Abstract
Patients with mutations in the polymerase gamma gene (POLG) may present with progressive ataxia and in such situations neuroimaging findings may suggest the diagnosis. Herein we report a patient with a POLG gene W748S homozygous mutation and characteristic lesions in the thalamus, cerebellum and inferior olivary nucleus seen on magnetic resonance imaging.
Keywords: Ataxia, MRI, POLG
Introduction
The nuclear POLG gene in chromosome 15q25.7 codes for polymerase gamma enzyme which is involved in replication and repair of mitochondrial DNA.1 Multiple mutations have been identified and are associated with diverse clinical syndromes called “POLG-related disorders” including progressive external ophthalmoparesis, Alpers’ syndrome, epilepsy, Parkinsonism, infertility in men, and ataxia-neuropathy spectrum (previously called SANDO and MIRAS).2–6
The hereditary ataxias are classified according to their causative genes and inheritance patterns (i.e. autosomal dominant, autosomal recessive, x-linked, or mitochondrial). A broad range of diagnostic considerations may be suggested by the family history, by findings on physical examination, and by magnetic resonance imaging (MRI) evidence of atrophy or abnormal signal intensity in the cerebellum, brainstem, spinal cord, and other brain structures. However, a definitive diagnosis relies on molecular genetic testing.7 Occasionally, as in the ataxia-neuropathy spectrum related to POLG mutation, characteristic MRI findings strongly suggest the diagnosis of ataxia-neuropathy. To illustrate this, here we report a patient with a POLG gene W748S homozygous mutation and a unique combination of lesions in the thalamus, cerebellum and inferior olivary nucleus seen on MRI.
Case report
A 29-year-old woman presented with a 5-year history of ataxia and dysarthria. There were no relevant personal or familial histories. Physical examination revealed external ophthalmoparesis, generalized areflexia, abnormal leg pallesthesia, wide-based gait, positive Romberg test, dysmetria, and a predominantly left dysdiadochokinesia. Laboratory studies were unremarkable. Nerve conduction velocity tests showed a distal symmetric sensorimotor neuropathy and somatosensory evoked potentials were absent.
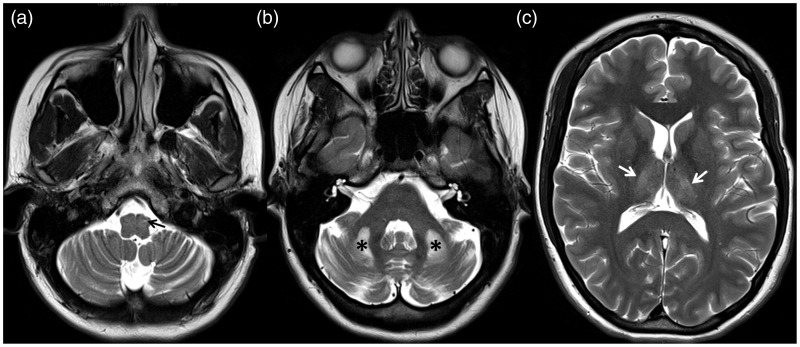
Brain MRI showed cerebellar atrophy and T2 bright bilateral lesions in the dorsal thalami, cerebellar white matter and left inferior olivary nucleus (Figure 1). Diffusion-weighted images showed no restricted diffusion in those areas. Contrast-enhanced images were not obtained. Neuroimaging findings prompted a search for POLG mutation and genetic testing confirmed a POLG W748S homozygous mutation.
Figure 1.
Axial T2-weighted images show cerebellar atrophy with prominent fissures and hyperintense lesions in left inferior olivary nucleus (arrow in A), cerebellar white matter (asterisks in B) and dorso-medial thalami (arrows in C).
Discussion
There are different phenotypes in ataxia related to POLG mutations including: (1) young-onset (<25 years old) with or without ataxia; (2) late-onset ataxia; (3) multiple system atrophy; (4) ataxia with epilepsy; (5) ataxia with cognitive impairment; (6) ataxia with elevated liver enzymes; and (7) ataxia with neuropathy.8 The clinical presentation of the last of these matches the symptoms present in our patient, which were mainly related to cerebellar signs and neuropathy. However, it is to be noted that the genotype–phenotype correlations may be variable and that mixed syndromes are possible.9
In 2001, Rantamäki et al. reported a Finnish family with adult-onset progressive ataxia, dysarthria, neuropathy, epilepsy, ophthalmoparesis and MRI findings similar to those of our patient with abnormal T2 signal intensity in the thalami and cerebellar white matter.10 Additional neuroimaging findings have been reported in other series, including cerebellar atrophy and olivary nucleus degeneration, and these findings can be present in patients with homozygous W748S/W748S and heterozygous W748S/A467T POLG mutations.1,5,11–13
The latest ACR appropriateness criteria guidelines classify ataxia in four groups as follows.7 Variant 1: Gradually progressive ataxia or ataxia of long duration (mass lesion, demyelinating disorders, congenital disorders, hereditary and idiopathic degenerative processes, superficial siderosis, spinal cord and peripheral nerve-related ataxia, nutritional deficiency, toxins and drugs); Variant 2: Acute ataxia as a possible manifestation of stroke; Variant 3: Acute or subacute ataxia as a manifestation of suspected infection; and Variant 4: Acute ataxia following head trauma. Occasionally MRI allows differentiation between these groups, but if a hereditary and idiopathic degenerative ataxia is suspected, neuroimaging findings are nonspecific in most patients. Nevertheless, awareness of specific MRI findings in the ataxia-neuropathy spectrum related to POLG mutation may be helpful to facilitate the diagnosis and lead to the appropriate genetic tests.
Conclusion
In the context of neurodegenerative ataxia, MRI findings of bright T2 lesions in the dorso-medial thalami, cerebellar white matter and the inferior olivary nuclei should prompt a search for POLG mutation and the ataxia-neuropathy spectrum-related disorders.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Habek M, Barun B, Adamec I, et al. Early-onset ataxia with progressive external ophthalmoplegia associated with POLG mutation: Autosomal recessive mitochondrial ataxic syndrome or SANDO? Neurologist 2012; 18: 287–289. [DOI] [PubMed] [Google Scholar]
- 2.Winterthun S, Ferrari G, He L, et al. Autosomal recessive mitochondrial ataxic syndrome due to mitochondrial polymerase gamma mutations. Neurology 2005; 64: 1204–1208. [DOI] [PubMed] [Google Scholar]
- 3.Horvath R, Hudson G, Ferrari G, et al. Phenotypic spectrum associated with mutations of the mitochondrial polymerase gamma gene. Brain 2006; 129: 1674–1684. [DOI] [PubMed] [Google Scholar]
- 4.Stumpf JD, Saneto RP, Copeland WC. Clinical and molecular features of POLG-related mitochondrial disease. Cold Spring Harb Perspect Biol 2013; 5: a011395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hakonen AH, Heiskanen S, Juvonen V, et al. Mitochondrial DNA polymerase W748S mutation: A common cause of autosomal recessive ataxia with ancient European origin. Am J Hum Genet 2005; 77: 430–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milone M, Brunetti-Pierri N, Tang LY, et al. Sensory ataxic neuropathy with ophthalmoparesis caused by POLG mutations. Neuromuscul Disord 2008; 18: 626–632. [DOI] [PubMed] [Google Scholar]
- 7.Broderick DF, Wippold FJ II, Cornelius RS, et al., Expert Panel on Neurologic Imaging. ACR Appropriateness Criteria® ataxia. [online publication]. Reston (VA): American College of Radiology (ACR), 2012. https://acsearch.acr.org/docs/69477/Narrative/ (2012, accessed 10 August 2015).
- 8.Synofzik M, Srulijes K, Godau J, et al. Characterizing POLG ataxia: Clinics, electrophysiology and imaging. Cerebellum 2012; 11: 1002–1011. [DOI] [PubMed] [Google Scholar]
- 9.Schulte C, Synofzik M, Gasser T, et al. Ataxia with ophthalmoplegia or sensory neuropathy is frequently caused by POLG mutations. Neurology 2009; 73: 898–900. [DOI] [PubMed] [Google Scholar]
- 10.Rantamäki M, Krahe R, Paetau A, et al. Adult-onset autosomal recessive ataxia with thalamic lesions in a Finnish family. Neurology 2001; 57: 1043–1049. [DOI] [PubMed] [Google Scholar]
- 11.Van Goethem G, Luoma P, Rantamaki M, et al. POLG mutations in neurodegenerative disorders with ataxia but no muscle involvement. Neurology 2004; 63: 1251–1257. [DOI] [PubMed] [Google Scholar]
- 12.Arkadir D, Meiner V, Karni A, et al. Teaching NeuroImages: Hypertrophic olivary degeneration in a young man with POLG gene mutation. Neurology 2015; 84: e59. [DOI] [PubMed] [Google Scholar]
- 13.Wong LJ, Naviaux RK, Brunetti-Pierri N, et al. Mutations of mitochondrial DNA polymerase gamma A are a frequent cause of autosomal dominant or recessive progressive external ophthalmoplegia. Ann Neurol 2002; 52: 211–219. [DOI] [PubMed] [Google Scholar]