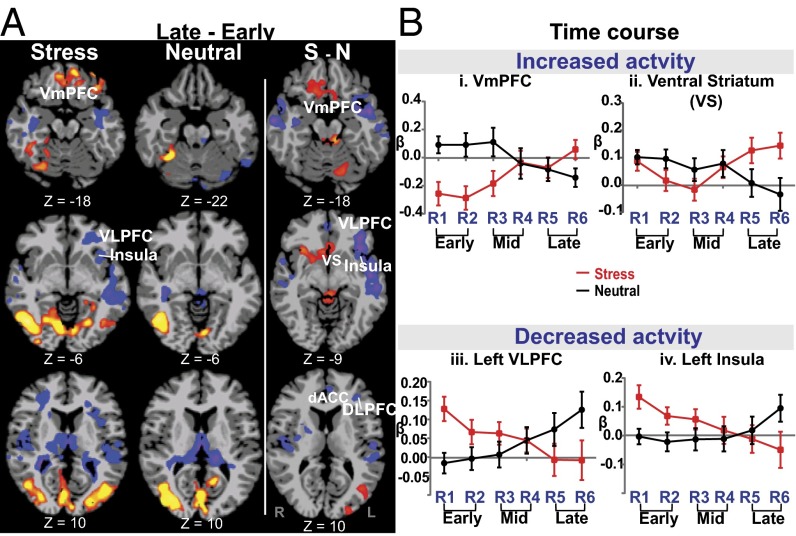
Significant condition (stress/neutral) by time period (early two, mid two, late two runs) interaction effects indicated that dynamic changes in neural activation in the late relative to early runs during stress versus neutral were observed in the VmPFC, ventral striatum (VS), left (L) insula, midbrain, L VLPFC, R hippocampus, temporal lobe, and L precuneus/IPL regions (P < 0.05, WBC; large effect sizes were seen for these activation clusters ranging from d = 1.21 to 1.85 (Fig. 3). (Also see SI Appendix, Fig. S2 and Table S3 for MNI coordinates of specific regions showing significant interaction effects across run comparisons and conditions.) Furthermore, secondary analyses showed that sex did not significantly influence these whole brain interaction effects. The time courses of these dynamic functional activations for representative and key specific ROIs are illustrated for the VmPFC and ventral striatum across runs in Fig. 3 B, i and ii (also see SI Appendix, Fig. S2). Interestingly, we found that the VmPFC showed significant deactivation in the early period (runs 1–2 relative to stress baseline) and then a remarkable recovery in the late period (runs 5–6 relative to the stress baseline) whereas the neutral condition runs showed minimal change in the early runs and a nonsignificant reduction in the later runs compared with the neutral baseline (Fig. 3 B, i). Remarkably, the VS (Fig. 3 B, ii) showed no changes in the early stress runs but a significant increase during the late period, relative to changes in the neutral runs, resulting in an overall interaction effect (Fig. 3A, columns 2 and 3). In contrast, we also found an opposite dynamic response (Fig. 3 A and B, iii and iv) during stress in the L VLPFC, L insula, bilateral middle temporal lobe (MTL), and R hippocampus, with increases in neural activity during the early period, followed by reduced activation during the late stress period relative to no statistically significant change from baseline in the early versus late runs of the neutral condition (Fig. 3A, column 2), thereby suggestive of an adaptive or habituation network representing the stress adaptation response.
Fig. 3.
Significant condition × time period interactions in whole brain analysis showing time-dependent neural changes in brain response to stress. (A) Late–early runs of stress (first column), neutral (second column), and their contrast of stress-neutral (S-N) (third column) show increased dynamic activity for stress in the VmPFC, posterior cingulate cortex, and middle occipital gyrus, but decreased activity in the L ventrolateral PFC (VLPFC), insula, and superior/middle temporal gyrus, and no similar late-versus-early run changes in the neutral condition (second column). The S-N (third column) contrast indicates increased activity in the VmPFC and ventral striatum, midbrain, and L middle occipital gyrus, but decreased activity in the L VLPFC, insula, and superior/middle temporal gyrus for stress (late–early)–neutral (late–early) contrasts (P < 0.05, whole brain corrected). (B, i–iv) Time courses of responses across runs in key regions of interest (VmPFC, ventral striatum, L VLPFC, and insula) are shown to illustrate simple effects assessed in the whole brain contrasts of the interaction effects shown in A. Early, first two runs; Late, last two runs (of the successive six-run block for each condition); red/yellow, increased relative activation; blue/purple, decreased relative activation.
Ventromedial PFC Functional Connectivity During Stress.
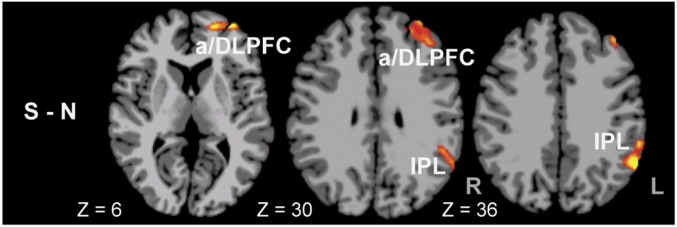
As hypothesized, we expected the VmPFC activation to show dynamic changes during stress and to show increased connectivity with other executive and attentional control regions during S vs. N states. To test this hypothesis, we extracted the functional VmPFC response during averaged stress response across all runs to assess functional connectivity with the rest of the brain during stress versus neutral average and stress compared with neutral conditions across all runs. We found increased functional connectivity between the VmPFC and the L anterior prefrontal cortex (aPFC) and the DLPFC [Brodmann area (BA) 9 and 10] and IPL regions during S-N condition (P < 0.05 whole brain corrected), indicating increased connectivity with executive control and attention regions in the face of continued stress (Fig. 4). On the other hand, we found negative inhibitory connectivity between the VmPFC and limbic and striatal regions of the amygdala, hippocampus, striatum, and insula but no differential connectivity between these regions across the stress and neutral conditions (SI Appendix, Fig. S3). The increased VmPFC connectivity with aPFC/DLPFC and IPL did not correlate with active coping or coping behaviors.
Fig. 4.
Whole brain functional connectivity with the VmPFC seed taken from the averaged brain stress response across all runs. Increased connectivity (shown in red/yellow) with the L anterior PFC (aPFC) and dorsolateral PFC (DLPFC) and L inferior parietal lobe (IPL) was found during stress average (S) compared with the neutral average (N) responses (P < 0.05, whole brain corrected).
VmPFC Plasticity and Individual Differences in Coping.
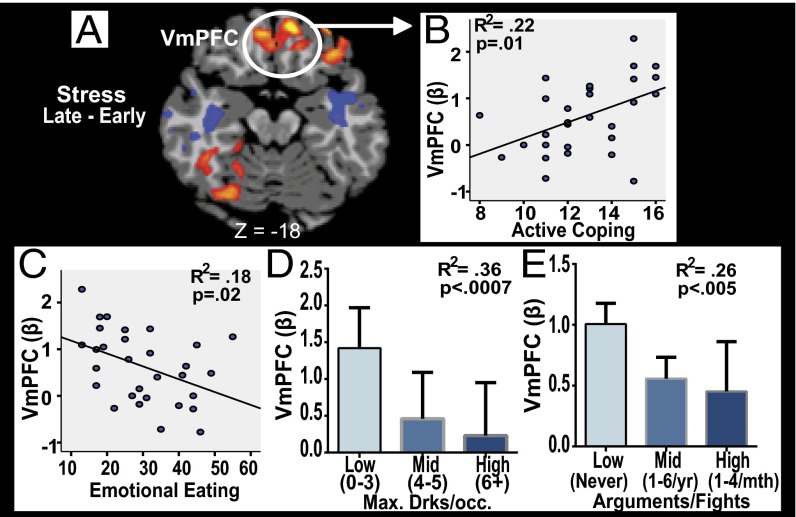
To test the hypothesis that the dynamic responses in VMPFC contribute to significant variations in stress coping, we used an individual differences approach to assess whether individual differences in VmPFC plasticity was associated with active coping and maladaptive coping behaviors. In a separate session, all subjects completed assessments on real life coping responses and also reported on common coping behaviors (such as emotional eating and frequency and number of alcohol drinks consumed) and on the frequency of interpersonal arguments and fights (see SI Appendix for description of these measures). The dynamic increase in VmPFC response during stress as shown in Fig. 3 B, i and presented in Fig. 5A (extracted beta weights from mean peak VmPFC increase in run 6 − mean lowest VmPFC response in run 2) was significantly associated with higher active coping scores (r = 0.47, R2 = 0.22, P = 0.01). Conversely, blunted change in the VmPFC during stress, representing failure of VmPFC neuroplasticity, was associated with greater emotional eating (P = 0.02), higher maximum amount of alcohol consumed per occasion (P < 0.0007), and increased frequency of interpersonal arguments and fights (P < 0.005) (Fig. 5). We also assessed whether the online average stressfulness and arousal ratings correlated with heart rate, with cortisol, and with VmPFC neuroplasticity. We found moderately significant positive correlations between VmPFC neuroplasticity and average stressfulness (r = 0.40, P < 0.03) and arousal (r = 0.45, P < 0.01) ratings. There were no significant correlations between heart rate and cortisol during stress and online stress and arousal ratings.
Fig. 5.
VmPFC functional plasticity and coping. Individuals showing greater VmPFC activity in the late compared with early runs (A) during stress report higher active coping scores (B), and lower scores on emotional eating behaviors (C), lower nonbinge levels of alcoholic drinks consumed per occasion (D), and low to never getting into arguments and fights with others (E) (see SI Appendix, Table S1 and Detailed Materials and Methods for measures used).
Discussion
Using a brief, sustained exposure paradigm in a stress versus no-stress neutral experiment, we demonstrated significant subjective, physiological, and endocrine stress responses, along with temporally related, dynamically distinct and separable patterns of neural activation in brain circuits underlying the stress response. First, sustained increases in the amygdala, striatum, hypothalamus, midbrain, R insula, and R dorsolateral prefrontal cortex (DLPFC) regions were observed, suggesting a neural pattern consistent with a stress reactivity and processing circuit representing the distress-signaling component of the stress response. A second neural pattern with dynamic temporally related changes during stress versus neutral showed early increases followed by later reductions in activation in the ventrolateral prefrontal cortex (VLPFC), dorsal anterior cingulate cortex (dACC), L DLPFC, hippocampus, and insula that pointed to a stress adaptation response network. Finally, opposite to the previous pattern, we found a mobilization of the VmPFC and VS response during stress marked by initial hypoactivity in VmPFC and no change in the VS, followed by increased VmPFC and VS activation in later runs, relative to the neutral response, supporting previous basic science research and our hypothesis that the VmPFC is a key region of a dynamic and flexible neural circuit that may underlie behavioral control and active, resilient coping.
Previous animal and human data indicate that psychosocial stress disrupts prefrontal and attentional circuits underlying executive functioning and cognitive coping and that there is significant brain plasticity such that these disruptions are reversible with stress removal (9). In addition, the VmPFC has been found to modulate behavioral control over stress (18) and is important for various affective and behavioral coping [including somatomotor control (31), behavioral flexibility (32), regulation of negative emotion and anxiety (22, 33), and persistence in the face of setbacks (21)] and in integrating these signals for appropriate decision-making and goal-directed behaviors (34). This previous work led us to specifically hypothesize that the VmPFC is a key locus in the behavioral control and resilience-coping network. In further support of this hypothesis, the average stress-specific VmPFC response across all runs resulted in positive strengthening connectivity with average activation in other key prefrontal cortical networks, such as the aPFC/DLPFC and attentional regions such as the IPL known to underlie executive attention and cognitive control. The aPFC/DLPFC and IPL are coactivated during working memory (35), and their role in cognitive control and executive attentional processes required for goal-directed behaviors and control of intended actions has been demonstrated (6, 36). Given the findings of dynamic mobilization of the VmPFC activation, we specifically tested whether this region in the coping network correlates with active coping and coping behaviors. We found that active coping self-report scores were positively correlated with VmPFC functional plasticity and, conversely, that failure of VmPFC plasticity was associated with maladaptive coping behaviors (greater reporting of emotional eating, binge alcohol consumption, and higher frequency of arguments and fights). Although these maladaptive coping behaviors may seem disparate at first, an underlying aspect of these maladaptive behaviors encompasses emotion dysregulation and/or loss of self-control, processes known to contribute to emotional and behavioral self-regulation (34, 37, 38). Together, the current findings indicate that VmPFC neuroplasticity during stress plays a significant role in adaptive and resilient coping.
The brief, sustained exposure task required participants to provide ongoing subjective stressfulness and arousal ratings using a button box, but not to actively regulate or reduce their stress levels or alter their stress via specific responses. Interestingly, we found a significant positive association between the dynamic VmPFC response during stress representing active coping and average stressfulness and arousal ratings. Although seeming counterintuitive at first glance, these positive associations may represent the conscious process of increasing interoceptive and subjective awareness of stress signals as an initial step toward regaining perceived and behavioral control over stress in healthy individuals. Increasing awareness of stress and emotions and appraisal of stress and emotional stimuli are key components of emotion regulation (37, 39) and are in contrast to the more quick and “autopilot” type of responding and emotion dysregulation associated with high stress states (40). Further support for this interpretation comes from the growing evidence indicating the use of mindfulness strategies that focus on increasing awareness of the perceptual and internal state as a strategy for increasing perceived and behavioral control over stress, pain, depression, and other stress-related conditions (41, 42).
Along with stress-related VmPFC plasticity, we found the ventral striatum to increase during late relative to early runs in the stress versus neutral contrast, consistent with its role in avoidant learning and motivation (43, 44). There are also direct connections between the VmPFC and the VS to facilitate processing of salient aversive and appetitive stimuli, reinforcement learning, and motivation for action and response selection (43, 45, 46). This research is consistent with the coordinated increase in activity seen in both the VmPFC and VS during the late runs of S vs. N exposure.
In addition to the distinct and separable dynamic VmPFC and VS activation suggestive of a neural circuit for active and resilient coping, we found evidence for two additional distinct patterns of neural activity underlying additional key components of the stress response (38, 47). First, sustained activity throughout the S vs. N period in regions including the amygdala, hippocampus, hypothalamus, thalamus, anterior insula, dorsal striatum, and midbrain was found. These regions make up the well-known limbic and striatal brain circuit underlying stress perception, experience, and conscious processing of stress (15). Because participants were viewing previously unseen aversive and highly stressful stimuli, these sustained increases may be representative of the stress “alarm” signal of the acute stress response to not only signal distress but also mobilize the habit circuit involving the dorsal striatum (12, 13). In addition, sustained increased right lateralized activity was also observed in regions involved in integrative processing and monitoring of negative emotional stimuli (e.g., anterior cingulate) (48) and in regions involved in cognitive appraisal and working memory (e.g., DLPFC) (25, 35) (shown in Fig. S1), suggesting that such cognitive processing of distress plays a key role in the brain stress reactivity and stress-signaling response in healthy individuals.
We also found that increased cortisol response to stress was positively associated with key limbic striatal regions involved in glucocorticoid stress-signaling regions such as the hypothalamus, amygdala/hippocampus (49, 50), and ventral striatum (51–53) whereas a highly significant negative association with the rostral ACC (rACC, BA 10 and 32) extending into the OFC (BA 11) of the VmPFC was observed. Of note, the center of mass for this negative association was more dorsal in the rACC than the center of the VmPFC dynamic activation. However, the extent of the negative association cluster extended ventrally into the dynamic VmPFC activation region (SI Appendix, Table S3). Acute stress-related increases in glucocorticoids are critical both for the alarm signal as well as in regaining control over the stress signal resulting in healthy stress coping. Recent animal data show a glucocorticoid role in modulation of the medial PFC involved in goal-directed behaviors (54). Interestingly, the highly significant negative association between stress-related cortisol increases and reduced rACC/VmPFC was for the S-N average rACC/VmPFC response and not for the dynamic changes that were found to be correlated with active coping. It follows then that those individuals showing lower dynamic activity and thus more maladaptive coping were likely to have higher cortisol reactivity. However, cortisol change did not directly predict coping behaviors, suggesting that individual differences in dynamic neural stress responses in the VmPFC-related behavioral control circuit may mediate the link between glucocorticoids and coping behaviors, and future research to further explore this relationship is warranted.
The final distinct neural stress pattern was a temporally related activation showing significantly increased activation in the insula and midtemporal regions, R ventral hippocampus, and L VLPFC in the early runs, followed by dynamic changes with decreased activity during the late runs during S vs. N conditions. Indeed, L midtemporal, ventral hippocampus, and the VLPFC are involved in processing interoceptive stress signals, matching to prior experience and in semantic and nonverbal representation, integration, and modulation (13). Although we did not predict dynamic decreases in these specific regions, reduction in activation of these regions during the later stress runs may represent acute neuroplasticity involved in adapting and habituating to decrease the impact of the stress experience. Well-known cognitive and behavioral coping strategies such as distraction, suppression, and mental distancing are often used to adapt and decrease the stressful experience, and one may speculate that the distinct temporally related decreases in neural activity in these specific regions during stress may involve such coping processes. Future studies that assess this neural component of the stress response may provide further insights into the neural mechanisms underlying stress-adaptive responses that reduce acute experiencing of unpredictable and uncontrollable stressful events.
Although the above findings are informative, an important caveat is that men constituted a minority of the sample, and thus the findings are more generalizable to women than men. Although secondary post hoc analyses did not show a significant influence of sex on dynamic neural responses, the smaller sample of men likely limited our ability to adequately explore sex differences. Future studies with a larger sample are needed to replicate current findings and to fully assess any potential sex differences in neural stress responses.
Despite the limited generalizability to men, the results may have clinical utility. Using a previously unused multimethod functional neuroimaging approach, they provide evidence for distinct dynamic neural activation consistent with acute functional neuroplasticity that may play a role in regaining behavioral control to support resilient coping during stress. The findings also suggest three related but separable components of neural signaling during stress, involving the VmPFC-related network playing a role in regaining perceptual and behavioral control and decision making (34), a distinct cortico-limbic-striatal circuit for stress perception, reactivity, and conscious processing, and a stress adaptation circuit possibly for reducing and adapting to the aversive stress experience (38). These components of the stress response may have implications for identifying stress-related vulnerabilities for mental and physical health disorders and may also provide target neural measures for assessing intervention effects. For example, blunted or disrupted VmPFC response has been associated with PTSD (14, 25) and alcoholism relapse (13), and also in depression and addiction and in individuals with high childhood trauma and cumulative adversity (13, 55, 56). However, whether the stress pathophysiology in each of these disorders and conditions was due to a failure of functional neuroplasticity in the dynamic VmPFC network or the stress adaptation network during stress, as shown in the current study, or due to an overreactivity or inability to reduce stress reactivity, or both, is not clear. The findings suggest the potential utility of the current experimental approach as a neurobehavioral assay of stress reactivity and resilience coping in testing novel behavioral and pharmacological strategies that may reduce stress reactivity and/or improve resilient coping in individuals with maladaptive coping and in those with stress-related disorders. On the other hand, the current experiment did not assess response selection and instrumental actions during stress, and future research is needed to understand and identify dynamic brain processes that underlie stress-related response selection or instrumental action and their link to coping behaviors.
Materials and Methods
Participants.
Thirty right-handed, nonsmoking, community adults [73% women; mean age = 25.7 (8.61) y; 76% Caucasian; years of education = 15.7 (2.17)] who did not meet criteria for any psychiatric disorders, including substance use disorders based on the assessment by the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders IV (DSM-IV-TR) (57) participated in the study. All study procedures were approved by the Human Investigation Committee of the Yale University School of Medicine, and all participants signed a written informed consent.
Overall Procedures.
On the scanning day, all subjects arrived at the Yale Stress Center between 1200 and 1400 hours and were given a standard lunch and then received training on fMRI experimental procedure. Between 1400 and 1600 hours, the subjects participated in the MRI scan where, upon arrival, an i.v. line was inserted in the nondominant (left) arm of subjects by a nurse for cortisol data collection. The subject then completed a practice task consisting of 10 trials using stimuli that were not used for the in-scan fMRI task. After a 45-min adaptation period, the subject entered the MRI, and a pulse oximeter was placed on the nondominant forefinger to obtain heart rate. The subject completed the functional scan, and repeated blood draws via the i.v. line were also obtained. The brief, sustained stress/neutral exposure task is presented in Fig. 1, and details about the visual stimuli, task procedures, the subjective, physiological, and endocrine measurements, assessment of coping behaviors, and the fMRI procedures and data analyses are presented in SI Appendix.
Supplementary Material
Acknowledgments
This research was supported by NIH and NIH Roadmap for Medical Research Common Fund Grants UL1-DE019586 (to R.S.), R01-AA13892 (to R.S.), R01-DK099039 (to R.S.), PL1-DA024859 (to R.S.), and K08-AA023545-01 (to D.S.), as well as by NIH-supported Yale Clinical and Translational Science Award (CTSA) UL1-RR024139.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1600965113/-/DCSupplemental.
References
- 1.Backé EM, Seidler A, Latza U, Rossnagel K, Schumann B. The role of psychosocial stress at work for the development of cardiovascular diseases: A systematic review. Int Arch Occup Environ Health. 2012;85(1):67–79. doi: 10.1007/s00420-011-0643-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosengren A, Orth-Gomér K, Wedel H, Wilhelmsen L. Stressful life events, social support, and mortality in men born in 1933. BMJ. 1993;307(6912):1102–1105. doi: 10.1136/bmj.307.6912.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007;298(14):1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- 4.Robbins TW. Controlling stress: How the brain protects itself from depression. Nat Neurosci. 2005;8(3):261–262. doi: 10.1038/nn0305-261. [DOI] [PubMed] [Google Scholar]
- 5.LeDoux JE, Gorman JM. A call to action: Overcoming anxiety through active coping. Am J Psychiatry. 2001;158(12):1953–1955. doi: 10.1176/appi.ajp.158.12.1953. [DOI] [PubMed] [Google Scholar]
- 6.Williams PG, Thayer JF. 2009. Executive functioning and health: Introduction to the special series. Ann Behav Med 37(2):101–105.
- 7.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 8.Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10(6):410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liston C, McEwen BS, Casey BJ. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc Natl Acad Sci USA. 2009;106(3):912–917. doi: 10.1073/pnas.0807041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnsten A, Mazure CM, Sinha R. This is your brain in meltdown. Sci Am. 2012;306(4):48–53. doi: 10.1038/scientificamerican0412-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McEwen BS, Morrison JH. The brain on stress: Vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 2013;79(1):16–29. doi: 10.1016/j.neuron.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plessow F, Kiesel A, Kirschbaum C. The stressed prefrontal cortex and goal-directed behaviour: Acute psychosocial stress impairs the flexible implementation of task goals. Exp Brain Res. 2012;216(3):397–408. doi: 10.1007/s00221-011-2943-1. [DOI] [PubMed] [Google Scholar]
- 13.Seo D, Tsou KA, Ansell EB, Potenza MN, Sinha R. Cumulative adversity sensitizes neural response to acute stress: Association with health symptoms. Neuropsychopharmacology. 2014;39(3):670–680. doi: 10.1038/npp.2013.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16(2):331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- 15.Sinha R, Lacadie C, Skudlarski P, Wexler BE. Neural circuits underlying emotional distress in humans. Ann N Y Acad Sci. 2004;1032:254–257. doi: 10.1196/annals.1314.032. [DOI] [PubMed] [Google Scholar]
- 16.Drevets WC, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386(6627):824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 17.Admon R, et al. Striatal hypersensitivity during stress in remitted individuals with recurrent depression. Biol Psychiatry. 2015;78(1):67–76. doi: 10.1016/j.biopsych.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maier SF, Watkins LR. Role of the medial prefrontal cortex in coping and resilience. Brain Res. 2010;1355:52–60. doi: 10.1016/j.brainres.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleshner M, Maier SF, Lyons DM, Raskind MA. The neurobiology of the stress-resistant brain. Stress. 2011;14(5):498–502. doi: 10.3109/10253890.2011.596865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hariri AR, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297(5580):400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- 21.Bhanji JP, Delgado MR. Perceived control influences neural responses to setbacks and promotes persistence. Neuron. 2014;83(6):1369–1375. doi: 10.1016/j.neuron.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Somerville LH, et al. Interactions between transient and sustained neural signals support the generation and regulation of anxious emotion. Cereb Cortex. 2013;23(1):49–60. doi: 10.1093/cercor/bhr373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seo D, et al. Disrupted ventromedial prefrontal function, alcohol craving, and subsequent relapse risk. JAMA Psychiatry. 2013;70(7):727–739. doi: 10.1001/jamapsychiatry.2013.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gee DG, et al. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci USA. 2013;110(39):15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shin LM, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62(3):273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- 26.McKlveen JM, et al. Role of prefrontal cortex glucocorticoid receptors in stress and emotion. Biol Psychiatry. 2013;74(9):672–679. doi: 10.1016/j.biopsych.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Butts KA, Weinberg J, Young AH, Phillips AG. Glucocorticoid receptors in the prefrontal cortex regulate stress-evoked dopamine efflux and aspects of executive function. Proc Natl Acad Sci USA. 2011;108(45):18459–18464. doi: 10.1073/pnas.1111746108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lang PJ, Bradley MM, Cuthbert BN. 2008. International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual (University of Florida, Gainesville, FL), Tech Rep A-8.
- 29.Phan KL, Liberzon I, Welsh RC, Britton JC, Taylor SF. Habituation of rostral anterior cingulate cortex to repeated emotionally salient pictures. Neuropsychopharmacology. 2003;28(7):1344–1350. doi: 10.1038/sj.npp.1300186. [DOI] [PubMed] [Google Scholar]
- 30.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 31.Bechara A, Damasio AR. The somatic marker hypothesis: A neural theory of economic decision. Games Econ Behav. 2005;52(2):336–372. [Google Scholar]
- 32.Fellows LK, Farah MJ. Ventromedial frontal cortex mediates affective shifting in humans: Evidence from a reversal learning paradigm. Brain. 2003;126(Pt 8):1830–1837. doi: 10.1093/brain/awg180. [DOI] [PubMed] [Google Scholar]
- 33.Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: Reappraisal and suppression of negative emotion. Biol Psychiatry. 2008;63(6):577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000;10(3):295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- 35.Friedman HR, Goldman-Rakic PS. Coactivation of prefrontal cortex and inferior parietal cortex in working memory tasks revealed by 2DG functional mapping in the rhesus monkey. J Neurosci. 1994;14(5 Pt 1):2775–2788. doi: 10.1523/JNEUROSCI.14-05-02775.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seeley WW, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gross JJ, John OP. Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. J Pers Soc Psychol. 2003;85(2):348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- 38.McEwen BS. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol Rev. 2007;87(3):873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 39.Gratz KL, Roemer L. Multidimensional assessment of emotion regulation and dysregulation: Development, factor structure and initial validation of the Difficulties of Emotion Regulation Scale. J Psychopathol Behav Assess. 2004;26(1):41–54. [Google Scholar]
- 40.Paulson S, Davidson R, Jha A, Kabat-Zinn J. Becoming conscious: The science of mindfulness. Ann N Y Acad Sci. 2013;1303:87–104. doi: 10.1111/nyas.12203. [DOI] [PubMed] [Google Scholar]
- 41.Teasdale JD, et al. Metacognitive awareness and prevention of relapse in depression: Empirical evidence. J Consult Clin Psychol. 2002;70(2):275–287. doi: 10.1037//0022-006x.70.2.275. [DOI] [PubMed] [Google Scholar]
- 42.Segal ZV, Walsh KM. Mindfulness-based cognitive therapy for residual depressive symptoms and relapse prophylaxis. Curr Opin Psychiatry. 2016;29(1):7–12. doi: 10.1097/YCO.0000000000000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30(5):220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 44.Schultz W. Dopamine neurons and their role in reward mechanisms. Curr Opin Neurobiol. 1997;7(2):191–197. doi: 10.1016/s0959-4388(97)80007-4. [DOI] [PubMed] [Google Scholar]
- 45.Goto Y, Grace AA. Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nat Neurosci. 2005;8(6):805–812. doi: 10.1038/nn1471. [DOI] [PubMed] [Google Scholar]
- 46.Price JL. Free will versus survival: Brain systems that underlie intrinsic constraints on behavior. J Comp Neurol. 2005;493(1):132–139. doi: 10.1002/cne.20750. [DOI] [PubMed] [Google Scholar]
- 47.Folkman S. Personal control and stress and coping processes: A theoretical analysis. J Pers Soc Psychol. 1984;46(4):839–852. doi: 10.1037//0022-3514.46.4.839. [DOI] [PubMed] [Google Scholar]
- 48.MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288(5472):1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- 49.de Kloet ER, Joëls M, Holsboer F. Stress and the brain: From adaptation to disease. Nat Rev Neurosci. 2005;6(6):463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 50.McEwen BS. Brain on stress: How the social environment gets under the skin. Proc Natl Acad Sci USA. 2012;109(Suppl 2):17180–17185. doi: 10.1073/pnas.1121254109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piazza PV, et al. Glucocorticoids have state-dependent stimulant effects on the mesencephalic dopaminergic transmission. Proc Natl Acad Sci USA. 1996;93(16):8716–8720. doi: 10.1073/pnas.93.16.8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pruessner JC, Champagne F, Meaney MJ, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: A positron emission tomography study using [11C]raclopride. J Neurosci. 2004;24(11):2825–2831. doi: 10.1523/JNEUROSCI.3422-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10(6):397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hollon NG, Burgeno LM, Phillips PE. Stress effects on the neural substrates of motivated behavior. Nat Neurosci. 2015;18(10):1405–1412. doi: 10.1038/nn.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mayberg HS. Limbic-cortical dysregulation: A proposed model of depression. J Neuropsychiatry Clin Neurosci. 1997;9(3):471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- 56.Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: Involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10(3):318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- 57.First MB, Spitzer RL, Gibbon M, Janet B. 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version (Biometrics Research, New York State Psychiatric Institute, New York)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.