Abstract
Objective
To evaluate the role of advanced magnetic resonance (MR) sequences (fast imaging employing steady-state acquisition (FIESTA), T2 star-weighted angiography (SWAN) and spoiled gradient recalled echo (SPGR)) in patients with single small enhancing computed tomography lesions and scolex demonstration in typical and atypical parenchymal neurocysticercosis.
Methods
In this study, 59 patients of new-onset seizures with single small enhancing computed tomography lesions of the brain were included. Along with routine MR sequences, advanced MR sequences, like SWAN, FIESTA, and pre and post-contrast SPGR, were performed. Follow-up MR studies focussing on the morphology of the lesions and demonstration of scolex were performed 6 monthly for 3 years.
Results
The majority of patients (62.7%) were men with partial seizure as the most common manifestation. On SPGR, contrast lesions were identified as either ‘typical’ (42, 71.2%) or ‘atypical’ (17, 28.8%). In the typical lesion group, SWAN and FIESTA sequences detected scolex in 30 (71.4%) and 32 (76.2%), respectively. The combination of SPGR-contrast, FIESTA and SWAN sequences detected scolex in 35 (83.3%) patients compared to 19 (45.2%) by routine sequences (P < 0.001). In the atypical lesion group, SWAN and FIESTA sequences detected scolex in 15 (88.2%) and 16 (94.1%) patients, respectively. The combination of SPGR-contrast, FIESTA and SWAN sequences detected scolex in 16 (94.1%) patients compared to 10 (58.8%) by routine sequences (P < 0.001). Follow-up showed greater resolution with lesser calcification in the typical group compared to the atypical group.
Conclusion
This study provides an insight into the natural course of typical and atypical solitary cysticercus granuloma lesions, and the utility of SPGR-contrast, FIESTA and SWAN MR sequences in scolex demonstration and identification of atypical lesions.
Keywords: Neurocysticercosis, epilepsy, solitary cysticercus granuloma
Introduction
A single enhancing computed tomographic lesion is a commonly encountered neuroimaging entity.1,2 Neurocysticercosis and tuberculoma constitute the two most important differential diagnoses of these lesions.3–6 It has also been reported in immigrants in western countries.7,8 A single cysticercal lesion is termed solitary cysticercus granuloma (SCG). Typically these lesions are small with eccentric scolex and perilesional oedema and have been described as ‘type A’ lesions. Some atypical or ‘type B’ lesions have also been described in those having bilobed, septated, or disc configurations. In regions where intracranial tuberculomas are equally frequent, it is often a challenge to differentiate between a focal tuberculoma and cysticercal lesions. In India, the reported incidence of a solitary lesion among patients with new-onset seizures varies from 23% to 72%.9–12
The detection of scolex is considered the gold standard for the diagnosis of neurocysticercosis. Computed tomography (CT) is comparatively a less effective imaging modality for detecting scolex.13–17 Magnetic resonance imaging (MRI) has been found to be more valuable in detecting lesions of neurocysticercosis especially in the subarachnoid space, ventricles, brainstem, cerebellum and spinal cord. Conventional MRI sequences such as T1, T2 and fluid attenuated inversion recovery (FLAIR) are better than CT in scolex demonstration.18,19 Individually, several advanced magnetic resonance (MR) sequences such as fast imaging employing steady-state acquisition (FIESTA), spoiled gradient recalled echo (SPGR) and T2 star-weighted angiography (SWAN) have been evaluated in patients with neurocysticercosis and have been found useful in the demonstration of scolex.20–23 The SPGR sequence has been shown to be a more accurate sequence for detecting cysts and scolex in the case of intraventricular neurocysticercosis. The SWAN sequence has also been validated and has been found better than conventional studies for diagnosing neurocysticercosis based on scolex detection.22 The SPGR-contrast sequence is better than T1-contrast in detecting small lesions and delineating their morphology. The time to resolution of these lesions is very variable as reported in the literature. Most studies are based on CT, which shows an early resolution, but considering the limitations of CT, the correct picture can only be ascertained with an MRI-based study with a long follow-up.
In this study, we studied the natural course of typical and atypical lesions in light of the special sequences, namely FIESTA, SWAN and SPGR. As lesions have been shown to persist beyond 1 year an extended follow-up of 3 years was planned to study the course better. During this period the recurrence of seizures was also systematically recorded.
Materials and methods
This prospective cohort study was conducted from September 2011 to March 2015. Patients were enrolled during the first 6 months, and were then followed for 3 years. All human and animal studies have been approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Patients were enrolled after written consent signed by the patient, their parents (in the case of minors) or their legal guardian, as applicable.
Inclusion criteria
We screened consecutive patients with new-onset seizures (within 14 days of first seizure) with a single ring enhancing brain lesion of less than 20 mm in diameter and fulfilling the diagnostic criteria of SCG on contrast-enhanced CT.24
Exclusion criteria
Patients with additional brain lesions on MRI were excluded. The patients with tuberculosis, immunocompromised state, diabetes and malignancy were also excluded to avoid heterogeneity in data acquisition. Patients taking steroids or anti-helmenthics were not included to aid the study of the natural course of the disease.
Evaluation
A detailed clinical evaluation was performed. The patients were subjected to a battery of laboratory tests including complete blood count, erythrocyte sedimentation rate, blood sugar, blood urea nitrogen, creatinine, chest radiograph and enzyme-linked immunosorbent assay for HIV. Tuberculin testing, cryptococcal latex antigen detection and cerebrospinal fluid examination were performed in addition in patients with doubtful lesions.
MR protocol
MRI brain was performed on a 3Tesla MR scanner (SignaHDxt; General Electric, Milwaukee, WI, USA) using a 12-channel HNS-head coil. MRI included T1 SPGR (TR/TE/inversion time 8.412 ms/3.328 ms/400 ms, acquisition matrix 288 × 288, interslice space of 1 mm and flip angle 13°, T2-weighted (TR/TE/number of averages (NEX) 6820 ms/71.8 ms/1, acquisition matrix 512 × 256,interslice space of 3 mm), and T2-weighted FLAIR (TR/TE/inversion time 8372 ms/84.7 ms/2150 ms, acquisition matrix 256 × 160, interslice space of 3 mm). In addition, SWAN imaging with TR/TE/NEX 46.8 ms/25.0 ms/0.69, acquisition matrix 320 × 224, interslice space of 1.2 mm with 2.4 mm slice thickness and flip angle 15° was also used. FIESTA imaging with TR/TE/NEX 4.7 ms/1.7 ms/1, acquisition matrix 320 × 256, interslice space of 0.5 mm with slice thickness 1 mm and flip angle of 55° was used. After the administration of gadolinium contrast agent at a dose of 0.1 mmol/kg body weight T1-weighted MRI was repeated using the same pre-contrast acquisition conditions.
Images were analysed independently by two authors who were not aware of the clinical details of the patients. Inter-observer differences were discussed and common consensus was reached. Routine and advanced sequences were used to detect scolex and analyse characteristics of scolex, cyst content, cyst wall and perilesional oedema. The scolex was defined as a central or eccentric nodule in the wall of the lesion. Conventional or routine sequences were defined as sequences currently used for imaging neurocysticercosis; we defined T2 and T2-FLAIR as routine sequences. Advanced sequences were defined as sequences not routinely used in MRI protocols for neurocysticercosis and were being used only in special situations. We considered SPGR, SPGR-contrast, SWAN and FIESTA as advanced MR sequences. The SWAN technique combines three-dimensional (3D) T2 star-based multi-echo acquisition with a reconstruction algorithm. SWAN generates multiple echoes, which have different TE times. The signals vary according to different tissues, which are then automatically reconstructed using a post-processing algorithm. FIESTA is a fast steady-state sequence that gives T2 information and is useful to demonstrate structures or lesions with high water content. FIESTA has a good signal to noise ratio that allows the evaluation of small structures or lesions.25
The patients were divided into two groups, based on lesion characteristics on the results of the SPGR-contrast sequence. The first group was labelled the ‘typical-lesion’ group, and consisted of patients demonstrating single round or oval ring enhancement with minimal perilesional oedema and no midline shift. Those patients who exhibited with an atypical morphology (coalesced lesions, lesions with septation or conglomerated lesions with scolex) were included in the second group, designated as the ‘atypical-lesion’ group.
Treatment
All patients were treated with antiepileptic drugs. Oxcarbazepine was administered according to their body weight (15 mg/kg body weight). No patient received corticosteroid or albendazole as per the departmental treatment protocol of SCG.
Follow-up
Follow-up imaging was performed at 6, 12, 24 and 36 months. The lesions were considered ‘resolved’ if the follow-up MR study did not show the lesion. Persistence of the lesion was defined as evidence of cyst in any MR sequence (with or without contrast). Homogenous calcification was defined as complete cyst hypointensity on T2 and SWAN. Mottled calcification was defined as patchy calcification on SWAN. Patients with either resolution of lesion or homogenously calcified lesion were not imaged beyond the last follow-up. Seizure recurrence was defined as one or more seizures occurring 1 week after the index seizure.
Statistical analysis
We used the statistical package for social sciences software (version 16.0; SPSS Inc., Chicago, IL, USA) for statistical analyses. Pearson chi-square and Fisher’s exact tests were used to compare the two groups. Non-parametric tests such as Cochrane’s Q test for related samples were used to compare the sequences of MRI for scolex detection. A P value of <0.05 was considered to be statistically significant.
Results
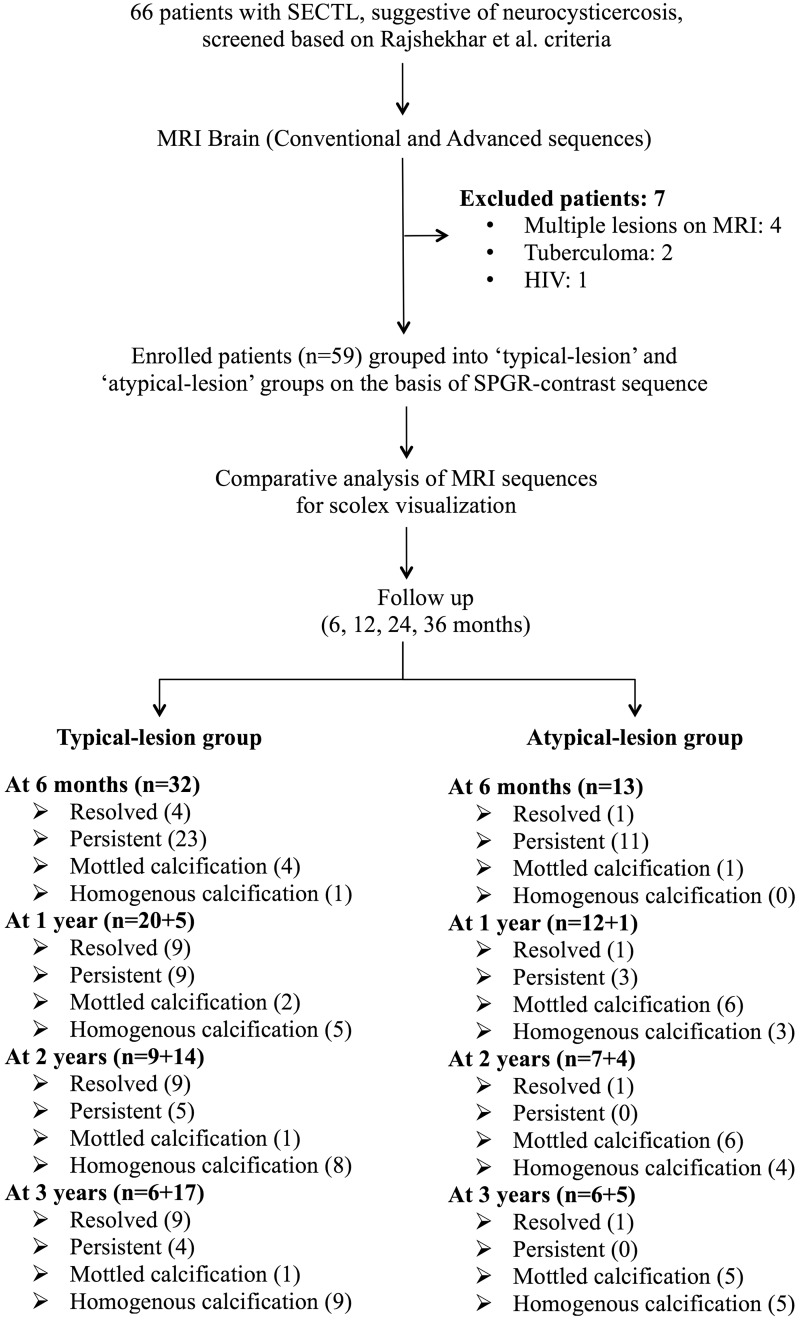
We screened 66 patients with SCG. Seven patients were excluded. Among the excluded patients two had tuberculoma with pulmonary tuberculosis; one was HIV positive and four had multiple brain lesions on MRI. Therefore, 59 patients were enrolled and followed for further analysis (Figure 1).
Figure 1.
Algorithm of the study.
SPGR: spoiled gradient recalled echo; SECTL: single enhancing computed tomographic lesion; MRI: magnetic resonance imaging.
Clinical characteristics
The majority of our patients (37, 62.7%) were boys or men. The mean age was 18.9 years (range 7–42). None of the patients had focal neurological deficits. Partial seizure was the most frequent seizure type encountered (Table 1).
Table 1.
Baseline clinical and neuroimaging characteristics of patients with solitary cysticercus granuloma (N = 59).
| Characteristics | Total (n = 59) | Typical lesion group (n = 42) | Atypical lesion group (n = 17) | p value | |
|---|---|---|---|---|---|
| 1 | Age mean (SD) in years | 18.9 (7-42) | 19.1 (7.34) | 18.3 (9.13) | |
| 2 | Sex (M:F) | 37:22 | 29:13 | 8:9 | 0.11 |
| 3 | Seizures | 49 (83.1%) | 34 (80.9%) | 15 (88.2%) | 0.49 |
| 4 | Seizures and headache | 10 (16.9%) | 8 (19.1%) | 2 (11.8%) | 0.49 |
| 5 | Type of seizures | ||||
| Partial seizures | 8 (13.5%) | 6 (14.3%) | 2 (11.8%) | 0.88 | |
| Complex partial seizures | 3 (5.1%) | 2 (4.8%) | 1 (5.9%) | ||
| Partial with secondary generalisation | 20 (33.9%) | 13 (30.9%) | 7 (41.2%) | ||
| Generalised tonic clonic seizures | 28 (47.5%) | 21 (50.0%) | 7 (41.2%) | ||
| 6 | MRI (hemisphere involved) | ||||
| Right hemisphere | 26 (44.1%) | 16 (38.1%) | 10 (58.8%) | 0.15 | |
| Left hemisphere | 33 (55.9%) | 26 (61.9%) | 7 (41.2%) | ||
| 7 | MRI (lobe involved) | ||||
| Frontal | 31 (52.5%) | 20 (47.6%) | 11 (64.7%) | 0.60 | |
| Parietal | 17 (28.8%) | 14 (33.3%) | 3 (17.6%) | ||
| Temporal | 3 (5.1%) | 2 (4.8%) | 1 (5.9%) | ||
| Occipital | 8 (13.6%) | 6 (14.3%) | 2 (11.8%) | ||
F: Female; M: Male; MRI: Magnetic Resonance Imaging; SD: Standard deviation.
Imaging characteristics: baseline
Out of 59 patients, 42 (71.2%) had typical lesions and 17 (28.8%) had atypical parenchymal ring lesions on SPGR sequences.
Typical lesion group (n = 42)
In our study, T2 and T2-FLAIR detected scolex in 17 (40.5%) and 11 (29.2%) patients, respectively, in the typical lesion group. Both routine sequences collectively detected scolex in 19 (45.2%) patients. SPGR, SPGR-contrast, SWAN and FIESTA sequences detected scolex in 14 (13.3%), 13 (30.9%), 30 (71.4%) and 32 (76.2%) patients, respectively. Advanced sequences collectively detected scolex in 35 (83.3%) patients. As compared to routine sequences, advanced sequences detected more scolices, which was statistically significant (P < 0.001). On comparing various sequences, we found the scolex detection rate in descending order of frequency to be advanced (combined) > FIESTA > SWAN > routine > T2 > SPGR non-contrast > SPGR-contrast > T2-FLAIR.
All the scolices detected on T2, SWAN and FIESTA were hypo or iso-intense. On T2-FLAIR, hypo or iso-intense scolices were seen in 81.8% and hyper-intense in 18.2%. On T1-SPGR non-contrast imaging, only 14.3% of scolex were hypo or iso-intense and 85.7% were hyperintense; on T1-SPGR-contrast, 30.8% of scolex were hypo or iso-intense while 69.2% were hyperintense. Similarly, the cyst content was hypo or iso-intense in all lesions on T2-FLAIR, SPGR non-contrast and SPGR-contrast sequences. In the SWAN sequence too, the majority of lesions (92.9%) were iso-intense and the rest (7.1%) were hyperintense. On the other hand, the majority of cyst content was hyperintense on T2 (85.7%) and FIESTA (95.2%) sequences. The cyst wall was hypo or iso-intense in all lesions on T2, T2-FLAIR, SPGR non-contrast, SWAN and FIESTA sequences. The cyst wall was hyperintense in the SPGR-contrast-enhanced sequence. Perilesional oedema was present in all patients.
Atypical lesion group (n = 17)
In the atypical lesion group, T2 and T2-FLAIR sequences detected scolex in nine (52.9%) and six (35.3%) patients, respectively. Both routine sequences collectively detected scolex in 10 (58.8%) patients. SPGR, SPGR-contrast, SWAN and FIESTA sequences detected scolex in nine (52.9%), seven (41.2%), 15 (88.2%) and 16 (94.1%) patients, respectively. Advanced sequences collectively detected scolex in 16 (94.1%) patients. As compared to routine sequences advanced sequences detected more scolices, which was statistically significant (P < 0.001). On comparing various sequences, we found the scolex detection rate in descending order of frequency to be ‘collective advanced sequences’ > FIESTA > SWAN > routine > T2 > SPGR non-contrast > SPGR-contrast > T2-FLAIR.
All scolices detected on T2, T2-FLAIR, SWAN and FIESTA were hypo or iso-intense. On the SPGR sequence, only 33.3% of scolex were hypo or iso-intense, and 66.7% were hyper-intense. On SPGR-contrast imaging, 14.3% of scolex were hypo or iso-intense while 85.7% were hyper-intense. Similarly, cyst content was hypo or iso-intense in all lesions in T2-FLAIR, SPGR non-contrast and SPGR-contrast sequences. In the SWAN sequence, too, the majority of lesions (94.1%) were hypo or iso-intense while the rest (5.9%) were hyperintense. On the other hand, hyperintense cyst content was seen in the majority of lesions onT2 (94.1%) and all lesions on the FIESTA sequence. The cyst wall was hypo or iso-intense in all lesions in T2, T2-FLAIR, SPGR non-contrast, SWAN and FIESTA sequences. The cyst wall was hyperintense in the SPGR-contrast sequence. Perilesional oedema was present in all patients.
Follow-up
Table 2 summarises the natural evolution of scolex and cyst characteristics on various MRI sequences during the 3-year follow-up period of typical and atypical lesions.
Table 2.
Natural evolution of scolex and cyst characteristics on various MRI sequences during 3 years follow-up.1
| Baseline |
6 Months |
1 Year |
2 Years |
3 Years |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Typical N = 42 | Atypical N = 17 | Typical N = 32 | Atypical N = 13 | Typical N = 20 | Atypical N = 12 | Typical N = 9 | Atypical N = 7 | Typical N = 6 | Atypical N = 6 | |||
| Scolex | T2 | S | 17 (40.5) | 9 (52.9) | 8 (25) | 3 (23.1) | 4 (20) | 0 (0) | 2 (22.2) | 0(0) | 1(16.7) | 0(0) |
| NS | 25 (59.5) | 8 (47.1) | 19 (59.4) | 8 (61.5) | 11 (55) | 11 (91.7) | 7 (77.8) | 7(100) | 5 (83.3) | 6 (100) | ||
| T2-FLAIR | S | 11 (29.2) | 6 (35.3) | 6 (18.8) | 1 (7.7) | 3 (15) | 0 (0) | 1 (11.1) | 0 (0) | 1 (16.7) | 0 (0) | |
| NS | 31 (70.8) | 11 (64.7) | 18 (56.2) | 11 (84.6) | 11 (55) | 8 (66.7) | 7 (77.8) | 7 (100) | 5 (83.3) | 6 (100) | ||
| Routine | S | 19 (45.2) | 10 (58.8) | 7 (21.9) | 3 (23.1) | 4 (20) | 0 (0) | 2 (22.2) | 0 (0) | 1 (16.7) | 0 (0) | |
| NS | 23 (54.8) | 7 (41.2) | 20 (62.5) | 10 (76.9) | 11 (55) | 11 (91.7) | 7 (77.8) | 7 (100) | 5 (83.3) | 6 (100) | ||
| T1 SPGR (NC) | S | 14 (33.3) | 9 (52.9) | 6 (18.8) | 2 (15.4) | 4 (20) | 0 (0) | 2 (22.2) | 0 (0) | 1 (16.7) | 0 (0) | |
| NS | 28 (66.7) | 8 (47.1) | 17 (53.1) | 10 (76.9) | 8 (50) | 7 (58.3) | 3 (33.3) | 7 (100) | 3 (50) | 6 (100) | ||
| T1 SPGR (contrast) | S | 13 (30.9) | 7 (41.2) | 4 (12.5) | 1 (7.7) | 2 (10) | 0 (0) | 2 (22.2) | 1 (14.3) | 1 (16.7) | 0 (0) | |
| NS | 29 (69.1) | 10 (58.8) | 22 (68.8) | 11 (84.6) | 12 (60) | 7 (58.3) | 4 (44.4) | 6 (85.7) | 5 (83.3) | 6 (100) | ||
| SWAN | S | 30 (71.4) | 15 (88.2) | 11 (34.4) | 4 (30.8) | 4 (20) | 0 (0) | 2 (22.2) | 0 (0) | 1 (16.7) | 0 (0) | |
| NS | 12 (28.6) | 2 (11.8) | 16 (50.0) | 8 (25.0) | 9 (45) | 9 (75) | 7 (77.8) | 7 (100) | 5 (83.3) | 6 (100) | ||
| FIESTA | S | 32 (76.2) | 16 (94.1) | 7 (21.9) | 5 (38.5) | 5 (25) | 0 (0) | 3 (33.3) | 0 (0) | 1 (16.7) | 0 (0) | |
| NS | 10 (23.8) | 1 (05.9) | 13 (40.6) | 7 (21.9) | 8 (40) | 6 (50) | 5 (55.5) | 7 (100) | 4 (66.7) | 6 (100) | ||
| Advanced | S | 35 (83.3) | 16 (94.1) | 10 (31.2) | 5 (38.5) | 5 (25) | 0 (0) | 3 (33.3) | 0 (0) | 1 (16.7) | 0 (0) | |
| NS | 7 (16.7) | 1 (05.9) | 19 (59.3) | 9 (69.2) | 14 (70) | 7 (58.3) | 6 (66.6) | 7 (100) | 5 (83.3) | 6 (100) | ||
| Scolex character | T2 | Ho/Iso | 17 (100) | 8 (88.9) | 8 (100) | 3 (100) | 4 (100) | NA | 2 (100) | NA | 1 (100) | NA |
| Hyr | 0 (0) | 1 (11.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||||
| T2-FLAIR | Ho/Iso | 9 (81.8) | 6 (100) | 6 (100) | 1 (100) | 3 (100) | NA | 1 (100) | NA | 1 (100) | NA | |
| Hyr | 2 (18.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||||
| T1 SPGR (NC) | Ho/Iso | 2 (14.3) | 3 (33.3) | 2 (33.3) | 2 (100) | 1 (25) | NA | 0 (0) | NA | 0 (0) | NA | |
| Hyr | 12 (85.7) | 6 (66.7) | 4 (66.7) | 0 (0) | 3 (75) | 2 (100) | 1 (100) | |||||
| T1 SPGR (contrast) | Ho/Iso | 4 (30.8) | 1 (14.3) | 1 (25) | 0 (0) | 0 (0) | NA | 0 (0) | 1 (100) | 0 (0) | NA | |
| Hyr | 9 (69.2) | 6 (85.7) | 3 (75) | 1 (100) | 2 (100) | 2 (100) | 0 | 1 (100) | ||||
| SWANa | Ho/Iso | 30 (100) | 15 (100) | 11 (100) | 4 (100) | 4 (100) | NA | 2 (100) | NA | 1 (100) | NA | |
| FIESTAa | Ho/Iso | 32 (100) | 16 (100) | 7 (100) | 5 (100) | 5 (100) | NA | 3 (100) | NA | 1 (100) | NA | |
| Cyst content | T2 | Ho | 0 (0) | 0 (0) | 2 (9.5) | 0 (0) | 1 (10) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Iso | 6 (14.3) | 1 (5.9) | 1 (4.8) | 2 (18.2) | 5 (50) | 1 (16.7) | 2 (40) | 1 (20) | 3 (75) | 0 (0) | ||
| Hyr | 36 (85.7) | 16 (94.1) | 18 (85.7) | 9 (81.8) | 4 (40) | 5 (83.3) | 3 (60) | 4 (80) | 1 (25) | 2 (100) | ||
| T2-FLAIRa | Ho | 31 (73.8) | 14 (82.4) | 14 (66.7) | 10 (83.3) | 4 (36.4) | 6 (85.7) | 4 (80) | 6 | 3 (75) | 3 (100) | |
| Iso | 11 (26.2) | 3 (17.6) | 7 (33.3) | 2 (16.7) | 7 (63.6) | 1 (14.3) | 1 (20) | 0 (0) | 1 (25) | 0 (0) | ||
| T1 SPGRa (NC) | Ho | 42 (100) | 17 (100) | 20 (90.9) | 11 (91.7) | 11 (100) | 6 (100) | 3 (100) | 4 (100) | 4 (100) | 3 (100) | |
| Iso | 0 (0) | 0 (0) | 2 (9.1) | 1 (8.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| T1 SPGRa (contrast) | Ho | 42 (100) | 17 (100) | 25 (100) | 11 (100) | 11 (100) | 0 (0) | 6 (100) | 6 (100) | 5 (100) | 3 (100) | |
| Iso | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 9 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| SWAN | Ho | 0 (0) | 2 (11.8) | 0 (0) | 0 (0) | 1 (11.1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Iso | 39 (92.9) | 14 (82.3) | 23 (100) | 10 (90.9) | 8 (88.9) | 1 (100) | 5 (100) | 2 (40) | 4 (100) | 3 (75) | ||
| Hyr | 3 (7.1) | 1 (5.9) | 0 (0) | 1 (9.1) | 0 (0) | 0 (0) | 0 (0) | 3 (60) | 0 (0) | 1 (25) | ||
| FIESTAa | Iso | 2 (4.8) | 0 (0) | 3 (15) | 3 (25) | 7 (58.3) | 5 (83.3) | 3 (60) | 6 (100) | 3 (75) | 0 (0) | |
| Hyr | 40 (95.2) | 17 (100) | 17 (85) | 9 (75) | 5 (41.7) | 1 (16.7) | 2 (40) | 0 (0) | 1 (25) | 4 (100) | ||
| Cyst wall | T2 | Ho | 26 (61.9) | 9 (52.9) | 16 (59.3) | 0 (0) | 11 (84.6) | 11 (100) | 9 (100) | 6 (85.7) | 6 (100) | 5 (100) |
| Iso | 16 (38.1) | 8 (47.1) | 11 (40.7) | 4 (36.4) | 2 (15.4) | 0 (0) | 0 (0) | 1 (14.3) | 0 (0) | 0 (0) | ||
| Hyr | 0 (0) | 0 (0) | 0 (0) | 7 (63.6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| T2-FLAIRa | Ho | 7 (16.7) | 3 (17.6) | 16 (66.7) | 4 (33.3) | 12 (92.3) | 11 (100) | 7 (87.5) | 5 (71.4) | 6 (100) | 6 (100) | |
| Iso | 35 (83.3) | 14 (82.4) | 8 (33.3) | 8 (66.7) | 1 (7.7) | 0 (0) | 1 (12.5) | 2 (28.6) | 0 (0) | 0 (0) | ||
| T1 SPGRa (NC) | Ho | 0 (0) | 0 (0) | 1 (4.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (60) | |
| Iso | 42 (100) | 17 (100) | 22 (95.7) | 12 (100) | 12 (100) | 10 (100) | 3 (100) | 6 (100) | 4 (100) | 2 (40) | ||
| T1 SPGRa (contrast) | Hyr | 42 (100) | 17 (100) | 26 (100) | 12 (100) | 13 (100) | 12 (100) | 8 (100) | 7 (100) | 6 (100) | 6 (100) | |
| SWANa | Ho | 29 (69.1) | 17 (100) | 13 (48.1) | 8 (66.7) | 10 (71.4) | 8 (88.9) | 9 (100) | 3 (42.9) | 6 (100) | 5 (83.3) | |
| Iso | 13 (30.9) | 0 (0) | 14 (51.9) | 4 (33.3) | 4 (28.6) | 1 (11.1) | 0 (0) | 4 (57.1) | 0 (0) | 1 (16.7) | ||
| FIESTAa | Ho | 16 (38.1) | 0 (0) | 20 (100) | 10 (83.3) | 12 (92.3) | 7 (87.5) | 8 (100) | 7 (100) | 5 (100) | 6 (100) | |
| Iso | 26 (61.9) | 17 (100) | 0 (0) | 2 (16.7) | 1 (7.7) | 1 (12.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
A data of sequences with zero values have not been shown in table. Values in parenthesis () are in percentages. Characteristics of non-visualised cyst cavities have not been included. FIESTA: Fast imaging employing steady-state acquisition, FLAIR: Fluid attenuated inversion recovery, Ho: Hypointense, Hyr: Hyperintense, Iso: Isointense, NA: Not applicable, NC: Non-contrast, NS: Not seen, S: Seen, SPGR: Spoiled gradient recalled echo, SWAN: T2 star-weighted angiography.
Typical lesion group
At 6 months, MRI was repeated in 32 patients, with 10 baseline patients refusing imaging. T2 and T2-FLAIR detected scolex in eight (25%) and six (18.8%) patients, respectively. Both routine sequences collectively detected scolex in eight (25%) patients. SPGR, SPGR-contrast, SWAN and FIESTA sequences detected scolex in six (18.8%), four (12.5%), 11 (34.4%) and seven (21.9%) patients, respectively. Advanced sequences collectively detected scolex in 11 (34.4%) patients. The cyst content, wall and scolex characteristics remained similar to baseline on different sequences. Lesions disappeared in four patients while homogenous calcification was observed in one patient.
At 1 year, MRI was performed in 20 patients, with seven patients refusing follow-up imaging. T2 and T2-FLAIR detected scolex in four (25%) and three (15%) patients, respectively. Both routine sequences collectively detected scolex in four (25%) patients. SPGR, SPGR-contrast, SWAN and FIESTA sequences detected scolex in four (20%), two (10%), four (20%) and five (25%) patients, respectively. Advanced sequences collectively detected scolex in five (25%) patients. The cyst content, wall and scolex characteristics remained similar to baseline on different sequences. Lesions disappeared in five more patients and homogenous calcification was observed in four patients.
At 2 years, MRI was performed in nine patients, with two patients refusing follow-up imaging. T2 and T2-FLAIR detected scolex in two (22.2%) and one (11.1%) patient, respectively. Both routine sequences collectively detected scolex in two (22.2%) patients. SPGR, SPGR-contrast, SWAN and FIESTA sequences detected scolex in two (22.2%), two (22.2%), two (22.2) and three (33.3%) patients, respectively. Advanced sequences collectively detected scolex in three (33.3%) patients. The cyst content, wall and scolex characteristics remained similar to baseline on different sequences. No new disappearance of lesions was observed but homogenous calcification was detected in three patients.
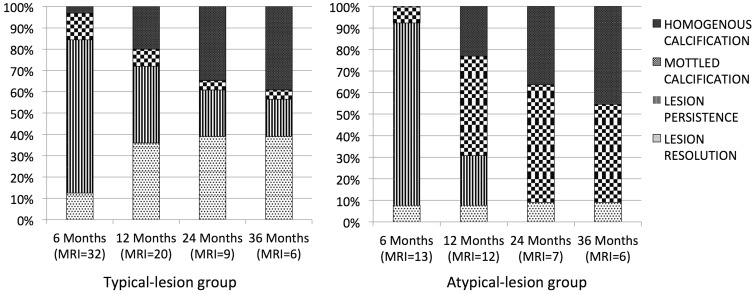
At 3 years, MRI was performed in six patients. Scolex was still visible in only one patient in all sequences. The cyst content, wall and scolex characteristics remained similar to baseline on different sequences. Further resolution of lesions was not observed while homogenous calcification was detected in three patients. Figure 2 summarises the outcomes in the typical lesion and the atypical lesion groups.
Figure 2.
Natural course of typical and atypical lesions during 3-year follow-up.
MRI: magnetic resonance imaging.
Atypical lesion group
At 6 months, MRI was repeated in 13 patients, with four patients refusing follow-up imaging.T2 and T2-FLAIR detected scolex in three (23.1%) and one (7.7%) patient, respectively. Both routine sequences collectively detected scolex in three (23.1%) patients. SPGR, SPGR-contrast, SWAN and FIESTA sequences detected scolex in two (15.4%), one (7.7%), four (30.8%) and five (38.5%) patients, respectively. Advanced sequences collectively detected scolex in five (38.46) patients. The cyst content, wall and scolex characteristics remained similar to baseline on different sequences. Lesions disappeared in one patient.
At 1 year, MRI was performed in 12 patients. Scolex was not detected in any patient on any imaging sequence. The cyst content, wall and scolex characteristics remained similar to baseline on different sequences. Lesions homogenously calcified in three patients.
At 2 years, MRI was performed in seven patients, with two patients refusing follow-up imaging. Scolex was not detected in any patient on any imaging sequence. The cyst content, wall and scolex characteristics remained similar to baseline on different sequences. Lesions homogenously calcified in one patient.
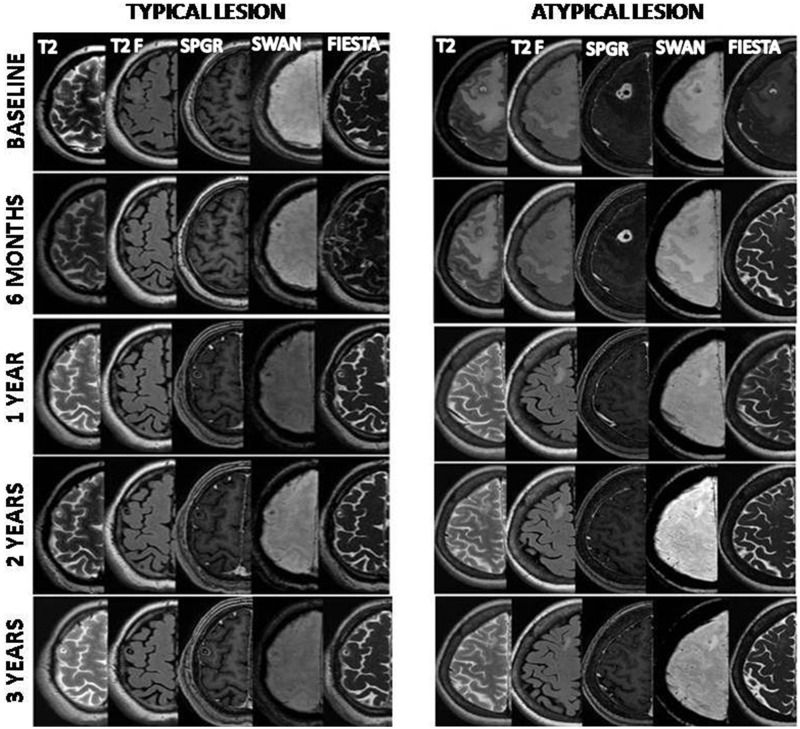
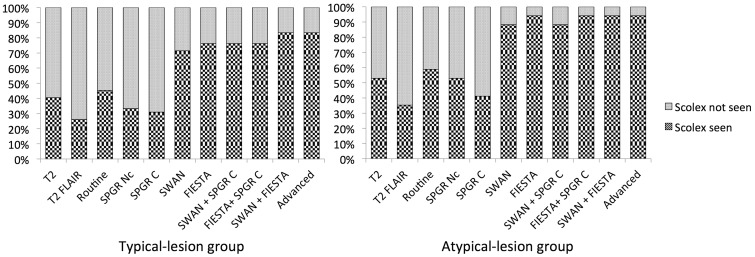
At 3 years, MRI was performed in six patients. Scolex was not detected in any patient on any imaging sequence. The cyst content, wall and scolex characteristics remained similar to baseline on different sequences. Lesions homogenously calcified in one patient. Figure 2 summarises the outcomes in the typical lesion and the atypical lesion groups. Figure 3 depicts the fate of a typical and an atypical lesion over 3 years, while figure 4 compares the ability of different MR sequences, and their combinations, in demonstrating scolex in the two groups.
Figure 3.
The fate of a typical and an atypical lesion over 3 years as seen on different magnetic resonance sequences (T2-T2-weighted, T2F-T2-weighted fluid attenuated inversion recovery, spoiled gradient recalled echo (SPGR) with contrast, T2 star-weighted angiography (SWAN), fast imaging employing steady-state acquisition (FIESTA)).
Figure 4.
Comparison of the ability of different magnetic resonance sequences, and their combinations, in demonstrating scolex in the typical lesion and the atypical lesion group.
FLAIR: fluid attenuated inversion recovery; SPGR: spoiled gradient recalled echo; SWAN: T2 star-weighted angiography; FIESTA: fast imaging employing steady-state acquisition
Seizure recurrence
Seizure recurrence occurred in four patients, three belonged to the typical lesion group while one had an atypical lesion. Recurrence occurred at 3, 6, 7 and 9 months. Two episodes of recurrence occurred in a patient with atypical lesions (3 and 9 months). The rate of recurrence was not statistically different in the two groups.
Discussion
We studied the relative efficacy of conventional MR sequences (T2 and T2-FLAIR) versus advanced MR sequences (SPGR, SPGR-contrast, SWAN and FIESTA) in detecting the scolex in 59 patients with SCG. We found that nearly one-fourth of patients had atypical lesions, associated with diagnostic uncertainty, which showed a lower rate of spontaneous resolution and a higher chance of calcification than the typical lesions. Advanced sequences detected significantly more scolices than conventional sequences. The natural course and fate was found to be different than those projected in previous studies.
In developing countries neurocysticercosis and tuberculoma are common causes of single ring enhancing lesions. Differentiation between these two entities is frequently difficult and a lot depends on the visualisation of the scolex on imaging. Rajshekhar and coworkers proposed and validated the criteria to diagnose SCG based on clinical and CT findings.13,24 Typical rounded, small ring lesions with an eccentric dot are often characteristic of neurocysticercosis. Atypical lesions are often of abnormal morphology (conglomerate or coalesced lesions) with or without significant perilesional oedema and size greater than 20 mm.13,14 These atypical lesions often pose a diagnostic dilemma, and this is where the identification and characterisation of the lesion requires going beyond CT and conventional MRI. Such atypical lesions may be seen in up to one in 10 patients (four/43) and have been referred to as type B lesions, compared with the classic rounded variety (type A) lesions.13 Advanced MR sequences help in demonstrating the scolex more frequently and thus in establishing a definitive diagnosis in patients with atypical enhancing lesions of neurocysticercosis. There is a paucity of data regarding atypical parenchymal cysticercal lesions and their natural fate.
We observed that approximately 29% of our patients with SCG had conglomerate, septated or coalesced appearance on the SPGR-contrast sequence and were analogous to the earlier described type B lesions. These atypical lesions often persist unresolved for a longer period, and may even be associated with a higher risk of seizure recurrence because of the persistence of a calcified focus.
We also observed that, compared to the conventional sequences, advanced MR sequences were more sensitive in demonstrating the scolex. Lucato and coworkers reported that the FLAIR sequence was a better technique to identify scolex.18 do Amaral and coworkers reviewed the value of various newer MRI sequences such as diffusion and perfusion-weighted imaging, spectroscopy, cisternography with FLAIR and supplemental oxygen and 3D-CISS (FIESTA) in atypical lesions.21 3D-CISS (FIESTA) was found to be the best non-invasive sequence to determine intraventricular and subarachnoid cystic lesions, and these imaging sequences demonstrated scolex efficiently in a cysticercal lesion.21 FIESTA sequences are increasingly being used in various medical disorders such as cardiac imaging, lumbar foraminal stenosis, abdominal imaging and cochlear and retro cochlear pathology. In terms of imaging for cysticercal lesions, its use in ventricular and subarachnoid space lesions has been advocated. In a study by Mont’Alverne Filho, FIESTA with SPGR-contrast sequences showed better detection of scolex in intraventricular neurocysticercosis.20 A similar sequence was included in the MRI protocol for suspected neurocysticercosis by Hingwala et al.26 To date, no prospective trial has evaluated the utility of the FIESTA sequence in intraparenchymal neurocysticercosis. In 2012, Verma and coworkers reported the improved detection rate of parenchymal cysticercal lesions with SWAN MRI.22
In our study, we found an increased and better detection of scolex using FIESTA in intraparenchymal lesions. FIESTA significantly detected 32% more scolices than T2 and T2-FLAIR. When combined with SWAN and enhanced SPGR, 37% more scolices were detected than with routine sequences. FIESTA detected scolex in all patients in the atypical lesion group and thus proved helpful in diagnosing such indeterminate lesions.
The exact time when imaging lesions of SCG disappear is not exactly known. A wide variation in the rates of complete resolution has been observed.6,10,27 A CT-based prospective follow-up study of 210 patients observed that at 3 months only 19% of lesions had completely resolved, at 1 year approximately 63% had disappeared, and in 89% patients resolution occurred within 2 years.28 In another prospective study, Singh and coworkers observed that approximately 73% of SCG had disappeared within 2 months on CT.29 As advocated for diagnosis, thin section CT (5 mm) should have been used for follow-up to look for actual lesion disappearance.30,31 It is very likely that one may miss the lesion (as size decreases in the phase of resolution) on follow-up by just performing a conventional CT instead of following a thin-slice protocol. Even with the thin-slice protocols some lesions again may be missed because of sectional inaccuracies and lack of proper differentiation from the background; this happens more commonly with lesions that are yet to calcify and project a proper hypodensity to the appreciated. Contrary to these studies, we found a higher lesion persistence rate on MRI study. In the typical group, spontaneous resolution occurred in 36% at 12 months, with half the resolution occurring before 6 months. Homogenous calcification occurred in 20% at 12 months. Later, follow-up imaging showed increasing calcification rather than resolution, and even at 3 years at least 9.5% had lesion persistence. In the atypical group, spontaneous resolution occurred in one patient at 6 months. The remaining lesions progressively changed from cystic to mottled calcification, and later to homogenous calcification. At 3 years, 45.5% had homogenous calcification and 45.5% had mottled calcification, with spontaneous resolution in only one patient. The atypical lesion group showed more calcification compared to the typical lesion group. Our findings that atypical solitary cysticercal lesions persist longer is important because seizure recurrence in neurocysticercosis has been attributed to persistence or calcification of the lesion.32,33
Conclusion
Advanced sequences such as SWAN, FIESTA and SPGR are very helpful techniques in scolex demonstration as well as in assessing the morphology of SCG. Follow-up with MRI may help in detecting patients with atypical lesions, which have a longer persistence and calcification rate. We advocate the use of advanced MRI sequences to be a part of the imaging protocol for single enhancing CT lesions suspected of SCG.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Yodnopaklow P, Mahuntussanapong A. Single small enhancing CT lesions in Thai patients with acute symptomatic seizures: a clinico-radiological study. Trop Med Int Health 2000; 5: 250–255. [PubMed] [Google Scholar]
- 2.Rajshekhar V, Joshi DD, Doanh NQ, et al. Taenia solium taeniosis/cysticercosis in Asia: epidemiology, impact and issues. Acta Tropica 2003; 87: 53–60. [DOI] [PubMed] [Google Scholar]
- 3.Garg RK. Single enhancing computerized tomography detected lesion in immunocompetent patients. Neurosurg Focus 2002; 12: e4. [DOI] [PubMed] [Google Scholar]
- 4.Garg RK, Malhotra HS. Solitary cysticercus granuloma. Expert Rev Anti Infect Ther 2012; 10: 597–612. [DOI] [PubMed] [Google Scholar]
- 5.Rajshekhar V and Chandy MJ (eds). Incidence of solitary cysticercus granulomas. In: Solitary Cysticercus Granuloma. Chennai: Oriental Longman Ltd., 2000, pp. 12–28.
- 6.Garg RK, Singh MK, Misra S. Single-enhancing CT lesions in Indian patients with seizures: a review. Epilepsy Res 2000; 38: 91–104. [DOI] [PubMed] [Google Scholar]
- 7.Del Brutto OH, Nash TE, Garcia HH. Cysticerci-related single parenchymal brain enhancing lesions in non-endemic countries. J Neurol Sci 2012; 319: 32–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ndimubanzi PC, Carabin H, Budke CM, et al. A systematic review of the frequency of neurocysticercosis with a focus on people with epilepsy. PLoS Negl Trop Dis 2010; 4: e870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murthy JMK, Yangala R, Srinivas M. The syndromic classification of the International League Against Epilepsy: a hospital-based study from south India. Epilepsia 1998; 39: 48–54. [DOI] [PubMed] [Google Scholar]
- 10.Garg RK, Nag D. Single ring- or disk-enhancing computed tomographic lesion in Indian children and adolescents after first seizure. Arch Pediatr Adolesc Med 1997; 157: 632–634. [DOI] [PubMed] [Google Scholar]
- 11.Garcia HH, Modi M. Helminthic parasites and seizures. Epilepsia 2008; 49(Suppl 6): 25–32. [DOI] [PubMed] [Google Scholar]
- 12.Carabin H, Ndimubanzi PC, Budke CM, et al. Clinical manifestations associated with neurocysticercosis: a systematic review. PLoS Negl Trop Dis 2011; 5: e1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rajshekhar V, Chacko G, Haran RP, et al. Clinicoradiological and pathological correlations in patients with solitary cysticercus granuloma and epilepsy: focus on presence of the parasite and edema formation. J Neurol Neurosurg Psychiatry 1995; 59: 284–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajshekhar V. Solitary cerebral cysticercus granuloma. Epilepsia 2003; 44(Suppl 1): 25–28. [DOI] [PubMed] [Google Scholar]
- 15.Agarwal PP, Gaikwad SB, Garg A, et al. Giant intraparenchymal neurocysticercosis: Unusual MRI findings. Neurol India 2004; 52: 259–260. [PubMed] [Google Scholar]
- 16.Singh G, Rajshekhar V, Murthy JM, et al. A diagnostic and therapeutic scheme for a solitary cysticercus granuloma. Neurology 2010; 75: 2236–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Del Brutto OH, Rajshekhar V, White AC, Jr., et al. Proposed diagnostic criteria for neurocysticercosis. Neurology 2001; 57: 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lucato LT, Guedes MS, Sato JR, et al. The role of conventional MR imaging sequences in the evaluation of neurocysticercosis: impact on characterization of the scolex and lesion burden. Am J Neuroradiol 2007; 28: 1501–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.García HH, Del Brutto OH. Imaging findings in neurocysticercosis. Acta Tropica 2003; 87: 71–78. [DOI] [PubMed] [Google Scholar]
- 20.Mont’Alverne Filho FE, Machado Ldos R, Lucato LT, et al. The role of 3D volumetric MR sequences in diagnosing intraventricular neurocysticercosis: preliminary results. Arq Neuropsiquiatr 2011; 69: 74–78. [DOI] [PubMed] [Google Scholar]
- 21.do Amaral LL, Ferreira RM, da Rocha AJ, et al. Neurocysticercosis: evaluation with newer MR techniques and atypical forms. Top Magn Reson Imaging 2005; 16: 127–144. [DOI] [PubMed] [Google Scholar]
- 22.Verma A, Awasthi R, Prasad KN, et al. Improved detection of parenchymal cysticercal lesions in neurocysticercosis with T2*-weighted angiography magnetic resonance imaging. Acad Radiol 2012; 19: 958–964. [DOI] [PubMed] [Google Scholar]
- 23.Robbani I, Razdan S, Pandita KK. Diagnosis of intraventricular cysticercosis by magnetic resonance imaging: improved detection with three-dimensional spoiled gradient recalled echo sequences. Australas Radiol 2004; 48: 237–239. [DOI] [PubMed] [Google Scholar]
- 24.Rajshekhar V, Chandy MJ. Validation of diagnostic criteria for solitary cysticercus granuloma in patients presenting with seizure. Acta Neurol Scand 1997; 96: 76–81. [DOI] [PubMed] [Google Scholar]
- 25.Kulkarni M. Constructive interference in steady-state/FIESTA-C clinical applications in neuroimaging. J Med Imaging Radiat Oncol 2011; 55: 183–190. [DOI] [PubMed] [Google Scholar]
- 26.Hingwala D, Chatterjee S, Kesavadas C, et al. Applications of 3D CISS sequence for problem solving in neuroimaging. Indian J Radiol Imaging 2011; 21: 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Souza A, Nalini A, Kovoor JM, et al. Natural history of solitary cerebral cysticercosis on serial magnetic resonance imaging and the effect of albendazole therapy on its evolution. J Neurol Sci 2010; 288: 135–141. [DOI] [PubMed] [Google Scholar]
- 28.Rajshekhar V. Rate of spontaneous resolution of a solitary cysticercus granuloma in patients with seizures. Neurology 2001; 57: 2315–2317. [DOI] [PubMed] [Google Scholar]
- 29.Singh MK, Garg RK, Nath G, et al. Single small enhancing computed tomographic (CT) lesions in Indian patients with new-onset seizures. A prospective follow-up in 75 patients. Seizure 2001; 10: 573–578. [DOI] [PubMed] [Google Scholar]
- 30.Souza AD, Nalini A, Srikanth SG. Solitary cerebral parenchymal cysticercosis: A prospective comparative study with computed tomography and magnetic resonance imaging. Neurol India 2013; 61: 639–643. [DOI] [PubMed] [Google Scholar]
- 31.Rajshekhar V, Chandy MJ. Comparative study of CT and MRI in patients with seizures and a solitary cerebral cysticercus granuloma. Neuroradiology 1996; 38: 542–546. [DOI] [PubMed] [Google Scholar]
- 32.Kishore D, Misra S. Short course of oral prednisolone on disappearance of lesion and seizure recurrence in patients of solitary cysticercal granuloma with single small enhancing CT lesion: an open label randomized prospective study. J Ass Physicians India 2007; 55: 419–424. [PubMed] [Google Scholar]
- 33.Rajshekhar V, Jeyaseelan L. Seizure outcome in patients with a solitary cerebral cysticercus granuloma. Neurology 2004; 62: 2236–2240. [DOI] [PubMed] [Google Scholar]