Abstract
The effect of respiratory gating on the major diffusion-imaging metrics and that of cardiac gating on mean kurtosis (MK) are not known. For evaluation of whether the major diffusion-imaging metrics—MK, fractional anisotropy (FA), and mean diffusivity (MD) of the brain—varied between gated and non-gated acquisitions, respiratory-gated, cardiac-gated, and non-gated diffusion-imaging of the brain were performed in 10 healthy volunteers. MK, FA, and MD maps were constructed for all acquisitions, and the histograms were constructed. The normalized peak height and location of the histograms were compared among the acquisitions by use of Friedman and post hoc Wilcoxon tests. The effect of the repetition time (TR) on the diffusion-imaging metrics was also tested, and we corrected for its variation among acquisitions, if necessary. The results showed a shift in the peak location of the MK and MD histograms to the right with an increase in TR (p ≤ 0.01). The corrected peak location of the MK histograms, the normalized peak height of the FA histograms, the normalized peak height and the corrected peak location of the MD histograms varied significantly between the gated and non-gated acquisitions (p < 0.05). These results imply an influence of respiration and cardiac pulsation on the major diffusion-imaging metrics. The gating conditions must be kept identical if reproducible results are to be achieved.
Keywords: Mean kurtosis, fractional anisotropy, mean diffusivity, respiratory gating, cardiac gating, diffusion-imaging
Introduction
Diffusion-imaging sequences are commonly applied in neuroimaging, both for research and diagnostic purposes.1–4 These sequences provide information about the microstructural architecture of the tissues through their high sensitivity to the motion of water molecules within or around the cells. Several metrics have been derived for quantifying the information provided by the diffusion-imaging sequences. Of these, fractional anisotropy (FA), mean diffusivity (MD), and mean kurtosis (MK) are the three most commonly used metrics.3,4 FA and MD are the metrics derived from diffusion tensor imaging (DTI); the former represents the degree of directional coherence and the latter the magnitude of diffusion.5 MK is the metric derived from diffusion kurtosis imaging (DKI), and it quantifies the degree of deviation from a Gaussian distribution of water diffusion.6,7
Precision and accuracy of diffusion measurements are important to allow reproducibility and correct interpretation of a tissue’s microstructural integrity. For achieving these, the imaging parameters that can influence the diffusion characteristics must be identified a priori and kept identical throughout the image acquisitions. There have been reports about the effect of cardiac pulsation and the benefit of cardiac gating on the quantification of FA and MD.8–11 However, the findings and conclusions drawn are inconsistent among the reports. Whereas some studies report a significant difference in uncertainty about the FA and MD values between gated and non-gated acquisitions, others suggest that the effect is minimal and negligible for group studies. The effect of cardiac pulsation and gating on MK—a recently introduced diffusion-imaging metric, is still not known. In addition, the effect of respiration (i.e. another major physiologic motion) and the benefit of respiratory gating on the major diffusion-imaging metrics have not been reported.
To understand the effect of respiration and cardiac pulsation on the major diffusion-imaging metrics and the benefit of gating, in this study we evaluated whether the MK, FA, and MD of the brain varied between respiratory or cardiac gated and non-gated acquisitions.
Materials and methods
Participants
This prospective study was approved by the local institutional review board. Written informed consent was obtained from all participants.
Eleven healthy volunteers (10 men and one woman; mean age ± standard deviation = 28.50 ± 5.97 years; age range = 22–41 years)—one for test of repeatability and 10 for tests of the effect of gating, were included in the study. No participants had any history of cardiac or respiratory diseases. Gross abnormalities of the brain were ruled out by T2-weighted images of the brain.
Imaging
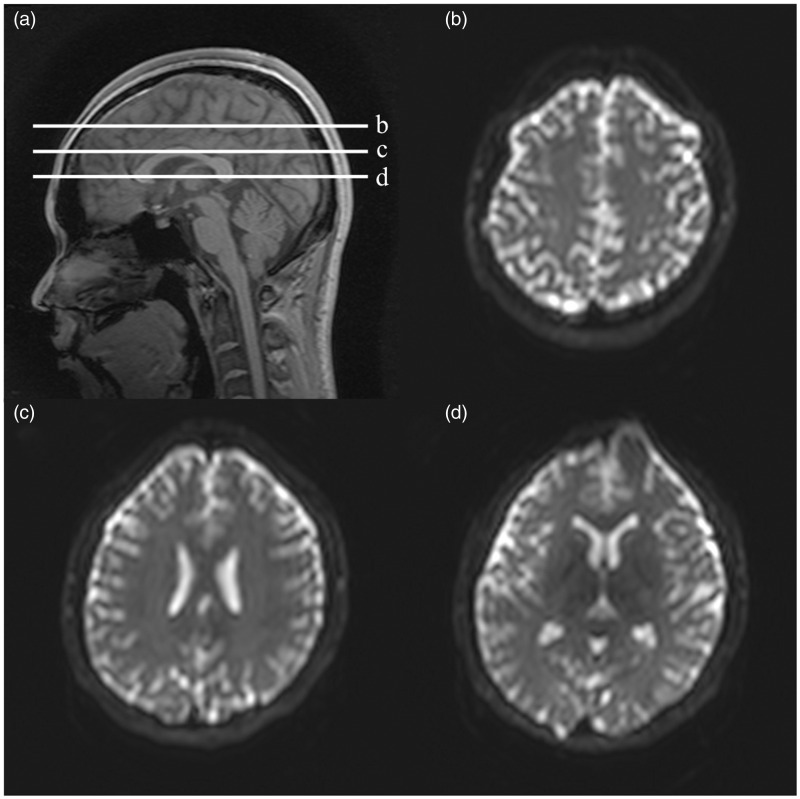
All magnetic resonance imaging (MRI) acquisitions were performed on a 3.0T scanner (Achieva TX, Philips Medical Systems, Best, The Netherlands) and a 32-channel head coil. Padding and a fixation device were used that minimized head motion during acquisitions and variations in head position among acquisitions, and the participants were asked to lie still and not to move during acquisitions. A fast spin-echo echo-planar imaging sequence was used for acquisition of diffusion-imaging. The major imaging parameters were as follows: repetition time (TR)/ echo time (TE) = 2000 ms (non-gated and cardiac-gated acquisitions) or 3000 ms (respiratory-gated acquisition)/ 35 ms, b-value (b) = 0, 1000, 2000 s mm–2, the number of diffusion gradient directions = 32, the number of excitation (NEX) = 1, field of view (FOV) = 224 × 224 mm, matrix size = 76 × 72. Gated and non-gated acquisitions were performed in 10 healthy volunteers (all men; mean age ± standard deviation = 27.30 ± 4.55 years; age range = 22–35 years). For gated acquisitions, the acquisitions were set to be performed at diastole and end expiration, so as to minimize pulsation and gross motion artifacts. Three 3-mm thick axial sections were obtained at the level of the centrum semiovale, the body of the bilateral lateral ventricles, and the foramina of Monro (Figure 1). The acquisition time for each condition ranged from 4.5 to 8 minutes. The gated and non-gated acquisitions were performed consecutively.
Figure 1.
A scout sagittal image showing the slice positions (a), and examples of the axial sections for diffusion imaging (b)–(d); (b) centrum semiovale level, (c) level of the body of bilateral lateral ventricles, (d) foramina of Monro level). For the axial sections, the echo-planar images with no diffusion weighting (b = 0 s mm–2) are given.
To assess the repeatability of diffusion measurements, we performed a total of 10 non-gated acquisitions in a volunteer (a 41-year-old woman) on different occasions. A total of 43 axial sections were obtained that covered the whole brain.
As the TR varied among the acquisitions, the effect of varying the TR on the major diffusion-imaging metrics was tested. For this purpose, non-gated diffusion-imaging with TRs ranging from 1000 to ∼5000 ms (with an interval of 500 ms) was performed in a volunteer (a 32-year-old man). All other imaging parameters were kept the same as those for comparison of gated and non-gated acquisitions.
Image processing and evaluation
The image sections were checked for consistency of the slice position among acquisitions to ensure that there was no head motion during acquisitions and to rule out gross artifacts. Correction for eddy current-related distortions was performed at the operator console. The MK, FA, and MD maps were then constructed from the diffusion images by use of Diffusion Kurtosis Estimator software version 2.5.1. Co-registration of the maps between the gated and non-gated acquisitions and among the repeated acquisitions was performed as correction for any minimal motion among acquisitions, with use of default parameters of Statistical Parametric Mapping (SPM8). A visual review of co-registered images was performed as a check for image registration. To avoid inclusion of the cerebrospinal fluid (CSF) from the evaluation, we created an exclusion mask of the CSF. We achieved this by setting up of a signal intensity threshold (i.e. the signal intensity of the CSF) to the echo-planar images with no diffusion weighting (b = 0 s mm–2). The mask was subsequently applied on the corresponding diffusion-imaging metric maps.
We employed histogram analysis to compare the major diffusion-imaging metrics. Histograms of brain parenchyma were constructed for each acquisition and metric with the use of ImageJ software.12 Two major measures of the histograms—the normalized peak height and the peak location—which are reported to be valuable determinants of histogram, were extracted.3,13 Repeatability of the diffusion measurements was determined by the intraclass correlation coefficient (ICC) values. An ICC >0.80 was considered to indicate repeatability. Variation in the major diffusion-imaging metrics between the gated and non-gated acquisitions was determined by use of Friedman and post hoc Wilcoxon tests. Corrected p < 0.05 was considered to indicate significance.
The effect of varying the TR on the major diffusion-imaging metrics was tested by use of Pearson’s product-moment correlation analysis. A p < 0.05 was considered to indicate significance.
Results
Repeatability of the diffusion measurements
The ICC values among 10 repeated acquisitions were 0.997, 0.966, and 0.999 for MK, FA, and MD, respectively. Excellent repeatability was achieved for all three diffusion-imaging metrics.
Effect of varying TR on the diffusion-imaging metrics
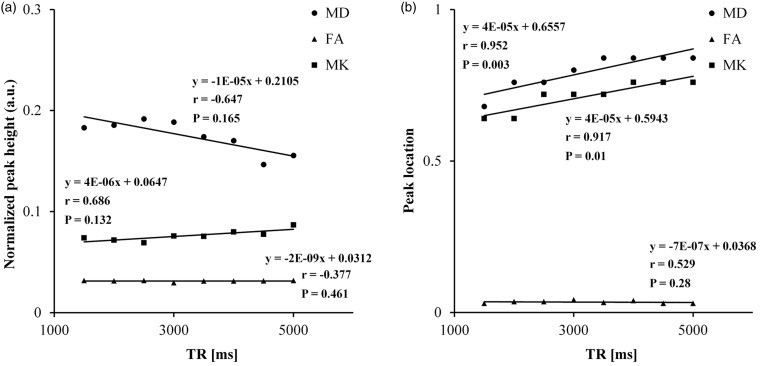
Figure 2 summarizes the results for the correlation between the major diffusion-imaging metrics and the TR. The peak location of MK and MD increased significantly with an increase in TR (p ≤ 0.01). The peak height of all imaging metrics and the peak location of FA did not vary significantly with variations in TR.
Figure 2.
Scatter plots showing correlation between the histogram measures of the major diffusion-imaging metrics—the normalized peak height (a) and peak location (b), and TR. a.u.: arbitrary unit; TR: repetition time; MD: mean diffusivity; FA: fractional anisotropy; MK: mean kurtosis.
Any further evaluation of the peak location of MK and MD was performed after correction for variation in TR.
Effect of respiratory and cardiac gating
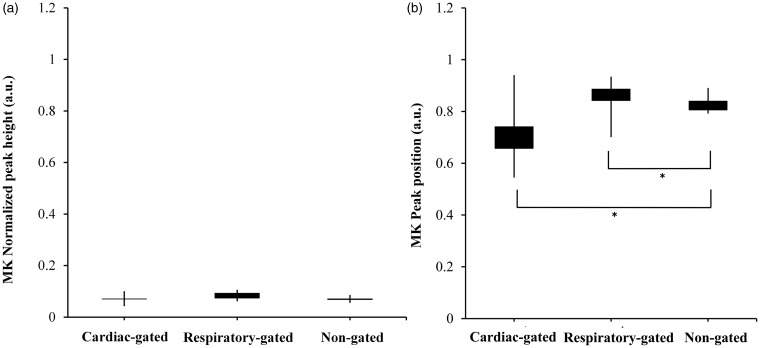
The histogram measures of the major diffusion-imaging metrics, for the gated and non-gated acquisitions, are given in Figures 3–5. The corrected peak location of the MK histogram for non-gated acquisition was significantly larger than that for respiratory- (p = 0.005) and cardiac-gated (p = 0.008) acquisitions. The normalized peak height of the FA histogram for non-gated acquisition was significantly lower than that for respiratory- (p = 0.007) and cardiac-gated (p = 0.009) acquisitions. The normalized peak height of the MD histogram for non-gated acquisitions was significantly higher than that for respiratory- (p = 0.005) and cardiac-gated (p = 0.007) acquisitions. The corrected peak location of the MD histogram for non-gated acquisition was significantly smaller than that for respiratory-gated acquisition (p = 0.004).
Figure 3.
Box whisker plots showing the normalized peak height (a) and peak location (b) of MK histograms for gated and non-gated acquisitions. The box in each box whisker plot indicates the interquartile range. The whiskers indicate standard error. *Statistical significance. a.u.: arbitrary unit; MK: mean kurtosis.
Figure 4.
Box whisker plots showing the normalized peak height (a) and peak location (b) of FA histograms for gated and non-gated acquisitions. The box in each box whisker plot indicates the interquartile range. The whiskers indicate standard error. *Statistical significance. a.u.: arbitrary unit; FA: fractional anisotropy.
Figure 5.
Box whisker plots showing the normalized peak height (a) and peak location (b) of MD histograms for gated and non-gated acquisitions. The box in each box whisker plot indicates the interquartile range. The whiskers indicate standard error. *Statistical significance. a.u.: arbitrary unit; TR: repetition time; MD: mean diffusivity.
Discussion
In this study, we evaluated whether the major diffusion-imaging metrics of the brain varied between respiratory or cardiac-gated and non-gated acquisitions. The results revealed a significant variation in the histogram measures between the gated and non-gated acquisitions for all three major diffusion-imaging metrics. As the repeatability of the diffusion measurements was excellent and there was no gross head motion or variation in slice position among acquisitions, the observed variations in the histogram measures are considered to be the effect of respiratory and cardiac gating.
In this study, the normalized peak height of the FA histograms was significantly lower with non-gated than with gated acquisitions. The reverse was also true for the peak height of the MD histograms. As variation in the peak height of a histogram indicates variation in the proportion of voxels that contribute the maximum frequency, the observation of a lower peak height of the FA histograms is thought to reflect a larger degree of FA heterogeneity with non-gated acquisition.3,13 On the contrary, the higher peak height of the MD histograms with non-gated acquisition is indicative of the presence in the histogram of a large proportion of voxels with the same MD value. This may further imply that the non-gated acquisition may not be able to identify correctly the brain regions with different MD values. The observation of a smaller peak location of the MD histograms with non-gated acquisition, compared to respiratory gating, may suggest that the non-gated acquisition underestimates the MD value in the largest proportion of voxels. A larger peak location of MK histograms with non-gated acquisition, compared to gated acquisitions, is indicative of a larger deviation from a Gaussian distribution with non-gated acquisition. Although the exact mechanisms that underlie variation in the histogram measures between the gated and non-gated acquisitions are not known, it is thought that pulsation-induced intravoxel phase dispersion and high signal intensity flow-related artifacts that propagate along the phase-encoding direction are responsible.2,14,15 The former results in signal attenuation of the affected voxels, whereas the latter gives rise to a signal increase within the voxels. The observation of a discrepancy among the results of previous studies and ours for non-gated acquisition may denote that both over- and underestimation of the major diffusion-imaging metrics are possible with non-gated acquisition.7–10
Whereas the results of this study reflect a significant impact of respiratory and cardiac gating on the major diffusion-imaging metrics, the observation of excellent repeatability among acquisitions may suggest that the diffusion measurements are repeatable and reproducible, provided the imaging parameters are kept identical. Although gated acquisition may be desired for improved accuracy, the non-gated acquisition with other imaging parameters being identical can serve as a precise alternative. Considering the increase in acquisition time with respiratory or cardiac gating, non-gated acquisition may be preferred for clinical studies.
This study has several limitations. First, the effect of gating on the major diffusion-imaging metrics was evaluated in three supratentorial sections only. The brainstem was not included in the evaluation because this structure is susceptible to several other confounding artifacts, such as susceptibility artifacts.16 It is possible that variation in the major diffusion-imaging metrics between the gated and non-gated acquisitions is more pronounced at the level of the brainstem because CSF pulsations are accentuated at the cisterns that surround the brainstem.17 Nevertheless, it is believed that, in this study, we were able to demonstrate the difference in the major diffusion-imaging metrics among gated and non-gated acquisitions, in areas thought to be less affected by CSF pulsations. Second, variation in the effect of gating among the brain sections was not evaluated; this is an ongoing project. According to a previous report, the effect of gating can be negligible at the cranial sections.18 Third, repeatability was tested only for non-gated acquisition.
In conclusion, in this study, we evaluated the effect of respiratory and cardiac gating on the major diffusion-imaging metrics. The major diffusion-imaging metrics are susceptible to artifacts related to respiration and cardiac pulsation. The knowledge that gating can affect the quantification of the major diffusion-imaging metrics would be valuable for correct interpretation of the metrics. The gating condition must be kept identical for reproducible results to be achieved.
Funding
This work was supported by (1) JSPS KAKENHI grant number 23791380 and (2) the Creation of Innovation Centers for Advanced Interdisciplinary Research Areas Program, Ministry of Education, Culture, Sports, Science and Technology, Japan.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Schaefer PW, Grant PE, Gonzalez RG. Diffusion-weighted MR imaging of the brain. Radiology 2000; 217: 331–345. [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi K, Tha KK, Terae S, et al. Improved detection of heat stroke-induced brain injury by high b-value diffusion-weighted imaging. J Comput Assist Tomogr 2011; 35: 498–500. [DOI] [PubMed] [Google Scholar]
- 3.Tha KK, Terae S, Nakagawa S, et al. Impaired integrity of the brain parenchyma in non-geriatric patients with major depressive disorder revealed by diffusion tensor imaging. Psychiatry Res 2013; 212: 208–215. [DOI] [PubMed] [Google Scholar]
- 4.Van Cauter S, Veraart J, Sijbers J, et al. Gliomas: Diffusion kurtosis MR imaging in grading. Radiology 2012; 263: 492–501. [DOI] [PubMed] [Google Scholar]
- 5.Moseley M. Diffusion tensor imaging and aging: A review. NMR Biomed 2002; 15: 553–560. [DOI] [PubMed] [Google Scholar]
- 6.Jensen JH, Helpern JA, Ramani A, et al. Diffusional kurtosis imaging: The quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med 2005; 53: 1432–1440. [DOI] [PubMed] [Google Scholar]
- 7.Tabesh A, Jensen JH, Ardekani BA, et al. Estimation of tensors and tensor-derived measures in diffusional kurtosis imaging. Magn Reson Med 2011; 65: 823–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim S, Pickup S, Poptani H. Effects of cardiac pulsation in diffusion tensor imaging of the rat brain. J Neurosci Methods 2010; 194: 116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Habib J, Auer DP, Morgan PS. A quantitative analysis of the benefits of cardiac gating in practical diffusion tensor imaging of the brain. Magn Reson Med 2010; 63: 1098–1103. [DOI] [PubMed] [Google Scholar]
- 10.Chung S, Courcot B, Sdika M, et al. Bootstrap quantification of cardiac pulsation artifact in DTI. Neuroimage 2010; 49: 631–640. [DOI] [PubMed] [Google Scholar]
- 11.Gui M, Tamhane AA, Arfanakis K. Contribution of cardiac-induced brain pulsation to the noise of the diffusion tensor in Turboprop diffusion tensor imaging (DTI). J Magn Reson Imaging 2008; 27: 1164–1168. [DOI] [PubMed] [Google Scholar]
- 12.Rasband WS. Image J, U.S. National Institutes of Health, Bethesda, MD, USA, http://imagej.nih.gov/ij/, 1997–2012 (accessed 9 September 2013).
- 13.Cercignani M, Inglese M, Pagani E, et al. Mean diffusivity and fractional anisotropy histograms of patients with multiple sclerosis. AJNR Am J Neuroradiol 2001; 22: 952–958. [PMC free article] [PubMed] [Google Scholar]
- 14.Zhuo J, Gullapalli RP. MR artifacts, safety, and quality control. Radiographics 2006; 26: 275–297. [DOI] [PubMed] [Google Scholar]
- 15.Luk Pat GT, Meyer CH, Pauly JM, et al. Reducing flow artifacts in echo-planar imaging. Magn Reson Med 1997; 37: 436–447. [DOI] [PubMed] [Google Scholar]
- 16.Nagae-Poetscher LM, Jiang H, Wakana S, et al. High-resolution diffusion tensor imaging of the brain stem at 3 T. AJNR Am J Neuroradiol 2004; 25: 1325–1330. [PMC free article] [PubMed] [Google Scholar]
- 17.Brant-Zawadzki M, Atkinson D, Detrick M, et al. Fluid-attenuated inversion recovery (FLAIR) for assessment of cerebral infarction. Initial clinical experience in 50 patients. Stroke 1996; 27: 1187–1191. [DOI] [PubMed] [Google Scholar]
- 18.Wirestam R, Greitz D, Thomsen C, et al. Theoretical and experimental evaluation of phase dispersion effects caused by brain motion in diffusion and perfusion MR imaging. J Magn Reson Imaging 1996; 6: 348–355. [DOI] [PubMed] [Google Scholar]