Abstract
We report a case of a 17-year-old man presenting with new onset psychiatric symptoms. Magnetic resonance imaging (MRI) and proton magnetic resonance (MR) spectroscopy revealed some lesions in the right cerebellar hemisphere and ipsilateral cerebellar tonsil suggestive of encephalitis. An extensive workup was negative for both infectious and neoplastic diseases and he was afterward diagnosed with anti-N-methyl-D-aspartate receptor (anti-NMDAR) encephalitis. This disorder is an autoimmune encephalitis, highly lethal but curable, predominantly found in young female with ovarian teratoma. He received methylprednisolone. His clinical findings gradually improve and he made a complete recovery. Accordingly, repeated brain MRI and proton MR spectroscopy showed a gradual reduction of the lesions; MRI taken six months after starting therapy showed complete resolution of the lesions. Our case shows that, although rare, anti-NMDAR encephalitis should be considered also in young men for whom a rapid onset of psychiatric neurological disorders cannot be explained by more frequent causes. Our report underlines also the usefulness of MRI and proton MR spectroscopic findings in the diagnosis and follow-up of this disease.
Keywords: anti-NMDAR encephalitis, Magnetic Resonance spectroscopy, Magnetic Resonance Imaging
Case report
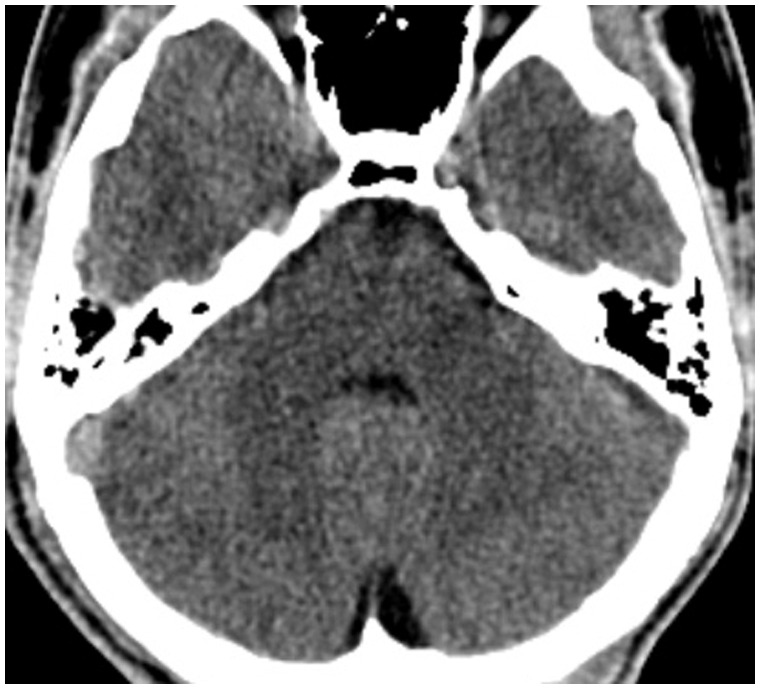
A 17-year-old man, with no significant past medical history, was referred to the emergency department of our hospital because of syncope while running; he had headache and mild fever for a few days before. He received a brain Computer Tomography (CT) (Figure 1) and a routine electroencephalography (EEG) with negative results. After some days, he was diagnosed with vasovagal syncope and discharged from the hospital.
Figure 1.
Non-contrast brain Computed Tomography (CT) at first admission, which does not show any significant alteration.
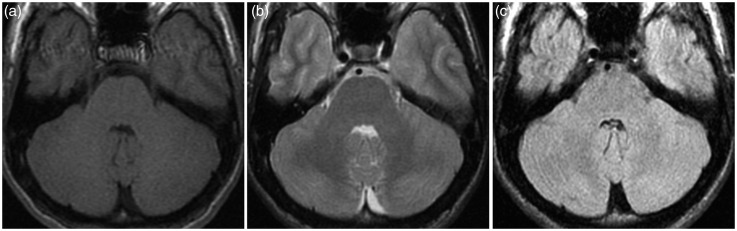
After about 10 weeks, the patient became progressively confused, agitated and sleepless, had weakness of the legs, walking difficulties, fluctuations in consciousness, and auditory hallucinations in the form of calling voices. Thus, he was again referred to the emergency department. On examination, he had normal vital signs, without evidence of any focal or global neurological deficit; a routine EEG did not show significant abnormalities. He was prescribed olanzapine and admitted to the inpatient psychiatry ward for a presumed acute psychosis. He had also a magnetic resonance imaging (MRI) brain scan (Figure 2), which was unremarkable.
Figure 2.
First brain magnetic resonance imaging (MRI). (a) T1, (b) T2 and (c) fluid-attenuated inversion recovery (FLAIR) axial images at the level of cerebellum do not reveal any significant alteration.
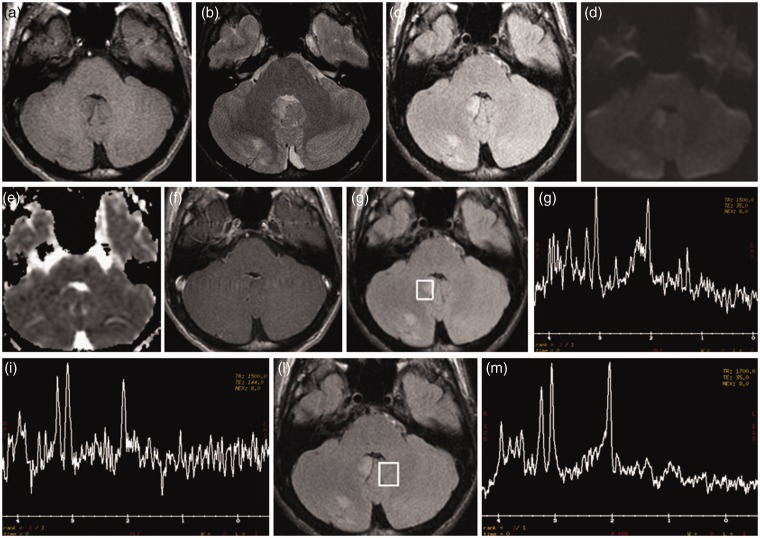
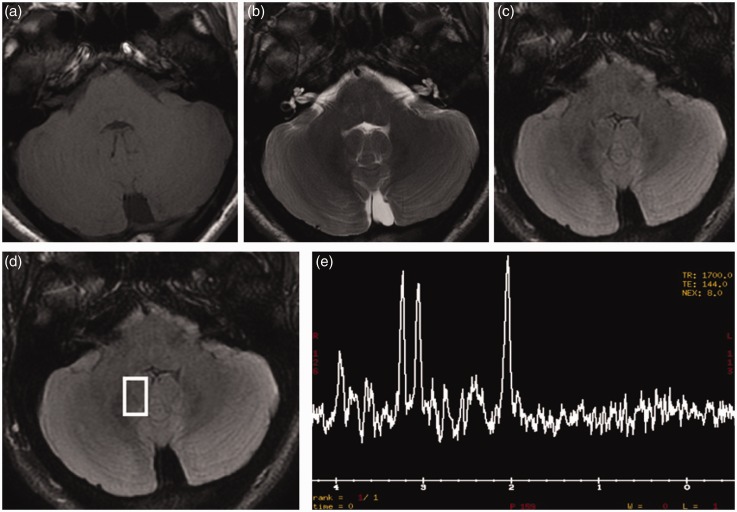
The patient progressed during the next seven days to an unresponsive, catatonic-like state, had a seizure with urine loss, mild fever (38℃), and, thus, had a second MRI brain scan (Figure 3). T2 and fluid-attenuated inversion recovery (FLAIR) showed some cortical-subcortical hyperintense areas in the right cerebellar hemisphere and ipsilateral cerebellar tonsil, which presented faint T1 hypointensity. These lesions did not demonstrate diffusion restriction on Diffusion Weighted Imaging/Apparent Diffusion Coefficient (DWI/ADC) images nor abnormal contrast enhancement. Spectroscopy, acquired with the single-slice single-voxel technique using the point-resolved spectroscopy (PRESS) sequence at both short Time Echo (TE) (TE = 35) and long (TE = 144) TEs within the abnormal areas, also in comparison with apparently normal contralateral cerebellum, showed a reduced N-acetyl aspartate (NAA) peak, with decreased NAA/Creatine (Cr) ratio, and a mildly increased choline (Cho) peak, with elevated Cho/Cr ratio.
Figure 3.
Second brain magnetic resonance imaging (MRI). T2 (b) and fluid-attenuated inversion recovery (FLAIR) (c) images reveal some cortical-subcortical hyperintense areas in the right cerebellar hemisphere and ipsilateral cerebellar tonsil, which demonstrate slight T1 hypointensity (a). These areas do not show diffusion restriction on DWI/ADC (Diffusion Weighted Imaging/Apparent Diffusion Coefficient) images (d) and (e) nor pathological contrast enhancement (f). Spectroscopy (g)−(i), acquired at both short Time Echo (TE) (TE = 35) and long (TE = 144) TEs within the abnormal areas, revealed a reduced N-acetyl aspartate (NAA) peak, with decreased NAA/creatina (Cr) ratio, and a slightly increased choline (Cho) peak, with elevated Cho/creatina (Cr) ratio. Spectroscopy, acquired within the apparently normal contralateral cerebellum (l), shows a normal spectrum (m).
Lesion distribution and MRI features allowed us to rule out other more frequent causes of encephalitis; given the MRI findings and the acute presentation of a severe psychotic state, a presumptive diagnosis of autoimmune encephalitis was made.
A lumbar puncture was performed. The cerebrospinal fluid (CSF) was sent for numerous studies including a full paraneoplastic panel, herpes simplex virus Polymerase Chain Reaction (PCR), varicella zoster virus PCR, Epstein–Barr virus PCR, cytomegalovirus PCR, bacterial colture (Mycobacterium tuberculosis, Mycoplasma pneumoniae, Listeria monocytogenes, Borrelia burgdoferi), that were all negative. Finally, anti–NMDA receptor (anti-NMDAR) antibodies were detected in the blood and CSF, and his diagnosis was therefore confirmed as anti-NMDAR encephalitis.
Since anti–NMDAR encephalitis is frequently associated with an underlying neoplasm, the patient had also a chest, abdomen and pelvis CT scan, that showed no evidence of malignancy.
High-dose intravenous methylprednisolone was started. His symptoms and clinical findings gradually improved: he needed to be hospitalised for one month and, gradually, made a complete recovery.
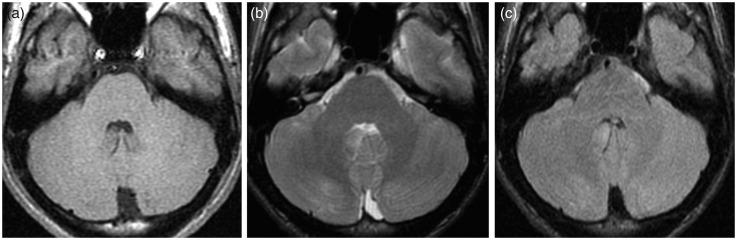
Repeated brain MRI imaging performed 10 days later showed a decrease in the width of the lesions (Figure 4); MRI taken six months after starting therapy revealed complete resolution of the lesions (Figure 5). Also repeated proton magnetic resonance (MR) spectroscopy, acquired with the single-slice single-voxel using the PRESS sequence at both short (TE = 35) and long (TE = 144) TEs within the affected areas, showed gradual normalisation of the spectra.
Figure 4.
Brain magnetic resonance imaging (MRI) performed 10 days after starting steroid treatment. (a) T1, (b) T2 and (c) fluid-attenuated inversion recovery (FLAIR) images demonstrate a significant decrease in the width of the cerebellar lesions.
Figure 5.
Brain magnetic resonance imaging (MRI) obtained six months after diagnosis. (a) T1, (b) T2, (c) fluid-attenuated inversion recovery (FLAIR) images show disappearance of the cerebellar lesions. Spectroscopy (d) and (e), acquired within the affected areas, showed normalisation of the spectrum.
Discussion
We report the MRI and proton MR spectroscopic findings in a 17-year-old man with anti-NMDAR encephalitis not associated with an underlying neoplasm and the resolution of the disorder after steroid treatment. Anti-NMDR encephalitis was described for the first time by Dalmau et al. in 2007.1 Since then, >600 cases of anti-NMDAR encephalitis have been reported:2–3 it is the second most frequent autoimmune encephalitis after acute disseminated encephalomyelitis (ADEM).4 A multicentre study showed a 91% female predilection, unlike our patient, and a mean age at the diagnosis of 23 years old (range 5–76 years).5
Anti-NMDAR encephalitis was initially classified as a paraneoplastic syndrome because it was first reported in women with an ovarian teratoma,6–8 unlike our patient. Further studies showed that 40% of subjects with anti-NMDAR encephalitis do not have a detectable tumour and that men can also be affected,2–3 Among patients with a tumour, 94% have an ovarian teratoma,8 while detection of other neoplasms is very rare.7
Anti-NMDAR encephalitis is a serious but curable neuroautoimmune disease caused by the aberrant production of immunoglobulin (Ig) G antibodies against the NMDAR in response to a number of possible stimuli (i.e. tumour, infection).5,9,10 Anti-NMDAR antibodies cross-react with the NMDARs and induce their internalisation,6 resulting in a progressive loss of surface receptors, which leads to decreased NMDAR function.11 Physiologically, the NMDAR is important for synaptic plasticity and, in turn, is responsible for higher functions such as learning and memory.5 Inhibition of NMDAR leads to a reduced gamma-aminobutyric acid (GABA) release in presynaptic neurons that in turn switches off the inhibitory effects of glutamate release in the postsynaptic neurons.9 Glutamate is the major excitatory neurotransmitter distributed throughout the brain12 and a diminished glutamatergic function has been implicated in the pathophysiology of schizophrenia and affective disorders, psychotic and neurocognitive symptoms similar to those in schizophrenia.12 This reduction in surface NMDARs is reversible after antibody removal.11
Due to the acute or subacute onset of the disease, overlap of the stages and clinical symptoms and signs are extremely common,8 Increasing clinical disease severity is thought to correlate with gradually decreasing availability of NMDAR function.13
Clinically, in 70% of patients, anti-NMDAR encephalitis begins with a prodromal phase lasting 5–14 days.10,14 During this phase, patients complain of mild fever, malaise, inability to concentrate, nausea, diarrhoea, myalgia, vomiting, headache with stiff neck, gastrointestinal symptoms.1,10
Later on, the psychotic and/or seizure phase (subacute onset behavioural change) is characterised by emotional and schizophrenia-like behavioural disturbances, including sleep disturbance, apathy, fear, depression, decreased cognitive skills, psychosis (delusions, and hallucinations), hallucinations, sudden onset of aggression or irritability10,14,15 paranoia.9 Multiple neurological deficits may be noted, such as seizure (most commonly generalised tonic clonic), ataxia, dystonias, autonomic instability, choreiform movements, clouding of consciousness, pathologic posturing, speech dysfunction, orofacial dyskinesias.9,10,14,15 Seizures may prove refractory to standard medical management, requiring multiple anticonvulsant medications.12 Such patients are often mistaken for psychiatric patients and thus admitted to psychiatric facilities.9,12
Patients often become non-verbal and non-responsive (unresponsive phase).12,14 Autonomic instability may manifest with cardiac arrhythmia, hypotension, hypertension, hyper- or hypothermia;12 central hypoventilation can occur in those with brainstem and/or hypothalamus involvement.9 Patients no longer follow verbal commands, may appear mute and akinetic;13 may maintain gaze as if in a catatonic state, smile inappropriately, or demonstrate stereotyped athetotic movements.13
EEG may be unremarkable or reveal non-specific slowing, disorganised activity or epileptiform discharge.10
CSF biochemistry, cytology and microbiology examinations are either unremarkable or nonspecific;10 in the latter case may show pleocytosis or increased protein concentration, suggesting an inflammatory or immune-mediated neurological process.9
Despite the severity of symptoms, conventional MRI of the brain is frequently normal, often referred to as the clinical-radiologic paradox:9 only 35% of the patients have abnormal brain MRI at disease onset, increasing to 50/55% when the entire course of the disease is considered.10,11 When abnormal, MRI usually reveals mild and non-specific changes,16,17 such as discrete lesions that are predominantly subtle9 may be single or multiple,10 and correlate poorly with symptoms.9,10
The abnormalities identified on routine MRI studies are preferentially seen on FLAIR or T2W images, usually involving the limbic system,14 cortical and subcortical regions of the brain (especially medial temporal lobes and frontobasal-insular regions),5,11 followed by brainstem and basal ganglion,9,10 and, infrequently, the spinal cord and cerebellum,6 as in our patient. The lesions can be non-enhancing, as in our case, or accompanied by subtle contrast enhancement in the affected areas or the meninges.16
To the best of our knowledge, only one case of proton MR spectroscopic evaluation has previously been reported.14 In the cited study,14 spectroscopy, acquired with the single-slice multi-voxel chemical-shift technique (TE = 30 and 135 ms), showed a reduced NAA peak and a slightly increased Cho peak, resulting in an inverted Cho/NAA ratio, as in our case. A reduced NAA peak is considered to depict the loss of neuronal functional activity; an increased Cho peak suggests cell destruction or high cellularity and may be seen in both inflammatory/infectious disease and in neoplastic lesions.18
The diagnostic workup is based on the definitive determination of anti-NMDAR antibodies in blood or preferably in CSF19 as in our patient.
The main priority in managing patients with suspected encephalitis is to rule out infectious causes, many of which are effectively treated using antimicrobial agents.16 An important differential diagnosis that should be kept in mind and excluded in a younger population with a rapid onset of cognitive and behavioural abnormalities is herpes encephalitis, which accounts for approximately 19% of encephalitis.17 However, herpes encephalitis frequently involves temporal lobes, presents diffusion restriction on DWI/ADC sequences and shows patchy enhancement on contrast-enhanced images.17
Another diagnostic priority is to exclude paraneoplastic aetiology as these syndromes may be life-threatening and may be treatable if diagnosed early.20 Thus, it is advisable to have an extensive diagnostic workup to exclude possible underlying malignancy, as in our patient.
Although anti-NMDAR encephalitis presents very severe clinical symptoms and is potentially lethal, more than 75% of all patients have a substantial recovery or only mild sequelae by appropriate treatment21 while all other patients remain severely disabled or die.22 Early recognition and treatment are predictive of a good outcome.11,12 Patients are treated with tumour resection if required, first-line immunotherapy (intravenous and/or oral steroids, intravenous immunoglobulin, and/or plasma exchange), as in our patient, and second-line immunotherapy (cyclophosphamide or rituximab) if indicated.2
Conclusion
The present case report describes the usefulness of MRI and proton MRI spectroscopy in the diagnosis of anti-NMDAR encephalitis and highlights the importance of considering this disease in the differential diagnosis of patients with unexplained psychiatric-neurological conditions.23–26 This disorder should be considered also in young men without neoplasm, although is more common in female with ovarian teratoma. This is especially important because this disorder is potentially treatable and early treatment is linked with a better prognosis while delays in treatment may allow patients to progress to coma and/or require intensive medical care including mechanical ventilation. Also, since the determination of anti-NMDAR antibodies in blood or in cerebrospinal fluid takes a few weeks, the therapy has to be considered before the availability of these results. Our report highlights also the usefulness of MRI, when abnormal at the diagnosis, in the follow-up of this disorder.
Acknowledgements
All authors contributed equally to the manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Dalmau J, Tüzün E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol 2007; 61(1): 25--36. [DOI] [PMC free article] [PubMed]
- 2.Wright S, Hacohen Y, Jacobson L, et al. N-methyl-D-aspartate receptor antibody-mediated neurological disease: Results of a UK-based surveillance study in children. Arch Dis Child 2015; 0: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adang LA, Lynch DR, Panzer JA. Pediatric anti-NMDA receptor encephalitis is seasonal. Ann Clin Transl Neurol 2014; 1: 921–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Titulaer MJ, Höftberger R, Iizuka T, et al. Overlapping demyelinating syndromes and anti-NMDA receptor encephalitis. Ann Neurol 2014; 75: 411–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yau MLY, Fung ELW. Early consideration of anti-NMDAR encephalitis in unexplained encephalopathy. Hong Kong Med J 2013; 19: 362–364. [DOI] [PubMed] [Google Scholar]
- 6.Peery HE, Day GS, Dunn S, et al. Anti-NMDA receptor encephalitis. The disorder, the diagnosis and the immunobiology. Autoimmun Rev 2012; 11: 863–872. [DOI] [PubMed] [Google Scholar]
- 7.Hayashi M, Motegi E, Honma K, et al. Successful laparoscopic resection of 7 mm ovarian mature cystic teratoma associated with anti-NMDAR encephalitis. Case Rep Obstet Gynecol. 2014. DOI:10.1155/2014/618742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Acién P, Acién M, Ruiz-Maciá E, et al. Ovarian teratoma-associated anti-NMDAR encephalitis: A systematic review of reported cases. Orphanet J Rare Dis 2014; 9: 157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hacohen Y, Absoud M, Hemingway C, et al. NMDA receptor antibodies associated with distinct white matter syndromes. Neurol Neuroimmunol Neuroinflammation 2014; 1: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kattepur AK, Patil D, Shankarappa A, et al. Anti-NMDAR limbic encephalitis–a clinical curiosity. World J Surg Oncol 2014; 12: 256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen B, Wang Y, Geng Y, et al. Marked improvement of anti-N-methyl-D-aspartate receptor encephalitis by large-dose methylprednisolone and plasmapheresis therapy combined with 18F-fluorodeoxyglucose positron emission tomography imaging: A case report. Exp Ther Med 2014; 8: 1167–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuppuswamy PS, Takala CR, Sola CL. Management of psychiatric symptoms in anti-NMDAR encephalitis: A case series, literature review and future directions. Gen Hosp Psychiatry 2014; 36: 388–391. [DOI] [PubMed] [Google Scholar]
- 13.Hayata Y, Hamada K, Sakurai Y, et al. Anti-glutamate ɛ2 receptor antibody-positive and anti-N-methyl-D-aspartate receptor antibody-negative lobar encephalitis presenting as global aphasia and swallowing apraxia. Case Rep Neurol 2014; 6: 291–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mariotto S, Tamburin S, Salviati A, et al. Anti-N-methyl-D-aspartate receptor encephalitis causing a prolonged depressive disorder evolving to inflammatory brain disease. Case Rep Neurol 2014; 6: 38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azizyan A, Albrektson JR, Maya MM, et al. Anti-NMDA encephalitis: An uncommon, autoimmune mediated form of encephalitis. J Radiol Case Rep 2014; 8: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki H, Kitada M, Ueno S, et al. Anti-NMDAR encephalitis preceded by dura mater lesions. Neurol Sci 2013; 34: 1021–1022. [DOI] [PubMed] [Google Scholar]
- 17.Saraya A, Mahavihakanont A, Shuangshoti S, et al. Autoimmune causes of encephalitis syndrome in Thailand: Prospective study of 103 patients. BMC Neurol 2013; 13: 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Umamaheswara Reddy V, Agrawal A, Murali Mohan KV, et al. The puzzle of choline and lipid peak on spectroscopy. The Egyptian Journal of Radiology and Nuclear Medicine 2014; 45: 903–907. [Google Scholar]
- 19.Dalmau J, Lancaster E, Martinez-Hernandez E, et al. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol 2011; 10: 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim SY, Choi SA, Ryu HW, et al. Screening autoimmune anti-neuronal antibodies in pediatric patients with suspected autoimmune encephalitis. J Epilepsy Res 2014; 4: 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: An observational cohort study. Lancet Neurol 2013; 12: 157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turner MR, Irani SR, Leite MI, et al. Progressive encephalomyelitis with rigidity and myoclonus. Glycine and NMDA receptor antibodies. Neurology 2011; 77: 439–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Catalucci A, Anselmi M, Splendiani A, et al. Pediatric inflammatory diseases. Part I: Multiple sclerosis. Neuroradiol J 2012; 25: 684–694. [DOI] [PubMed] [Google Scholar]
- 24.Splendiani A, Catalucci A, Limbucci N, et al. Pediatric inflammatory diseases. Part III: Small vessels vasculitis. Neuroradiol J 2012; 25: 715--724. [DOI] [PubMed]
- 25.Paonessa A, Limbucci N, Tozzi E, et al. Radiological strategy in acute stroke in children. Eur J Radiol 2010; 74(1): 277--385. [DOI] [PubMed]
- 26.Felli V, Catalucci A, Splendiani A, et al. Progressive Multifocal Leukoencephalopathy (PML) following treatment with Rituximab in a HIV-negative patient with non-Hodgkin lymphoma: case report and review of the literature. Neuroradiol J 2014; 27: 657--664. [DOI] [PMC free article] [PubMed]