Abstract
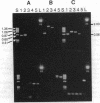
The phylogenetic affinities of the grass family (Poaceae) have long been debated. The chloroplast genomes of at least some grasses have been known to possess three inversions relative to the typical gene arrangement found in most flowering plants. We have surveyed for the presence of these inversions in grasses and other monocots by polymerase chain reaction amplification with primers constructed from sequences flanking the inversion end points. Amplification phenotypes diagnostic for the largest inversion (28 kilobase pairs) were found in genera representing all grass subfamilies, and in the nongrass families Restionaceae, Ecdeiocoleaceae, and Joinvilleaceae, but not in any other monocots--notably, Flagellariaceae, Anarthriaceae, Cyperaceae, or Juncaceae. This finding is consistent with one of the two principal views of grass phylogeny in suggesting that Poaceae and Cyperaceae (sedges) are not closest relatives. A second (approximately 6 kilobases) inversion appears to occur in a subset of the families possessing the 28-kilobase inversion and links Joinvilleaceae and Poaceae, while the smallest inversion appears unique to grasses. These inversions thus provide a nested set of phylogenetic characters, indicating a hierarchy of relationships in the grasses and allies, with Joinvilleaceae identified as the likely sister group to the Poaceae.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Del Sal G., Manfioletti G., Schneider C. The CTAB-DNA precipitation method: a common mini-scale preparation of template DNA from phagemids, phages or plasmids suitable for sequencing. Biotechniques. 1989 May;7(5):514–520. [PubMed] [Google Scholar]
- Hiratsuka J., Shimada H., Whittier R., Ishibashi T., Sakamoto M., Mori M., Kondo C., Honji Y., Sun C. R., Meng B. Y. The complete sequence of the rice (Oryza sativa) chloroplast genome: intermolecular recombination between distinct tRNA genes accounts for a major plastid DNA inversion during the evolution of the cereals. Mol Gen Genet. 1989 Jun;217(2-3):185–194. doi: 10.1007/BF02464880. [DOI] [PubMed] [Google Scholar]
- Howe C. J., Barker R. F., Bowman C. M., Dyer T. A. Common features of three inversions in wheat chloroplast DNA. Curr Genet. 1988 Apr;13(4):343–349. doi: 10.1007/BF00424430. [DOI] [PubMed] [Google Scholar]
- Howe C. J. The endpoints of an inversion in wheat chloroplast DNA are associated with short repeated sequences containing homology to att-lambda. Curr Genet. 1985;10(2):139–145. doi: 10.1007/BF00636479. [DOI] [PubMed] [Google Scholar]
- Palmer J. D., Thompson W. F. Chloroplast DNA rearrangements are more frequent when a large inverted repeat sequence is lost. Cell. 1982 Jun;29(2):537–550. doi: 10.1016/0092-8674(82)90170-2. [DOI] [PubMed] [Google Scholar]
- Quigley F., Weil J. H. Organization and sequence of five tRNA genes and of an unidentified reading frame in the wheat chloroplast genome: evidence for gene rearrangements during the evolution of chloroplast genomes. Curr Genet. 1985;9(6):495–503. doi: 10.1007/BF00434054. [DOI] [PubMed] [Google Scholar]
- Rodermel S., Orlin P., Bogorad L. The transcription termination region between two convergently-transcribed photoregulated operons in the maize plastid chromosome contains rps14, trnR (UCU) and a putative trnfM pseudogene. Nucleic Acids Res. 1987 Jul 10;15(13):5493–5493. doi: 10.1093/nar/15.13.5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]