Abstract
Objective
Recent studies have shown the efficacy of mechanical thrombectomy in acute ischemic stroke. We sought to identify prognostic parameters for clinical and radiological outcome after mechanical thrombectomy.
Methods
In 34 patients (age 72 ± 13 years, 64.7% women) with acute occlusion of the distal ICA and/or M1 segment who were treated with mechanical thrombectomy, the Spearman correlation was performed to assess potential prognostic outcome parameters (age, NIHSS, ASPECT, thrombus length (TL), clot burden score (CBS), relative filling time delay (rFTD), time to recanalization (TTR) and TICI score). The modified Rankin scale (mRS) and the Alberta Stroke Program Early CT (ASPECT) score were used for clinical and radiological outcome, respectively. Receiver operating characteristic (ROC) analysis was performed to assess parameters predicting favorable clinical (ΔmRS ≤ 2) and radiological outcome (ΔASPECT ≤ 2).
Results
Variables associated with favorable clinical outcome included NIHSS, TL, TTR and TICI score (p ≤ 0.01) with NIHSS ≤ 15 (p = 0.001, area under the curve (AUC) 0.87), TL ≤ 2 cm (p = 0.017, AUC 0.75), TTR ≤ 231 min (p = 0.001 AUC 0.88) and TICI ≥ 2b (p = 0.050, AUC 0.70). Shorter TTR and higher TICI scores were associated with favorable radiological outcome (p < 0.001) with TTR ≤ 224 min (p = 0.023, AUC 0.77) and TICI ≥ 2b (p = 0.000, AUC 0.86).
Conclusion
Fast and complete recanalization is essential to achieve a favorable radiological and functional outcome after mechanical thrombectomy in acute ischemic stroke. Age, CBS and collateral supply play a subordinate role.
Keywords: Stroke, mechanical thrombectomy, outcome, 4D computed tomography, perfusion imaging, collateral circulation
Introduction
Many clinical and radiological variables affect the clinical course in acute ischemic stroke.1 But because of the narrow therapeutic window treatment decisions need to be made rapidly. Site and extent of occlusion give information on the probability of successful recanalization with intravenous thrombolysis.2–4 Proximal occlusions of the major cerebral arteries account for more than one-third of cases of acute anterior-circulation stroke.5 However, the efficacy of intravenous thrombolysis in these cases is limited.2 Recent trials have shown the significant benefit of endovascular treatment in patients with acute ischemic stroke due to proximal cerebral arterial occlusion in the anterior circulation when performed within six hours after stroke onset.6–9 The proportion of patients with independent functional outcome after three months is significantly higher compared to intravenous therapy alone. Many clinical and radiological variables have been analyzed to help predict favorable outcome after acute anterior circulation stroke. However, the heterogeneity in treatment has often been a limiting factor in the definition of relevant outcome predictors.4,10 The purpose of this study was to define relevant parameters for clinical and radiological outcome after acute anterior-circulation stroke on the basis of a homogenous study population, where endovascular mechanical thrombectomy was consistently performed with stent retriever devices.
Materials and methods
Study population
In 121 patients with acute thromboembolic large-vessel occlusion within the anterior circulation (internal carotid artery (ICA) and/or M1 segment) presenting to our institution between September 2011 and November 2014, mechanical thrombectomy was performed. Thirty-four of these patients, who underwent pre-interventional multimodal computed tomography (MMCT), including non-enhanced CT (NECT), volume perfusion CT (VPCT) and bolus-tracked CT angiography (CTA), were enrolled in this retrospective study. National Institutes of Health Stroke Scale (NIHSS) score on admission as well as modified Rankin scale (mRS) score before symptom onset (pre-mRS) and after 90 days (three-month mRS) were obtained from the database. Aside from three patients with unclear onset, duration of symptoms on admission was <4.5 hours. In case of unclear onset, the “last seen normal” time point was considered as onset. All patients were treated with endovascular mechanical thrombectomy; 76.5% of patients received a bridging with intravenous thrombolysis with recombinant tissue plasminogen activator (rt-PA), 14.7% additional intra-arterial thrombolysis with rt-PA. Alberta Stroke Program Early CT (ASPECT) score was assessed on NECT on admission and after 24 hours. Patients with signs of hemorrhage on NECT, already demarcated infarction or proximal occlusion of the ICA were excluded from the study.
The study was performed in compliance with the local ethics committee. All participants or next of kin gave informed consent according to legal requirements.
Imaging and image reconstruction
In all patients thin-section helical NECT (reconstructed section thickness 0.6 mm, increment 0.4 mm, reconstruction kernel H31s), VPCT (reconstructed section thickness 0.6 mm, increment 0.4 mm, reconstruction kernel H20f) and arterial helical CTA (section thickness 0.6 mm, increment 0.4 mm, reconstruction kernel B20f) were performed on a 128-section CT scanner (Somatom Definition AS+, Siemens, Forchheim, Germany) on admission. Follow-up NECT was performed 24 hours after admission.
Dynamic four-dimensional (4D)-CTA was reconstructed from the VPCT data set (80 kV, 180 mAs, collimation 128 × 0.6 mm, rotation time 0.3 s) acquired with 9.6 cm coverage in the z-axis.11 A total of 30 ml of iodinated contrast agent (Imeron 400; Bracco Imaging, Konstanz, Germany) followed by 50 ml of saline flush were administrated intravenously at a rate of 5 ml/s using a double-piston power injector (Medtron, Saarbruecken, Germany). A pulsed full-rotation scan beginning 4 s after contrast injection with 35 scans over 68 s was used. Temporal maximum intensity projections (tMIP; slice thickness 25 mm) were calculated combining all 35 spiral scans of the 4D-CTA examination through temporal fusion and displaying maximal enhancement over the 68-second scan time for each voxel.
Arterial head and neck CTA was performed from the aortic arch to the cranial vertex (120 kV, 160 mAs, collimation 128 × 0.6 mm, rotation time 0.3 s). 60 ml of iodinated contrast agent (Imeron 400; Bracco Imaging, Konstanz, Germany) followed by 50 ml of saline flush were injected intravenously at a rate of 5 ml/s. Monitoring started with a delay of 5 s. Bolus tracking was performed in the ascending thoracic aorta with a fixed start delay of 4 s after exceeding 100 HU.
Imaging analysis
Baseline thin-slice NECT was assessed for the presence of hyperdense middle cerebral artery sign (HMCAS). Early ischemic changes were defined on axial reconstructions (slice thickness 4.8 mm) with the ASPECT score (range 10–0).
On CTA the site of occlusion was identified.
Relative filling time delay (rFTD) was defined on the unprocessed 4D-CTA images as the time difference between the first contrast opacification in the middle cerebral artery (MCA) branches in the sylvian fissure of the affected hemisphere and in the corresponding contralateral non-affected MCA branches.
The 4D-CTA analysis was performed with commercial software (syngo.via CT Dynamic Angio, Siemens, Forchheim, Germany). The thrombus length (TL) was measured on 4D-CTA tMIP images by connecting straight lines between the proximal and distal clot end in axial or coronal planes. In five cases in which the distal clot end could not be defined, the longest measured clot extent (3 cm) in the study population was set as arbitrary TL.
Semiquantitative extent of thromboembolic vessel occlusion was assessed with the Clot Burden Score (CBS).
Follow-up CT scans after 24 hours were assessed for the extent and location of infarction using the ASPECT score.
Mechanical thrombectomy
In all patients mechanical thrombectomy was performed by using stent retriever devices (Solitaire FR (Covidien), Revive SE (Codman) or pREset (Phenox)). The model used or a combination was at the discretion of the performing neurointerventionalist. The thrombus was passed with a microcatheter and the distal stent retriever end was deployed distally to the cerebral artery thrombus. After a minimum delay of 5 min the stent retriever was removed under negative-pressure aspiration at the guiding catheter in the ICA. Thrombectomy was repeated in case of persistent occlusion or incomplete vessel recanalization with the option to switch to another thrombectomy device. The thrombolysis in cerebral infarction (TICI) score (0–3) was used to define the grade of reperfusion in postinterventional angiography series after completion of the procedure. Successful vessel recanalization was defined by a grade of reperfusion of 2b or 3 and was achieved in 27 patients (79.4%). The mean time from symptom onset to recanalization was 312 min (260–368). All interventions were performed by experienced interventional neuroradiologists with 10–15 years of operating experience in neurointerventional treatment of stroke. Procedure-related complications included embolization into a new territory (anterior cerebral artery) during successful recanalization of an M1 occlusion in one patient (2.9%) and periprocedural dissection of the ICA in two patients (5.9%).
Outcome analysis
Outcome analysis was subdivided into clinical and radiological outcomes.
Clinical outcome was determined by the difference between the three-month mRS and pre-mRS (ΔmRS), radiological outcome by the difference between the ASPECT score on baseline CT and at 24 hours (ΔASPECT). Favorable clinical outcome was defined as a degradation of the mRS score not exceeding two points; favorable radiological outcome was defined according to the calculated threshold in the ROC analysis for favorable clinical outcome as a change in the ASPECT score not exceeding two points.
Statistical analysis
For statistical data analysis, commercial software (SPSS 20, IBM, Chicago, IL, USA) was used. The data evaluation was performed by two raters in consensus, one with 15 and one with three years of experience in stroke imaging. Results are described as mean ± standard deviation or as median with interquartile range. A p value of <0.05 was considered statistically significant. Clinical and radiological outcome was dichotomized into favorable (ΔmRS ≤ 2, ΔASPECT ≤ 2) and poor (ΔmRS > 2, ΔASPECT > 2) outcome. The Spearman nonparametric rank correlation was used to examine the relationship between clinical outcome (ΔmRS) at three months and the following parameters: 1) NIHSS score; 2) ASPECT score; 3) TL; 4) CBS; 5) Age; 6) rFTD; 7) TICI score; and 8) TTR. In an analogous manner the variables were assessed for correlation with radiological outcome (ΔASPECT). Variables with p values < 0.05 were included in a receiver-operating characteristics (ROC) analysis to assess parameters predicting favorable clinical and radiological outcome. For variables with p values < 0.05, the optimal threshold was determined by the Youden index. Sensitivity and specificity at the optimal threshold and the area under the curve (AUC) were calculated. The confidence interval (CI) was set at 95%.
Results
Baseline data
Demographic and clinical baseline data are summarized in Table 1.
Table 1.
Patient characteristics at baseline.
| Baseline characteristics | |
|---|---|
| Patient demographics | |
| Number of patients, n | 34 |
| Age, mean (SD), (years) | 72 (±13) |
| Female, n (%) | 22 (64.7) |
| Clinical characteristics | |
| Admission NIHSS score, median (interquartile range) | 15 (13–18) |
| Onset-to-imaging time, median (interquartile range), (minutes) | 161 (118–206) |
| Onset-to-recanalization time, median (interquartile range), (minutes) | 312 (260–368) |
| Pre-stroke mRS, median (interquartile range) | 0 (0–2) |
| Pre-stroke mRS, n (%) | |
| 0 | 20 (58.8) |
| 1 | 6 (17.6) |
| 2 | 3 (8.8) |
| >2 (3 or 4) | 5 (14.7) |
| Imaging characteristics | |
| Location of occlusion in left hemisphere, n (%) | 15 (44.1) |
| Site of occlusion, n (%) | |
| ICA with involvement of the M1 segment | 4 (11.8) |
| Carotis-T | 5 (14.7) |
| M1 segment | 25 (73.5) |
| TL, mean (SD), (cm) | 1.4 (±0.6) |
| CBS, median (interquartile range) | 6 (4–7) |
| HMCAS, n (%) | 23 (67.6) |
| Admission ASPECT, median (interquartile range) | 10 (9–10) |
| Relative filling time delay, mean (SD), (seconds) | 9.69 (± 5.47) |
| Treatment characteristics | |
| Mechanical thrombectomy, n (%) | 34 (100%) |
| Treatment with i.v. rt-PA, n (%) | 26 (76.5) |
| Treatment with i.a. thrombolysis, n (%) | 5 (14.7) |
NIHSS: National Institutes of Health Stroke Scale; mRS: modified Rankin scale; ICA: internal carotid artery; TL: thrombus length; CBS: clot burden score; HMCAS: hyperdense middle cerebral artery sign; ASPECT: Alberta Stroke Program Early Computed Tomography score; i.v.: intravenous; rt-PA: recombinant tissue plasminogen activator; i.a.: intra-arterial.
Median NIHSS at presentation was 15 (interquartile range: 13–18). Mean duration of symptoms at time of initial CT was 161 (118–206) min. Mean time from symptom onset to recanalization was 312 (260–368) min. HMCAS was present in 23 patients (67.6%). Mean TL was 1.4 (±0.6) cm. Median CBS was 6 (interquartile range, 4–7). Mean rFTD was 9.69 (±5.47) seconds.
Outcome analysis
General outcome results are summarized in Table 2.
Table 2.
Clinical, radiological and technical outcome data.
| Outcome characteristics | |
|---|---|
| Clinical outcome | |
| Three-month mRS, median (interquartile range) | 3 (2–4) |
| Three-month mRS, n (%) | |
| 0–2 | 12 (35.3) |
| 3–5 | 15 (44.1) |
| 6 | 6 (17.6) |
| ΔmRS (three-month mRS–pre-mRS), median (interquartile range) | 2 (1–3) |
| Favorable clinical outcome (ΔmRS ≤ 2), n (%) | 20 (58.8) |
| Radiological outcome | |
| ASPECT at 24 hours, median (interquartile range) | 6.5 (4.75–10) |
| ΔASPECT (Admission ASPECT–ASPECT at 24 hours), median (interquartile range) | 2 (0–4.25) |
| Favorable radiological outcome (ΔASPECT ≤ 2), n (%) | 21 (61.8) |
| Technical outcome | |
| TICI score, n (%) | |
| 0 | 1 (2.9) |
| 1 | 3 (8.8) |
| 2a | 3 (8.8) |
| 2b | 14 (41.2) |
| 3 | 13 (38.2) |
| Favorable technical outcome (TICI score ≥ 2b), n (%) | 27 (79.4) |
mRS: modified Rankin scale; ASPECT: Alberta Stroke Program Early Computed Tomography score; TICI: thrombolysis in cerebral infarction.
Clinical outcome
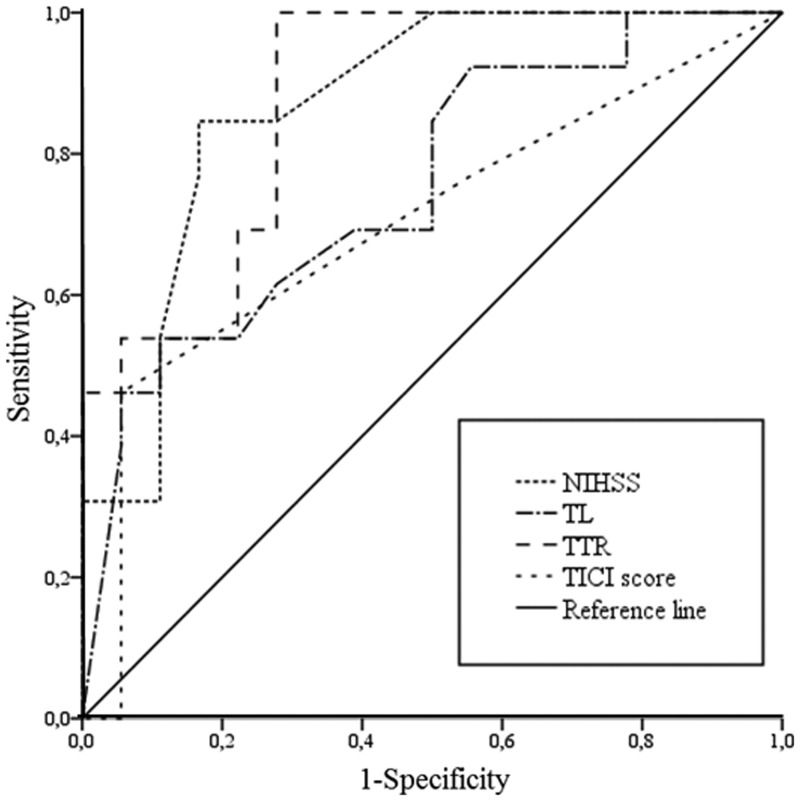
Clinical outcome was measured on the basis of the comparison of mRS before admission and after 90 days. Median pre-mRS was 0 (interquartile range: 0–2), median three-month mRS was 3 (interquartile range: 2–4). Median ΔmRS was 2 (range 0–6, interquartile range 1–3). In one patient the three-month mRS was not available because of loss to follow-up. Favorable clinical outcome (ΔmRS ≤ 2) was found in 20 patients (58.8%). There was no change in mRS from admission to three-month follow-up in five patients (14.7%). Variables associated with clinical outcome included 1) NIHSS (R = 0.41; p = 0.011); 2) TL (R = 0.44; p = 0.014); 3) TTR (R = 0.46; p = 0.004); and 4) TICI score (R = –0.42; p = 0.008). In ROC analysis, favorable clinical outcome was correlated with NIHSS ≤ 15 (AUC 0.87, p = 0.001, sensitivity 85%, specificity 83%, Youden index 0.68), TL ≤ 2 cm (AUC 0.75, p = 0.017, sensitivity 54%, specificity 99%, Youden-index 0.53), TTR ≤ 231 min (AUC 0.88, p = 0.000, sensitivity 100%, specificity 65%, Youden index 0.65) and TICI score ≥ 2b (AUC 0.70, p = 0.05, sensitivity 94%, specificity 46%, Youden-index 0.40). Results are summarized in Table 3 and shown in Figure 1.
Table 3.
Data from the ROC analysis respective favorable clinical outcome.
| Clinical outcome |
Youden- index | |||
|---|---|---|---|---|
| Predictor | AUC | Threshold | p value | |
| NIHSS | 0.87 | 15.5 | 0.001 | 0.68 |
| TL | 0.75 | 2.1 cm | 0.017 | 0.53 |
| TTR | 0.88 | 231 min | 0.000 | 0.65 |
| TICI score | 0.70 | 2b | 0.050 | 0.40 |
ROC: receiver operating characteristic; NIHSS: National Institutes of Health Stroke Scale; TL: thrombus length; TTR: time to recanalization; TICI: thrombolysis in cerebral infarction; AUC: area under the curve.
Figure 1.
Variables associated with clinical outcome.
NIHSS: National Institutes of Health Stroke Scale; TL: thrombus length; TTR: time to recanalization; TICI: thrombolysis in cerebral infarction.
Radiological outcome
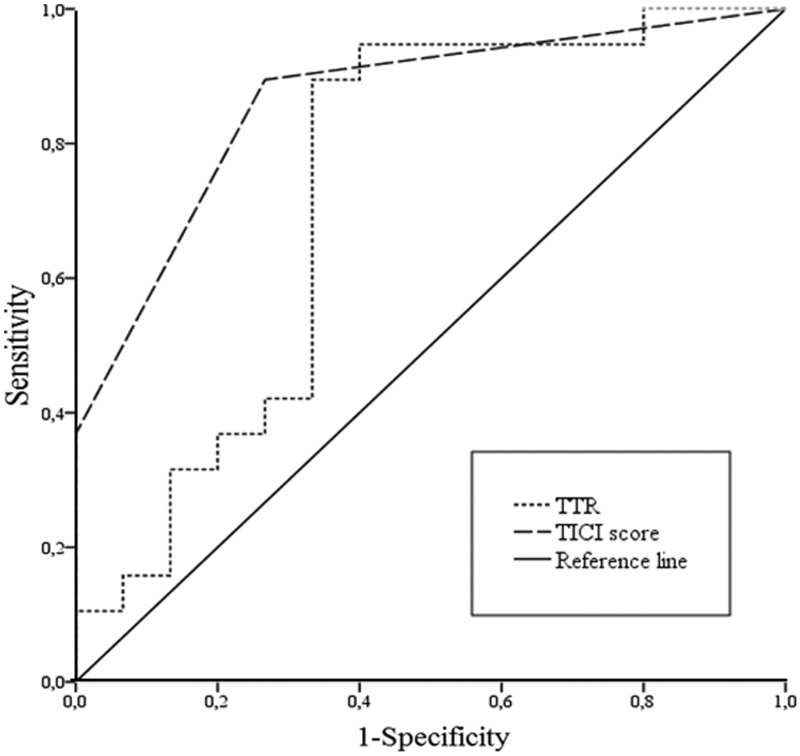
Radiological outcome was measured on the basis of the ASPECT score. Median ΔASPECT was 2 (range 0–8, interquartile range 0–4.25). Favorable radiological outcome was seen in 21 patients (61.8%). In 10 patients (29.4%) ASPECT score remained the same at the 24-hour follow-up examination. One symptomatic intracerebral hemorrhage was observed. Variables associated with radiological outcome included 1) TTR (R = 0.43; p = 0.006); and 2) TICI score (R = −0.76; p = 0.000). In ROC analysis, favorable radiological outcome was correlated with TTR ≤ 224 min (AUC 0.77, p = 0.023, sensitivity 90%, specificity 67%, Youden index 0.66) and TICI score ≥ 2b (AUC 0.86, p = 0.000, sensitivity 73%, specificity 89%, Youden index 0.63). Results are summarized in Table 4 and shown in Figure 2.
Table 4.
Data from the ROC analysis respective favorable radiological outcome.
| Radiological outcome |
Youden- index | |||
|---|---|---|---|---|
| Predictor | AUC | Threshold | p value | |
| TTR | 0.77 | 224 minutes | 0.023 | 0.66 |
| TICI score | 0.86 | 2b | 0.000 | 0.63 |
ROC: receiver operating characteristic; TTR: time to recanalization; TICI: thrombolysis in cerebral infarction; AUC: area under the curve.
Figure 2.
Variables associated with radiological outcome.
TTR: time to recanalization; TICI: thrombolysis in cerebral infarction.
rFTD was marginally insignificant both for clinical (p = 0.06) and radiological outcome (p = 0.08).
Discussion
The beneficial effect of intra-arterial treatment on functional outcome compared to intravenous therapy in patients with acute anterior circulation large-vessel occlusion has recently been proven in several trials.6–9 So the question is no longer whether endovascular treatment should be performed in these patients but how we can further improve patients’ outcome. This is why we sought to evaluate relevant parameters for clinical and radiological outcomes in an exclusive patient cohort. This cohort invariably underwent multimodal CT including CTA and whole-brain perfusion imaging before mechanical thrombectomy was performed. As an exact measurement of TL and precise information on clot burden require a time-resolved imaging analysis, 4D-CTA was reconstructed from time-resolved CTA data.11–13 Collateral status was assessed on 4D-CTA source images with a recently introduced method by measuring the rFTD.10
Outcome analysis
In our study clinical outcome was defined as the difference between mRS before admission and after three months as we considered a preexisting limitation in the patients’ functional status as an important factor for the determination of favorable outcome. Favorable clinical outcome was imputed to 20 patients (58.8%). Aside from NIHSS and TL as external factors beyond medical control, TTR and the grade of reperfusion remained the only influencing variables with effect on clinical outcome and the only variables that significantly correlated with radiological outcome at all. Revascularization with subsequent complete reperfusion (TICI 2b or 3) was an independent predictor of favorable outcome and was achieved in 79.4% of cases in our study group. In 23.5% there was a mismatch between complete revascularization and favorable clinical outcome. Analyzing this “mismatch” group, we found that the duration from symptom onset until complete recanalization was in mean much longer in this subgroup compared with the whole study population (405 (273–451) min versus 312 (260–368) min), indicating that favorable outcome after technically successful mechanical thrombectomy is time dependent.14–16 We found the duration from symptom onset until recanalization to be the strongest independent predictor of good clinical outcome with a threshold of four hours.1 In accordance with previous studies, NIHSS strongly correlated with clinical outcome with a score of more than 15 indicating unfavorable outcome.14 A TL of less than 2 cm was associated with favorable clinical outcome. In previous studies TL has been detected to be a main factor for recanalization after intravenous thrombolysis, with a TL exceeding 8 mm minimizing the chance of successful recanalization.2 This proposed threshold for successful recanalization is, however, considerably lower than the calculated threshold in our patient cohort, which might indicate that only very large thrombi exceeding 2 cm limit the efficacy of mechanical thrombectomy. The integration of the site of occlusion in the analysis of occlusion extent through the CBS was only of minor relevance for the functional and radiological outcome in our study, while it was found to be of major relevance for the efficacy of recanalization and for clinical outcome after intravenous therapy.1,3,4 Analysis of the collateral blood flow through calculation of the rFTD marginally didn’t show a significant correlation with clinical and radiological outcomes, while it was found to be an independent predictor of clinical outcome in previous studies.10,17 However, in these studies the inhomogeneity of the study population respective to intra-arterial or intravenous treatment or the effect of recanalization might have influenced the outcome. According to our results it seems likely that CBS and collateral status might be of major relevance for outcome estimations after intravenous thrombolysis, but their significance after endovascular treatment is restricted. The gist of our study is clear: To maintain the functional independence of a patient after acute stroke due to proximal cerebral artery occlusion within the anterior circulation, recanalization has to be fast and complete.
Outlook
We see a chance in improving the outcome after thrombectomy in two points: First, the amelioration of patient management from the point of admission until intervention to speed up the time interval until recanalization.18,19 A future option could be referring the patient directly to the angiographic room to perform an intravenous DynaCT to confirm a proximal large-vessel occlusion, locate the site of occlusion and to exclude intracerebral hemorrhage.20 The intervention could then be started immediately. Second, the further development of the thrombectomy devices should be directed to achieve even higher rates of complete recanalization and effective reperfusion and to reduce complication rates.21–25
Conclusion
Our study emphasizes the role of fast and complete revascularization in acute ischemic stroke within the anterior circulation to achieve a favorable functional outcome after mechanical thrombectomy. Age, CBS and collateral supply play a subordinate role. It is time to recall the basics – “Time is brain.”
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Zhu G, Michel P, Jovin T, et al. Prediction of recanalization in acute stroke patients receiving intravenous and endovascular revascularization therapy. Int J Stroke 2015; 10: 28–36. [DOI] [PubMed] [Google Scholar]
- 2.Riedel CH, Zimmermann P, Jensen-Kondering U, et al. The importance of size: Successful recanalization by intravenous thrombolysis in acute anterior stroke depends on thrombus length. Stroke 2011; 42: 1775–1777. [DOI] [PubMed] [Google Scholar]
- 3.Puetz V, Dzialowski I, Hill MD, et al. Intracranial thrombus extent predicts clinical outcome, final infarct size and hemorrhagic transformation in ischemic stroke: The clot burden score. Int J Stroke 2008; 3: 230–236. [DOI] [PubMed] [Google Scholar]
- 4.Tan IYL, Demchuk AM, Hopyan J, et al. CT angiography clot burden score and collateral score: Correlation with clinical and radiologic outcomes in acute middle cerebral artery infarct. AJNR Am J Neuroradiol 2009; 30: 525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heldner MR, Zubler C, Mattle HP, et al. National Institutes of Health Stroke Scale score and vessel occlusion in 2152 patients with acute ischemic stroke. Stroke 2013; 44: 1153–1157. [DOI] [PubMed] [Google Scholar]
- 6.Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015; 372: 11–20. [DOI] [PubMed] [Google Scholar]
- 7.Cohen JE, Rabinstein AA, Ramirez-de-Noriega F, et al. Excellent rates of recanalization and good functional outcome after stent-based thrombectomy for acute middle cerebral artery occlusion. Is it time for a paradigm shift? J Clin Neurosci 2013; 20: 1219–1223. [DOI] [PubMed] [Google Scholar]
- 8.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015; 372: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 9.Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015; 372: 1009–1018. [DOI] [PubMed] [Google Scholar]
- 10.Cao W, Campbell BC, Dong Q, et al. Relative filling time delay based on CT perfusion source imaging: A simple method to predict outcome in acute ischemic stroke. AJNR AM J Neuroradiol 2014; 351: 1683–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaschka IN, Kloska SP, Struffert T, et al. Clot burden and collaterals in anterior circulation stroke: Differences between single-phase CTA and multi-phase 4D-CTA. Clin Neuroradiol. Epub ahead of print 20 November 2014. DOI: 10.1007/s00062-014-0359-6. [DOI] [PubMed] [Google Scholar]
- 12.Frölich AM, Schrader D, Klotz E, et al. 4D CT angiography more closely defines intracranial thrombus burden than single-phase CT angiography. AJNR Am J Neuroradiol 2013; 34: 1908–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baxa J, Rohan V, Tupy R, et al. Determination of the middle cerebral artery occlusion length in acute stroke: Contribution of 4D CT angiography and importance for thrombolytic efficacy prediction. Clin Neuroradiol 2015; 25: 257–265. [DOI] [PubMed] [Google Scholar]
- 14.Abilleira S, Cardona P, Ribó M, et al. Outcomes of a contemporary cohort of 536 consecutive patients with acute ischemic stroke treated with endovascular therapy. Stroke 2014; 45: 1046–1052. [DOI] [PubMed] [Google Scholar]
- 15.Khatri P, Abruzzo T, Yeatts SD, et al. Good clinical outcome after ischemic stroke with successful revascularization is time-dependent. Neurology 2009; 73: 1066–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenthal ES, Schwamm LH, Roccatagliata L, et al. Role of recanalization in acute stroke outcome: Rationale for CT angiogram-based “benefit of recanalization” model. AJNR AM J Neuroradiol 2008; 29: 1471–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beyer SE, von Baumgarten L, Thierfelder KM, et al. Predictive value of the velocity of collateral filling in patients with acute ischemic stroke. J Cereb Blood Flow Metab 2015; 35: 206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun CH, Nogueira RG, Glenn BA, et al. “Picture to puncture”: A novel time metric to enhance outcomes in patients transferred for endovascular reperfusion in acute ischemic stroke. Circulation 2013; 127: 1139–1148. [DOI] [PubMed] [Google Scholar]
- 19.Mazighi M, Chaudhry SA, Ribo M, et al. Impact of onset-to-reperfusion time on stroke mortality: A collaborative pooled analysis. Circulation 2013; 127: 1980–1985. [DOI] [PubMed] [Google Scholar]
- 20.Struffert T, Deuerling-Zheng Y, Kloska S, et al. Flat detector CT in the evaluation of brain parenchyma, intracranial vasculature, and cerebral blood volume: A pilot study in patients with acute symptoms of cerebral ischemia. AJNR Am J Neuroradiol 2010; 31: 1462–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bae GS, Kwon HJ, Kang CW, et al. Mechanical thrombectomy using a solitaire stent in acute ischemic stroke; initial experience in 40 patients. J Cerebrovasc Endovasc Neurosurg 2012; 14: 164–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen JE, Gomori JM, Leker RR, et al. Recanalization with stent-based mechanical thrombectomy in anterior circulation major ischemic stroke. J Clin Neurosci 2012; 19: 39–43. [DOI] [PubMed] [Google Scholar]
- 23.Saver JL, Jahan R, Levy EI, et al. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): A randomised, parallel-group, non-inferiority trial. Lancet 2012; 380: 1241–1249. [DOI] [PubMed] [Google Scholar]
- 24.Stampfl S, Hartmann M, Ringleb PA, et al. Stent placement for flow restoration in acute ischemic stroke: A single-center experience with the Solitaire stent system. AJNR AM J Neuroradiol 2011; 32: 1245–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dorn F, Stehle S, Lockau H, et al. Endovascular treatment of acute intracerebral artery occlusions with the Solitaire Stent: Single-centre experience with 108 recanalization procedures. Cerebrovasc Dis 2012; 34: 70–77. [DOI] [PubMed] [Google Scholar]