Abstract
Preterm neonates represent a high-risk population for abnormal neuropsychological development. But presently, an accurate method for identifying those at risk is not available. This study evaluated the association between the microstructural organization measured with Diffusion Tensor Imaging (DTI) in term-corrected preterm neonates and subsequent motor performance. Fractional anisotropy (FA), axial diffusion (AD), mean diffusivity (MD) and radial diffusivity (RD) were determined in two regions of interest (ROIs) corresponding to the posterior limb of the internal capsule (PLIC) and cortico-spinal tract (CST). The Griffiths Mental Developmental Scales (GMDS) were longitudinally administered at 3, 6 and 15 months; and correlations between the metrics of diffusivity and the motor subscale of the GMDS were assessed using the Spearman correlation. A statistically significant negative correlation was observed between the AD of PLIC of the left hemisphere and the 3-month GMDS Locomotor Subscale. These results suggested that AD is a valid indicator of the stage of maturation of the motor pathway in preterm neonates, but not of later motor outcome.
Keywords: Axial diffusion, diffusion tensor imaging, fetal development, motor outcome, motor system, neurological development, neurological indicators, preterm neonates
Introduction
Recent World Health Organization (WHO) estimates of global rates of preterm births indicate that 14.9 million (11.1%) of the 135 million live births worldwide in 2010 were preterm.1 Preterm neonates represent a high-risk population for brain damage that can result in neurodevelopmental disabilities of variable severity, including cerebral palsy (CP).2
Despite improving rates of survival for preterm infants over the past 2 decades, the prevalence of disability has remained relatively constant, with up to 50% of these infants exhibiting motor, cognitive or behavioral impairments and 5–15% presenting cerebral palsy later in life.3 Motor difficulties are often associated with minor neurological dysfunction, low intelligence quotient (IQ), slow processing speed and hyperactivity.4 To limit this unfavorable outcome, several preventive neuromotor therapies can be implemented during the early stages of development, when dendritic outgrowth and synapse formation are highly active. In order for therapies to be effective,5 the identification of high-risk neonates is essential. Clinical risk factors and cranial ultrasound, currently used to study preterm neonates around birth, are not optimal predictors for long-term outcome.6
Alternatively, magnetic resonance imaging (MRI) is currently considered the most sensitive neuroimaging modality for assessing brain myelination and perinatal brain injuries (e.g. cerebral hemorrhage, periventricular leukomalacia and reduction of white matter volume), which when present, may be associated with subsequent motor deficits;7 however, up to 18% of preterm infants can present cerebral palsy with no concurrent parenchymal abnormality.8 Thus, even white matter lesions detectable with standard MRI sequences have a suboptimal sensitivity and specificity for subsequent neurological abnormalities. Therefore, a method to accurately identify which preterm neonates have a higher risk for poor developmental outcome is currently lacking.
The implementation of specific sequences for evaluating microstructural organization of the white matter in preterm neonates could further improve the prognostic value of MRI. Weiss et al.9 hypothesized that both the macro- and micro-structural modifications occurring during the late second and third trimesters of gestation are responsible for the subsequent neuromotor deficits seen in those born preterm. In this context, diffusion tensor imaging (DTI) has the crucial ability to evaluate the integrity (myelination) of white matter tracts even in the absence of major focal lesions in standard MRI images. In particular, DTI measures the degree of diffusion and direction of water molecules at each voxel.10 A series of indices of diffusion and anisotropy can then be calculated.
Axial diffusivity (AD) represents the largest eigenvalue of the tensor (lower values indicate loss of coherent axonal organization11 associated with axon damage and degeneration). Mean diffusivity (MD) represents the average of the three eigenvalues describing the tensor (lower values indicate a decrease in water content and an increase in complexity of white matter, thus increasing myelination12). Radial diffusivity (RD) represents the average of the two minor eigenvalues of the tensor (increase indicates demyelination or loss of myelin sheet integrity11). Fractional anisotropy (FA) is the main index of anisotropy and it is determined by fiber diameter and density, myelination, extracellular diffusion, inter-axonal spacing and intra-voxel fiber-tract coherence.13
Since the relationship between various DTI indices across different physiological/pathological conditions is still lacking, studies should use multiple DTI indices. Previous DTI studies on preterm neonates, infants or adolescents focused on potential alterations of FA. For example, Arzoumanian et al.14 and Rose et al.15 found that the FA of the posterior limb of the internal capsule (PLIC) was significantly lower in preterm infants with cerebral palsy. Lower FA values of PLIC were found in preterm infants with psychomotor delay and cerebral palsy.16
When focusing on preterm infants studied at a term-equivalent age, Krishnan et al.17 conclude that an increased diffusivity in white matter is predictive of adverse neurodevelopmental outcome. A retrospective analysis of DTI studies of preterm neonates evaluated at term-corrected age demonstrated lower FA values in the PLIC in children with abnormal neurologic examinations, at 1 year of age.15 Lower FA in the corpus callosum of preterm infants was shown to correlate with a lower psychomotor developmental index.18 Pogribna et al.19 report that DTI in extremely low-birth-weight infants with impaired cognitive and language functions at 18–22 months of corrected age correlate with a significantly higher MD and lower FA in six specific regions (PLIC, frontal periventricular zone, occipital periventricular zone, corpus callosum, centrum semiovale and subventricular zone). Overall, these studies indicate the importance of considering diffusion metrics other than FA in neonatal investigations.
During the early stages of neonatal brain maturation, increasing complexity of white matter organization determines a reduction of the total water content.20 This, in turn, causes a reduction of water diffusion along each of the three axes that may not necessarily be associated with a concomitant variation of FA.21 This has three critical implications for the study of neonatal patients:
First, measures of water diffusivity, such as AD or MD, may be more sensitive markers of perinatal white matter maturation.
Second, measures of diffusivity may correlate with the motor and neuromotor status of the neonate, and be a possible predictor of developmental outcome.
Third, AD and FA are not expected to be necessarily concordant in preterm neonates, as typically observed in the adult brain,22 therefore AD may exhibit an inverse correlation.
The aim of the present study was to assess the relationship between measures of diffusivity, in the absence of severe white matter abnormalities at conventional MRI; and their association with neuromotor outcome, assessed longitudinally within the first 15 months of life. Unlike previous studies,23,24 we aimed to establish a specific anatomical-clinical association, focusing on the motor domain, which can be more easily and selectively evaluated at the anatomical and clinical level, even in the earliest stages of development. Therefore, we assessed the presence of significant correlations between the microstructural status in regions of interest typically used to evaluate the integrity of the motor pathway, using a complete set of DTI parameters (FA, AD, MD and RD); and a measure of motor performance, assessed with the Development Quotient for motor sub-scale A (DQA) of the Griffiths Mental Developmental Scales (GMDS) at 3, 6 and 15 months of corrected age.
Patients and methods
Patients
We included 38 consecutive preterm neonates (20 males) born during the 28th to the 36th week of gestation, into this prospective study. Legal guardians of the neonates gave written informed consent prior to enrollment. This study was approved by our local ethics committee (Ethics Committee of G D’Annunzio University of Chieti-Pescara). Gestational age, weight at birth, APGAR (backronym for Appearance, Pulse, Grimace, Activity and Respiration) score, pH base excess at birth, hours of physiotherapy and days of O2 therapy were recorded. Participants underwent a routine single-session MRI examination (scan duration: 18 min) at the term-corrected age. This included conventional clinical and DTI sequences. Neonates presenting a normal brain or only periventricular punctate white matter lesions at conventional MRI were enrolled in our study. We excluded neonates with cystic periventricular leukomalacia and germinal matrix hemorrhage on their MRI; or that presented brain abnormalities on MRI other than periventricular punctate lesions.
Periventricular punctate lesions are a classic MRI sign of periventricular leukomalacia and their physiopathological explanation is not completely clear.25 The evaluation of conventional MRI sequences and the count of the punctate white matter lesions on T1-weighted images were performed by a neuroradiologist (MC) with 10 years of experience in pediatric neuroradiology. Behavioral assessment was conducted at 3, 6 and 15 months using the GMDS.26 We excluded subjects whom were lost to follow-up from this study (patient flow chart in Supplemental materials). Our analysis was conducted on the 27 preterm neonates who met the inclusion criteria.
MRI procedure
MRI was performed using a 3T scanner (Philips Achieva, The Netherlands) with an 8-channel head-coil. Neonates were sedated with oral chloral hydrate (50 mg/Kg). Adapted earplugs and Minimuffs (Natus Medical Incorporated, San Carlos, CA, USA) were used to minimize noise exposure. Neonates were placed on their left side within a MRI-compatible vacuum pillow, in order to minimize movements. A neonatologist (EC, RS) constantly monitored the blood oxygen saturation and cardiac frequency.
Standard MRI examination included a three-dimensional (3D) T1-weighted Fast Field Echo (FFE) (section thickness 1 mm, intersection 0 mm, Repetition Time (TR) 9.2 ms and Echo Time (TE) 4.3 ms), 3 mm axial and coronal Turbo Spin Echo (TSE) T2-weighted (section thickness 3 mm, intersection 0.5 mm, TR 3 s and TE 80 ms) and Inversion Recovery (IR) sequence (section thickness 3mm, intersection 0.5 mm, TR 4938 ms, TE 13 ms and Inversion Time (TI) 400 ms). Diffusion magnetic resonance images were acquired using a couple of diffusion-weighted multi-slice spin echo Echo Planar Imaging (EPI) pulse sequence with enhanced gradient mode and six gradient directions, rotated in the subject’s space. Other sequence parameters were: Field of View (FOV) 18 cm, slice thickness 2 mm with no gap, imaging matrix 92 × 88 (for an iso-voxel size of 2 × 2 × 2 mm3), 40 slices, b-value 1000 s/mm2, EPI factor 47, Sensivity Encoding (SENSE) factor 2 and Number of Excitations (NEX) 1. For each subject, two consecutive DTI scans were acquired to improve the signal-to-noise ratio (by averaging) and avoid patient elimination due to excessive movement. A couple of six gradient directions DTI sequence were used, in order to reduce acquisition time, decrease the number of directions; doubling the number of acquisitions in order to increase the possibility of acquiring at least one valid scan in each subject. A higher accuracy with more gradient directions such as typically required for tractography was not required.
DTI data processing
DTI pre-processing was performed using FMRIB Software Library FSL Tools 5.0.27 Volumes were registered (rigid affine) to the first b0 image, to correct for movement artifacts and eddy current distortions. Corrected DTI sequences were averaged. A brain mask was obtained using BET on b0 volume. DTIFIT was used to fit diffusion tensors to each voxel and compute standard indices of diffusion and anisotropy.8 All FA volumes were aligned to the FA Oishi human neonatal brain,28 using the FMRIB Nonlinear Registration Tool, following a standard procedure for aligning three distinct diffusion maps29 (images in the supplemental materials). An average of the individual co-registered FA maps was obtained and the first mask was generated at the threshold of 0.15.
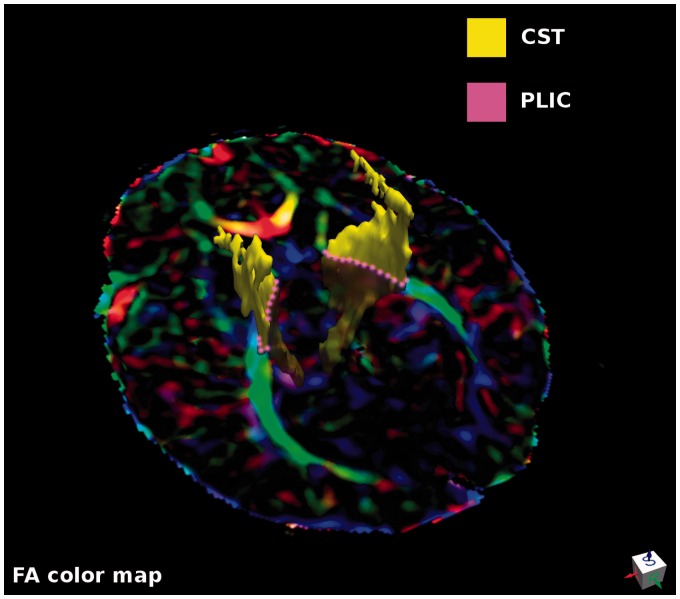
Regions of interest (ROIs) corresponding to the left and right Cortical Spinal Tract (CST) were automatically obtained by combining four Oishi parcels (i. the cortico-spinal tract, ii. the cerebral peduncle, iii. the posterior limb of internal capsule and iv. the superior corona radiata), and masking the resulting ROIs with the individual FA mask (Figure 1, yellow). ROIs corresponding to the left and right PLIC were identified using the corresponding Oishi parcels (Figure 1, pink). ROIs were masked with the individual FA skeletonized masks, thus limiting the inter-subject variability in the number of included voxels. DTI measures were the average of voxels in each ROI.
Figure 1.
An axial slice of FA colored map indicating the two ROIs for each side used to extract FA, AD, MD and RD averages. ROIs corresponding to the left and right CST (yellow) were obtained by combining four Oishi parcels (i. cortico-spinal tract, ii. cerebral peduncle, iii. posterior limb of internal capsule and iv. superior corona radiata) and masking the resulting ROIs with individual FA masks. ROIs corresponding to the left and right PLIC were identified, using the corresponding Oishi parcels (pink) and masking the ROIs with the individual FA mask.
AD: axial diffusivity/diffusion; CST: cortical spinal tract; FA: fractional anisotropy; MD: mean diffusivity; PLIC: posterior limb of the internal capsule; RD: radial diffusivity; ROI: regions of interest
Griffiths scales
The Griffiths scales of mental development (GMDS) Revision 2.0, measure the rate of development of infants and young children.26 GMDS have been used since the 1930s and continue to be recognized as a valuable tool for assessing development in this age group.30
The components of this test evaluate five areas of development providing five specific (corresponding to each subscale) and one general developmental quotient (DQ). The subscales refer to specific functional domains: locomotor, personal and social, hearing and language, eye and hand coordination, and performance. For the present study, we focused on the locomotor subscale, which assesses gross motor skills, including the ability to balance and to co-ordinate/control movements. The rationale for focusing on subscale A was to investigate the specific relationship between the anatomical substrate of motor functions, measured in terms of DTI parameters from specific regions of the motor pathway (CST and PLIC), and motor performance assessed at multiple time points after birth (at 3, 6 and 15 months). GMDS were administered at 3, 6 and 15 months by two trained neonatologists (EC and RS) in consensus. The GMDS score for DQA starts from 50 (corresponding to <1 percentile, worst) to 150 (>99 percentile) with a mean score of 100.
Statistical analysis
Data were analyzed with the SPSS Statistics for Linux, Version 20.0 (International Business Machines (IBM), Armonk, NY, USA).
Significant differences between the left and right CST and PLIC were assessed separately for FA, AD, MD and RD measures, using a 2-way repeated measures analysis of variance (ANOVA) with both anatomical structure (CST and PLIC) and hemisphere (left or right) as factors.
Significant correlations between the values of FA, AD, MD and RD; and the motor subscale of the GMDS at 3, 6 and 15 months, were assessed using a Spearman correlation coefficient and a threshold of p < 0.05, with the FDR adjusted for all MRI measurements and performance tests, at three time-points.
Partial correlation analyses were conducted to test whether the correlation between measures of AD within the left PLIC and the DQA were statistically significant; also, when controlling for important clinical variables (gestational age, age at scan, weight at birth and number of punctate lesions in MRI, APGAR, pH, Base Excess; and the duration of physiotherapy and O2 therapy).
Results
Patients
Analysis was conducted on 27 out of the original 38 patients (median gestational age was 33.6 weeks). We excluded four neonates because conventional MRI showed extensive white matter involvement (cystic periventricular leukomalacia and germinal matrix hemorrhage), two neonates due to excessive movement during DTI acquisitions and five of the patients were lost to follow-up. A summary of the clinical and conventional neuroimaging data of the 27 neonates is in Supplemental Table S1.
DTI indices
Indices of central tendency and dispersion of values of FA, AD, MD and RD from each side (left and right) and anatomical structures (CST and PLIC) are presented in Supplemental Table S2. A 2-way analysis of variance (ANOVA) for FA anatomical structure (CST and PLIC) and hemisphere (left and right) as factors revealed that there was a significant effect of structure (F(1,26) = 1525.7; p < 0.0001), but it was not significant (n.s.) for side and interaction (F(1,26) = 3.2; p = n.s.; F(1,26) = 4.2; p = n.s.). Therefore, the values of FA were significantly greater in the PLIC versus the CST.
Two-way ANOVA of AD revealed a significant effect of interaction (F(1,26) = 5.3; p < 0.03), but not of structure (F(1,26) = 2.8; p = n.s.) and of side (F(1,22) = 0.3; p = n.s.). A 2-way ANOVA of MD revealed a significant effect of structure (F(1,26) = 608.0; p < 0.0001), but not for side (F(1,22) = 0.2; n.s.) and for interaction (F(1,26) = 0.3; p = n.s.). Thus, the values of MD were significantly smaller in the PLIC, compared to CST. Finally, a 2-way ANOVA of RD revealed a significant effect of structure (F(1,26) = 337.4; p < 0.0001) but not for side (F(1,26) = 1.0; n.s.) and for interaction (F(1,26) = 1.3; p = n.s.).
Griffiths locomotor subscale (DQA)
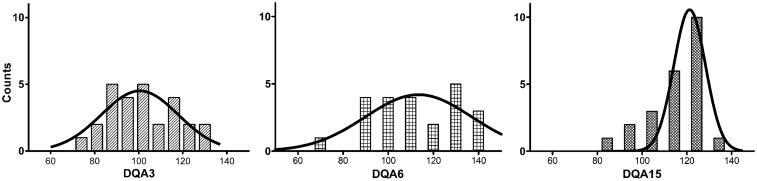
Measures of DQA were 104 ± 15, 109 ± 20 and 111 ±16; at 3, 6 and 15 months, respectively. During development, the kurtosi increased from −0.8 to 1.1, while the skewedness decreased from 0.2 to −1.0 (Figure 2).
Figure 2.
The distribution of DQA at 3, 6 and 15 months (DQA3, DQA6 and DQA15 unit-less scores, respectively). Superimposed Gaussian fits denote temporally decreasing inter-subject variability as: (a) the peak of the distribution narrowed while shifting towards higher values, (b) kurtosi increased and (c) skewedness decreased.
DQA: Developmental Quotient for motor sub-scale A
DTI-GMDS correlations
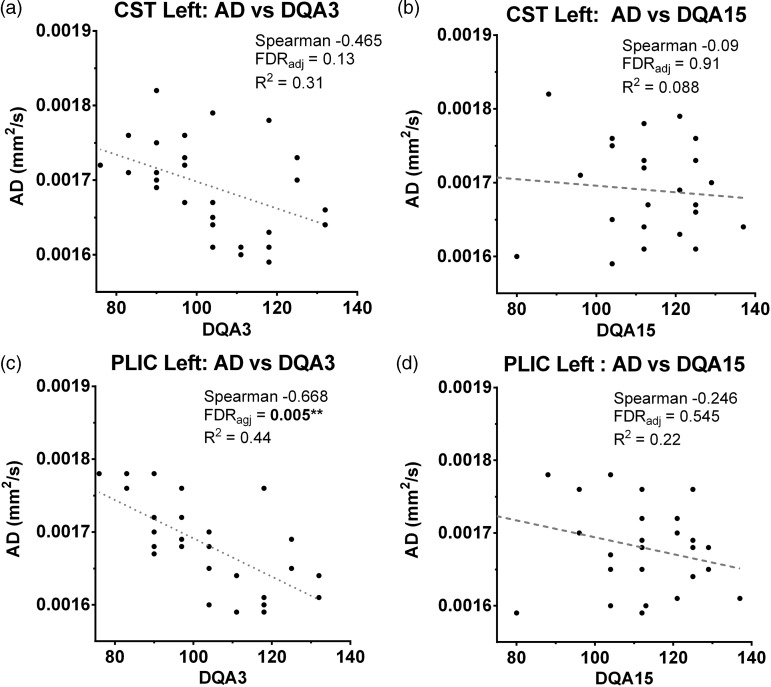
Results of the Spearman correlation coefficient of FA, AD, MD and RD of the left and right CST and PLIC, versus the Locomotor Subscale of the GMDS, are presented in Table 1. A significant negative correlation (2-tailed, FDR corrected) was observed between DQA values obtained at 3 months and the AD measured the PLIC (rho = − .678; p < 0.005) of the left hemisphere (Figure 3). We observed no significant correlations between DQA at 3 months versus measures of FA, MD and RD; and between DQA at 6 and 15 months, versus all the indices of diffusion.
Table 1.
Correlations between DTI measures (FA, AD, MD and RD) and DQA values at 3, 6 and 15 months of age.a
| TI data | DQA3 | FDRadj | DQA6 | FDRadj | DQA15 | FDRadj |
|---|---|---|---|---|---|---|
| FA CST left | 0.192 | 0.729 | 0.188 | 0.729 | 0.187 | 0.729 |
| FA CST right | 0.301 | 0.471 | 0.199 | 0.729 | 0.180 | 0.737 |
| FA PLIC left | 0.013 | 0.968 | 0.059 | 0.912 | 0.071 | 0.912 |
| FA PLIC right | 0.074 | 0.912 | 0.055 | 0.912 | 0.084 | 0.912 |
| AD CST left | −0.465 | 0.132 | −0.068 | 0.912 | −0.099 | 0.912 |
| AD CST right | −0.448 | 0.132 | 0.008 | 0.968 | 0.027 | 0.968 |
| AD PLIC left | −.678b | 0.005 | −0.271 | 0.483 | −0.246 | 0.545 |
| AD PLIC right | −0.329 | 0.414 | 0.016 | 0.968 | 0.052 | 0.912 |
| MD CST left | −0.449 | 0.132 | −0.107 | 0.912 | −0.103 | 0.912 |
| MD CST right | −0.523 | 0.123 | −0.100 | 0.912 | −0.038 | 0.949 |
| MD PLIC left | −0.455 | 0.132 | −0.328 | 0.414 | −0.320 | 0.417 |
| MD PLIC right | −0.362 | 0.339 | −0.075 | 0.912 | −0.009 | 0.968 |
| RD CST left | −0.367 | 0.339 | −0.113 | 0.912 | −0.115 | 0.912 |
| RD CST right | −0.475 | 0.132 | −0.158 | 0.826 | −0.063 | 0.912 |
| RD PLIC left | −0.284 | 0.483 | −0.274 | 0.483 | −0.272 | 0.483 |
| RD PLIC right | −0.253 | 0.539 | −0.083 | 0.912 | −0.052 | 0.912 |
FDR correction applied for n = 27 (q = 0.05).
Correlation is significant at the 0.01 level (2-tailed).
AD: axial diffusivity; CST: cortico-spinal tract; DQA: Griffiths Locomotor Subscale; DTI: diffusion tensor imaging; FA: fractional anisotropy; FDR: False Discovery Rate; MD: mean diffusivity; PLIC: posterior limb of the internal capsule; RD: radial diffusivity
Figure 3.
DTI/DQA correlations. Upper row: Correlations between (a) AD values of the left CST and values of the Griffiths locomotor subscale were statistically significant at 3 months (DQA3) and (b) n.s. at 15 months (DQA15). (c) Lower row: Correlation between AD values of the left PLIC and values of the Griffiths locomotor subscale were statistically significant at 3 months (DQA3), (d) but n.s. at 15 months (DQA15).
AD: axial diffusivity; CST: cortico-spinal tract; DQA: Developmental Quotient for motor sub-scale A; DTI: diffusion tensor imaging; FDR:; n.s.: not significant; PLIC: posterior limb of the internal capsule
Partial correlations
Partial correlation analysis revealed a significant negative correlation between AD of the left PLIC and DQA at 3 months, even when the clinical and anatomical variables were taken into account (Table 2).
Table 2.
Partial correlations between DTI parameters of left PLIC and DQA at 3 months (DQA3), taking the effect of weight at birth, number of lesions at standard MRI, gestational age, age at scan, APGAR, pH, base excess, hours of physiotherapy and days of O2 therapy into account.
| PLIC left AD | p value | |
|---|---|---|
| Weight at birth | −0.662a | <0.0001 |
| Punctuate lesions in convectional MRI | −0.623a | 0.001 |
| Gestational age at birth | 0.682a | <0.0001 |
| APGAR | −0.580a | 0.002 |
| pH | −0.661a | <0.0001 |
| Base excess | −0.651a | <0.0001 |
| Physiotherapy | −0.624a | 0.001 |
| Age at scan | −0.682a | <0.0001 |
| O2 therapy | −0.587a | 0.002 |
Correlation is significant at the 0.01 level (2-tailed)
AD: axial diffusivity; APGAR: Appearance, Pulse, Grimace, Activity and Respiration; CST: cortico-spinal tract; DQA: Griffiths Locomotor Subscale; DTI: diffusion tensor imaging; FA: fractional anisotropy; FDR: False Discovery Rate; MD: mean diffusivity; MRI: magnetic resonance imaging; PLIC: posterior limb of the internal capsule; RD: radial diffusivity
Discussion
The present study evaluated the microstructural organization of the motor pathway in preterm neonates and investigated a potential relationship with the motor outcome, assessed longitudinally up to 15 months. Multiple measures of diffusivity (FA, AD, MD and RD) differed across two anatomical structures: Specifically, greater FA and smaller AD, MD and RD values were found in the PLIC, as compared to the CST. This result might reflect the regional reduction of diffusivity in the PLIC compared to the CST, due to the higher fiber packaging. Furthermore, a negative correlation between diffusivity and motor outcome was only observed for AD and values from the earliest neuromotor follow-up. This correlation was stronger and reached statistical significance in the left hemisphere. These results suggested that AD is a sensitive indicator of the structural maturation of the motor pathway in neonates, although it did not appear to be a valid indicator of later motor performance.
Differences between indices of diffusivity
Compared to FA, other DTI parameters, although recently used in neonatal studies,23,24 have not been commonly used to assess the status of white matter structures. During the early stages of development, the progressive formation of more complex white matter structures determines a reduction of water content19 associated with a global decrease in water diffusivity along all directions,31 but not necessarily with a simultaneous variation of FA.32 This may explain the absence in the present study of a correlation between measures of AD and FA, which is instead typically observed in adults. As a consequence, AD and MD may provide a measure of the initial maturation of white matter structures, since it is sensitive to the physiological decrease of water content and global diffusivity. The maturation of key structures of the motor pathway that are assessable with a neuropsychological evaluation should yield a negative correlation with AD and motor performance. AD reflects a more general index of fiber organization;33 while the latter might be considered a more sensitive indicator of white matter maturation, especially in a subclinical population.
Hemispherical and regional differences
Although there are indications that motor and language system specialization has an early onset,34 it is unknown whether lateralized systems are already established at birth, or instead continue to develop during postnatal life. The observed higher anisotropic organization (FA) in the motor pathway of the left hemisphere supports the former hypothesis and is consistent with previous evidence for the presence of a handedness preference in neonates.35 In addition, Gupta et al.36 report significantly higher FA in the right compared with left frontal cortex in the prenatal period, a relationship that subsequently reversed in the postnatal period, probably due to the faster development of the left hemisphere in later phases of gestation.37 A trend towards lateralization of handedness to the left hemisphere in neonates was also demonstrated in a fMRI study of cortical activation induced by passive extension and flexion of the hand.38 Notably, the hypothesis of a higher maturation of the left hemisphere is in accordance with the fact that statistically significant correlations between measure of diffusivity (AD) and motor behavior were observed only for structures of the left hemisphere.
The results of the partial correlation analysis suggested that the relationship with the PLIC was less dependent on the contribution of other clinical and anatomical variables. In general, we think that the present PLIC ROI may have been more sensitive and more specific for the motor pathway, because it was directly positioned on a definite anatomical structure, rather than derived from an existing atlas. Instead, the CST was defined by combining multiple Oishi parcels, a procedure that may have resulted in a greater spatial blurring and in the inclusion of unrelated voxels.39 Alternatively, the different indices of anisotropy that we found in the two structures may reflect different patterns of fiber packing and/or myelination.22 Our data do not permit the identification of which, if either, is the explanation.
Early versus late follow-up
Correlations between DTI indices and behavioral outcome were only observed with measures obtained at the first follow-up. A hypothesis for the lack of a correlation with later follow-up assessments is that a compensatory recovery of white matter structural integrity occurred in the intervening time interval. Notably, the analysis of the behavioral data showed that the distribution of behavioral values tended to narrow from the first to the last follow-up, which means that behavioral variability decreased progressively in time. While this could reflect a potential functional recovery due to later compensation, the larger variability at the earliest time point may more simply reflect lower reliability of the assessment near birth. Nonetheless, the strong correlation observed between AD and motor performance at 3 months, which survived correction for multiple comparisons and control analysis to estimate the contribution of potential confounding variables, indicated that this result was the result of pure chance. Therefore, we reasoned that the higher variability of the scores at 3 months does not simply reflect more noise (i.e. less reliability), but at least in part, true inter-subject differences; however, these results suggested that the study of the relationship between brain and behavioral development may benefit from a parallel DTI/neuromotor follow-up, which was not feasible in the present study, due to ethical considerations.
Study limitations
Prematurity is associated with a general alteration/reduction of the normal maturation of brain structures, inducing the presence of a high inter-subject variability. In the present study, we selected a conservative approach in order to minimize the contribution of uncontrolled variables on the correlation analysis. Therefore, a large number of subjects were excluded from the original sample according to clinical, technical and behavioral criteria; thus, we used non-parametric statistical tests. Whereas this choice limited statistical power, especially for the evaluation of covariate factors, it provided a more homogenous patient group. Furthermore, we were interested in a neonatal population for which the risk of adverse neurological outcome cannot be easily predicted on the basis of clinical data and conventional MRI sequences.
The study lacked clinical or MRI findings that would have permitted a more accurate identification of the neonate population with the highest risk of adverse neurological outcomes.
Another potential limitation of the current study concerns the focus on the motor system/performance, which prevents a generalization of our findings to other neuromotor domains; however, we reasoned that the motor domain is an optimal model to test the presence of a correlation between neurostructural and neuromotor data, for several reasons:
First, the motor pathway can be more easily identified and segmented in MRI images of preterm babies, compared to other systems or pathways40,41 that are less myelinated at birth.42
Second, the deficits of the motor domain are a frequent clinical consequence that is associated with preterm birth.43
Third, motor performance can be more easily assessed, even in a very early stage (i.e. 3 months) of neuromotor development, using standardized tests.
Fourth, the analysis method used in this study was geared towards an easier translation of results into clinical practice, rather than being based on group analysis, such as TBSS.44
Another limitation of this study was the relatively limited follow-up period (15 months). This was another reason this study was focused on the neuromotor functionality that is more easily explored in the first months of life.
A further issue of the present work concerns the parameters of the DTI acquisition. The voxel size, which is appropriate for the adult population, may be considered large for neonatal brain size, preventing the possibility to exclude partial volume effects; however, the choice of the voxel size, as the number of directions, was the result of a compromise between SNR and acquisition time, which was critical for our lightly sedated population.
A final limitation pertains to the use of the present DTI measures. Therefore, these results must be confirmed in a multicenter study using more directions.
Conclusions
The present study demonstrated the relationship between indices of neurostructural development and motor performance, suggesting that DTI, and especially AD, is a valid indicator of the maturation of motor pathway in preterm neonates, but not of later motor outcome. These results also highlighted the limits of using only microstructural data as a biomarker for predicting subsequent neuromotor development. The ability to predict long-term outcome will probably require the inclusion of other clinical and functional neuroimaging parameters, using a multivariate approach.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Authors' contribution
RN designed the research, drafted the manuscript, analyzed and interpreted the data, performed the statistical analysis, made the critical revision of the manuscript for important intellectual content and gave final approval. CS designed the research, analyzed and interpreted the data, made a critical revision of the manuscript for important intellectual content and gave final approval. EC acquired the data, analyzed and interpreted the data, made a critical revision of the manuscript for important intellectual content and gave final approval. RS acquired the data, analyzed and interpreted the data, made a critical revision of the manuscript for important intellectual content and gave final approval. PAM designed the research, analyzed and interpreted the data, performed the statistical analysis. GLR handled funding and supervision, made critical revision of the manuscript for important intellectual content and gave final approval. SD made critical revision of the manuscript for important intellectual content and gave final approval. MC designed the research, conceived the study, handled funding and supervision, made a critical revision of the manuscript for important intellectual content and gave final approval.
Ethical standards and patient consent
We declare that all human and animal studies were approved by the Ethics committee of ‘G d'Annunzio' University and ASL N.2 Lanciano-Vasto-Chieti, Italy; and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that all patients gave informed consent prior to inclusion in this study.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Supplemental material
The online supplemental materials are available at http://neu.sagepub.com/supplemental.
References
- 1.Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: A systematic analysis and implications. Lancet 2012; 379: 2162–2172. [DOI] [PubMed] [Google Scholar]
- 2.Marlow N, Wolke D, Bracewell MA, et al. Neurologic and developmental disability at six years of age after extremely preterm birth. New Engl J Med 2005; 352: 9–19. [DOI] [PubMed] [Google Scholar]
- 3.Doyle LW. Victorian Infant Collaborative Study G. Evaluation of neonatal intensive care for extremely low birth weight infants in Victoria over two decades: I. Effectiveness. Pediatrics 2004; 113: 505–509. [DOI] [PubMed] [Google Scholar]
- 4.Van Hus JW, Potharst ES, Jeukens-Visser M, et al. Motor impairment in very preterm-born children: Links with other developmental deficits at 5 years of age. Develop Med Child Neurol 2013; 56: 587–594. [DOI] [PubMed] [Google Scholar]
- 5.Spittle AJ, Orton J, Doyle LW, et al. Early developmental intervention programs post hospital discharge to prevent motor and cognitive impairments in preterm infants. Cochrane Database Syst. Rev 2007; 2. Article CD005495. [DOI] [PubMed]
- 6.Ment LR, Hirtz D, Hüppi PS. Imaging biomarkers of outcome in the developing preterm brain. Lancet Neurol 2009; 8: 1042–1055. [DOI] [PubMed] [Google Scholar]
- 7.Tortora D, Panara V, Mattei PA, et al. Comparing 3T T1-weighted sequences in identifying hyperintense punctate lesions in preterm neonates. Am J Neuroradiol 2014; 36: 581–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woolrich MW, Jbabdi S, Patenaude B, et al. Bayesian analysis of neuroimaging data in FSL. NeuroImage 2009; 45: S173–186. [DOI] [PubMed] [Google Scholar]
- 9.Weiss J, Takizawa B, McGee A, et al. Neonatal hypoxia suppresses oligodendrocyte Nogo-A and increases axonal sprouting in a rodent model for human prematurity. Exp Neurol 2004; 189: 141–149. [DOI] [PubMed] [Google Scholar]
- 10.Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magnet Reson 1994; 103: 247–254. [DOI] [PubMed] [Google Scholar]
- 11.Song S-K, Sun S-W, Ju W-K, et al. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. NeuroImage 2003; 20: 1714–1722. [DOI] [PubMed] [Google Scholar]
- 12.Neil JJ, Shiran SI, McKinstry RC, et al. Normal brain in human newborns: Apparent diffusion coefficient and diffusion anisotropy measured by using diffusion tensor MR imaging. Radiology 1998; 209: 57–66. [DOI] [PubMed] [Google Scholar]
- 13.Rose J, Vassar R, Cahill-Rowley K, et al. Neonatal physiological correlates of near-term brain development on MRI and DTI in very-low-birth-weight preterm infants. NeuroImage 2014; 5: 169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arzoumanian Y, Mirmiran M, Barnes PD, et al. Diffusion tensor brain imaging findings at term-equivalent age may predict neurologic abnormalities in low birth weight preterm infants. Am J Neuroradiol 2003; 24: 1646–1653. [PMC free article] [PubMed] [Google Scholar]
- 15.Rose J, Mirmiran M, Butler EE, et al. Neonatal microstructural development of the internal capsule on diffusion tensor imaging correlates with severity of gait and motor deficits. Developm Med Child Neurol 2007; 49: 745–750. [DOI] [PubMed] [Google Scholar]
- 42.De Bruine FT, Van Wezel-Meijler G, Leijser LM, et al. Tractography of white-matter tracts in very preterm infants: A 2-year follow-up study. Develop Med Child Neurol 2013; 55: 427–433. [DOI] [PubMed] [Google Scholar]
- 16.Krishnan ML, Dyet LE, Boardman JP, et al. Relationship between white matter apparent diffusion coefficients in preterm infants at term-equivalent age and developmental outcome at 2 years. Pediatrics 2007; 120: e604–609. [DOI] [PubMed] [Google Scholar]
- 17.Van Kooij BJM, Van Pul C, Benders MJNL, et al. Fiber tracking at term displays gender differences regarding cognitive and motor outcome at 2 years of age in preterm infants. Pediatr Res 2011; 70: 626–632. [DOI] [PubMed] [Google Scholar]
- 18.Pogribna U, Burson K, Lasky RE, et al. Role of diffusion tensor imaging as an independent predictor of cognitive and language development in extremely low-birth-weight infants. Am J Neuroradiol 2014; 35: 790–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKinstry RC, Mathur A, Miller JH, et al. Radial organization of developing preterm human cerebral cortex revealed by non-invasive water diffusion anisotropy MRI. Cerebral Cortex 2002; 12: 1237–1243. [DOI] [PubMed] [Google Scholar]
- 20.Burzynska AZ, Preuschhof C, Backman L, et al. Age-related differences in white matter microstructure: Region-specific patterns of diffusivity. Neuroimage 2010; 49: 2104–2112. [DOI] [PubMed] [Google Scholar]
- 21.Beaulieu C. The basis of anisotropic water diffusion in the nervous system: A technical review. NMR Biomed 2002; 15: 435–455. [DOI] [PubMed] [Google Scholar]
- 22.Ball G, Counsell SJ, Anjari M, et al. An optimised tract-based spatial statistics protocol for neonates: Applications to prematurity and chronic lung disease. NeuroImage 2010; 53: 94–102. [DOI] [PubMed] [Google Scholar]
- 23.Geng X, Gouttard S, Sharma A, et al. Quantitative tract-based white matter development from birth to age 2 years. NeuroImage 2012; 61: 542–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cornette LG, Tanner SF, Ramenghi LA, et al. Magnetic resonance imaging of the infant brain: Anatomical characteristics and clinical significance of punctate lesions. Arch Dis Child Fetal Neonatal Ed 2002; 86: F171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huntley M. The Griffiths Mental Developmental Scales Manual from birth to two years. Thames: Bucks, Association for Research in Infant and Child Development, The Test Agency, 1996, pp. 5–39.
- 25.Jenkinson M, Beckmann CF, Behrens TE, et al. FSL. NeuroImage 2012; 62: 782–790. [DOI] [PubMed] [Google Scholar]
- 26.Oishi K, Mori S, Donohue PK, et al. Multi-contrast human neonatal brain atlas: Application to normal neonate development analysis. NeuroImage 2011; 56: 8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith SM, et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. NeuroImage 2006; 31: 1487–1505. [DOI] [PubMed] [Google Scholar]
- 28.Bayley N. California infant scale of mental development, Berkeley: University of California Press, 1936. [Google Scholar]
- 29.Mellergard P, Bengtsson F, Smith ML, et al. Time course of early brain edema following reversible forebrain ischemia in rats. Stroke 1989; 20: 1565–1570. [DOI] [PubMed] [Google Scholar]
- 30.Barkovich AJ. Concepts of myelin and myelination in neuroradiology. Am J Neuroradiol 2000; 21: 1099–1109. [PMC free article] [PubMed] [Google Scholar]
- 31.Budde MD, Kim JH, Liang HF, et al. Toward accurate diagnosis of white matter pathology using diffusion tensor imaging. Magnet Reson Med 2007; 57: 688–695. [DOI] [PubMed] [Google Scholar]
- 32.Husson B, Hertz-Pannier L, Renaud C. Motor outcomes after neonatal arterial ischemic stroke related to early MRI data in a prospective study. Pediatrics 2010; 126: 912–918. [DOI] [PubMed] [Google Scholar]
- 33.Cioni G, Pellegrinetti G. Lateralization of sensory and motor functions in human neonates. Perceptual & Motor Skills 1982; 54: 1151–1158. [DOI] [PubMed] [Google Scholar]
- 34.Gupta RK, Hasan KM, Trivedi R, et al. Diffusion tensor imaging of the developing human cerebrum. J Neurosci Res 2005; 81: 172–178. [DOI] [PubMed] [Google Scholar]
- 35.Hellge J. Hemispheric Asymmetry: What's Right and What's Left, Harvard University Press, 1993.
- 36.Erberich SG, Panigrahy A, Friedlich P, et al. Somatosensory lateralization in the newborn brain. NeuroImage 2006; 29: 155–161. [DOI] [PubMed] [Google Scholar]
- 37.Vos SB, Jones DK, Viergever MA, et al. Partial volume effect as a hidden covariate in DTI analyses. NeuroImage 2011; 55: 1566–1576. [DOI] [PubMed] [Google Scholar]
- 38.Counsell SJ, Maalouf EF, Fletcher AM, et al. MR imaging assessment of myelination in the very preterm brain. Am J Neuroradiol 2002; 23: 872–881. [PMC free article] [PubMed] [Google Scholar]
- 39.Cowan FM, De Vries LS. The internal capsule in neonatal imaging. Semin Fetal Neonat Med 2005; 10: 461–474. [DOI] [PubMed] [Google Scholar]
- 40.Yakovlev P and Lecours A. The myelogenetic cycles of regional maturation of the brain in Minkowski, A: Regional Development of the brain in early life, Oxford, Blackwell 1967.
- 41.De Kieviet JF, Piek JP, Aarnoudse-Moens CS, et al. Motor development in very preterm and very low-birth-weight children from birth to adolescence: A meta-analysis. J Am Med Ass 2009; 302: 2235–2242. [DOI] [PubMed] [Google Scholar]
- 43.Duerden EG, Foong J, Chau V, et al. Tract-based spatial statistics in preterm-born neonates predicts cognitive and motor outcomes at 18 months. Am J Neurol Radiol Pediatr 2015; 36: 1565–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.