Significance
The expansion and differentiation of human mesenchymal stem cells (hMSCs), an important cell type that is frequently used in stem-cell–based therapies, are regulated by interactions with their local extracellular microenvironment. Matrix stiffness is one of the key regulators, but often varies in space at a range of length scales in the local niche. To better understand and harness cell–matrix interactions, we used an innovative phototunable hydrogel platform to provide compelling evidence that the magnitude and spatial organization of matrix mechanics influence hMSCs through systematic regulation of cytoskeletal tension and transcriptional activation. These findings may have important implications in deepening our fundamental understanding of the role of extracellular matrix organization on disease, aging, and regenerative processes.
Keywords: photodegradable hydrogel, human mesenchymal stem cell, spatial matrix stiffness
Abstract
There is a growing appreciation for the functional role of matrix mechanics in regulating stem cell self-renewal and differentiation processes. However, it is largely unknown how subcellular, spatial mechanical variations in the local extracellular environment mediate intracellular signal transduction and direct cell fate. Here, the effect of spatial distribution, magnitude, and organization of subcellular matrix mechanical properties on human mesenchymal stem cell (hMSCs) function was investigated. Exploiting a photodegradation reaction, a hydrogel cell culture substrate was fabricated with regions of spatially varied and distinct mechanical properties, which were subsequently mapped and quantified by atomic force microscopy (AFM). The variations in the underlying matrix mechanics were found to regulate cellular adhesion and transcriptional events. Highly spread, elongated morphologies and higher Yes-associated protein (YAP) activation were observed in hMSCs seeded on hydrogels with higher concentrations of stiff regions in a dose-dependent manner. However, when the spatial organization of the mechanically stiff regions was altered from a regular to randomized pattern, lower levels of YAP activation with smaller and more rounded cell morphologies were induced in hMSCs. We infer from these results that irregular, disorganized variations in matrix mechanics, compared with regular patterns, appear to disrupt actin organization, and lead to different cell fates; this was verified by observations of lower alkaline phosphatase (ALP) activity and higher expression of CD105, a stem cell marker, in hMSCs in random versus regular patterns of mechanical properties. Collectively, this material platform has allowed innovative experiments to elucidate a novel spatial mechanical dosing mechanism that correlates to both the magnitude and organization of spatial stiffness.
Tissue homeostasis is maintained by a complex interplay between cells and their surrounding extracellular matrix (ECM), where the ECM is dynamically remodeled and organized on numerous length scales from the subcellular to the macroscopic (1). Although a complex milieu of chemical signals, such as chemokines and cytokines, provide a wealth of cues directing these processes, there is a growing appreciation that contextual presentation of these molecules within the surrounding physical environment can have a dramatic impact on cellular fate as well (2–4). For example, changes in matrix mechanics are important in many biological processes including the recruitment of cells to an injury site during wound healing (5) and during disease development (6, 7). For instance, local ECM organization plays a vital role in breast tumorigenesis, as it is up-regulated by the development of highly cross-linked and linearized local collagen structures, and such abnormal organization can lead to severe tissue stiffening that sustains cancer cell survival and promotes aggressive cellular invasion (8–10). This phenomenon is also observed in heart tissue, where organized ECM is a critical regulator that maintains normal valve function, and disarray of local mechanics provokes pathophysiology such as cardiac hypertrophy or valve calcification (7, 11).
Beyond basic tissue stiffening, matrix spatial organization and the resulting mechanics appear to matter, but how they coordinate a functional stimulus that facilitates intracellular signaling of healthy or diseased phenotypes is poorly understood. Intricate regulation of spatial matrix mechanics is implicated in tissue regeneration during wound healing (12, 13); however, it is challenging to identify individual events of subcellular mechanical stimulation, and investigate how such diverse variations in spatial stiffness integrate to direct local cellular activity. In order to elucidate some of these cell–matrix signaling processes in space, we applied a biomaterial system that allows for the systematic introduction of spatial variations in matrix mechanics. A tunable in vitro platform was developed by exploiting a photolabile chemistry to manipulate material properties of an initially isotropic hydrogel in situ and then examine how spatially patterned elasticity directs the cell fate of human mesenchymal stem cells (hMSCs).
Specifically, we explore whether activating (stiff) and deactivating (soft) mechanical signals, when presented together, obstruct one another, or if there is a threshold at which one signal dominates. To interrogate these hypotheses and their effect on individual stem cell fate, we use a poly(ethylene glycol) (PEG) hydrogel with photolabile linkages that allows for in situ softening of the material modulus on subcellular length scales by controlled light exposure through a photomask. Patterning is achieved on a micrometer scale to create soft (∼2 kPa) and stiff (∼10 kPa) regions in the hydrogel (14). This versatile, user-tunable platform allows one to elucidate how hMSCs respond to mechanical microenvironmental signals that often vary in space, and whether or not cell–matrix interactions lead to mechanotransduction relationships that are linear, step functions, or something more complex.
Results
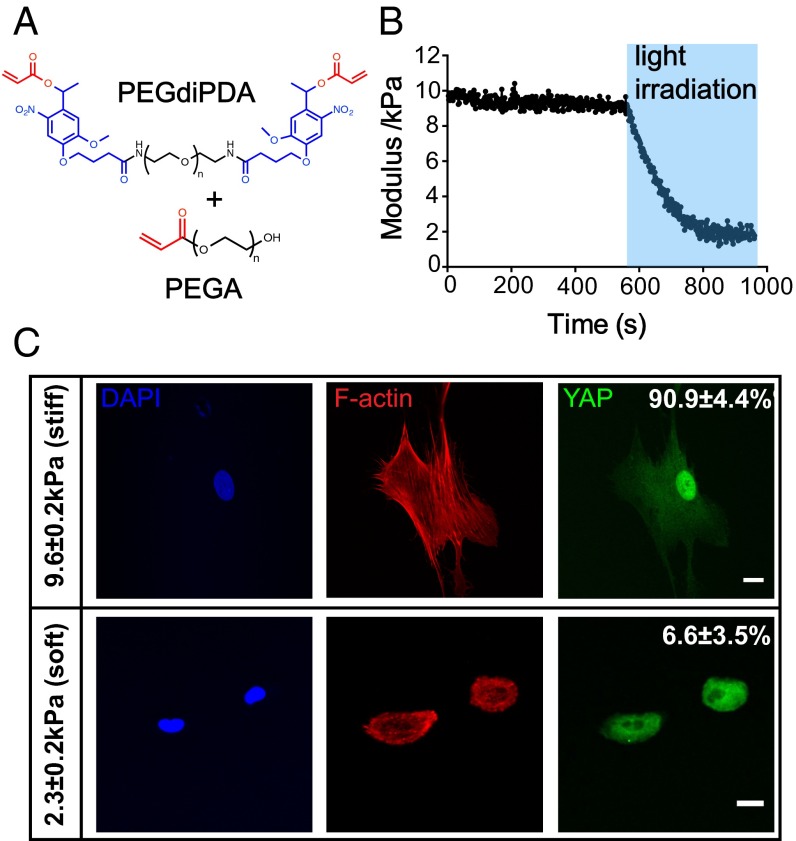
Hydrogels with tunable mechanical properties were synthesized by copolymerizing PEG monoacrylate (PEGA) with a photodegradable PEG diacrylate (PEGdiPDA) (Fig. 1A). The photolabile cross-linker allows in situ softening of the gel stiffness from an initial Young’s modulus (E) of 9.6 ± 0.2 kPa to 2.3 ± 0.2 kPa by exposure to 365 nm light at 10 mW/cm2 for 360 s (Fig. 1B). Cytoskeletal organization of hMSCs seeded on the hydrogel formulation with the highest modulus (subsequently referred to as stiff, E = 9.6 ± 0.2 kPa) exhibited more organized actin bundles, as indicated by F-actin staining, and tensile actomyosin fibers compared with those cultured on gel with the lowest modulus (subsequently referred to as soft; E = 2.3 ± 0.2 kPa) (Fig. 1C, Middle). In addition, seeding on uniformly stiff substrates led to 90.9 ± 4.4% nuclear Yes-associated protein (YAP) (15) activation, although YAP was mostly deactivated in the cytoplasm with only 6.6 ± 3.5% nuclear localization for hMSCs cultured on the softer gel (Fig. 1C, Right), indicating that two opposite intracellular signals are induced in this range of matrix mechanical properties.
Fig. 1.
(A) Chemical structures of the photodegradable cross-linker (PEGdiPDA) and PEGA. Acrylate functional groups are labeled in red and the photodegradable nitrobenyzl ether is labeled in blue. (B) Photodegradable hydrogels polymerized from PEGdiPDA and PEGA monomers can be softened from stiff (E = 9.6 ± 0.2 kPa) to soft (E = 2.3 ± 0.2 kPa) moduli by irradiation of 365 nm light for 360 s. (C) Immunostaining of hMSCs on stiff and soft hydrogel surfaces. On stiff hydrogels, hMSCs expressed tensile F-actin bundles and had 90.9 ± 4.4% nuclear YAP activation; whereas, on soft hydrogels, hMSCs only had 6.6 ± 3.5% nuclear YAP activation with less organized F-actin structure. DAPI (4’,6-diamidino-2-phenylindole; blue) F-actin (red), YAP (green). Scale bars = 20 μm; n = 5 with over 100 cells analyzed for each condition.
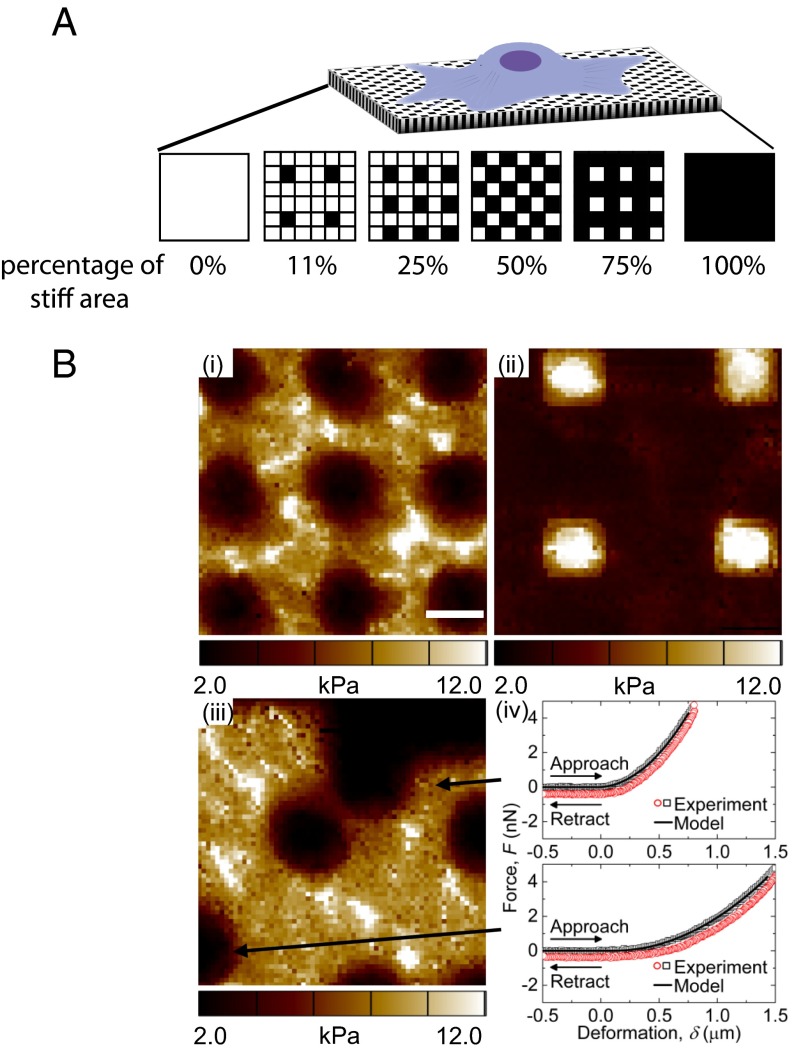
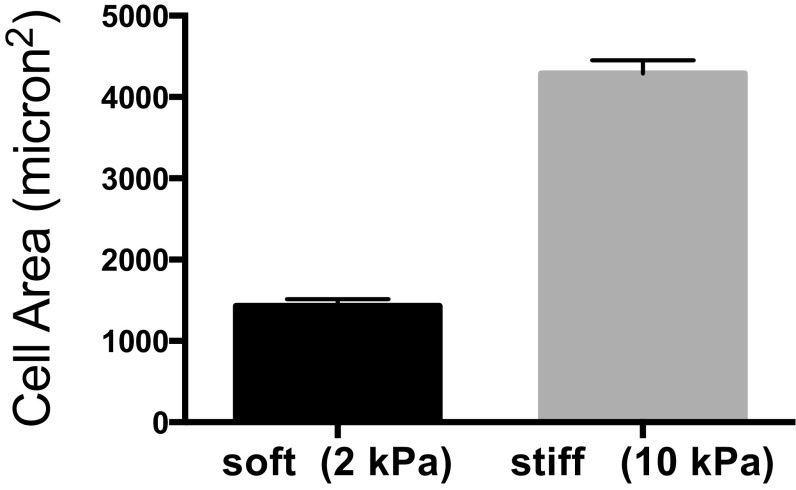
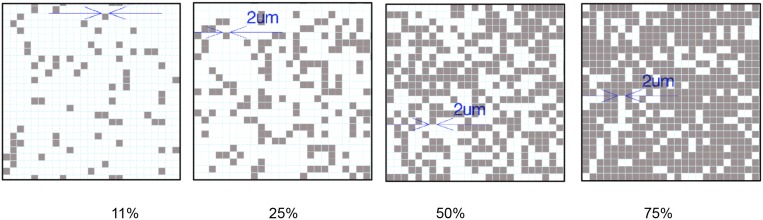
Subsequently, the aforementioned stiff and soft signals were introduced to hMSCs on the same surface through controlled softening of the hydrogel via spatial degradation of the nitrobenzyl ether cross-linker by passing light through a photomask and examining how spatial variations in mechanical signaling influences hMSC response. We aimed to pattern mechanical regions that were comparable in size to a mature focal adhesion (1 to 5 μm2) (16, 17); so that spatial mechanical variations would be introduced at a length scale that can potentially direct focal adhesion assembly and subsequently affect cellular attachment and internal mechanotransduction. Uniform patterns of 2 μm by 2 μm squares were created to introduce regions with distinct mechanical properties; specifically, after light exposure, the ratio of stiff to soft regions was varied as indicated in Fig. 2A. Also, because the average area of a spread hMSC is ∼1,500 μm2 on uniformly soft substrates (Fig. S1), the cells should sample a large number of both stiff and soft regions. Thus, statistically, any single hMSC on the substrate should experience a similar composition of mechanical cues.
Fig. 2.
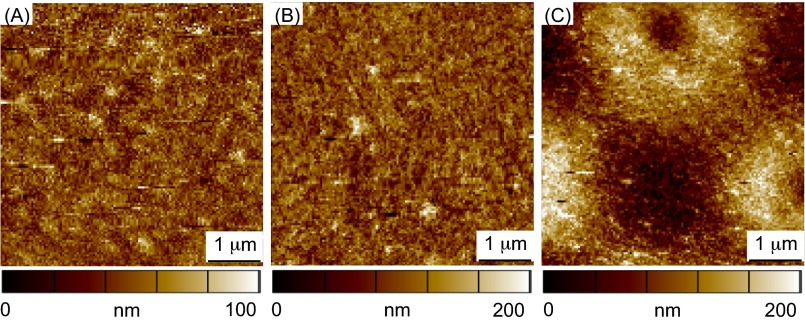
(A) An illustration of hMSCs seeded on mechanically patterned hydrogel surfaces with different stiff-to-soft ratios. Black indicates chrome-covered areas that will remain stiff, and white squares indicate areas exposed to light that will be degraded to soft regions. (B) AFM elastic moduli maps of (i) 75% stiff (regular pattern), (ii) 11% stiff (regular pattern), and (iii) 75% stiff (random pattern) hydrogels. (iv) Representative force-deformation data and model fits for two regions in iii. Scale bars = 2 μm.
Fig. S1.
hMSCs spreading area on 2-kPa hydrogels. The average area of a spread hMSC is about 1,500 μm2 on uniformly soft substrates; n > 5 with over 100 cells analyzed in each condition. Mean ± SE.
Using several different lithographic masks, cell culture substrates were synthesized with varying patterns to study the effects of a range of different stiff to soft ratios (3:1, 1:1, 1:3, 1:8) and corresponding areas of stiffness (75%, 50%, 25%, and 11%, respectively) on hMSC matrix interactions and signaling. The resulting patterned substrates were characterized by atomic force microscopy (AFM) as shown in Fig. 2B; representative images for the regular 75% and 11% stiff, as well as random 75% stiff substrates are shown in Figs. 2B, i, ii, and iii, respectively. As evident from the AFM data, the spatial variations in the moduli values are consistent with the patterns found on the lithographic masks, indicating successful pattern transfer to the intended regions of the hydrogels using photosoftening. In addition, the magnitude of the moduli measured using AFM are in good agreement with rheological bulk moduli measurements (Fig. 1B, Fig. S2) of the uniformly stiff and soft substrates, with values ranging from ∼2–3 kPa in the soft regions to ∼10–12 kPa in the stiff regions. For reference, two AFM force (F)–deformation (δ) datasets from Fig. 2B, iii are shown in Fig. 2B, iv. In both the soft and stiff regions, the AFM F–δ data are well-described by the model used to extract the moduli values (i.e., F is proportional to δ2), suggesting that the contact geometry between the AFM tip and hydrogel surface is well approximated by the analytical solution for a rigid conical indenter in contact with an elastic half-space.
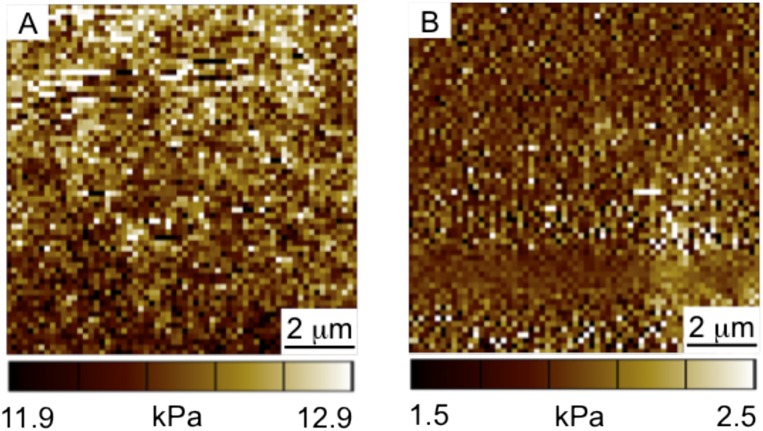
Fig. S2.
AFM elastic moduli maps of uniformly stiff and soft hydrogels. (A) Elastic modulus of the uniformly stiff hydrogel is 12.4 ± 0.2 kPa. (B) Elastic modulus of the uniformly soft hydrogel is 2.0 ± 0.2 kPa. Scale bar = 2 μm.
Complementary to the mechanical property measurements, the surface roughness after gel patterning was also analyzed by AFM, as surface topography has been shown to influence cell fate (18, 19). After photopatterning, the hydrogel surface (75% stiff) had an rms roughness of 43 ± 2 nm, which is on the same order of magnitude compared with the rms roughness of 12 ± 1 nm on the uniformly stiff hydrogel, and 23 ± 2 nm on the uniformly soft hydrogel (Fig. S3). Therefore, hydrogels were fabricated with distinctly varying mechanical regions with minimal changes in the surface topography that maintain a constant adhesive area among conditions of different stiff-to-soft ratios.
Fig. S3.
Surface roughness measured by AFM. Images are color coded by surface topographic features, with darker colors representing lower z positions and brighter color represents higher z positions. (A) Root mean square roughness of the uniformly stiff hydrogel is 12 ± 1 nm. (B) Root mean square roughness of the uniformly soft hydrogel is 23 ± 2 nm. (C) Root mean square roughness of the patterned hydrogel (75% stiff) is 43 ± 2 nm. Scale bar = 1 μm.
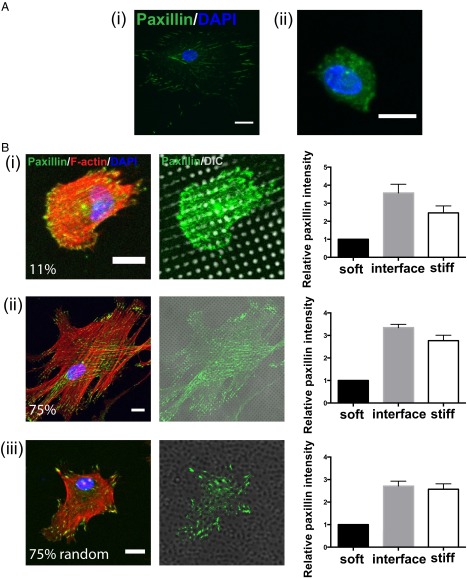
Subsequently, markers of cell–matrix interactions were examined to determine whether hMSCs can distinguish between differences in mechanical properties on the μm scale, or if they simply sense the overall average stiffness. Both the F-actin structure and the focal adhesion protein paxillin were stained for hMSCs cultured on surfaces with spatially varying mechanical moduli (Fig. 3). In general, punctate paxillin staining, indicating mature focal adhesions (20), was observed at the cell–matrix interface when hMSCs were cultured on the uniformly stiff substrates (Fig. 3A, i). In contrast, hMSCs on the soft substrate (Fig. 3A, ii) had uniform basal paxillin expression throughout the cell body instead of forming clustered structures at the interface, indicating that fewer mature focal adhesions were formed. It is interesting to note that when hMSCs were cultured on patterned surfaces, punctate paxillin staining appeared to concentrate in the stiff regions and along the border of stiff-to-soft regions. This observation was further quantified by using image analysis that first identified stiff and soft regions of the hydrogel surface based on bright field images [differential interference contrast (DIC channel)] and then calculated the relative focal adhesion intensity (normalized to area) in both regions with mechanically distinct properties, as well as within an interfacial edge between them (Fig. 3B; Fig. S4). For hMSCs on both 11% (Fig. 3B, i) and 75% (Fig. 3B, ii) stiff substrates, the focal adhesion intensities within the interfacial region and stiff regions were almost threefold higher, compared with that measured in the soft regions. However, with distinct clustered paxillin structures, many more tensile F-actin fibers were observed to initiate from focal adhesion sites in the stiff regions when hMSCs were on 75% stiff substrates than those cultured on 11% stiff substrates. This observation implies that cytoskeletal tension in hMSCs, which is much stronger on substrates with a higher fraction of stiff regions, is generated as a function of the mechanical property patterning (Fig. 3B, i and ii).
Fig. 3.
(A, i) Paxillin staining (green) of hMSCs on stiff hydrogels illustrates clustered structures, which indicate the formation of mature focal adhesion. (ii) However, paxillin staining of hMSCs seeded on soft hydrogels results in a basal uniform expression throughout the cell body. Paxillin (green); DAPI (blue). Scale bars = 20 μm. (B) Relative localization of focal adhesions in hMSCs to mechanical regions on the patterned hydrogel. (i) Immunostaining of hMSCs on a regularly patterned hydrogel with 11% stiff area. Focal adhesion formation was observed by staining for paxillin (green), and cytoskeletal organization was observed by staining for F-actin (red). The localization of focal adhesions relative to the mechanically patterned regions was analyzed to identify the relative paxillin intensity within stiff, soft, and interfacial regions based on the fluorescent and DIC channels (Right). For quantification, all paxillin intensities for each image were normalized to the absolute value found in the soft regions. (ii) Immunostaining of hMSCs on regularly patterned hydrogel with 75% stiff area. (iii) Immunostaining of hMSCs on randomized patterned hydrogel with 75% stiff area. In all three conditions, paxillin intensities were about threefold higher in the stiff and interfacial regions relative to the soft regions. Paxillin (green), F-actin (red), DAPI (blue). Scale bars = 20 μm; n > 10 with over 50 cells analyzed for each condition. Data plotted as mean ±SE.
Fig. S4.
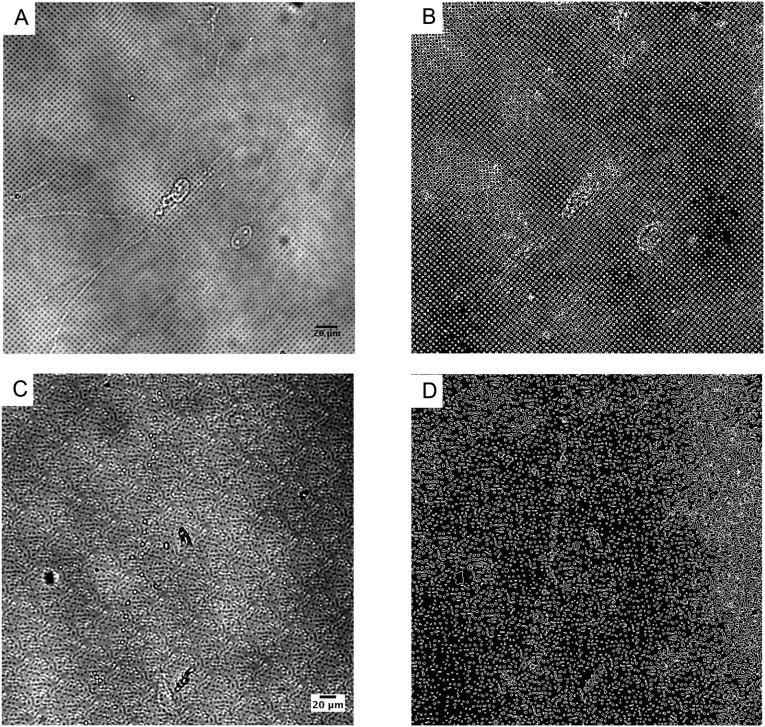
Outlining stiff, soft, and interfacial edge regions by image analysis. (A) A representative bright field image of a regularly patterned 75% stiff hydrogel. Image analysis was performed on A to generate B, which was segmented into three regions: interface (white), soft (black inside the interface), and stiff (black outside of the interface) areas. (C) A representative bright field image of a randomly patterned 75% stiff hydrogel. Image analysis was performed on C to generate D, which was segmented into three regions: interface (white), soft (black inside the interface), and stiff (black outside of the interface) areas. Scale bar = 20 μm.
In addition, we hypothesized that the spatial organization of the stiff versus soft regions, which plays an important role in the regulation of actin structure formation in hMSCs, would also affect cell–matrix signal transduction. Therefore, we created materials with the same ratio of soft and stiff regions, but distributed the regions in a random (or disorganized) manner as shown in Fig. S5 and validated in Fig. 2B, iii to investigate the corresponding cell responses. Specifically, hydrogels were fabricated by a repeating 50 μm by 50 μm (2,500 μm2) square pattern, in which the locations of the 2 μm by 2 μm stiff and soft regions were randomized. This patterned region is on the same size scale as a spread hMSC (Fig. S1), so the repeat unit is large enough for a single cell to sense these variations. When hMSCs were cultured on the random 75% stiff substrate (Fig. 3B, iii), punctate paxillin was again observed to colocalize with the stiff regions and with a similar distribution on stiff, soft, and interfacial regions as that found on the regular patterns (Fig. 3B, iii, Right). F-actin fibers stemmed from these focal adhesion sites; however, due to the differences in spatial organization of the focal adhesions, hMSCs on the random patterns had less organized tensile actin bundles than those on the regular pattern, even on hydrogels with the same percentage of stiff regions.
Fig. S5.
Design of the random patterns of 11%, 25%, 50%, and 75% stiff regions. The stiffness of each 2 μm by 2 μm regions was randomly decided based on a random number generator (1 as stiff and 0 as soft) with a defined stiff to soft ratio (11–75% stiff) in the 50 μm by 50 μm (2,500 μm2) square.
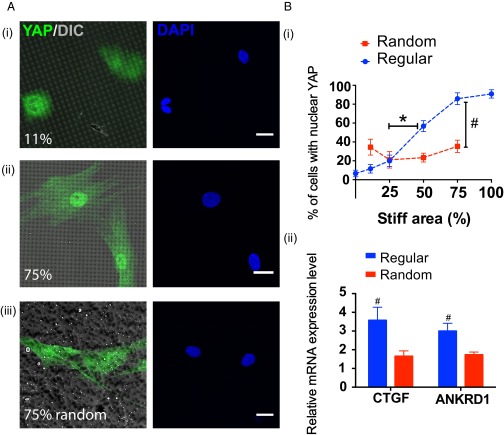
Based on the differences observed in hMSC–matrix interactions on substrates with varying percentages of stiff and soft regions and between regular and random patterns, we next sought to investigate whether or not these differences in cytoskeletal tension would lead to activation of the subcellular transcriptional coactivator YAP, the mediator that gauges extracellular mechanical stimulations into genetic events that can eventually affect hMSCs differentiation (15, 21, 22). Specifically, when hMSCs sense competing mechanical signals, does a threshold or critical interfacial area exist that a cell needs to sense in order for it to accumulate sufficient cytoskeletal tension to sustain nuclear YAP activation? Moreover, how will the spatial organization of mechanical signals play a role in such regulation? To answer these questions, hMSCs were studied on substrates patterned with regular 11% and 75% stiff regions, which represent two distinct stiff and soft ratios regimes from the cytoskeleton tension analysis (Fig. 4A, i and ii). Intracellular YAP localization was visualized by immunostaining, and more prominent nuclear YAP localization was observed on the substrate with 75% stiff moduli; however on 11% stiff substrates, YAP mainly remained inactive and localized in the cytoplasm (Fig. 4A, i and ii). This activation level was quantified based on the percentage of cells that had nuclear YAP staining, which was 83.4 ± 6.9% and 11.6 ±4.5%, respectively (Fig. 4B, i).
Fig. 4.
(A, i) Intracellular YAP (green) localization within hMSCs on regularly patterned hydrogels with 11% stiff area. Image was overlaid with the DIC channel to indicate the underlying pattern. YAP was mainly deactivated in the cytoplasm on 11% stiff hydrogels. (ii) Intracellular YAP localization of hMSCs on regularly patterned hydrogels with 75% stiff area. YAP was observed to primarily be activated in the nuclei. (iii) Intracellular YAP localization of hMSCs on randomly patterned hydrogels with 75% stiff area. YAP was found to be mainly deactivated in the cytoplasm. YAP (green), DAPI (blue). Scale bars = 20 μm. (B, i) The percent of hMSCs with intracellular YAP activation was quantified based on immunostaining analysis and found to increase correspondingly to stiff percentages on regular stiff patterns, indicated by the blue dash line and symbols. A sigmoidal response was observed with a significant increase in YAP activation from 25% stiff to 50% stiff. However, on randomly patterned hydrogels intracellular YAP activation in hMSCs was insensitive to the change of underlying stiff percentages, indicated by the red dash line and symbols; n > 5 with over 100 cells analyzed for each condition; * compared with regular 25% stiff, P < 0.01 based on one-way ANOVA analysis using Turkey’s multiple comparisons test; # compared with random 75% stiff, P < 0.001 based on unpaired t test. (ii) Comparison of relative mRNA expression levels of the genes CTGF and ANKRD1 for hMSCs on regularly and randomly patterned hydrogels with 75% stiff regions. For both genes, hMSCs on regular patterns had significantly higher expression levels than those on the random patterns. n = 3. # P < 0.05, based on unpaired t test.
Based on this analysis, further investigations were performed to study whether there was a functional relationship that might dictate intracellular YAP activation in hMSCs when they experience these variations in spatial stiffness. To this end, hMSCs were seeded on hydrogels with regularly patterned 0%, 11%, 25%, 50%, 75%, and 100% stiff regions as shown in Fig. 2A. A sigmoidal response was observed (Fig. 4B, i), in which less than 20% activation was observed on surfaces with 0%, 11%, and 25% stiff regions; whereas, a statistically significant higher level of YAP activation was induced when the underlying substrates were increased from 25% to 50% stiffness, but the trend plateaus near 75% stiffness.
Because of the differences in cellular attachment and cell–matrix interactions on regular and randomly patterned surfaces (Fig. 3B), an additional investigation was performed to characterize YAP localization when hMSCs were seeded onto substrates with randomized stiffness regions. To our surprise, when hMSCs were cultured on the 75% random stiff pattern, YAP was mostly deactivated in the cytoplasm with only 35.4 ± 6.5% nuclear localization (Fig. 4 A, iii, and B, i), which was significantly lower than the 83.4 ± 6.9% intracellular YAP activation in hMSCs on 75% stiff regular patterns. Additional random patterns were generated from 11% to 75% spatial stiffness; however, no statistically significant elevation in YAP activation was observed as the spatial stiffness increased, in contrast to the cellular response on the gels with regularly distributed stiffness (Fig. 4B, i). These results implied to us that a mechanism other than a dose response may be regulating the mechanotransduction process when the spatial organization of the matrix stiffness is disrupted. These differences were further quantified by comparing gene expression levels of ANKRD1 and CTGF, two of the genes that are up-regulated by the activity of YAP (15). Results show that there is significantly higher expression of both ANKRD1 and CTGF for hMSCs cultured on regularly spaced 75% stiff patterns compared with 75% randomized stiff patterns (Fig. 4B, ii).
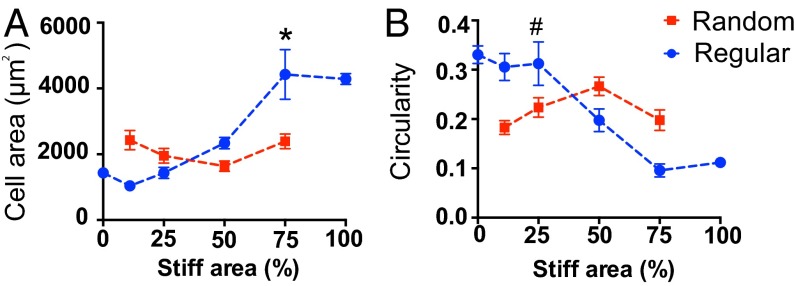
Complementary to these experiments, it was then investigated whether cell morphology correlated with the activation trend observed with the mechanosensor YAP in response to spatial mechanical dosing for both the regular and random patterns. On uniformly stiff substrates, hMSCs spread to an area of 4,290 ± 163 μm2, which is approximately a threefold increase relative to those on uniformly soft microenvironments (1,431 ± 84 μm2) (Fig. 5A). Previous reports have noted that YAP activation can be regulated by cell geometry through differences in cytoskeletal tension (15). When cultured on hydrogels with regularly spaced patterns of mechanical properties, the hMSC area was observed to increase in a manner that corresponded to the percentage of stiff regions, and a dramatic increase in cell area (2,338 ± 173 μm2 to 4,429 ± 757 μm2) was observed when the hydrogel mechanics increased from 50% to 75% stiff (Fig. 5A, blue dashed line and symbols). In contrast, cell spreading remained around 2,400 μm2 on hydrogels with random patterns: from 2,430 ± 265 μm2 on the 11% stiff to 2,483 ± 221 μm2 on the 75% stiff samples regardless of the stiff to soft ratio (Fig. 5A, red dashed line and symbols). Beyond measurements of cell spreading, circularity was also examined, as it can be a quantitative indicator of cytoskeletal tension (23) (Fig. 5B). Consistent with other measurements, circularity of hMSCs decreased correspondingly to the spatial stiffness area on the regular patterned surfaces (Fig. 5B, blue dashed line and symbols); but was insensitive to increases in stiff regions on the randomly patterned gels (Fig. 5B, red dashed line and symbols).
Fig. 5.
(A) Cell spreading area increased correspondingly to stiff percentages on regularly patterned hydrogels, indicated by the blue dashed line and symbols, consistent with the trend of intracellular YAP activation. A significant increase of cell area was observed from 50% to 75% stiff gels. On the other hand, cell spreading was insensitive to the change of underlying stiff percentages on randomly patterned hydrogels, indicated by the red dashed line and symbols; * compared with regular 50% stiff, P < 0.01 based on one-way ANOVA using Tukey’s multiple comparisons test. (B) Complimentary, cellular circularity decreased relative to stiff percentages on regularly patterned hydrogels, indicated by the blue dashed line and symbols. However, circularity did not change significantly with increased stiff area on randomly patterned hydrogels, indicated by the red dash line and symbols; # compared with 75% regular stiff, P < 0.01 based on one-way ANOVA; n > 5 with over 100 cells analyzed for each condition. Mean ± SE.
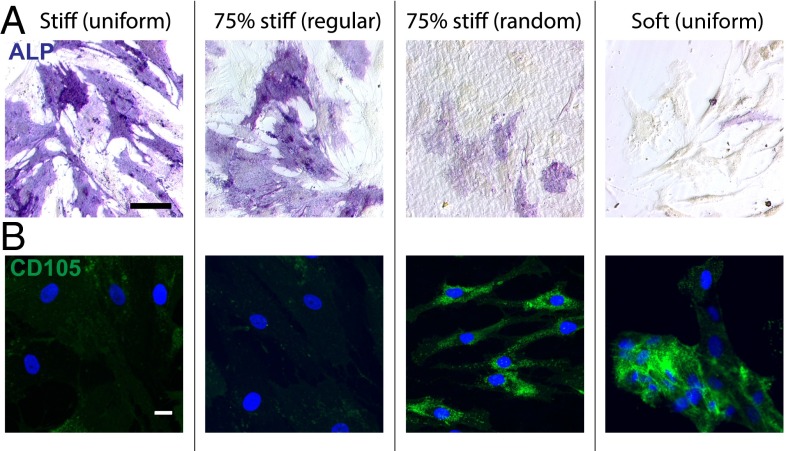
The presented data suggest that the magnitude and spatial organization of a cell’s mechanical matrix environment are coupled and converge to be a functional regulator of hMSCs by influencing their morphology and activating internal mechanotransduction sensors. Based on this supposition, we hypothesized that the effect of regular and random patterns of matrix mechanics in a cell’s niche can lead to divergence in hMSC fate. To this end, we investigated hMSC osteognesis by culturing them on regularly and randomly patterned hydrogels with 75% stiff regions. First, alkaline phosphatase (ALP) staining was performed (Fig. 6A), and cells on the regularly patterned gels were more spread, larger in area, and stained darker, indicating a higher expression of the osteogenic marker ALP, and found to be similar to hMSCs cultured on the uniformly stiff gels. In contrast, minimal staining was observed in hMSCs cultured on randomly patterned surfaces and uniformly soft surfaces, suggesting a significantly lower level of osteogenesis (Fig. 6A). It is intriguing to note that a significantly higher level of expression of the stem cell marker CD105 was observed in hMSCs that were cultured on either the random patterned gels or the uniformly soft hydrogels (Fig. 6B), implying that the randomized stiff cues were arranged in a manner that inhibit hMSCs osteogenesis.
Fig. 6.
(A) ALP staining of hMSCs on uniformly stiff, 75% stiff with regular and random patterns and uniformly soft hydrogels after 7 d culture in mixed media. hMSCs on uniformly stiff and regularly patterned 75% stiff samples had prominent ALP expression; but the expression levels were significantly lower on the randomly patterned 75% stiff and uniformly soft samples. ALP (purple). Scale bar = 100 μm. (B) CD105 staining of hMSCs on uniformly stiff, 75% stiff with regular and random patterns and uniformly soft hydrogels after 7 d culture in mixed media. hMSCs on uniformly soft and randomly patterned 75% stiff samples had significantly higher CD105 expressions than those on uniformly stiff and regularly patterned 75% stiff samples. CD105 (green), DAPI (blue). n = 3. Scale bar = 20 μm.
Discussion
Previous investigations of subcellular adhesive ligand organization (17, 24–26) and surface nanotopography features (18, 19, 27) on stem cell behavior demonstrated the fundamental role of ECM structure in directing cell–matrix interactions. However, the effect of matrix mechanics is still largely unknown. Specifically, it is challenging to precisely define and elucidate the spatial organization and presentation of matrix mechanics in vivo and in cellular culture platforms. For example, the heterogeneous chemical composition of native bone tissue leads to varied mechanical properties on a wide range of length scales. This homeostasis is disrupted by physical deformity upon injury and subsequent inflammatory response, leading to disorganized chemical and physical structures in the local cell microenvironment (28). Other examples in liver pathology further emphasize the regulatory role of matrix organization in fibrotic diseases through the modulation of tissue morphogenesis, in which the functional hepatic architecture is disturbed by an abnormal accumulation of ECM proteins and lead to fibrous scar and subsequent cirrhosis (29–33). Elucidating how cells, especially stem cells that are recruited to the wound site, distinguish and respond to these discrepancies in the mechanical environment is critical for the field to deepen our collective understanding of the tissue regeneration process. Here, we applied a unique phototunable hydrogel system to introduce and characterize the effects of spatial variations in mechanical properties on cells cultured on the matrices. We observed that spatially altering mechanical stiffness affects hMSCs in a dose-dependent manner through the regulation of cytoskeletal tension and intracellular transcriptional activation. Specifically, focal adhesion formation colocalized with activating, stiff regions on hydrogel substrates, which in turn directed the formation of the actin cytoskeleton structure. Increasing the stiffness ratio on regularly patterned hydrogels promoted YAP activation, as well as increased cell spreading and led to more elongated cell morphologies. It is interesting to note that hMSCs became insensitive to increases in the spatial stiffness of their microenvironment if the regions were arranged in a randomized manner. Further, the randomization was observed to disrupt the actin structure, reduce YAP activation, and decrease cell spreading.
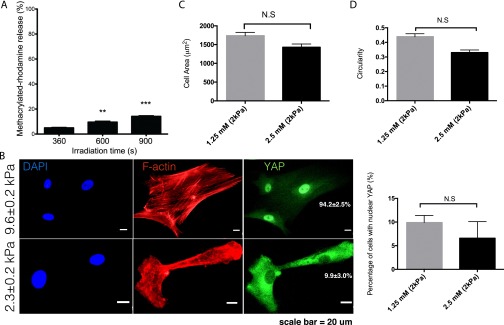
This dosing response was found to be specific to the presentation of spatially patterned mechanical regions, but independent of similar spatial variations in adhesive area among the different patterns. As previously reported, 95% of the adhesive ligand RGD remains attached after cleavage of the cross-linker because it was conjugated to the nondegradable region of the network (21), so that the mechanically patterned hydrogel has a relatively homogenous adhesive ligand concentration across stiff and soft regions. Additional experiments were conducted to ensure that the ∼5% decrease in RGD concentration in the soft area after irradiation did not affect cellular morphology and downstream transcriptional activation events (Fig. S6). As another control experiment, we introduced the adhesive protein fibronectin onto hydrogels of a uniform modulus of elasticity, in this case the stiffer formulation, and then created patterns of adhesive and nonadhesive areas (24). In this system, no significant differences in YAP activation (∼90%; Fig. S7) were observed in hMSCs when they were exposed to adhesive ligands that were regularly patterned in 25%, 87%, or randomly patterned in 25% of the area. These results further support the conclusion that the observed trend in YAP activation is not regulated through variations in ECM contact area, but is defined by spatial variations in mechanical signals that induce different magnitudes of cytoskeletal tension.
Fig. S6.
Characterization of RGD concentration after irradiation. (A) quantification of the percent release of methacrylated-rhodamine from PEGdiPDA after 360, 600, and 900 s of irradiation. **P < 0.01; ***P < 0.001. (B) Representative images of YAP activation on both 10 and 2 kPa hydrogel with 1.25 mM RGD peptide. (C) Cell spread area on 2 kPa hydrogel with 1.25 and 2.5 mM RGD. (D) Circularity of cell shape on 2 kPa hydrogel with 1.25 and 2.5 mM RGD. N.S. = not significant based on unpaired t test.
Fig. S7.
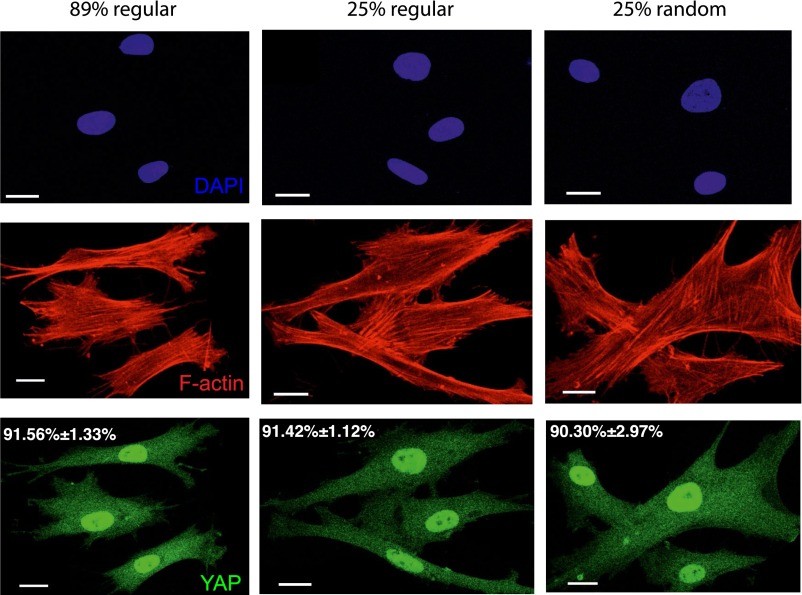
YAP activation in hMSCs on fibronectin patterned surface. Fibronectin was patterned onto regular 89%, 25%, and randomized 25% surface area in nondegradable PEG-diacrylate hydrogel. YAP was observed to be activated in hMSCs independently of the percentage or organization of the patterned adhesive area. Scale bar = 20 μm.
Previous literature has indicated that surface roughness is a potent physical cue that affects cell–ECM interactions (27, 34, 35). As we aimed to define and independently vary spatial stiffness, we carefully designed and optimized the photodegradation procedure to minimize significant differences in surface topography. In multiple examples, significant changes in cellular behavior and stem cell markers have been observed when MSCs sense topographical features > 100 nm, e.g., between Rq 1 and 100 nm (35); on 100-nm topography features (19) and on 300-nm topography features (34). Therefore, we expected the effect of rms roughness (43 ± 2 nm) on hMSC responses to patterned surfaces to be minimized, because the compliant and flexible nature of the hydrogel rendered systems with minimal changes in nanoscale topography feature that would significantly alter cellular attachment and cytoskeletal structure.
Finally, osteogenic differentiation of hMSCs cultured on hydrogels with regularly patterned mechanical regions was significantly higher than those cultured on random patterns, as measured by ALP staining. Complementary, the randomized stiffness patterns promoted a higher CD105 expression in hMSCs, thus demonstrating the regulatory role of the spatial organization of the local environmental stiffness in determining cell fate decisions. Collectively, these observations support the notion that mechanical dosing influences hMSCs, but in a manner that correlates to both spatial distribution and organization of the matrix mechanics.
In previous work by Dalby et al. (19), it was reported that nanotopography features that were randomly introduced on poly (methyl methacrylate) surfaces promoted cell adhesion (long fibrillarlike adhesions) and subsequent higher osteogenic differentiation than surfaces with more orderly and organized nanotopography features. Although we are using two quite different platforms, e.g., rigid poly (methyl methacrylate) surfaces vs. compliant hydrogel substrates; nanoscale topography vs. micrometer-scale mechanical patterns, our findings support similar conclusions, which are: organization of extracellular matrix impacts stem cell signaling through systematic regulation of cytoskeletal structure, transcriptional activation, and gene expression profile. In our study, we observed that the regularly spaced micrometer-scale mechanical pattern promoted a focal adhesion structure that induced higher cytoskeletal tension (Fig. S8), which further led to YAP activation and enhanced osteogenesis. On the other hand, Dalby et al. observed that certain disordered nanotopographical features alter cellular cytoskeletal structure in a way that lead to distinct gene expression profiles, which promote osteogenesis. It is clear that the morphological changes in cytoskeletal structure upon cell–ECM interaction initiates the downstream transduction of extracellular signal, which implies that a complex interplay between integrin expression, surface chemistry, protein conformation, topography, and mechanics most likely intersect to influence the overall cell–ECM contact.
Fig. S8.
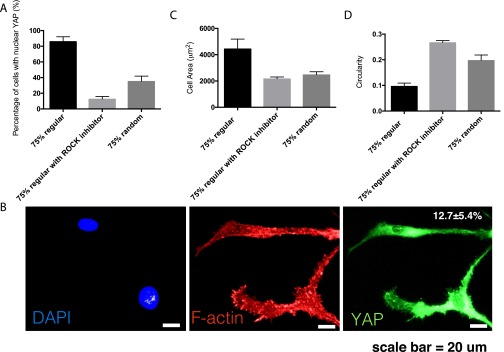
YAP activation and cell morphology of hMSCs on regular 75% stiff substrate treated with Y-27632 ROCK inhibitor. (A) Nuclear YAP activation in hMSCs was significantly reduced from 83.4 ± 6.9% to 12.7 ± 5.4% upon ROCK inhibition. (B) Representative images of YAP localization and F-actin structure in hMSCs on regular 75% stiff substrate treated with Y-27632 ROCK inhibitor. Scale bar = 20 μm. (C) Cell area reduced from 4429 ± 757 to 2173 ± 235 μm2 upon ROCK inhibition. (D) Circularity increased from 0.096 ± 0.013–0.2674 ± 0.007 upon ROCK inhibition.
Taken as a whole, our results begin to provide insight into stem cell behavior during dynamic events that occur during matrix remodeling, as to whether the organization of the matrix environment acts as a mechanical “switch” for cell lineage decisions. These findings highlight the importance of new biomaterial matrices that allow experimenters intimate control of cell-matrix interactions and signaling. Innovations in biomaterial chemistry are enabling novel experiments to be performed that are improving the field’s understanding of mechanotransduction, especially as it relates to changes in cell behavior that occurs during tissue remodeling in development, disease processes, and wound healing.
Materials and Methods
Human mesenchymcal stem cells (hMSCs) were isolated from fresh human bone marrow (Lonza). P2 hMSCs were used in these experiments. Distinct mechanical regions were introduced on the photodegradable hydrogels by photopatterning using 36 nm light and characterized by AFM. Cellular morphology, gene expressions, and differentiations on the patterned substrates were analyzed similar to previous studies (21). For complete details of materials and methods, please refer to Supporting Information.
SI Materials and Methods
Synthesis of Photodegradable Hydrogel Components.
Poly(ethylene glycol) di-photodegradable acrylate (PEGdiPDA) was synthesized by coupling 4-[4-(1-Acrylethyl)-2-methoxy-5-nitrophenoxy]butanoic acid with poly(ethylene glycol) bis-amine (Mn ∼3400 Da; Laysan Bio, Inc.) with (1-[Bis (dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxid hexafluorophosphate) (HATU) activation as previously described in refs. 36 and 14. Purification of the resultant PEGdiPDA was conducted by dialysis in water to ensure over 90% purity (characterized by 1H-NMR). The adhesive peptide, OOGRGDSG (diethylene glycol-diethylene glycol-glycine-arginine-glycine-aspartic acid-serine-glycine), was synthesized on a Tribute peptide synthesizer (Protein Technologies) using standard Fmoc solid phase peptide synthesis protocols (21), where the N-terminal amine was later modified with acrylic acid to render Acryl-OOGRGDSG as described in ref. 21. Peptide purification and characterization were conducted as previously described using semipreparative reversed-phase high performance liquid chromatography (RP-HPLC; Waters 2767, 2489, 2545) and matrix-assisted laser desorption-ionization time-of-flight mass spectrometry (MALDI; Applied Biosystems DE Voyage) (21).
Photodegradable Hydrogel Formulation.
Photodegradable hydrogels were polymerized as described in ref. 21. In brief, in PBS solution, 2.5 wt% PEGdiPDA was copolymerized with 10 wt% PEGA [poly(ethylene glycol) monoacrylate, Mn ∼400 Da; Monomer-Polymer and Dajac Laboratories, Inc.] and 2.5 mM Acryl-OOGRGDSG in the presence of 0.2 M ammonium persulfate (APS) and 0.1 M tetramethylethylenediamine (TEMED). Gels were formed on acrylated cover glass with an average thickness of 100 μm.
Characterization of Hydrogel Mechanical Properties.
A shear rheometer (ARES; TA) was used to measure the modulus of the PEGdiPDA hydrogel before and after degradation. In brief, optically thin PEGdiPDA hydrogels (thickness = 50 μm) were polymerized in situ between an 8-mm diameter tool. Network evolution was tracked using a dynamic time sweep (γ = 10%; ω = 10 rad/s; determined to be in the LVE regime for this material) until the storage modulus (G′) reached a plateau. Degradation by light irradiation (λ = 365 nm; I0 = 10 mW/cm2; Omnicure 1000, Lumen Dynamics) started after the plateau, and was monitored using the same dynamic time sweep parameters.
Photo Patterning Mechanical Regions in Hydrogel.
Hydrogel surfaces were covered by chrome photomasks (Photoscience) to control the irradiation area, and exposed to collimated 365 nm light for 360 s, to produce materials with spatially varying moduli between 2 and 10 kPa. Photomasks were used to create regular and randomized patterns of 2 μm by 2 μm squares, and the area of stiff regions was varied from 0%, 11%, 25%, 50%, 75–100% of the total area, respectively.
Characterization of Patterned Hydrogel Mechanical Properties by AFM.
An atomic force microscope (Cypher; Asylum Research) was used to measure the spatially varying elastic moduli of the PEGdiPDA hydrogels with regular and randomized patterns of 2 μm by 2 μm squares. In brief, force spectroscopy (force–volume mapping) was conducted at each node of a square grid (64 × 64) distributed over a 10 μm × 10 μm area; the measurements were carried out in PBS solution at 25 °C in the fluid cell for the Cypher AFM. The AFM probes were Bruker SNL-10 triangular SiN cantilevers with Si tips. Each probe was calibrated using the thermal fluctuation method (37); resulting values for the spring constant kc varied from 0.11 to 0.14 N/m. Moreover, each probe was inspected before and after force spectroscopy with scanning electron microscopy (SEM) to assess the half-angle α and check for damage or material transfer; α varied from 15° to 30° and minimal transfer was observed. AFM force–displacement (F–d) data were acquired with a peak load of 5 nN at a displacement rate of 10 µm/s. Force–deformation (F–δ) data were derived from the raw F–d curves by subtracting the AFM cantilever deflection, F/kc. To assess Young’s modulus E at each node, the loading portion of each F–δ curve was fit to an analytical model for a rigid conical tip in contact with an elastic half-space (38), i.e., F=(2/π)(E/1−ν2)(tanα)δ2, using an average value for α from SEM, an assumed Poisson’s ratio for the hydrogel (ν = 0.5), and E as the sole fitting parameter.
Adhesive Ligand Patterning in Hydrogels.
Nondegradable PEG hydrogels (E = 8.6 ± 1. 4 kPa) were polymerized with 2.5 wt% PEG diacrylate (MW: 6,000 Da) and 7 wt% PEG monoacrylate (MW: 400 Da) in PBS with 0.05 M APS and 0.025 M TEMED. After polymerization, hydrogels were incubated in 0.2 mg/mL Sulfo-SANPAH (Sulfosuccinimidyl 6-(4′-azido-2′-nitrophenylamino)hexanoate; Life Technologies) solution in water at 4 °C overnight. Sulfo-SANPAH was covalently conjugated into the gels by reacting the aryl azide group with the pendant acrylate group found in the gels initiated by irradiation of 365 nm light at 5 mW/cm2 for 10 min. A photomask was used during irradiation to control the spatially patterned area. After conjugation, gels were rinsed in PBS at 4 °C overnight, and three additional rinses, each for 10 min were performed at room temperature. Subsequently, 10 μg/mL fibronectin solution in 50 mM Hepes buffer (pH = 8.5, 1M Hepes (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid in water; MP Biomedicals) was applied to the patterned hydrogels and incubated at 4 °C overnight to allow covalent conjugation between the primary amine found on fibronectin and the N-Hydroxysuccinimide esters (NHS Esters) present on Sulfo-SANPAH. An overnight wash in PBS was performed at 4 °C, followed by three additional washes in room temperature to eliminate nonspecific fibronectin conjugation. The patterned hydrogels were kept at 4 °C until cell seeding.
Human Mesenchymal Stem Cell Culture and Reagents.
Cell culture reagents were purchased from Invitrogen, except as noted. Human mesenchymcal stem cells (hMSCs) were isolated from fresh human bone marrow (Lonza) following the protocol described in (39). Isolated hMSCs were labeled as P1 and frozen down in 95% (vol/vol) FBS and 5% (vol/vol) DMSO and stored in liquid nitrogen. P1 hMSCs were expanded in culture media (low-glucose DMEM supplemented with 50 μg/mL penicillin, 50 μg/mL streptomycin, and 1 μg/mL fungizone) containing 20% (vol/vol) FBS to generate P2 cells, which were used in all the reported experiments in this manuscript. P2 hMSCs were cultured on tissue culture polystyrene (TCPS) in culture media supplemented with 10% (vol/vol) FBS and 1 ng/mL FGF at 1,000 cells/cm2 for 2 d prior to experimentation. Cell seeding density on hydrogels was 1,000 cells/cm2 for immunostaining, and 4,000 cells/cm2 for RT-qPCR and differentiation assays. In the differentiation assays, 72 h after cell seeding, the media was changed to a bipotential adipogenic/osteogenic inductive medium and cultured for 7 d until assay performance. The bipotential inductive medium (mixed media) was made by combining adipogenic and osteogenic inductive media at a 1:1 ratio (21). Adipogenic media consisted of high-glucose DMEM supplemented with 15% (vol/vol) FBS, 50 μg/mL penicillin, 50 μg/mL streptomycin, 1 μg/mL fungizone, 10 μg/mL insulin, 10 μM dexamethasone, and 0.5 mM 3-isobutyl-1-methylxanthine (IBMX). Osteogenic media consisted of high-glucose DMEM supplemented with 10% FBS, 50 U/mL penicillin, 50 μg/mL streptomycin, 1 μg/mL fungizone, 100 nM dexamethasone, 50 μM ascorbic acid, and 20 mM β-glycerophosphate.
In other assays and immunostaining, 24 h after cell seeding, media was changed to culture media containing 1% FBS to inhibit cell growth and minimize background signals. Samples were harvested or fixed at day 3 (3 d after cell seeding).
Immunostaining.
Samples were fixed in 2% (vol/vol) paraformaldehyde for 30 min. The fixation solution was then removed and samples were rinsed in PBS twice before permeabilization using 0.1% TritonX-100 in PBS for 1 h. Following a quick rinse in PBS after permeabilization, samples were blocked in 5% (wt/vol) BSA for 1 h to minimize nonspecific binding. All the aforementioned steps were conducted at room temperature. Anti-YAP (1:250, mouse; Santa Cruz), anti-CD105 (1:150, mouse, abcam), and anti-paxillin (1:400, rabbit; Millipore) primary antibodies in 5% (wt/vol) BSA were applied to samples and incubated at 4°C overnight. Before the addition of secondary antibody (1:1000, goat anti-rabbit AlexaFluor 488; goat anti-mouse AlexaFluor 555; Invitrogen), phalloidin (1:1,000; Sigma Aldrich) and DAPI (1 μg/mL; Sigma) in 1% BSA, primary antibody was removed and rinsed in PBST (0.5 wt% Tween-20 in PBS) 3 times. After 1 h incubation at room temperature, the secondary antibody solution was removed and rinsed 3 times with PBST. Samples were kept in PBS at 4°C until imaging (LSM 710 NLO; Carl Zeiss AG).
Cell Morphology Analysis.
Cell spreading area and circularity were analyzed by ImageJ based on the F-actin staining. Fluorescent intensity and contrast were adjusted to show the whole cell body uniformly. Then, a binary representation of each image was made, and the area was outlined and measured by the Analyze Particles function. More than 100 cells were analyzed for each condition.
Focal Adhesion Colocalization Analysis.
Bright field images from the DIC channel were segmented by intensity to generate a mask of both stiff and soft regions on the hydrogel. A 2-pixel wide (1 pixel from the stiff mask and 1 pixel from the soft mask; 1 pixel = 0.423 μm) edge segmentations were generated at the interface between the stiff and soft masks. Focal adhesions, based on paxillin staining within the cell, were segmented with an intensity threshold to eliminate nonspecific background signals. The average intensity of the focal adhesions was then quantified within each mechanical region. These intensities were then normalized to the total area for each mechanical region and plotted relative to the average intensity found in the soft regions.
Gene Expression Analysis.
Relative mRNA expression levels of CTGF and ANKRD1 were analyzed by quantitative real-time PCR (qRT-PCR). In brief, RNA was extracted and pooled from four hydrogel samples of the same condition by TRI Reagent (Sigma-Aldrich) following manufacturer’s instructions. RNA was precipitated in isopropanol, washed by 75% (vol/vol) ethanol/25% (vol/vol) water, and quantified by spectrophotometry (ND-1000; NanoDrop). Reverse transcription was conducted to synthesize cDNA from total RNA using iScript Synthesis kit (Bio-Rad). qRT-PCR was then performed using SYBR Green reagents (Bio-Rad) on an iCycler (Bio-Rad) to measure relative mRNA expression level, which was normalized to the housekeeping gene GAPDH. Primer sequences are listed below:
GAPDH-FWD: GCAAGAGCACAAGAGGAAGAG;
GAPDH-REV: AAGGGGTCTACATGGCAACT.
CTGF-FWD: AGGAGTGGGTGTGTGACGA;
CTGF-REV: CCAGGCAGTTGGCTCTAATC.
ANKRD1-FWD: AGTAGAGGAACTGGTCACTGG;
ANKRD1-REV: TGGGCTAGAAGTGTCTTCAGAT.
Alkaline Phosphatase and Oil Red O Staining.
Samples were rinsed in PBS and fixed in citrate buffered, 60% (vol/vol) acetone for 30 s, then incubated in FAST BLUE RR SALT solution supplemented with naphthol AS-MX phosphate (85L1; Sigma-Aldrich) for 30 min at room temperature. Another batch of samples were processed with Oil red O staining after fixation by incubating in 0.3% Oil-Red-O solution for 20 min at room temperature. Bright field (20× objective) on a Nikon inverted microscope was used to capture the blue and red staining, respectively.
Statistics.
A Student’s t test and one-way ANOVA were used to compare data sets as indicated in individual experiment. A P value of 0.05 or less was used to determine statistical significance.
Acknowledgments
We thank Eric Bunker for developing the Matlab code for image analysis, Dr. Joseph Grim for manuscript editing, and Prof. Virginia Ferguson and Dr. Emi Tokuda for helpful discussions on the work, as well as Dr. Joe Dragavon for assistance in image acquisition. This work was supported by the National Institutes of Health (Grants R01 DE016523 and R21 AR067469) and the Howard Hughes Medical Institute (K.S.A.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1609731113/-/DCSupplemental.
References
- 1.Engler AJ, Humbert PO, Wehrle-Haller B, Weaver VM. Multiscale modeling of form and function. Science. 2009;324(5924):208–212. doi: 10.1126/science.1170107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 3.Lutolf MP, Gilbert PM, Blau HM. Designing materials to direct stem-cell fate. Nature. 2009;462(7272):433–441. doi: 10.1038/nature08602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilbert PM, et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329(5995):1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morrison SJ, Spradling AC. Stem cells and niches: Mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132(4):598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15(12):786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hinton RB, Yutzey KE. Heart valve structure and function in development and disease. Annu Rev Physiol. 2011;73:29–46. doi: 10.1146/annurev-physiol-012110-142145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egeblad M, Rasch MG, Weaver VM. Dynamic interplay between the collagen scaffold and tumor evolution. Curr Opin Cell Biol. 2010;22(5):697–706. doi: 10.1016/j.ceb.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levental KR, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139(5):891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Provenzano PP, et al. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006;4(1):38. doi: 10.1186/1741-7015-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schoen FJ. Evolving concepts of cardiac valve dynamics: The continuum of development, functional structure, pathobiology, and tissue engineering. Circulation. 2008;118(18):1864–1880. doi: 10.1161/CIRCULATIONAHA.108.805911. [DOI] [PubMed] [Google Scholar]
- 12.Augat P, Simon U, Liedert A, Claes L. Mechanics and mechano-biology of fracture healing in normal and osteoporotic bone. Osteoporos Int. 2005;16(Suppl 2):S36–S43. doi: 10.1007/s00198-004-1728-9. [DOI] [PubMed] [Google Scholar]
- 13.Klein P, et al. The initial phase of fracture healing is specifically sensitive to mechanical conditions. J Orthop Res. 2003;21(4):662–669. doi: 10.1016/S0736-0266(02)00259-0. [DOI] [PubMed] [Google Scholar]
- 14.Kloxin AM, Kasko AM, Salinas CN, Anseth KS. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science. 2009;324(5923):59–63. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dupont S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474(7350):179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 16.Scales TME, Parsons M. Spatial and temporal regulation of integrin signalling during cell migration. Curr Opin Cell Biol. 2011;23(5):562–568. doi: 10.1016/j.ceb.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Chen CS. Geometric control of cell life and death. Science. 1997;276(5317):1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 18.McMurray RJ, et al. Nanoscale surfaces for the long-term maintenance of mesenchymal stem cell phenotype and multipotency. Nat Mater. 2011;10(8):637–644. doi: 10.1038/nmat3058. [DOI] [PubMed] [Google Scholar]
- 19.Dalby MJ, et al. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat Mater. 2007;6(12):997–1003. doi: 10.1038/nmat2013. [DOI] [PubMed] [Google Scholar]
- 20.Turner CE. Paxillin and focal adhesion signalling. Nat Cell Biol. 2000;2(12):E231–E236. doi: 10.1038/35046659. [DOI] [PubMed] [Google Scholar]
- 21.Yang C, Tibbitt MW, Basta L, Anseth KS. Mechanical memory and dosing influence stem cell fate. Nat Mater. 2014;13(6):645–652. doi: 10.1038/nmat3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halder G, Dupont S, Piccolo S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat Rev Mol Cell Biol. 2012;13(9):591–600. doi: 10.1038/nrm3416. [DOI] [PubMed] [Google Scholar]
- 23.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6(4):483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 24.Trappmann B, et al. Extracellular-matrix tethering regulates stem-cell fate. Nat Mater. 2012;11(7):642–649. doi: 10.1038/nmat3339. [DOI] [PubMed] [Google Scholar]
- 25.Lee TT, et al. Light-triggered in vivo activation of adhesive peptides regulates cell adhesion, inflammation and vascularization of biomaterials. Nat Mater. 2015;14(3):352–360. doi: 10.1038/nmat4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petrie TA, et al. Multivalent integrin-specific ligands enhance tissue healing and biomaterial integration. Sci Transl Med. 2010;2(45):45ra60. doi: 10.1126/scitranslmed.3001002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dalby MJ, Gadegaard N, Oreffo ROC. Harnessing nanotopography and integrin-matrix interactions to influence stem cell fate. Nat Mater. 2014;13(6):558–569. doi: 10.1038/nmat3980. [DOI] [PubMed] [Google Scholar]
- 28.Rho J-Y, Kuhn-Spearing L, Zioupos P. Mechanical properties and the hierarchical structure of bone. Med Eng Phys. 1998;20(2):92–102. doi: 10.1016/s1350-4533(98)00007-1. [DOI] [PubMed] [Google Scholar]
- 29.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115(2):209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ingber DE. Cancer as a disease of epithelial-mesenchymal interactions and extracellular matrix regulation. Differentiation. 2002;70(9-10):547–560. doi: 10.1046/j.1432-0436.2002.700908.x. [DOI] [PubMed] [Google Scholar]
- 31.Bosman FT, Stamenkovic I. Functional structure and composition of the extracellular matrix. J Pathol. 2003;200(4):423–428. doi: 10.1002/path.1437. [DOI] [PubMed] [Google Scholar]
- 32.Ingber DE. Mechanobiology and diseases of mechanotransduction. Ann Med. 2003;35(8):564–577. doi: 10.1080/07853890310016333. [DOI] [PubMed] [Google Scholar]
- 33.Cox TR, Erler JT. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis Model Mech. 2011;4(2):165–178. doi: 10.1242/dmm.004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pan F, et al. Topographic effect on human induced pluripotent stem cells differentiation towards neuronal lineage. Biomaterials. 2013;34(33):8131–8139. doi: 10.1016/j.biomaterials.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 35.Chen W, et al. Nanotopography influences adhesion, spreading, and self-renewal of human embryonic stem cells. ACS Nano. 2012;6(5):4094–4103. doi: 10.1021/nn3004923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kloxin AM, Tibbitt MW, Anseth KS. Synthesis of photodegradable hydrogels as dynamically tunable cell culture platforms. Nat Protoc. 2010;5(12):1867–1887. doi: 10.1038/nprot.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hutter JL, Bechhoefer J. Calibration of atomic-force microscope tips. Rev Sci Instrum. 1993;64(7):1868–1873. [Google Scholar]
- 38.Sneddon IN. The relation between load and penetration in the axisymmetric boussinesq problem for a punch of arbitrary profile. Int J Eng Sci. 1965;3(1):47–57. [Google Scholar]
- 39.Mariner PD, Johannesen E, Anseth KS. Manipulation of miRNA activity accelerates osteogenic differentiation of hMSCs in engineered 3D scaffolds. J Tissue Eng Regen Med. 2012;6(4):314–324. doi: 10.1002/term.435. [DOI] [PMC free article] [PubMed] [Google Scholar]