Abstract
Objective
The purpose of this report was to discuss the overall limitations, safety and efficacy of flow-diverter stenting for intracranial aneurysms.
Methods
The authors performed a meta-analysis from January 2009 to September 2014 using the terms “flow diverter” and “intracranial aneurysms.” Additional studies were identified through references in each reviewed article. Data extraction, performed independently by the authors, included demographic data, technical and clinical complications, morbidity and mortality, aneurismal occlusion rates related to flow-diverter devices. The analysis was performed using a fixed effect.
Results
Twenty-nine studies with 1524 patients and three to 62 months of follow-up were identified for analysis. The overall technical failure and complication rate was 9.3% (95% CI 6%–12.6%). The rate of procedure-related complication was 14% (95% CI 10.2%–17.9%) and 6.6% (95% CI 4%–9.1%) for morbidity and mortality. Fusiform, dissecting and circumferential aneurysm (OR 3.10, 95% CI 0.93–10.37) were significant risk factors for technical failure and complication. Posterior circulation location (OR 4.03, 95% CI 2.45–6.61), peripheral location (OR 2.74, 95% CI 1.52–4.94) and fusiform, dissecting and circumferential aneurysm (OR 1.95, 95% CI 1.15–3.30) were statistically significant risk factors for procedure-related complications. Posterior circulation location (OR 4.39, 95% CI 2.44–7.90) and peripheral location (OR 3.64, 95% CI 1.74–7.62) were statistically significant risk factors for morbidity and mortality.
Conclusions
Fusiform, dissecting and circumferential aneurysm, posterior circulation and peripheral locations have greater procedure-related complications.
Keywords: Intracranial aneurysm, endovascular treatment, flow-diverter stenting
Introduction
Complex unruptured aneurysms such as fusiform, large and/or giant, or wide neck, as well as very small aneurysms or blood blister aneurysms that might be untreatable by conventional coiling and recurrences, can be considered amenable to flow-diverter devices.1 However, flow-diverter devices may cause bleeding or ischemic complications, which are a considerable source of neurological morbidity and mortality.2 With an accumulating number of studies of flow-diverter device treatment of intracranial aneurysms, our understanding of its role in endovascular treatment of intracranial aneurysms continues to evolve.2,3 Unfortunately, there is a remarkable degree of heterogeneity in previous studies regarding results and outcomes of flow-diverter devices in the treatment of intracranial aneurysms.3 We performed a meta-analytical review of the literature about their efficacy, safety, limitations and developments.
Methods
A PubMed, Embase and Web of Science search using the terms “flow diverter” and “intracranial aneurysms” was performed from January 2009 to September 2014. We incorporated English-language studies including more than five patients treated with flow-diverter devices and follow-up outcome. References in the reviewed studies were also incorporated if the inclusion criteria were met. Case reports, review studies and studies pertinent to lab experiments and animal experiments were excluded.
Data extraction
We extracted clinical data including patient age, patient sex, aneurysm location, aneurysm size, prior rupture, antiplatelet medication, flow-diverter type, aneurysm occlusion rate, procedure-related complications, morbidity and mortality. We calculated the aneurysm occlusion rate, procedure-related complications, morbidity and mortality across these studies, also making note of subgroup analyses for risk factors. Hazard ratios were extracted for each risk factor.
Data analysis
Statistical analysis was performed using the Meta package in RevMan 5. The primary outcome measure was the procedure-related complications. Procedure-related complications were classified as early (≤30 days) and delayed (>30 days). To explain variability in the primary outcome, we defined priori variables: patient sex, patient age, aneurysm size (<15 mm, ≥ 15 mm), aneurysm location (internal carotid artery (ICA), vertebrobasilar (VB), or peripheral (including anterior cerebral artery (ACA), middle cerebral artery (MCA), posterior cerebral artery (PCA), or ICA bifurcation), and antiplatelet medication. The meta-analyses of odds ratios (ORs) and p values were performed using a fixed-effects approach.
Results
Literature search
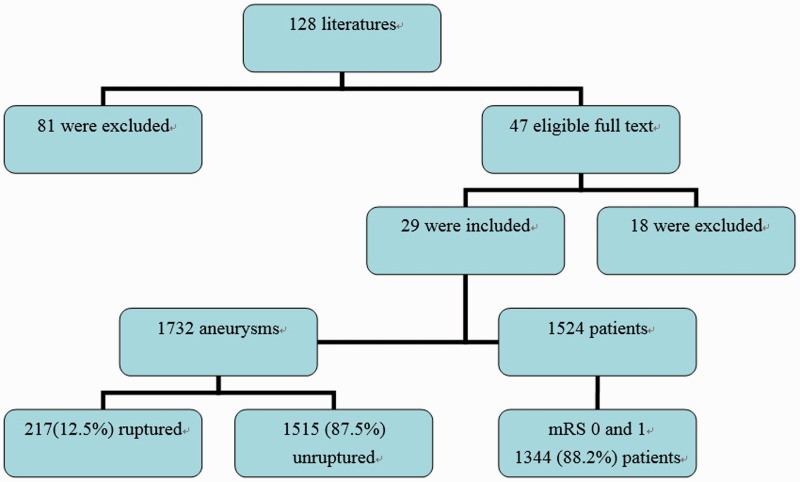
After an initial screening of 128 articles, 47 full-text articles were perused for eligibility, affording 29 studies providing adequate information about treated patients. Eighteen studies were excluded for missing detailed demographic and follow-up data. Across the 29 reviewed studies, there were 1524 patients followed up for three to 62 months4–32 (Figure 1). Twenty-five studies were retrospective and four studies were prospective; two analyses were multicenter and 27 were single-center. Silk (Balt Extrusion, Montmorency, France) was used in 16 studies, Pipeline (Pipeline embolization device(PED), Covidien, Mansfield, MA, USA) was used in 16 studies, flow redirection endoluminal device (FRED) Microvention, Tustin, CA, USA) was used in two studies and Surpass (Stryker, Neurovascular, Fremont, CA, USA) was used in two studies.
Figure 1.
Chart of the systematic review.
mRS: modified Rankin Scale score.
Demographic details
In 1524 patients with 1732 aneurysms, only 12.5% were treated after acute rupture (95% confidence interval (CI) 3.1%–21.9%)). The mean age at presentation was 53.7 ± 5.9 years old (range 10–89 years old). A total of 75.3% of patients were female (95% CI 69%–81.6%). Of aneurysms, 85.3% were anterior circulation (95% CI 78%–92.6%), 13.9% were posterior circulation (95% CI 6.7%–21.1%) and 6.2% were peripheral location, including ICA bifurcation, anterior communicating artery (Acoma), MCA, ACA and PCA (95% CI 2.6%–9.7%). A total of 54.7% of aneurysms were small (<10 mm, 95% CI 36.9%–72.5%) and 42.1% of aneurysms were large and giant (≥10 mm, 95% CI 24.6%–59.5%). There were 1656 flow diverters deployed, with an average of 1.1 per patient. Dual-antiplatelet regimens (aspirin and clopidogrel) were used in 26 studies and a mono-antiplatelet regimen (ticlopidine or clopidogrel) was used in three studies. The loading doses varied and more than 300 mg of aspirin and clopidogrel for at least six hours or 75 mg clopidogrel and less than 300 mg aspirin for three to seven days. Platelet inhibition testing was not performed.
Technical failure and complications
The overall technical failure and complication rate was 9.3% (95% CI 6–12.6). The rate of incomplete deployment was 5.2% (95% CI 3.1–7.4), failure was 1.2% (95% CI 0.2–2.3), vessel perforation was 0.9% (95% CI 0.1–1.6), misplacement was 0.9% (95% CI 0–1.7), stent migration was 0.5%(95% CI 0.2–1.2), ICA dissection was 0.5% (95% CI 0–1.0), and wire fracture was 0.1% (95% CI 0–0.2).
Early procedure-related complications
The rate of early procedure-related complication was 14% (95% CI 10.2–17.9), including 5% (95% CI 3–6.9) acute ischemic events (such as side branch and intra-stent thrombosis), 4.2% (95% CI 2.0–6.3) delayed ischemic events, 2.3% (95% CI 0.4–4.2) massive effect, 1.8% (95% CI 0.7–2.9) delayed hemorrhage, and 1.0% (95% CI 0.3–1.7) acute hemorrhage.
Delayed procedure-related complications
The delayed procedure-related complication rate was 6.6% (95% CI 4–9.1), including 3.3% (95% CI 1.4–5.3) morbidity and 3.2% (95% CI 1.8–4.7) mortality. The reasons for morbidity were 2.5% (95% CI 0.5–4.4) thromboembolic, 0.6% (95% CI 0.1–1.1) hemorrhagic and 0.3% (95% CI 0–0.6) mass effect. Reasons for mortality were 1.6% (95% CI 0.6–2.7) hemorrhagic, 0.5% (95% CI 0.1–1.0) thromboembolic, 0.5% (95% CI 0.2–1.2) infection, 0.3% (95% CI 0.1–0.7) myocardial infarct, and 0.2% (95% CI0–0.5) mass effect.
Clinical and follow-up imaging outcome
After three to 62 months of follow-up, the overall favorable clinical outcome (modified Ranking Scale score (mRS) 0 and 1) rate33 was 88.2% (95% CI 80.9–95.4), and complete or nearly complete (Raymond I and II34) aneurysmal occlusion rate was 84.4% (95% CI 79.9–88.9).
Risk factors for technical failure and complications, procedure-related complications, morbidity and mortality (Table 1)
Table 1.
Odd ratios and p values for potential risk factors for technical failure and complications, procedure-related complications, morbidity and mortality.
| Technical failure and complications OR (95% CI), p value | Procedure-related complications OR (95% CI), p value | Morbidity and mortality OR (95% CI), p value | |
|---|---|---|---|
| Female sex | 0.89 (0.18, 4.35), 0.89 | 1.59 (0.68, 3.73), 0.29 | 1.0 5(0.38, 2.90), 0.92 |
| Posterior circulation location | 0.73 (0.27, 2.0), 0.54 | 4.03 (2.45, 6.61), <0.0001 | 4.39 (2.44, 7.90), <0.0001 |
| Peripheral location | 1.45 (0.45, 4.73), 0.54 | 2.74 (1.52, 4.94), 0.0008 | 3.64 (1.74, 7.62), 0.0006 |
| Fusiform, dissecting and circumferential | 3.10 (0.93, 10.37), 0.07 | 1.95 (1.15, 3.30), 0.01 | 1.54 (0.73, 3.24), 0.26 |
| Unruptured | 0.45 (0.08, 2.44), 0.36 | 1.89 (0.80, 4.44), 0.15 | 1.00 (0.33, 3.00), 1.00 |
| Large and giant size | 1.71 (0.17, 17.15), 0.65 | 1.75 (0.72, 4.26), 0.22 | 1.04 (0.29, 3.80), 0.95 |
OR: odds ratio; CI: confidence interval.
Statistically significant risk factors for procedure-related complications were posterior circulation location (OR 4.03, 95% CI 2.45–6.61, p < 0.0001), peripheral location (OR 2.74, 95% CI 1.52–4.94, p = 0.0008) and fusiform, dissecting and circumferential aneurysm (OR 1.95, 95% CI 1.15–3.30, p = 0.01). Posterior circulation location (OR 4.39, 95% CI 2.44–7.90, p < 0.0001) and peripheral location (OR 3.64, 95% CI 1.74–7.62, p = 0.0006) were statistically significant risk factors for morbidity and mortality. Fusiform, dissecting and circumferential aneurysm (OR 1.54, 95% CI 0.73–3.24, p = 0.26) demonstrated a trend toward an increased risk of morbidity and mortality that was not statistically significant. In addition, fusiform, dissecting and circumferential aneurysm (OR 3.10, 95% CI 0.93–10.37, p = 0.07) and peripheral location (OR 1.45, 95% CI 0.45–4.37, p = 0.54) demonstrated a trend toward an increased risk of technical failure and complication. Female sex, unruptured aneurysm and large and giant size were not statistically significant risk factors for any adverse events.
Discussion
Flow-diverter devices in the treatment of intracranial aneurysms has been associated with 88.2% (95% CI 80.9%–95.4%) favorable clinical outcome, 84.4% (95% CI 79.9–88.9%) complete or nearly complete aneurysmal occlusion rate, 3.3% (95% CI 1.4–5.3%) morbidity and 3.2% (95% CI 1.8–4.7%) mortality. Two meta-analyses were attempted to clearly delineate their risks and efficacy in 2013, and they continue to present a considerable challenge owing to a remarkable degree of heterogeneity and publication biases not only in study design but also in results.3 Published aneurysmal complete occlusion rates are often variable, ranging anywhere from 55% to 95%.22,28 Among larger studies, mortality25,35 rates have ranged from 0% to 7%, whereas morbidity25,32 has ranged from 0% to 12%. This is probably in part due to the limited statistical power of an individual study, prompting our meta-analysis.
Overall complication rate
Arrese et al.3 collected 15 studies, and their early mortality rate was 2.8% (95% CI 1.7–3.8) and late mortality rate was 1.3% (95% CI 0.2–2.3). The early neurological morbidity rate was 7.3% (95% CI 5.7–9) and the late morbidity rate was 2.6% (95% CI 1.1–4). In the Brinjikji et al. analysis2 of 29 studies, aneurysmal complete occlusion rate was 76% (95% CI 70–81), procedure-related morbidity and mortality rates were 5% (95% CI 4–7) and 4% (95% CI 3–6), respectively. Our analysis of 29 studies demonstrated an technical failure and complication rate of 9.3% (95% CI 6–12.6), procedure-related complication rate of 14% (95% CI 10.2–17.9), morbidity and mortality rate of 6.6% (95% CI 4–9.1), favorable clinical outcome rate of 88.2% (95% CI 80.9–95.4) and complete or nearly complete aneurysmal occlusion rate of 84.4% (95% CI 79.9–88.9). Our results were comparable to two previous studies. This blanket value is highly influenced by the factors discussed below and is crucial to the therapeutic option for intracranial aneurysms.
Posterior circulation location
Flow-diverting stents may be an option for basilar artery (BA) aneurysms that are either difficult to treat by conventional endovascular methods, recur after previous endovascular treatments, or are candidates for high recurrence rates and disease progression. There is a lack of extensive data regarding the use of flow diverters for VB aneurysms. In a series of 12 flow-diverter cases for BA aneurysms, 66% had good functional outcomes, but there were five strokes (41%).29 The first and major concern of using a high-structural-profile device for vessel reconstruction in the BA is the patency of the perforators.29 Patients with posterior circulation aneurysms are at higher risk of ischemic stroke, particularly perforator infarction.2 Treatment by endovascular means with flow diversion is one option but is not necessarily the safest or most definitive treatment modality.36 We found that posterior circulation location was a risk factor for ischemic and hemorrhagic procedure-related complications and morbidity and mortality. Matching our experience, the use of flow-diverter devices for posterior circulation aneurysms was associated with more complications, and extra caution is required.
Fusiform, dissecting and circumferential aneurysms
Flow-diverter implantation offers a way to treat fusiform, dissecting and circumferential aneurysms that cannot be treated conventionally.26 These aneurysms are often located as posterior circulations and have a giant size.1 Those patients usually present with ischemic stroke, mass effect and have a dismal natural history37,38 that will rapidly deteriorate after treatment, most commonly from progressive massive effect, ischemic symptoms and brain-stem infarcts as well as ruptures.36 Currently, we are still at a loss for finding an optimal safe and effective treatment for large or giant VB fusiform aneurysms. Fusiform, dissecting and circumferential aneurysms are risk factors for flow diverter-related complications and show a trend to morbidity and mortality. Strategies applied thus far have had mixed results at best,36 including most recently flow diversion; a careful determination of the risks and benefits of intervention is appropriate.
Peripheral location
Flow diverter devices were originally designed for side-wall aneurysms and its use in bifurcation aneurysms had been limited. Nelson et al.39 reported a left basal ganglion infarct secondary to two pipeline embolization devices placed in the M1 segment of the left MCA with a pre-existing Neuroform stent. Van Rooij and Sluzewski reported another case of left basal ganglion infarction after placement of two telescoping pipeline embolization devices in the A1 segment of the ACA.40 Both cases were thought to be related to occlusion of lenticulostriate arteries from M1 and A1 segments of the MCA and ACA, respectively. Nossek et al.41 found that deployment of a pipeline embolization device from the distal supraclinoid ICA to the M1 may result in reversal of flow in the ACA/Acoma complex and regression of the ipsilateral A1. These reports raise concern about using flow-diverter devices in segments of vessels distal to Willis circle.
Technical complications
Device deployment is successful consistently in 87% to 100% across many studies,22,26,31,42 technical complications occurred in a range of 3.1% to 33.3% depending on the definition of technical complications in different studies.43 In our analysis, there was a 9.3% (95% CI 6–12.6) technical failure and complication rate, including incomplete deployment, failure, vessel perforation, misplacement, stent migration, ICA dissection, and wire fracture. Incomplete deployment of the flow-diverter device was mainly observed in vessel segments with tight or acute curves.26 Allowance for the anticipated foreshortening of approximately 50% and possible device shift during deployment is critical when choosing the length of the device.27 There is a potential risk of an endoleak-like phenomenon with implantation of an undersized device, which results in poor wall apposition. Similarly, implantation of an oversized device may result in poor coverage of the lesion because of an incomplete compaction of the strands.26 The diameter of the proximal segment of the target vessel and the diameter at the origin of the lesion determine the diameter of the flow-diverter device.44 The distal vessel diameter is of lesser importance. Selection of a stent diameter 0.25 to 0.5 mm larger than the distal parent diameter is also recommended.42 Most of these complications did not result in clinically significant sequelae.43
Study limitations
Although the incorporated studies adhered to a similar endovascular approach using flow-diverter devices, all drew from patients presenting to a selected hospital or group of hospitals and were not population based. All studies were limited by selection bias, as patients with an anticipated difficulty or failure of conventional microsurgical or endovascular techniques should particularly be considered for flow-diverter device treatment. The follow-up of patients treated by flow-diverter devices was short term and may be more limited. In addition, patients lost to follow-up were often not addressed. According to the publication bias analysis, small studies have reported more adverse outcomes than large studies.3 Our methods were limited by our English-language restriction. We intentionally provide 2009 as a start date for our PubMed search and used very broad search terms because of the learning curve and more technical problems in early cases.43
Conclusions
We demonstrated an overall morbidity rate 3.3% (95% CI 1.4–5.3) and mortality rate of 3.2% (95% CI 1.8–4.7) for flow-diverter devices in the treatment of intracranial aneurysms. Posterior circulation, fusiform, dissecting and circumferential aneurysm and peripheral location are significant risk factors for greater procedure-related complications.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Lv X, Jiang C, Li Y, et al. Treatment of giant intracranial aneurysms. Interv Neuroradiol 2009; 15: 135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brinjikji W1, Murad MH, Lanzino G, et al. Endovascular treatment of intracranial aneurysms with flow diverters: A meta-analysis. Stroke 2013; 44: 442–447. [DOI] [PubMed] [Google Scholar]
- 3.Arrese I, Sarabia R, Pintado R, et al. Flow-diverter devices for intracranial aneurysms: Systematic review and meta-analysis. Neurosurgery 2013; 73: 193–199. [DOI] [PubMed] [Google Scholar]
- 4.Kocer N, Islak C, Kizilkilic O, et al. Flow re-direction endoluminal device in treatment of cerebral aneurysms: Initial experience with short-term follow-up results. J Neurosurg 2014; 120: 1158–1171. [DOI] [PubMed] [Google Scholar]
- 5.Tan LA, Keigher KM, Munich SA, et al. Thromboembolic complications with Pipeline Embolization Device placement: Impact of procedure time, number of stents and pre-procedure P2Y12 reaction unit (PRU) value. J Neurointerv Surg 2015; 7: 217–221. [DOI] [PubMed] [Google Scholar]
- 6.Aydin K, Arat A, Sencer S, et al. Treatment of ruptured blood blister-like aneurysms with flow diverter SILK stents. J Neurointerv Surg 2015; 7: 202–209. [DOI] [PubMed] [Google Scholar]
- 7.Benaissa A, Januel AC, Herbreteau D, et al. Endovascular treatment with flow diverters of recanalized and multitreated aneurysms initially treated by endovascular approach. J Neurointerv Surg 2015; 7: 44–49. [DOI] [PubMed] [Google Scholar]
- 8.Kim LJ, Tariq F, Levitt M, et al. Multimodality treatment of complex unruptured cavernous and paraclinoid aneurysms. Neurosurgery 2014; 74: 51–56. [DOI] [PubMed] [Google Scholar]
- 9.Puffer RC, Piano M, Lanzino G, et al. Treatment of cavernous sinus aneurysms with flow diversion: Results in 44 patients. AJNR Am J Neuroradiol 2014; 35: 948–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen JE, Gomori JM, Moscovici S, et al. Delayed complications after flow-diverter stenting: Reactive in-stent stenosis and creeping stents. J Clin Neurosci 2014; 21: 1116–1122. [DOI] [PubMed] [Google Scholar]
- 11.Shankar JJ, Vandorpe R, Pickett G, et al. SILK flow diverter for treatment of intracranial aneurysms: Initial experience and cost analysis. J Neurointerv Surg 2013; 5(Suppl 3): iii11–iii15. [DOI] [PubMed] [Google Scholar]
- 12.Delgado Almandoz JE, Crandall BM, Scholz JM, et al. Pre-procedure P2Y12 reaction units value predicts perioperative thromboembolic and hemorrhagic complications in patients with cerebral aneurysms treated with the Pipeline Embolization Device. J Neurointerv Surg 2013; 5(Suppl 3): iii3–iii10. [DOI] [PubMed] [Google Scholar]
- 13.Diaz O, Gist TL, Manjarez G, et al. Treatment of 14 intracranial aneurysms with the FRED system. J Neurointerv Surg 2014; 6: 614–617. [DOI] [PubMed] [Google Scholar]
- 14.Malatesta E, Nuzzi NP, Divenuto I, et al. Endovascular treatment of intracranial aneurysms with flow-diverter stents: Preliminary single-centre experience. Radiol Med 2013; 118: 971–983. [DOI] [PubMed] [Google Scholar]
- 15.Chalouhi N, Tjoumakaris S, Starke RM, et al. Comparison of flow diversion and coiling in large unruptured intracranial saccular aneurysms. Stroke 2013; 44: 2150–2154. [DOI] [PubMed] [Google Scholar]
- 16.De Vries J, Boogaarts J, Van Norden A, et al. New generation of Flow Diverter (surpass) for unruptured intracranial aneurysms: A prospective single-center study in 37 patients. Stroke 2013; 44: 1567–1577. [DOI] [PubMed] [Google Scholar]
- 17.Çinar C, Bozkaya H, Oran I. Endovascular treatment of cranial aneurysms with the pipeline flow-diverting stent: Preliminary mid-term results. Diagn Interv Radiol 2013; 19: 154–164. [DOI] [PubMed] [Google Scholar]
- 18.Piano M, Valvassori L, Quilici L, et al. Midterm and long-term follow-up of cerebral aneurysms treated with flow diverter devices: A single-center experience. J Neurosurg 2013; 118: 408–416. [DOI] [PubMed] [Google Scholar]
- 19.Lin LM, Colby GP, Kim JE, et al. Immediate and follow-up results for 44 consecutive cases of small (<10 mm) internal carotid artery aneurysms treated with the pipeline embolization device. Surg Neurol Int 2013; 4: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kulcsár Z, Augsburger L, Reymond P, et al. Flow diversion treatment: Intra-aneurismal blood flow velocity and WSS reduction are parameters to predict aneurysm thrombosis. Acta Neurochir (Wien) 2012; 154: 1827–1834. [DOI] [PubMed] [Google Scholar]
- 21.Briganti F, Napoli M, Tortora F, et al. Italian multicenter experience with flow-diverter devices for intracranial unruptured aneurysm treatment with periprocedural complications—a retrospective data analysis. Neuroradiology 2012; 54: 1145–1152. [DOI] [PubMed] [Google Scholar]
- 22.Saatci I, Yavuz K, Ozer C, et al. Treatment of intracranial aneurysms using the pipeline flow-diverter embolization device: A single-center experience with long-term follow-up results. AJNR Am J Neuroradiol 2012; 33: 1436–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kizilkilic O, Kocer N, Metaxas GE, et al. Utility of VasoCT in the treatment of intracranial aneurysm with flow-diverter stents. J Neurosurg 2012; 117: 45–49. [DOI] [PubMed] [Google Scholar]
- 24.Maimon S, Gonen L, Nossek E, et al. Treatment of intra-cranial aneurysms with the SILK flow diverter: 2 years’ experience with 28 patients at a single center. Acta Neurochir (Wien) 2012; 154: 979–987. [DOI] [PubMed] [Google Scholar]
- 25.Berge J, Biondi A, Machi P, et al. Flow-diverter silk stent for the treatment of intracranial aneurysms: 1-year follow-up in a multicenter study. AJNR Am J Neuroradiol 2012; 33: 1150–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fischer S, Vajda Z, Aguilar Perez M, et al. Pipeline embolization device (PED) for neurovascular reconstruction: Initial experience in the treatment of 101 intracranial aneurysms and dissections. Neuroradiology 2012; 54: 369–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lubicz B, Collignon L, Raphaeli G, et al. Flow-diverter stent for the endovascular treatment of intracranial aneurysms: A prospective study in 29 patients with 34 aneurysms. Stroke 2010; 41: 2247–2253. [DOI] [PubMed] [Google Scholar]
- 28.Byrne JV, Beltechi R, Yarnold JA, et al. Early experience in the treatment of intra-cranial aneurysms by endovascular flow diversion: A multicentre prospective study. PLoS One 2010; 5: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kulcsár Z, Ernemann U, Wetzel SG, et al. High-profile flow diverter (silk) implantation in the basilar artery: Efficacy in the treatment of aneurysms and the role of the perforators. Stroke 2010; 41: 1690–1696. [DOI] [PubMed] [Google Scholar]
- 30.Briganti F, Napoli M, Leone G, et al. Treatment of intracranial aneurysms by flow diverter devices: Long-term results from a single center. Eur J Radiol 2014; 83: 1683–1690. [DOI] [PubMed] [Google Scholar]
- 31.Lylyk P, Miranda C, Ceratto R, et al. Curative endovascular reconstruction of cerebral aneurysms with the pipeline embolization device: The Buenos Aires experience. Neurosurgery 2009; 64: 632–643. [DOI] [PubMed] [Google Scholar]
- 32.Leonardi M, Cirillo L, Toni F, et al. Treatment of intracranial aneurysms using flow-diverting silk stents (BALT): A single centre experience. Interv Neuroradiol 2011; 17: 306–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Haan R, Limburg M, Bossuyt P, et al. The clinical meaning of Rankin ‘handicap’ grades after stroke. Stroke 1995; 26: 2027–2030. [DOI] [PubMed] [Google Scholar]
- 34.Raymond J, Guilbert F, Weill A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke 2003; 34: 1398–1403. [DOI] [PubMed] [Google Scholar]
- 35.Velioglu M, Kizilkilic O, Selcuk H, et al. Early and midterm results of complex cerebral aneurysms treated with Silk stent. Neuroradiology 2012; 54: 1355–1365. [DOI] [PubMed] [Google Scholar]
- 36.Siddiqui AH, Abla AA, Kan P, et al. Panacea or problem: Flow diverters in the treatment of symptomatic large or giant fusiform vertebrobasilar aneurysms. J Neurosurg 2012; 116: 1258–1266. [DOI] [PubMed] [Google Scholar]
- 37.Lv X, Li Y, Jiang C, et al. Endovascular treatment using stents for vertebral artery fusiform aneurysms. Neurol Res 2010; 32: 792–795. [DOI] [PubMed] [Google Scholar]
- 38.Lv X, Lv M, Li Y, et al. Endovascular treatment of ruptured and unruptured vertebral artery aneurysms. Digital-Neuroradiol J 2011; 1: 797–806. [DOI] [PubMed] [Google Scholar]
- 39.Nelson PK, Lylyk P, Szikora I, et al. The pipeline embolization device for the intracranial treatment of aneurysms trial. AJNR Am J Neuroradiol 2011; 32: 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Rooij WJ, Sluzewski M. Perforator infarction after placement of a pipeline flow-diverting stent for an unruptured A1 aneurysm. AJNR Am J Neuroradiol 2010; 31: E43–E44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nossek E, Chalif DJ, Chakraborty S, et al. Modifying flow in the ICA bifurcation: Pipeline deployment from the supraclinoid ICA extending into the M1 segment-clinical and anatomic results. AJNR Am J Neuroradiol 2014; 35: 2125–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lubicz B, Collignon L, Raphaeli G, et al. Pipeline flow-diverter stent for endovascular treatment of intracranial aneurysms: Preliminary experience in 20 patients with 27 aneurysms. World Neurosurg 2011; 76: 114–119. [DOI] [PubMed] [Google Scholar]
- 43.Tse MM, Yan B, Dowling RJ, et al. Current status of pipeline embolization device in the treatment of intracranial aneurysms: A review. World Neurosurg 2013; 80: 829–835. [DOI] [PubMed] [Google Scholar]
- 44.Estrade L, Makoyeva A, Darsaut TE, et al. In vitro reproduction of device deformation leading to thrombotic complications and failure of flow diversion. Interv Neuroradiol 2013; 19: 432–437. [DOI] [PMC free article] [PubMed] [Google Scholar]