Abstract
Objective
Obstructive sleep apnoea (OSA) has been linked to cardiovascular risk factors, such as hypertension, and clinical cardiovascular endpoints. Our aim was to assess whether OSA is independently associated with atherosclerosis and vascular dysfunction as assessed by cardiovascular magnetic resonance (CMR).
Methods
58 patients with OSA and 39 matched control subjects without OSA underwent CMR of the aorta and carotid arteries. Carotid and aortic wall thickness and aortic distensibility were measured. Multi-weighted, high resolution CMR imaging was used for carotid atheroma characterization according to the American Heart Association (AHA) atheroma classification, modified for CMR.
Results
Carotid [1.47 ± 0.03 mm vs. l.26 ± 0.05 mm, (P < 0.01)] and aortic wall thickness [2.95 ± 0.09 mm vs. 2.05 ± 0.07mm, (P < 0.001)]were increased in patients with OSA compared to controls. Aortic distensibility was decreased in patients with OSA [3.62 ± 0.3 vs. 4.75 ± 0.2 mmHg−1 × 10−3, (P < 0.05)]. Prevalence of carotid plaque, average carotid atheroma class, and prevalence of high risk features of carotid atheroma were increased in patients with OSA (P < 0.005 for all). On multivariate analysis, Oxygen desaturation index (ODI) emerged as an independent predictor of carotid and aortic wall thickness, but not of aortic stiffness.
Conclusions
OSA is associated with increased carotid and aortic atheroma burden and with advanced, high risk carotid atherosclerotic plaques, but not with aortic stiffening.
Keywords: Atherosclerosis, Obstructive sleep apnoea, Aorta, Carotid, Cardiovascular magnetic resonance, Arterial Stiffness
1. Introduction
Symptomatic obstructive sleep apnoea (OSA) (termed obstructive sleep apnoea syndrome) poses a complex challenge in determining its potential contribution to cardiovascular risk. Obesity is a key aetiological factor, and this condition affects 2–4% of adult men and 1% of women [1]. The prevalence of sleep apnoea features, as detected by sleep studies, in the absence of symptoms is reportedly even higher [1]. Many mechanisms independently linking OSA to vessel wall damage and atherogenesis. Many mechanisms independently linking OSA to vessel wall damage and atherogenesis (such as sympathetic activation, blood pressure surges, endothelial dysfunction, reactive oxygen species, dyslipidemia and impairment and impaired lipid metabolism [2]) have been proposed.
Recent animal studies have demonstrated an association between OSA and aortic atherosclerosis [3,4]. In human studies, OSA has been linked to arterial stiffening, endothelial dysfunction and increased Carotid intima media thickness [5,6]. Whilst large epidemiological studies and meta-analyses have linked OSA to hypertension and clinical cardiovascular endpoints (e.g. coronary artery disease, heart failure [7], stroke [8] and arrhythmias) [9], many of these studies failed to control for hidden confounders, in particular intra-abdominal obesity.
CMR is a powerful and well validated tool for non invasive carotid and aortic atheroma burden measurement and carotid atheroma characterization. CMR not only allows for the assessment of atheroma burden of the aorta but also provides the most direct and complete non-invasive assessment of central aortic stiffness [10–12], which is considered to be a key step in the pathophysiology of cardiovascular disease as it is thought to predispose to diastolic and eventually systolic heart failure as well as to micro-vascular end-organ damage [11,13].
In this study, we investigated whether OSA in middle-aged to older subjects with cardiovascular risk factors is independently associated with atherosclerosis and vascular dysfunction as assessed by CMR. Furthermore, in order to clarify the effect of obesity on the association between OSA and cardiovascular disease, we measured intra-abdominal adiposity, a well-recognized independent cardiovascular risk marker [14], which is accurately measured using magnetic resonance imaging (MRI).
2. Methods
2.1. Patients and control subjects
The study was approved by the local ethics committee, in accordance with the declaration of Helsinki, and participants gave informed consent. Subjects were between 45 and 75 years of age and were all assessed by our sleep clinic.These were usually snorers deemed to be at risk of having sleep apnoea. Patients were eligible if they had OSA proven by an overnight sleep study. Control subjects were eligible if they had no history of OSA symptoms with confirmed disease absence on overnight oximetry.
58 patients with OSA were recruited. 42 patients from this group were matched for age, gender, height and weight [and consequently body surface area and body mass index (BMI)] and cardiovascular risk factors to a group of 39 control subjects.
All participants underwent MRI of the aorta and carotid arteries and abdominal adipose tissue. Data from all patients with OSA (n = 58) were used for all correlation analyses, while the data from the matched OSA group (n = 42) were used for all comparisons of OSA vs. controls.
2.2. Sleep study
OSA was diagnosed from a one-night in-hospital respiratory multi-channel sleep study as previously described [15]. OSA severity was quantitatively expressed in the form of ODI (i.e. the number of oxygen desaturations exceeding 4% per hour of study). Subjects were characterized as patients with OSA if they were found to have ODI > 7.5. Control subjects were only included if the overnight tracing showed no evidence of OSA or nocturnal hypoventilation, an ODI < 5/h, and there was no history of snoring or witnessed apnoeas. This cut-off was chosen to reduce the possibility of overlap between the two groups.
2.3. Cardiovascular risk Score
The Framingham risk score was used to objectively assess an individual’s 10-year risk of death due to cardiovascular events [16].
2.4. Anthropometric measurements and indices
The waist, hip and neck circumferences were measured using standard techniques. Waist hip ratio, body surface area and BMI were calculated according to standard formulas [17].
2.5. CMR protocol
All CMR scans were performed on a 1.5 T MR system (40 mT/m; Siemens Healthcare, Erlangen, Germany).
2.5.1. Carotid and aortic atheroma burden quantification
For assessment of atherosclerosis of the aorta, transverse black blood, Turbo spin echo images of the descending thoracic aorta were acquired during diastole (Sequence parameters: repetition time: 750 ms, echo time: 11 ms, resolution: 0.8 mm × 0.8 mm, slice thickness: 5 mm).
For assessment of atherosclerosis of the carotids, we obtained black blood Turbo spin echo cross-sectional images of both carotid arteries (Sequence parameters: repetition time: 2 cardiac cycles, echo time: 81 ms, resolution: 0.5 mm × 0.5 mm, slice thickness: 3 mm). These images were segmented using semi-automated border detection algorithms developed using Matlab software (Mathworks Inc.) [11,13,18], in order to define the inner and outer vessel wall boundaries.
These images were analysed to produce maximal thickness for each vascular cross-section. Vascular wall thickness was defined as the distance between the points at which radial lines originating from the centre of the vessel meet the inner and outer vessel wall boundaries. Maximal thicknesses were averaged for the slices corresponding to the carotid arteries and the slices corresponding to the descending thoracic aorta.
2.5.2. Central aortic stiffness
ECG-gated, Steady state free precession ‘cine’ images were acquired during breath-hold to determine aortic distensibility (CMR parameters: repetition time (TR) 2.8 ms, echo time (TE) 1.4 ms, in-plane resolution 2 mm, slice thickness 7 mm, temporal resolution 40 ms). Maximum and minimum aortic cross-sectional areas over the cardiac cycle were determined using semi-automated edge detection algorithms developed using Matlab software (Mathworks Inc.) [19,20] and distensibility was calculated as the relative change in cross-sectional vascular lumen area divided by the pulse pressure, as previously described [19,20]. Distensibility of the proximal and distal descending thoracic aorta was averaged for analysis.
2.5.3. Carotid atheroma characterization
High resolution, black blood, proton density T1 and T2 weighted Turbo spin echo (repetition time/echo time: 1200/12, 700/12 and 3200/81 respectively) and white blood Steady state free precession imaging was used to characterize carotid plaque composition. Each carotid vessel was surveyed for atherosclerosis of the common carotid, carotid bulb, carotid bifurcation and internal carotid artery. For each subject the worst plaque was identified and allocated a rank based on the previously published Modified American Heart Association (AHA) Atherosclerotic Plaque Classification for MRI (ranging from 1–8) (Fig. S1-supplementary file). This system has been validated against histology of carotid endarterectomy in a large cohort, demonstrating very good agreement with histological carotid atheroma classification [21].
See Fig. S1-supplementary file as supplementary file. Supplementary material related to this article found, in the online version, at http://dx.doi.org/10.1016/j.atherosclerosis.2012.03.036.
A single experienced physician, blinded to the subject’s patient vs. control status, reviewed the carotid images determining atheroma class for all subjects. The same observer analyzed a subset of 10 subjects twice in order to determine intra-rater reproducibility. An additional experienced observer analyzed the same subset of subjects in order to assess inter-rater agreement. Intra-rater reproducibility was excellent (kappa = 0.815) with a good inter-rater agreement (kappa = 0.712).
2.5.4. Abdominal adipose tissue and neck measurements
Water suppression (WS) T1 weighted TSE imaging was used for abdominal visceral and subcutaneous adipose tissue measurements as previously described [22]. For each participant, 5 WS TSE slices (6 mm slice thickness, 4 mm gap) were obtained at the level of the midline of the 5th lumbar vertebra. VAT and SCAT areas were measured on each slice by manual contouring. The corresponding VAT and SCAT volumes were calculated by multiplying these areas by the slice and gap thickness and adding the slice volumes produced [22]. We also used Steady state free precession images of the neck to measure mean neck diameters.
2.6. Biochemical tests
Fasting blood samples for the determination of circulating glucose and lipids were obtained. Adiponectin, high sensitivity C-reactive protein (hsCRP) and Insulin concentrations were measured using commercially available ELISA kits (Millipore Corporation). An estimate of insulin resistance was calculated using the HOMA-IR (Homeostasis Model Assessment (for Insulin Resistance) equation [22].
2.7. Statistical analysis
Statistical analyses were carried out using SPSS version 15 (SPSS Inc.). Normal distribution of data was confirmed using the Kolmogorov–Smirnov test. Non-normally distributed data were logarithmically transformed for analysis as appropriate. Normally distributed data are presented as mean ± standard error while non-normally distributed data are presented as median (25th–75th percentile). Independent t test was used to compare numerical variables between groups as appropriate, while the chi-squared test was used for categorical variables. Carotid atheroma class between patients with OSA and controls was compared using the Mann–Whitney test, without transformation.
Pearson’s or Spearman’s coefficient regression was used for univariate correlation analysis as appropriate. Statistical significance for all analyses was assigned at P < 0.05.
Partial correlation analysis was applied for adjustment of correlations for a single parameter while stepwise regression models with backward elimination of independent variables were applied for multivariate analysis. Independent variables were entered in the model if the level of significance of their correlation with the dependent variable was P < 0.5, while correlations were regarded as independent for variables retained in the model and reaching a P < 0.05 level of significance.
3. Results
3.1. Group matching
Close matching between the group of patients with OSA and the group of controls with regard to demographics, anthropometric indices (including waist hip ratio), medication (including statin use) and exposure to cardiovascular risk factors was achieved (Table 1). Comparison of visceral adipose tissue and subcutaneous adipose tissue measurements confirmed the absence of any significant differences between the groups regarding the amount and distribution of abdominal adiposity. Neck size was also similar for the two groups.
Table 1.
Matching between OSA patients and controls.
| OSA patients n = 42 | Controls n = 39 | P | |
|---|---|---|---|
| Oxygen desaturation index (ODI) | 23.7 ± 1.9 | 2.5 ± 1.6 | P <0.0001 |
| Age in years | 56.5 ± 1.7 | 58.9 ± 2.5 | NS |
| Gender | 21% male | 23% male | NS |
| Hypertension | 55% | 49% | NS |
| Systolic blood pressure in mmHg | 130 ± 2.5 | 129 ± 3.9 | NS |
| Diastolic blood pressure in mmHg | 78.9 ± 2.4 | 81.6 ± 3.4 | NS |
| Body mass index (BMI) in kg/m2 | 30.4 ± 0.7 | 30.9 ± 0.8 | NS |
| Body surface area (BSA) in m2 | 2.03 ± 0.03 | 2.07 ± 0.03 | NS |
| Waist hip ratio | 0.98 ± 0.03 | 0.99 ± 0.04 | NS |
| Visceral adipose tissue (VAT) (ml) | 715 ± 43 | 748 ± 76 | NS |
| Subcutaneous adipose tissue (SCAT) (ml) | 1051 ± 65 | 1278 ± 118 | NS |
| Mean neck diameter (cm) | 13.7 ± 2.2 | 13.4 ± 2.6 | NS |
| Diabetes mellitus | 21% | 18% | NS |
| Fasting glucose | 5.7 ± 0.2 | 5.5 ± 0.4 | NS |
| Hyperlipidaemia | 53% | 51% | NS |
| Total cholesterol in mmol/L | 5.4 ± 0.2 | 5.6 ± 0.5 | NS |
| Low density lipoprotein (LDL) | 3.2 ± 0.17 | 3.3 ± 0.19 | NS |
| High density lipoprotein (HDL) | 1.27 ± 0.07 | 1.37 ± 0.08 | NS |
| Triglycerides in mmol/L | 1.99 ± 0.24 | 1.97 ± 0.4 | NS |
| Triglyceride/HDL ratio | 1.57 ± 0.18 | 1.44 ± 0.2 | NS |
| Smokers | 15% | 16% | NS |
| Pack years | 7.8 ± 1.8 | - | - |
| Cardiovascular disease (stroke, coronary artery disease, peripheral arterial disease) | 9% | 16% | NS |
| Family history of cardiovascular disease | 19% | 18% | NS |
| Framingham risk score | 22.1 ± 1.7 | 22.3 ± 2.1 | NS |
| Aspirin | 21.4% | 20.5% | NS |
| Clopidogrel | 2.4% | 0% | NS |
| Beta-blocker | 11.9% | 12.8% | NS |
| ACE inhibitors/angiotensin blockers | 26.2% | 30.8% | NS |
| Statins | 35.7% | 30.8% | NS |
| Diuretics | 14.2% | 20.5% | NS |
| Aspirin | 21.4% | 20.5% | NS |
| Clopidogrel | 2.4% | 0% | NS |
| Beta-blocker | 11.9% | 12.8% | NS |
| Nitrates | 0% | 5.1% | NS |
| Calcium channel blockers | 11.9% | 15.4% | NS |
| Insulin | 0% | 0% | NS |
| Thiazolinediones | 0% | 0% | NS |
| Sulfonylureas | 2.4% | 0% | NS |
| Metformin | 9.6% | 10.2% | NS |
3.2. Carotid/aortic atheroma burden and central aortic stiffness in OSA compared to control subjects
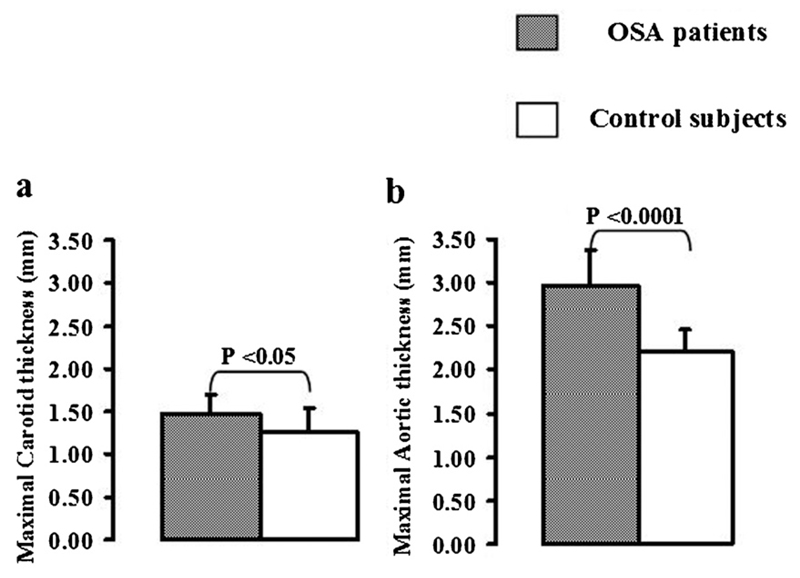
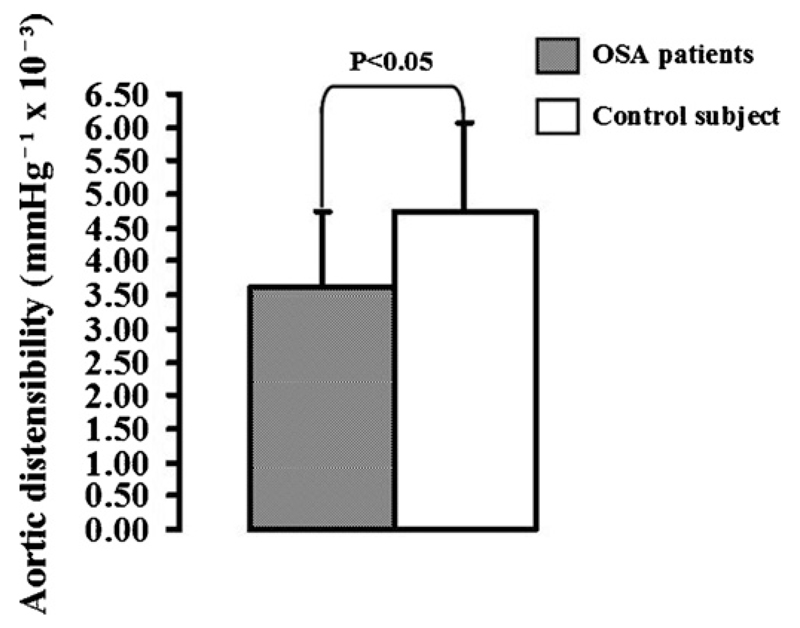
Maximal carotid wall thickness was increased in patients with OSA in comparison with controls [1.47 ± 0.03 mm vs. 1.26 ± 0.05 mm, (P < 0.01) (Fig. 1a)]. Maximal aortic wall thickness was also increased in patients with OSA [2.95 ± 0.09 mm vs. 2.05 ± 0.07 mm, (P < 0.001) (Fig. 2b)]. Aortic distensibility was decreased in patients with OSA in comparison with controls [3.62 ± 0.3 vs. 4.75 ± 0.2 mmHg−1 × 10−3, P < 0.05] (Fig. 2).
Fig. 1.
Atheroma burden in OSA: Maximal carotid (a) and aortic (b) wall thicknesses were increased in patients with OSA in comparison with controls.
Fig. 2.
Aortic stiffness in OSA: Aortic distensibility was decreased in patients with OSA in comparison with controls.
3.3. Carotid atheroma composition
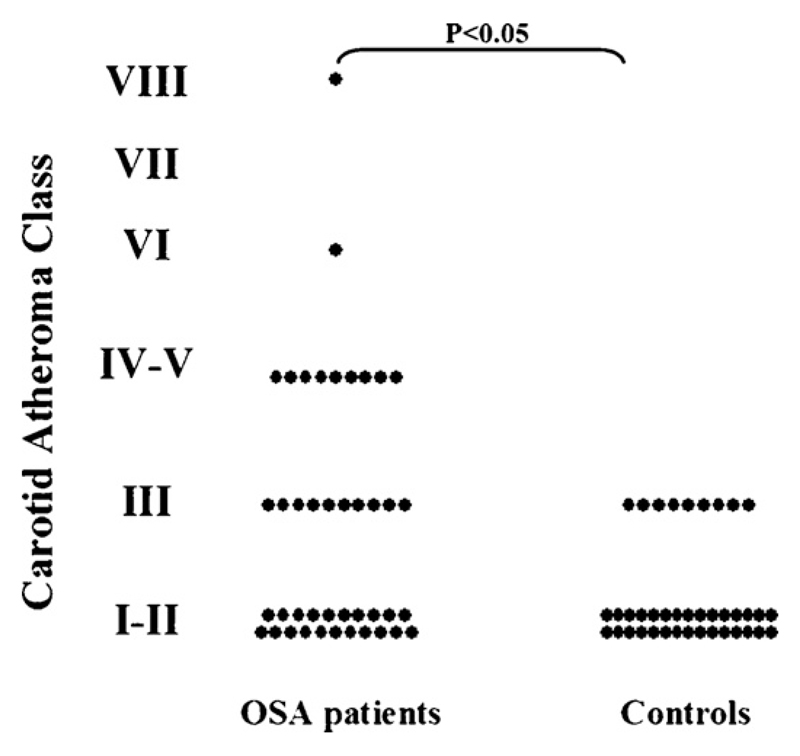
There was a significantly higher prevalence of advanced carotid plaque presence (i.e. vessels ranked as having plaques classified as type III and above) in patients with OSA in comparison with controls [21/42 (50%) vs. 9/39 (23%), (P < 0.05)]. Average carotid atheroma class was higher among patients with OSA in comparison with controls [2.3 (1.5–4.5) vs. 1.5 (1.5–1.5), (P < 0.05)]. Carotid atheroma with features of vulnerability (intra-plaque haemorrhage, plaque rupture, lipid rich necrotic core, i.e. vessels classified as having plaques type IV–VI), was present in 10/42 individuals suffering from OSA but in none of the controls (P < 0.005) (Fig. 3).
Fig. 3.
Carotid atheroma composition (according to the American Heart Association classification system as adopted for CMR) among patients with OSA and controls: Prevalence of carotid plaque (i.e. carotid atheroma class III and above), average carotid atheroma class and prevalence of ‘high risk’ features of carotid atheroma (carotid atheroma class IV–V and VI), were all increased in patients with OSA in comparison with controls.
3.4. Association between OSA severity and atheroma burden
3.4.1. Carotid artery wall thickness
Among all 58 patients with OSA maximal carotid thickness was associated with waist hip ratio (r = 0.32, P < 0.05), mean arterial blood pressure (r = 0.38, P < 0.05), Framingham risk score (r = 0.45, P < 0.005), high-density lipoprotein levels (r = −0.33, P < 0.05), HOMA IR (r = 0.33, P < 0.05), insulin (r = 0.31, P < 0.05) and CRP levels (r = 0.35, P < 0.05).
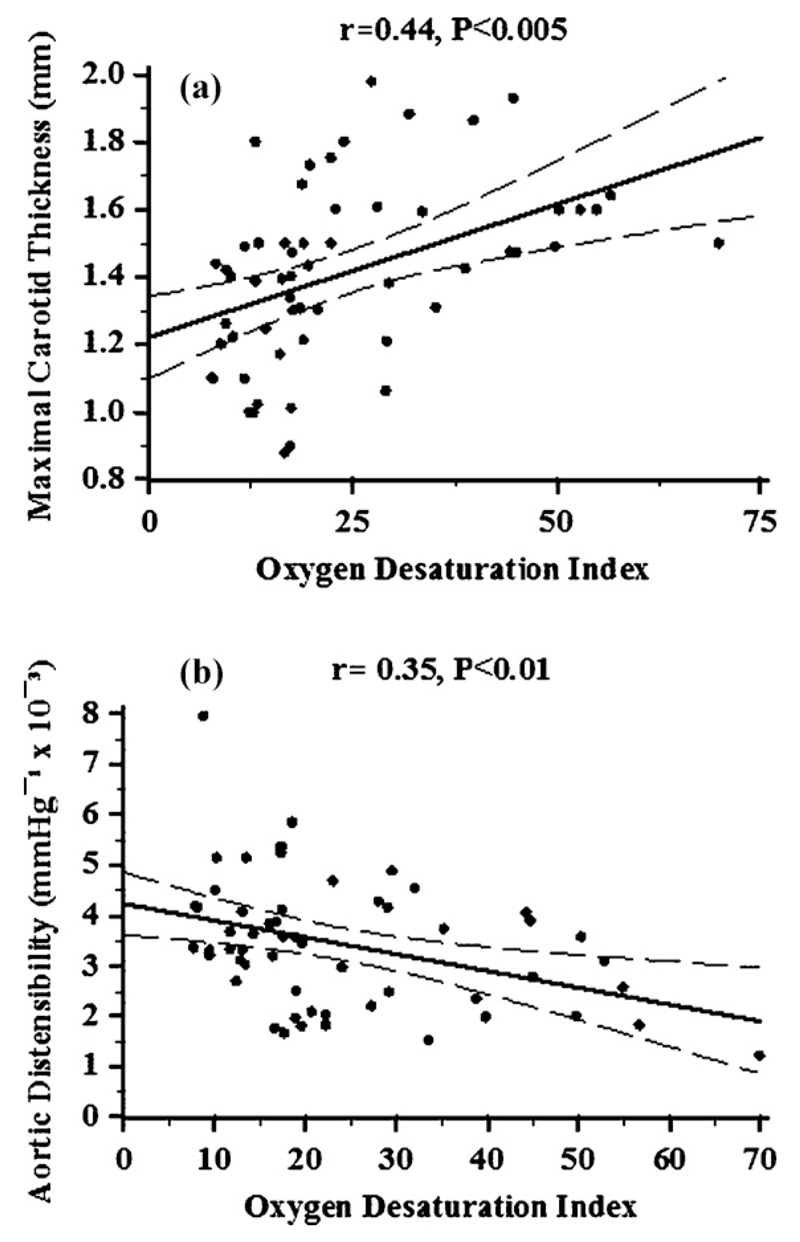
ODI positively correlated with maximal carotid thickness [r = 0.44, P < 0.005, respectively (Fig. 4a)]. This association remained significant after adjustment for Framingham risk score [(β = 0.3 ± 0.09), (P < 0.005)].
Fig. 4.
Oxygen desaturation index (ODI) vs. carotid atheroma burden and aortic stiffness: Oxygen desaturation index (ODI) correlated positively with maximal carotid thickness (a) and inversely with aortic distensibility (b) among patients with OSA.
In a linear multivariate regression analysis model with maximal carotid thickness designated as the dependent variable and including waist hip ratio, mean arterial pressure, HOMA IR, high-density lipoprotein, CRP and ODI as independent variables; ODI [β = 0.41 ± 0.15, P < 0.01] emerged as an independent predictor of maximal carotid thickness (R2 of the statistical model = 0.49).
3.4.2. Aortic wall thickness
Among the 58 patients with OSA maximal aortic thickness was associated with age (r = 0.4, P < 0.005), smoking (pack years) (r = 0.28, P < 0.05), mean arterial pressure (r = 0.31, P < 0.05), Framingham risk score (r = 0.47, P < 0.0001), HOMA IR (r = 0.29, P < 0.05), insulin (r = 0.28, P < 0.05), adiponectin (r = −0.3, P < 0.05), and CRP levels (r = 0.39, P < 0.005). ODI positively correlated with maximal aortic thickness (r = 0.38, P < 0.01). The association remained significant after adjustment for Framingham risk score (β = 0.63 ± 0.2, P < 0.005).
In a linear multivariate regression analysis model with maximal aortic thickness designated as the dependent variable of the model and age and including pack years, mean arterial pressure, HOMA IR, adiponectin, CRP and ODI as independent variables, CRP and ODI were independently associated with maximal aortic thickness [β = 0.031(0.015), (P < 0.05) and β = 0.61(0.21), (P < 0.01) respectively, (R2 = 0.39)].
3.4.3. Aortic stiffness
Among the 58 patients with OSA aortic distensibility was associated with age (r = −0.44, P < 0.001), mean arterial pressure (r = −0.52, P < 0.0001), insulin levels (r = −0.29, P < 0.05) and Framingham risk score (r = −0.33, P < 0.0005). Aortic distensibility correlated with ODI [r = −0.35, P < 0.01] (Fig. 4b) and this association remained significant after adjustment for Framingham risk score [β = −0.22 (0.09), (P < 0.01)].
In a linear multivariate regression analysis model with aortic distensibility as the designated dependent variable and including mean arterial pressure, age, insulin levels and ODI as independent variables; age [β = −0.013 (0.003), P < 0.0005] and mean arterial pressure [β = 0.008 (0.001), P < 0.0005] emerged as independent predictors of aortic stiffness in patients with OSA (R2 = 0.59).
4. Discussion
4.1. OSA and atherosclerosis
In this study, we have demonstrated increased atheroma burden in patients suffering from OSA in comparison with controls that were matched for known cardiovascular risk factors. We detected increased carotid and aortic wall thickness, suggesting the coexistence of both increased vascular remodelling and increased plaque vulnerability in OSA. These findings substantially expand previous observations in animal models of OSA and in OSA patients [3–5].
In animal studies, intermittent hypoxia – an experimental model of OSA- has been linked to atherogenesis and vascular dysfunction [3,4,23]. Proatherogenic effects of intermittent hypoxia have been shown to precede blood pressure increase and affect the vasculature independently [24]. Activation of the inflammatory NFκB pathway by intermittent hypoxia has been proposed as the basis of this phenomenon [3]. In the clinical setting, OSA has been associated with markers of increased inflammation [25]. Given the central role that systemic inflammation plays in atherogenesis and atheroma progression it is possible that nocturnal intermittent hypoxia caused by OSA in the clinical setting may contribute to cardiovascular risk via a systemic pro-inflammatory effect. More recently intermittent hypoxia has been shown to inhibit clearance of triglyceride-rich lipoproteins and inactivate adipose lipoprotein lipase in a mouse model [2,26]. Thus it is also possible that OSA is linked to atherogenesis and atheroma progression by independently contributing to impaired lipid metabolism.
Importantly, after adjustment for known cardiovascular risk factors -both individually considered and incorporated in the Framingham risk score - we detected an independent association between OSA severity, expressed in the form of ODI, and atheroma burden both of the aorta and the carotids. Thus, our data strongly indicate an independent link between OSA and the development and/or progression of vascular disease and suggest that intermittent hypoxia may be contributing to this relationship.
Exploiting one of the unique capabilities of CMR we also demonstrated, for the first time, the presence of more advanced atheroma affecting the carotid vessels of patients with OSA in comparison with controls. We detected both a higher prevalence of carotid plaques and the presence of more complicated and advanced plaques in OSA. Importantly, we identify significantly increased prevalence of carotid atheroma bearing features of ‘vulnerability’ (e.g. intra-plaque haemorrhage, lipid rich necrotic core) in OSA. These plaque characteristics are considered features of ‘high risk’ atheroma, i.e. predisposing the plaque to rupture, resulting in ischemic stroke [27,28]. Given the strong and reportedly independent link between OSA and stroke, this observation is scientifically and potentially clinically important [9].
4.2. OSA and central aortic stiffness
We were able to demonstrate increased central aortic stiffness in patients with OSA. Although increased arterial stiffness, as estimated by peripheral pulse waveform analysis, in patients with OSA has been previously reported [5,29], we were able to describe, for the first time, an increase in central aortic stiffness by direct assessment. Central aortic as opposed to peripheral arterial stiffening is considered especially important in the pathogenesis of cardiovascular complications. The normally elastic thoracic aorta, through the process of stiffening, loses its ability to moderate the transmission of detrimental pulsatile energy to the microvasculature of end-organs (e.g. brain, kidneys) and to reduce the afterload of the left ventricle. Thus, stiffening of the central aorta is thought to specifically predispose to diastolic and eventually systolic heart failure as well as to micro-vascular end-organ damage [11,13].
Aortic stiffness significantly correlated with ODI and this association remained significant after adjustment for Framingham risk score. However, adjustment for individual risk factors, as part of multivariate analysis, indicated that there was no independent ‘dose’ effect of OSA severity as expressed by ODI on aortic stiffness. Interestingly, it was blood pressure alongside age, which emerged as an independent predictor of aortic stiffness. This finding would be consistent with the presence of an indirect effect of OSA on aortic stiffness, mediated by the well established relationship between OSA and increased blood pressure. However, much larger clinical studies would be required to fully dissect the mechanisms underlying this relationship.
4.3. The effect of intra-abdominal adiposity
Having matched our groups for weight and height and having considered all indices of obesity (i.e. body surface area, BMI, waist hip ratio and neck circumference) in both univariate and multivariate analyses as appropriate, we are confident to have neutralized the effects of obesity [30]. Importantly, CMR also allowed us to confirm the absence of significant visceral and subcutaneous adipose tissue differences between patients and control subjects. Including these measurements in our analysis we were able to control for potential effects of excess intra-abdominal adiposity on our results.
5. Limitations
Our study design is limited in its ability to establish independence of our results from the effects of increased blood pressure. Blood pressure measurements did not emerge as an independent predictor of atheroma burden and severity on either univariate or multivariate analysis. Similarly, both systolic and diastolic (and thus mean) blood pressure was not significantly different between patients with OSA and controls. Although an increase in systolic blood pressure has been associated with OSA in large epidemiological cohorts, failure to detect such a difference in this group is not surprising, given the relatively small sample size and the fact that all patients and controls, who had a history of known high blood pressure, were under antihypertensive treatment at the point of assessment. Although this may reflect more realistically what happens in the ‘real world’ regarding individuals at high cardiovascular risk, such as the ones selected for this study, it means that a potential increase in blood pressure among patients with OSA in comparison to controls during the time preceding the clinical recognition of hypertension could exert a statistically undetected influence on our results. More importantly, large and repeated increases in blood pressure following each apnoea and arousal have been reported in association with OSA [31]. Such a phenomenon would also be unaccounted for as part of the present study analysis.
Although overnight pulse oximetry has been shown to have adequate sensitivity in the detection of sleep apnoea among asymptomatic individuals [32,33] and was used for the identification of controls in this study, polysomnography remains the gold standard for excluding OSA. Nonetheless, a formal sleep study for likely OSA patients as identified by both oximetry and a history of symptoms was deemed necessary to provide increased specificity in the diagnosis as well as improved accuracy in the assessment of severity of OSA.
6. Conclusions
In this study, we demonstrated an independent association between the presence and severity of OSA and increased carotid and aortic atheroma burden, and reported an association between OSA and the presence of advanced and high risk carotid atheroma. We detected an association between OSA and central aortic stiffening, which appears to be dependent on the increase in blood pressure associated with OSA. These findings were independent of both obesity and intra-abdominal adiposity. Our results suggest that OSA is an independent contributor to cardiovascular risk.
Supplementary Material
Acknowledgements
This work was supported by the Oxford NIHR Biomedical Research Centre programme and by the British Heart Foundation (London, UK). Funding for IK was provided by the State Scholarship Foundation (Greece). Funding for SC was partly provided by the Oxford Health Services Research Committee. The authors would also like to thank Charis Antoniades MD PhD for his contribution to this manuscript.
Footnotes
Conflicts of interest
None declared
Contributor Information
Ilias Kylintireas, Email: ilias_kylintireas@yahoo.co.uk.
Sonya Craig, Email: sonya.craig@orh.nhs.uk.
Richard Nethononda, Email: richard.nethononda@cardiov.ox.ac.uk.
Malcolm Kohler, Email: malcolm.k@bluewin.ch.
Jane Francis, Email: jane.francis@cardiov.ox.ac.uk.
Robin Choudhury, Email: robin.choudhury@cardiov.ox.ac.uk.
John Stradling, Email: john.stradling@orh.nhs.uk.
Stefan Neubauer, Email: stefan.neubauer@cardiov.ox.ac.uk.
References
- [1].Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. The New England Journal of Medicine. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- [2].Drager LF, Li J, Shin MK, et al. Intermittent hypoxia inhibits clearance of triglyceride-rich lipoproteins and inactivates adipose lipoprotein lipase in a mouse model of sleep apnoea. Eur Heart J. 2012;33:783–90. doi: 10.1093/eurheartj/ehr097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dematteis M, Julien C, Guillermet C, et al. Intermittent hypoxia induces early functional cardiovascular remodeling in mice. American Journal of Respiratory and Critical Care Medicine. 2008;177:227–35. doi: 10.1164/rccm.200702-238OC. [DOI] [PubMed] [Google Scholar]
- [4].Savransky V, Nanayakkara A, Li J, et al. Chronic intermittent hypoxia induces atherosclerosis. American Journal of Respiratory and Critical Care Medicine. 2007;175:1290–7. doi: 10.1164/rccm.200612-1771OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Drager LF, Bortolotto LA, Krieger EM, Lorenzi-Filho G. Additive effects of obstructive sleep apnea and hypertension on early markers of carotid atherosclerosis. Hypertension. 2009;53:64–9. doi: 10.1161/HYPERTENSIONAHA.108.119420. [DOI] [PubMed] [Google Scholar]
- [6].Doonan RJ, Scheffler P, Lalli M, et al. Increased arterial stiffness in obstructive sleep apnea: a systematic review. Hypertension Research. 2011;34:23–32. doi: 10.1038/hr.2010.200. [DOI] [PubMed] [Google Scholar]
- [7].Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122:352–60. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. The New England Journal of Medicine. 2005;353:2034–41. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- [9].Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. Journal of the American College of Cardiology. 2008;52:686–717. doi: 10.1016/j.jacc.2008.05.002. [DOI] [PubMed] [Google Scholar]
- [10].van der Meer RW, Diamant M, Westenberg JJ, et al. Magnetic resonance assessment of aortic pulse wave velocity, aortic distensibility, and cardiac function in uncomplicated type 2 diabetes mellitus. Journal of Cardiovascular Magnetic Resonance. 2007;9:645–51. doi: 10.1080/10976640601093703. [DOI] [PubMed] [Google Scholar]
- [11].Lee JM, Robson MD, Yu LM, et al. Effects of high-dose modified-release nicotinic acid on atherosclerosis and vascular function: a randomized, placebo-controlled, magnetic resonance imaging study. Journal of the American College of Cardiology. 2009;54:1787–94. doi: 10.1016/j.jacc.2009.06.036. [DOI] [PubMed] [Google Scholar]
- [12].Taniguchi H, Momiyama Y, Fayad ZA, et al. In vivo magnetic resonance evaluation of associations between aortic atherosclerosis and both risk factors and coronary artery disease in patients referred for coronary angiography. American Heart Journal. 2004;148:137–43. doi: 10.1016/j.ahj.2004.03.008. [DOI] [PubMed] [Google Scholar]
- [13].Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. Journal of Applied Physiology. 2008;105:1652–60. doi: 10.1152/japplphysiol.90549.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rosito GA, Massaro JM, Hoffmann U, et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham heart study. Circulation. 2008;117:605–13. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- [15].Kohler M, Craig S, Nicoll D, Leeson P, Davies RJ, Stradling JR. Endothelial function and arterial stiffness in minimally symptomatic obstructive sleep apnea. American Journal of Respiratory and Critical Care Medicine. 2008;178:984–8. doi: 10.1164/rccm.200805-717OC. [DOI] [PubMed] [Google Scholar]
- [16].Pocock SJ, McCormack V, Gueyffier F, Boutitie F, Fagard RH, Boissel JP. A score for predicting risk of death from cardiovascular disease in adults with raised blood pressure, based on individual patient data from randomised controlled trials. BMJ. 2001;323:75–81. doi: 10.1136/bmj.323.7304.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Krejza J, Arkuszewski M, Kasner SE, et al. Carotid artery diameter in men and women and the relation to body and neck size. Stroke. 2006;37:1103–5. doi: 10.1161/01.STR.0000206440.48756.f7. [DOI] [PubMed] [Google Scholar]
- [18].Wang Q, Robson MD, Francis JM, et al. Accuracy of quantitative MR vessel wall imaging applying a semi-automated gradient detection algorithm—a validation study. Journal of Cardiovascular Magnetic Resonance. 2004;6:895–907. doi: 10.1081/jcmr-200036198. [DOI] [PubMed] [Google Scholar]
- [19].Lee JM, Shirodaria C, Jackson CE, et al. Multi-modal magnetic resonance imaging quantifies atherosclerosis and vascular dysfunction in patients with type 2 diabetes mellitus. Diabetes and Vascular Disease Research. 2007;4:44–8. doi: 10.3132/dvdr.2007.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jackson CE, Shirodaria CC, Lee JM, et al. Reproducibility and accuracy of automated measurement for dynamic arterial lumen area by cardiovascular magnetic resonance. The International Journal of Cardiovascular Imaging. 2009;25:797–808. doi: 10.1007/s10554-009-9495-5. [DOI] [PubMed] [Google Scholar]
- [21].Cai JM, Hatsukami TS, Ferguson MS, Small R, Polissar NL, Yuan C. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation. 2002;106:1368–73. doi: 10.1161/01.cir.0000028591.44554.f9. [DOI] [PubMed] [Google Scholar]
- [22].Rider OJ, Francis JM, Ali MK, et al. Beneficial cardiovascular effects of bariatric surgical and dietary weight loss in obesity. Journal of the American College of Cardiology. 2009;54:718–26. doi: 10.1016/j.jacc.2009.02.086. [DOI] [PubMed] [Google Scholar]
- [23].Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. 2005;112:2660–7. doi: 10.1161/CIRCULATIONAHA.105.556746. [DOI] [PubMed] [Google Scholar]
- [24].Allahdadi KJ, Walker BR, Kanagy NL. Augmented endothelin vasoconstriction in intermittent hypoxia-induced hypertension. Hypertension. 2005;45:705–9. doi: 10.1161/01.HYP.0000153794.52852.04. [DOI] [PubMed] [Google Scholar]
- [25].Ohga E, Tomita T, Wada H, Yamamoto H, Nagase T, Ouchi Y. Effects of obstructive sleep apnea on circulating ICAM-1, IL-8, and MCP-1. Journal of Applied Physiology. 2003;94:179–84. doi: 10.1152/japplphysiol.00177.2002. [DOI] [PubMed] [Google Scholar]
- [26].Phillips CL, Yee BJ, Marshall NS, Liu PY, Sullivan DR, Grunstein RR. Continuous positive airway pressure reduces postprandial lipidemia in obstructive sleep apnea: a randomized, placebo-controlled crossover trial. American Journal of Respiratory and Critical Care Medicine. 2011;184:355–61. doi: 10.1164/rccm.201102-0316OC. [DOI] [PubMed] [Google Scholar]
- [27].U K-I JM, Tang TY, Patterson A, et al. Characterisation of carotid atheroma in symptomatic and asymptomatic patients using high resolution MRI. Journal of Neurology, Neurosurgery, and Psychiatry. 2008;79:905–12. doi: 10.1136/jnnp.2007.127969. [DOI] [PubMed] [Google Scholar]
- [28].Strong JP, McGill H., Jr The natural history of coronary atherosclerosis. American Journal of Pathology. 1962;40:37–49. [PMC free article] [PubMed] [Google Scholar]
- [29].Drager LF, Bortolotto LA, Figueiredo AC, Silva BC, Krieger EM, Lorenzi-Filho G. Obstructive sleep apnea, hypertension, and their interaction on arterial stiffness and heart remodeling. Chest. 2007;131:1379–86. doi: 10.1378/chest.06-2703. [DOI] [PubMed] [Google Scholar]
- [30].Kawaguchi Y, Fukumoto S, Inaba M, et al. Different impacts of neck circumference and visceral obesity on the severity of obstructive sleep apnea syndrome. Obesity (Silver Spring) 2011;19:276–82. doi: 10.1038/oby.2010.170. [DOI] [PubMed] [Google Scholar]
- [31].Kario K. Obstructive sleep apnea syndrome and hypertension: ambulatory blood pressure. Hypertension Research. 2009;32:428–32. doi: 10.1038/hr.2009.56. [DOI] [PubMed] [Google Scholar]
- [32].Gurubhagavatula I, Maislin G, Nkwuo JE, Pack AI. Occupational screening for obstructive sleep apnea in commercial drivers. American Journal of Respiratory and Critical Care Medicine. 2004;170:371–6. doi: 10.1164/rccm.200307-968OC. [DOI] [PubMed] [Google Scholar]
- [33].Vazquez JC, Tsai WH, Flemons WW, et al. Automated analysis of digital oximetry in the diagnosis of obstructive sleep apnoea. Thorax. 2000;55:302–7. doi: 10.1136/thorax.55.4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.