Abstract
This study aimed to verify whether brain abnormalities, previously described in patients with myotonic dystrophy type 1 (DM1) by magnetic resonance imaging (MRI), progressed over time and, if so, to characterize their progression. Thirteen DM1 patients, who had at least two MRI examinations, were retrospectively evaluated and included in the study. The mean duration (± standard deviation) of follow-up was 13.4 (±3.8) years, over a range of 7–20 years. White matter lesions (WMLs) were rated by semi-quantitative method, the signal intensity of white matter poster-superior to trigones (WMPST) by reference to standard images and brain atrophy by ventricular/brain ratio (VBR). At the end of MRI follow-up, the scores relative to lobar, temporal and periventricular WMLs, to WMPST signal intensity and to VBR were significantly increased compared to baseline, and MRI changes were more evident in some families than in others. No correlation was found between the MRI changes and age, onset, disease duration, muscular involvement, CTG repetition and follow-up duration. These results demonstrated that white matter involvement and brain atrophy were progressive in DM1 and suggested that progression rate varied from patient to patient, regardless of age, disease duration and genetic defect.
Keywords: Brain, magnetic resonance imaging, myotonic dystrophy type 1, white matter disease
Introduction
Myotonic dystrophy type 1 (DM1) is an autosomal dominant multisystem disorder, whose genetic defect consists of an abnormal expansion of cytosine-thymine-guanine (CTG) trinucleotide within the 3′ untranslated region of myotonic dystrophy protein kinase (DMPK) gene on chromosome 19q13.3.1–4 CTG expansion ranges from 50 to 4000 repeats, and usually correlates well with the age of onset, whereas normal individuals have fewer than 50 repeats.5,6 However, several questions including the etiopathogenesis underlying the multisystemic nature and the variable expressivity of signs and symptoms in DM1 remain unresolved.7–9
The DM1 phenotype is extremely variable and can range from the frequently severe, often fatal, congenital form to the asymptomatic or nearly asymptomatic carrier of genetic defect.10 The adult form is the most common form of DM1 and typically presents at 15–40 years of age with distal muscle weakness, myotonia, progressive muscular atrophy, hatchet face and ptosis, cognitive impairment and neuropsychiatric symptoms.5,6,10
Interest in the neurological aspects of DM1 has increased in the last several years,11 and several brain magnetic resonance imaging (MRI) studies have reported findings ranging from no or minimal abnormalities to marked brain atrophy and severe white matter involvement.12–38 However, there is great disagreement about the nature (developmental or degenerative) and the clinical and genetic significance of brain MRI abnormalities.39 Some authors, in fact, have found a significant association between age, disease duration, muscular impairment or CTG expansion and the severity of white matter involvement20,32–38 or brain atrophy,19,24,32,36 while others have found no significant relationships for white matter abnormalities25–29,35 nor for brain atrophy.26,28–31 Ota et al.28 have reported a significant negative correlation with CTG repeats only for the motor and prefrontal cortical atrophy. Romeo et al.35 have found that the severity of white matter lesions (WMLs) significantly correlated with age, but not with muscular impairment or CTG repeats. Di Costanzo et al.34 have reported a familial aggregation of WMLs, while others did not find a characteristic WML pattern among members of the same families.26,27 Longitudinal MRI studies are expected to settle these issues.11,39
This study verified if the abnormalities described in the brain MRIs of patients with DM1 progressed over time and tried to characterize such progression correlating the MRI findings with the clinical and genetic features. To the best of our knowledge, this is the first long-term longitudinal MRI study of the DM1 brain.
Materials and methods
Patients
Thirteen patients with the adult form of DM1, who had undergone over time two or more brain MRIs, were retrospectively included in this study. The patients (eight men and five women) belonged to five families (Table 1). The diagnosis was based on clinical features, family history, electromyography and genetic analysis of leukocytes. At the first observation, age ranged between 16 and 60 years and symptoms duration between 0 (patients without complaints detected in family examinations) and 26 years.
Table 1.
Clinical features of patients with myotonic dystrophy type 1.
| Family. | Patient | Age at baseline | Disease duration at baseline | Duration of follow-up | MIRS |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| no. | no. | Sex | Relationship | (years) | (years) | Inheritance | CTG repeats | (years) | Basal | Follow-up |
| 1 | 1 | M | Proband | 60 | 17 | Paternal | 100 | 7 | 4 | 5 |
| 1 | 2 | F | Parent | 44 | 24 | Paternal | 500 | 20 | 4 | 5 |
| 1 | 3 | F | Sibling | 22 | 8 | Paternal | 300 | 19 | 2 | 4 |
| 2 | 4 | F | Sibling | 34 | 4 | Paternal | 625 | 15 | 3 | 3 |
| 2 | 5 | M | Sibling | 27 | 8 | Paternal | 625 | 16 | 2 | 3 |
| 3 | 6 | M | Proband | 24 | 12 | Paternal | 467 | 12 | 2 | 3 |
| 3 | 7 | F | Sibling | 24 | 10 | Paternal | 467 | 13 | 3 | 4 |
| 3 | 8 | M | Parent | 54 | 0 | Unknown | 66 | 12 | 1 | 2 |
| 4 | 9 | M | Proband | 43 | 10 | Maternal | 210 | 12 | 3 | 4 |
| 4 | 10 | M | Sibling | 16 | 2 | Paternal | 650 | 15 | 2 | 3 |
| 5 | 11 | M | Proband | 39 | 26 | Maternal | 405 | 9 | 4 | 5 |
| 5 | 12 | F | Sibling | 29 | 0 | Maternal | 255 | 16 | 2 | 4 |
| 13 | M | 39 | 10 | Maternal | 467 | 8 | 3 | 4 | ||
MIRS: Muscular impairment rating scale.40
The severity of muscular involvement, assessed by the Muscular Impairment Rating Scale (MIRS),40 ranged from 2 (minimal signs) to 5 (severe proximal weakness). When it was not possible to perform a clinical, genetic and instrumental evaluation, parents were considered affected on the basis of family history and photographic evidence; for one patient, it was not possible to identify the affected parent. No patient had history of cerebrovascular disease, multiple sclerosis, meningoencephalitis, hypoxic insult, head trauma with loss of consciousness, progressive dementia or other neurological diseases that could explain the MRI findings. Furthermore, no patients had hypertension, hyperglycemia or hypercholesterolemia; two patients (no. 9 and 10; family no. 4) presented a body mass index (BMI) > 30, and two (no. 8 and 10) were smokers. Six patients (no. 2, 4, 5, 8, 10, 13) had hypertriglyceridemia, and two of them belonged to the same family (no. 2).
The study was reviewed and approved by the local ethics committee and all patients provided written informed consent.
Imaging
At baseline, brain MRI was performed on a 0.5 T scanner (Vectra, General Electric, Milwaukee, WI, USA). Routine examinations included sagittal T1-weighted images (repetition time (TR) = 500 ms, echo time (TE) = 20 ms) and axial proton density- (PD-) and T2-weighted spin-echo images (TR = 2000 ms, TE1 = 40 ms and TE2 = 100 ms). Slice thickness was 5 mm with 2 mm gaps; field of view/matrix was 24 cm/192 × 256 for sagittal and 20 cm/160 × 192 for axial series. At follow-up, brain MRI was performed on a 1.5 T scanner (Vectra, General Electric) and the protocol included axial PD- and T2-weighted spin-echo images (TR = 48,400 ms, TE1 = 85 ms, TE2 = 116 ms) and fluid-attenuation inversion recovery (FLAIR) images (TR = 8890 ms, TE = 123 ms, IR = 1240 ms). Slice thickness was 5 mm with 2 mm gaps.
WMLs were identified as areas with high signal on both PD- and T2-weighted images. They were recorded as periventricular (abutting the ventricular lining), lobar (outside the periventricular and temporal regions) or temporal WMLs and scored according to a semiquantitative method.41 The total score was the sum of products between the number of lesions and the category indicating their size (1 < 0.5 cm; 2 = 0.5–1.0 cm; 3 = 1.0–1.5 cm; 4 = 1.5–2.0 cm; 5 = 2.0–2.5 cm; 6 = 2.5–3.0 cm; 7 ≥ 3.0 cm). The periventricular WMLs were scored by measuring their greatest thickness on PD-weighted images at the frontal and occipital horns (“caps”) and at the body of the lateral ventricle (“rims” or “bands”).
The signal intensity of white matter postero-superior to trigones (WMPST) was evaluated by reference to standard images (see Figure 1(a) and (c) in Di Costanzo et al.24), showing different degrees of signal increase. It was scored as follows: 1 = mild = signal intensity less than standard image no. 1; 2 = moderate = signal intensity equal to or more than standard no. 1, but less than standard no. 2; 3 = severe = equal to or more than standard no. 2.42
Brain atrophy was assessed by the ventricular/brain ratio (VBR), calculated in the PD-weighted image showing the pars centralis of lateral ventricles at the point of longest visible antero-posterior septal connection. The width of the ventricular bodies halfway from the anterior to posterior margin was divided by the maximum width of the brain at that level.42
Two blinded experienced examiners, R.C. and A.S., independently evaluated the images and their results were compared. When they disagreed, a consensus meeting was held to reach an unequivocal agreement. Inter- and intra-rater reliabilities, measured by intraclass correlation coefficient, varied from 0.79 to 0.84 and from 0.84 to 0.87, respectively. Considering that small repositioning errors43 and image quality differences are inevitable across scans, and that intra-observer agreement may vary between 79% and 92% in scoring systems,44 only differences more than 20% from baseline were considered significant changes. Furthermore, longitudinal changes were also estimated based on qualitative radiological impression. Qualitative analysis is less sensitive than quantitative, but reduces the probability of a false-positive report.45
Genetic analysis
Genetic analysis was performed from leukocyte DNA to assess the length of CTG repeat sequence. Standard procedure consisted of DNA separation from leukocytes, digestion with NcoI and BamHI restriction enzymes, separation of DNA fragments by gel electrophoresis, transfer onto nylon membranes by Southern blotting, hybridization with DNA probe MDY1 and additional polymerase chain reaction using described primers.1 The CTG repeat size was estimated at the point of the most heavily staining band or at the midpoint of the smear for a very diffuse band.
Statistical analysis
Data were analyzed using the statistical package for the social sciences (SPSS)/personal computer (PC) statistical software package. Variables were examined for outliers and extreme values by box and normal quantile-quantile plots, and for distribution by the Kolmogorov-Smirnov test. When a normal distribution could not be accepted, variable transformations (square, square root, logarithmic, reciprocal of square root or reciprocal transformations) were reviewed. Since the normality could not be reached for most examined variables, a nonparametric test was used. The differences in the scores obtained at baseline and at the end of follow-up were evaluated with Wilcoxon test with a significance level of p < 0.05. The Spearman rank correlation coefficient (rs) measured the linear association between WMLs, WMPST hyperintensity and VBR score variations, detected at the end of MRI follow-up, and other examined variables (age, age at onset, disease duration, CTG repeats, follow-up duration, MIRS, cardiovascular risk factors). The Bonferroni’s correction was used to account for multiple comparisons.
Results
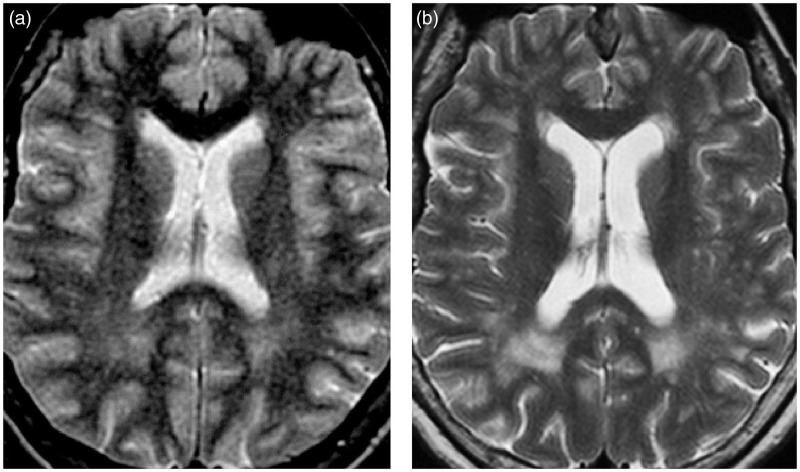
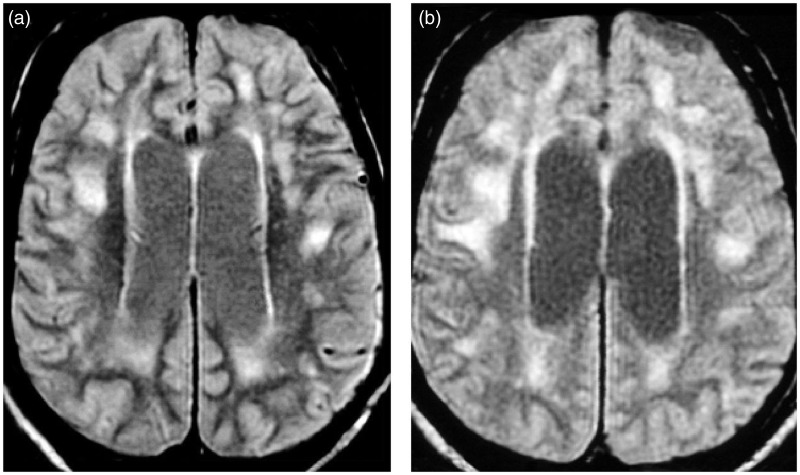
Clinical and MRI features of each DM1 patient are reported in Tables 1 and 2, respectively. During the follow-up, WMLs showed significant changes in five (no. 1, 2, 3, 4, 5) out of 13 patients (38.4%). In particular, for three (no. 1, 2, 3) of five patients (no. 1, 2, 3, 7, 11) who presented with lobar WMLs at the baseline, we observed the extension and the confluence of pre-existent WMLs and the appearance of new WMLs during follow-up (Table 2; Figure 1). In two (no. 4, 5) of eight patients (no. 4, 5, 6, 8, 9, 10, 12, 13) who did not present lobar WMLs at baseline, we detected the appearance of lobar WMLs during the follow-up (Table 2; Figure 2). Among the seven patients (no. 1, 2, 3, 4, 7, 11, 12) showing the characteristic temporopolar WMLs at baseline, three (no. 2, 3, 4) presented with an enlargement of lesions (Table 2; Figure 3). In one (no. 5) of six patients (no. 5, 6, 8, 9, 10, 13) who did not show such kind of WMLs at baseline, a temporopolar WML was detectable at the end of follow-up (Table 2; Figure 3). An extension of “bands” and/or “caps” signal was revealed in three patients (no. 1, 2, 3), and in two (no. 5, 12) there was the appearance of periventricular WMLs (Table 2; Figure 4). WMPST hyperintensity (Table 2; Figure 5) increased in six patients (no. 2, 3, 4, 5, 12, 13). VBR heightened (Table 2; Figure 6) in all, but in six (no. 1, 2, 3, 5, 8, 13) out of 13 patients (46.1%) the difference from baseline was more than 20%. The scores of lobar (Z = 2.0; p = 0.043), temporal (Z = 2.1; p = 0.034) and periventricular WMLs (Z = 2.1; p = 0.038), WHPST hyperintensity (Z = 2.4; p = 0.014) and VBR (Z = 3.1; p = 0.002) were significantly increased compared to the basal ones. No relationship was found between MRI score variations and age, age at onset, inheritance, disease duration, CTG repeats, MIRS, presence of cardiovascular risk factors and follow-up duration.
Table 2.
MRI findings at baseline and at the end of follow-up with results of statistical analysis.
| Patient |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | Median | p value | |
| WML-L | Baseline | 29 | 14 | 7 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 0.043 |
| Follow-up | 43a | 30a | 36a | 6a | 8a | 0 | 2 | 0 | 0 | 0 | 6 | 0 | 0 | 2 | ||
| WML-T | Baseline | 12 | 10 | 5 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 14 | 10 | 0 | 2 | 0.034 |
| Follow-up | 14 | 12a | 10a | 4a | 0 | 0 | 4 | 0 | 0 | 0 | 14 | 10 | 0 | 4 | ||
| WML-PV | Baseline | 6 | 6 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0.038 |
| Follow-up | 8a | 8a | 6a | 0 | 3a | 0 | 0 | 0 | 0 | 0 | 4 | 2a | 0 | 0 | ||
| HWMPST | Baseline | 3 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 0.014 |
| Follow-up | 3 | 3a | 3a | 2a | 2a | 1 | 1 | 1 | 1 | 1 | 2 | 2a | 2a | 2 | ||
| VBR | Baseline | 0.30 | 0.24 | 0.19 | 0.12 | 0.14 | 0.19 | 0.19 | 0.18 | 0.20 | 0.16 | 0.20 | 0.17 | 0.20 | 0.19 | 0.002 |
| Follow-up | 0.37a | 0.29a | 0.24a | 0.14 | 0.22a | 0.21 | 0.21 | 0.22a | 0.23 | 0.17 | 0.22 | 0.18 | 0.24a | 0.22 | ||
Increases more than 20% from baseline. MRI: magnetic resonance imaging; WML: white matter lesion; L: lobar; T: temporal; PV: periventricular; HWMPST: hyperintensity of white matter postero-superior to trigones; VBR: ventricular/brain ratio.
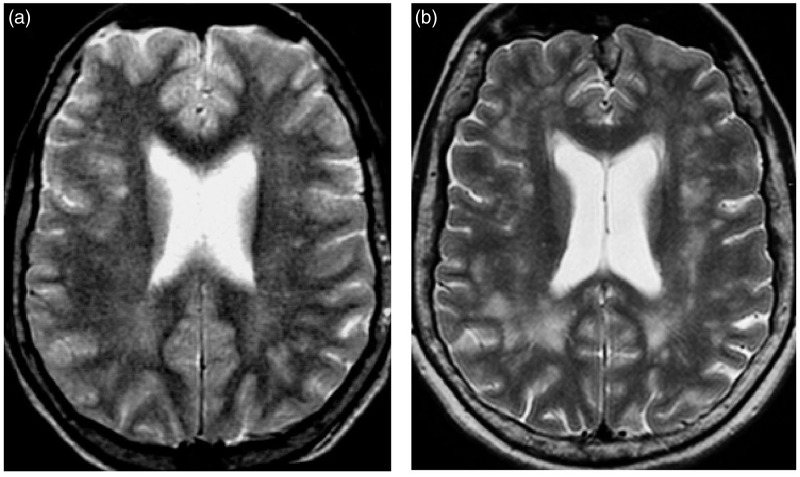
Figure 1.
Axial T2-weighted images showing the extension and confluence of pre-existent white matter lesions (WMLs) and the appearance of new WMLs in patient 3 (Table 2) during follow-up: (a) (baseline) and (b) (19 years later).
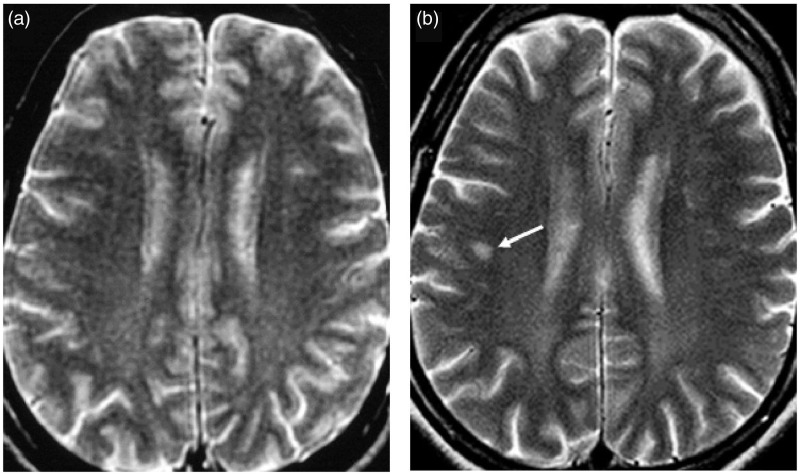
Figure 2.
Axial T2-weighted images showing the appearance of a lobar white matter lesion (WML) (arrow) in patient 5 during follow-up: (a) (baseline) and (b) (16 years later).
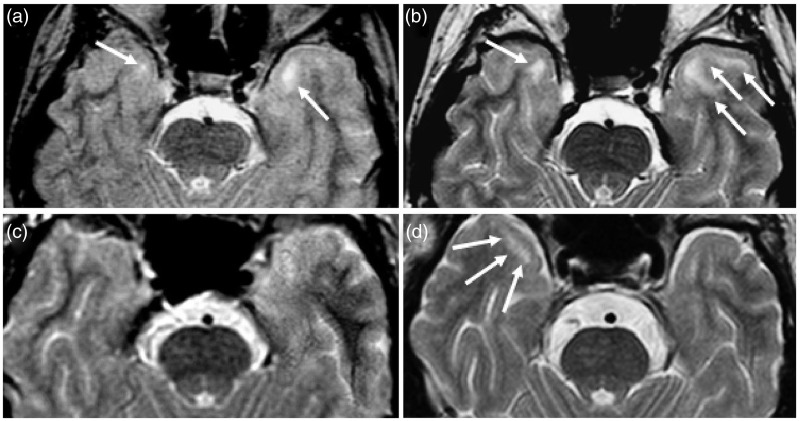
Figure 3.
Axial T2-weighted images showing the enlargement of pre-existent temporopolar white matter lesions (WMLs) (a) and (b) in patient 3 (Table 2) and the development of a temporopolar WML (c) and (d) in patient 5 (Table 2) during follow-up: (a) and (c) (baseline), (b) (19 years later) and (d) (16 years later).
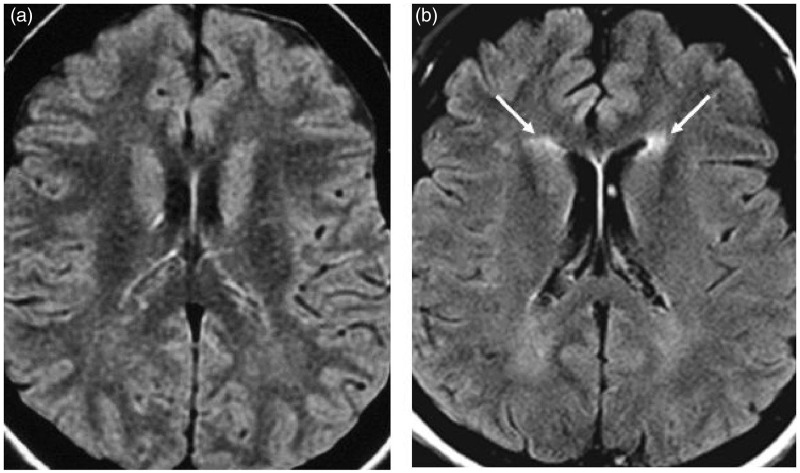
Figure 4.
Axial T2-weighted images showing the appearance of frontal “caps” (arrows) in patient 12 (Table 2) during follow-up: (a) (baseline) and (b) (16 years later).
Figure 5.
Axial T2-weighted images showing the increase of white matter postero-superior to trigones (WMPST) hyperintensity in patient 3 (Table 2) during follow-up: (a) (baseline) and (b) (19 years later).
Figure 6.
Axial PD-weighted images showing the ventricular enlargement in patient 1 (Table 2) during follow-up: (a) (baseline) and (b) (seven years later).
Discussion
The present study represents, to the best of our knowledge, the first long-term follow-up study of brain MRI features in patients with the adult form of DM1. The main previously unreported findings are progression over time of WML number and extent and brain atrophy, appearance of WMLs in patients previously devoid of MRI abnormalities, progressive increase of signal intensity of WMPST, significant MRI changes in some families and not in others, and absence of correlation between MRI changes and clinical or genetic features.
The progression over time of WML severity in the present study suggests that white matter involvement in the adult form of MD1 is probably more degenerative (and therefore progressive) in nature than developmental (and therefore stable over time). Previous cross-sectional studies reported conflicting results since some authors found a significant correlation between WMLs and disease duration,20,24,32–34,36 while others failed to find any significant correlation.25–27,29 The significant changes in the number and extent of WMLs occurred in the members of two (no. 1 and 2) out of five families (Tables 1 and 2), while in the remaining families the white matter changes were less evident or absent. This finding settles the issue about the familial recurrence of WMLs in DM1. Some authors, in fact, reported a familial aggregation of WMLs in DM1,34 while others26,27 did not find any characteristic pattern of brain MRI abnormalities in the members of the same families.
Another issue addressed by the present study is the progression of cerebral atrophy in the adult form of DM1. Previous cross-sectional studies reported conflicting results since some authors reported a significant correlation between brain atrophy and disease duration,24,32,33,36 while others denied such a correlation.26,28–31 In effect, our result of progressive brain atrophy is in strict agreement with a previous longitudinal pneumoencephalographic study46 that demonstrated a slowly progressive ventricular enlargement in muscular dystrophic (MD) patients followed for 20–97 months. Furthermore, considering that brain atrophy rate in healthy individuals is 0.2% per year after age 35 years and more than 0.5% per year over 60 years,47 we can consider abnormal the ventricular enlargement in about half of our patients.
The absence of correlation with age, age at onset, disease duration, CTG repeats, MIRS and follow-up duration suggests that the rate and extent of MRI abnormality progression vary from patient to patient and are independent from genetic analysis on leukocytes. The variability of rate of disease progression is a known feature of DM1. Mathieu et al.40 demonstrated a great variability of the rate of MD process comparing the disease duration with the MIRS scores in a large population of patients. The MIRS, in fact, was developed in concordance with the usual distal to proximal progression of muscular involvement in DM1.40 Di Costanzo et al.33 inferred the variable rate of progression of white matter involvement in DM1 in a cross-sectional study, by dividing their patient population in four groups on the basis of disease duration and severity of brain MR findings. The variable rate of progression of white matter involvement in DM1 could be explained by the huge variability of disease expression.7,10 The lack of correlation with CTG repeats is in agreement with some authors21,29–31,34,35 and in disagreement with others, who have found positive correlations between brain MR abnormalities and CTG repeats.28,32 The somatic mosaicism might explain the lack of correlation between the number of CTG repeats in blood leukocytes and brain abnormalities.
The etiopathogenesis of brain abnormalities in DM1 has been recently reviewed and a complex interaction between RNAopathy, spliceopathy and tauopathy has been implicated.9 In brief, mutated DMPK transcripts accumulate in RNA nuclear inclusions called foci and alter the alternative splicing of various transcripts, including those encoding for tau. This protein is expressed in neurons and glia, and is involved in microtubules assembly and stabilization, and in intracellular transport regulation.48 In DM1, the changes in alternative splicing might result in a dysfunction of tau protein, with a variation of microtubule organization and axonal transport.9 The consequence might be the breakdown of cytoskeleton, the impairment of neuronal structure and function, the formation of neurofibrillary tangles (NFTs), and hence the onset and progression of neurodegeneration.9,48 A number of neuropathological studies have shown NFT and altered level of tau isoforms in several DM1 brain regions.49–54 RNA foci have also been reported in oligodendrocytes of subcortical white matter and the corpus callosum, even if less intense than in cortical neurons,54,55 and pathological tau proteins have been detected in frontal white matter.51 Considering that cytoskeletal integrity is also important for the function and survival of oligodendrocytes and myelin sheaths,56 tau mis-splicing might be a unifying pathomechanism underlying the brain atrophy and the white matter abnormalities of DM1. However, the familial aggregation in the occurrence, severity and progression of WMLs in DM1 (Di Costanzo et al.34 and the present study) suggest that other genetic or environmental factors might be implicated. Neuropathological studies have reported loss of myelin and axons, dilated perivascular spaces, fibrillary gliosis, absence of cellular infiltrates or typical features of active breakdown myelin sheets, and presence of few fatty granular cells.14,50,54,57,58 These neuropathological findings associated with the progressive white matter involvement and the increasing ventricular enlargement, detected in the present study, suggest that a slow demyelinating process might underlie WMLs in the adult form of DM1.
An important limitation of this study is that we did not use any brain volumetric techniques, which are currently widely used for assessing the progression of several neurological diseases.59 Unfortunately, these techniques were not yet available at the time of our first MRI observations. However, visual rating scales correlate well with quantitative volumetric measures in the assessment of WML progression.60,61 Another limitation is the differences in image quality and spatial resolution across scans. These differences were inevitable given that the first MRI studies were performed with a 0.5 T scanner. However, visual rating scales, being rather easily applicable and requiring limited technical efforts, are particularly useful in studies where different machinery with different image quality have been used.62
Conclusion
In conclusion, the results of this longitudinal case series study suggest that white matter abnormalities described in patients with adult-type DM1 might be progressive and, if absent at baseline, might appear during the course of the disease. The rate and extent of progression vary from person to person, regardless of CTG length in peripheral blood, and tend to be more pronounced in some families than others, suggesting the involvement of other genetic or environmental factors. These findings, together with previously reported neuropathological findings and pathogenetic hypotheses, suggest that white matter abnormalities in DM1 might be an expression of a slow demyelination process.
Acknowledgments
The authors are grateful to Mariella Filangieri for her valuable assistance. This study was approved by the ethics committee of the university and therefore has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All patients gave their informed consent prior to their inclusion in the study.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author contributions are as follows: Guarantor of integrity of the entire study (G. Tedeschi and S. Cirillo); study concepts (A. Di Costanzo, R. Conforti, G. Tedeschi and S. Cirillo); study design (A. Di Costanzo, R. Conforti, G. Tedeschi); definition of intellectual content (A. Di Costanzo, R. Conforti, G. Tedeschi and S. Cirillo); literature research (A. Cristofano, M. de Cristofaro, R. Conforti, B. Brogna and A. Di Costanzo); clinical studies (A. Di Costanzo, M. de Cristofaro and R. Conforti); data acquisition (R. Conforti, A. Sardaro and A. Di Costanzo); data analysis (A. Di Costanzo, R. Conforti and G. Tedeschi); statistical analysis (A. Di Costanzo and A. Cristofano); manuscript preparation (A. Di Costanzo, A. Cristofano, R. Conforti and B. Brogna); manuscript editing (A. Di Costanzo); and manuscript review (R. Conforti and G. Tedeschi).
References
- 1.Brook JD, McCurrach ME, Harley HG, et al. Molecular basis of myotonic dystrophy: Expansion of a trinucleotide (CTG) repeat at the 3′ end of a transcript encoding a protein kinase family member. Cell 1992; 68: 799–808. [DOI] [PubMed] [Google Scholar]
- 2.Mahadevan M, Tsilfidis C, Sabourin L, et al. Myotonic dystrophy mutation: An unstable CTG repeat in the 3′ untranslated region of the gene. Science 1992; 255: 1253–1255. [DOI] [PubMed] [Google Scholar]
- 3.Fu YH, Pizzuti A, Fenwick RG, Jr, et al. An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science 1992; 255: 1256–1258. [DOI] [PubMed] [Google Scholar]
- 4.The International Myotonic Dystrophy Consortium (IDMC). New nomenclature and DNA testing guidelines for myotonic dystrophy type 1 (DM1). Neurology 2000; 54: 1218–1221. [DOI] [PubMed] [Google Scholar]
- 5.Johnson NE, Heatwole CR. Myotonic dystrophy: From bench to bedside. Semin Neurol 2012; 32: 246–254. [DOI] [PubMed] [Google Scholar]
- 6.Meola G, Cardani R. Myotonic dystrophies: An update on clinical aspects, genetic, pathology, and molecular pathomechanisms. Biochim Biophys Acta 2014; 1852: 594–606. [DOI] [PubMed] [Google Scholar]
- 7.Udd B, Krahe R. The myotonic dystrophies: Molecular, clinical, and therapeutic challenges. Lancet Neurol 2012; 11: 891–905. [DOI] [PubMed] [Google Scholar]
- 8.Kumar A, Agarwal S, Agarwal D, et al. Myotonic dystrophy type 1 (DM1): A triplet repeat expansion disorder. Gene 2013; 522: 226–230. [DOI] [PubMed] [Google Scholar]
- 9.Caillet-Boudin ML, Fernandez-Gomez FJ, Tran H, et al. Brain pathology in myotonic dystrophy: When tauopathy meets spliceopathy and RNAopathy. Front Mol Neurosci 2014; 6: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harper P. Myotonic dystrophy, 3rd edn London: W.B. Saunders, 2001. [Google Scholar]
- 11.Bugiardini E, Meola G. DM-CNS Group. Consensus on cerebral involvement in myotonic dystrophy: Workshop report: May 24–27, 2013, Ferrere (AT), Italy. Neuromuscul Disord 2014; 24: 445–452. [DOI] [PubMed] [Google Scholar]
- 12.Glantz RH, Wright RB, Huckman MS, et al. Central nervous system magnetic resonance imaging findings in myotonic dystrophy. Arch Neurol 1988; 45: 36–37. [DOI] [PubMed] [Google Scholar]
- 13.Huber SJ, Kissel JT, Shuttleworth EC, et al. Magnetic resonance imaging and clinical correlates of intellectual impairment in myotonic dystrophy. Arch Neurol 1989; 46: 536–540. [DOI] [PubMed] [Google Scholar]
- 14.Abe K, Fujimura H, Toyooka K, et al. Involvement of the central nervous system in myotonic dystrophy. J Neurol Sci 1994; 127: 179–185. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto T, Tayama M, Miyazaki M, et al. Neuroimaging study of myotonic dystrophy. I. Magnetic resonance imaging of the brain. Brain Dev 1995; 17: 24–27. [DOI] [PubMed] [Google Scholar]
- 16.Ogata A, Terae S, Fujita M, et al. Anterior temporal white matter lesions in myotonic dystrophy with intellectual impairment: An MRI and neuropathological study. Neuroradiology 1998; 40: 411–415. [DOI] [PubMed] [Google Scholar]
- 17.Miaux Y, Chiras J, Eymard B, et al. Cranial MRI findings in myotonic dystrophy. Neuroradiology 1997; 39: 166–170. [DOI] [PubMed] [Google Scholar]
- 18.Abe K, Fujimura H, Soga F, et al. The fluid-attenuated inversion-recovery pulse sequence in assessment of central nervous system involvement in myotonic dystrophy. Neuroradiology 1998; 40: 32–35. [DOI] [PubMed] [Google Scholar]
- 19.Akiguchi I, Nakano S, Shiino A, et al. Brain proton magnetic resonance spectroscopy and brain atrophy in myotonic dystrophy. Arch Neurol 1999; 56: 325–330. [DOI] [PubMed] [Google Scholar]
- 20.Di Costanzo A, Di Salle F, Santoro L, et al. T2 relaxometry of brain in myotonic dystrophy. Neuroradiology 2001; 43: 198–204. [DOI] [PubMed] [Google Scholar]
- 21.Kuo HC, Hsiao KM, Chen CJ, et al. Brain magnetic resonance image changes in a family with congenital and classic myotonic dystrophy. Brain Dev 2005; 27: 291–296. [DOI] [PubMed] [Google Scholar]
- 22.Kornblum C, Reul J, Kress W, et al. Cranial magnetic resonance imaging in genetically proven myotonic dystrophy type 1 and 2. J Neurol 2004; 251: 710–714. [DOI] [PubMed] [Google Scholar]
- 23.Wozniak JR, Mueller BA, Ward EE, et al. White matter abnormalities and neurocognitive correlates in children and adolescents with myotonic dystrophy type 1: A diffusion tensor imaging study. Neuromuscul Disord 2011; 21: 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Costanzo A, Di Salle F, Santoro L, et al. Brain MRI features of congenital- and adult-form myotonic dystrophy type 1: Case-control study. Neuromuscul Disord 2002; 12: 476–483. [DOI] [PubMed] [Google Scholar]
- 25.Sinforiani E, Sandrini G, Martelli A, et al. Cognitive and neuroradiological findings in myotonic dystrophy. Funct Neurol 1991; 6: 377–384. [PubMed] [Google Scholar]
- 26.Censori B, Provinciali L, Danni M, et al. Brain involvement in myotonic dystrophy: MRI features and their relationship to clinical and cognitive conditions. Acta Neurol Scand 1994; 90: 211–217. [DOI] [PubMed] [Google Scholar]
- 27.Fierro B, Daniele O, Aloisio A, et al. Neurophysiological and radiological findings in myotonic dystrophy patients. Eur J Neurol 1998; 5: 89–94. [DOI] [PubMed] [Google Scholar]
- 28.Ota M, Sato N, Ohya Y, et al. Relationship between diffusion tensor imaging and brain morphology in patients with myotonic dystrophy. Neurosci Lett 2006; 407: 234–239. [DOI] [PubMed] [Google Scholar]
- 29.Giorgio A, Dotti MT, Battaglini M, et al. Cortical damage in brains of patients with adult-form of myotonic dystrophy type 1 and no or minimal MRI abnormalities. J Neurol 2006; 253: 1471–1477. [DOI] [PubMed] [Google Scholar]
- 30.Kassubek J, Juengling FD, Hoffmann S, et al. Quantification of brain atrophy in patients with myotonic dystrophy and proximal myotonic myopathy: A controlled 3-dimensional magnetic resonance imaging study. Neurosci Lett 2003; 348: 73–76. [DOI] [PubMed] [Google Scholar]
- 31.Antonini G, Mainero C, Romano A, et al. Cerebral atrophy in myotonic dystrophy: A voxel based morphometric study. J Neurol Neurosurg Psychiatry 2004; 75: 1611–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bachmann G, Damian MS, Koch M, et al. The clinical and genetic correlates of MRI findings in myotonic dystrophy. Neuroradiology 1996; 38: 629–635. [DOI] [PubMed] [Google Scholar]
- 33.Di Costanzo A, Di Salle F, Santoro L, et al. Pattern and significance of white matter abnormalities in myotonic dystrophy type 1: An MRI study. J Neurol 2002; 249: 1175–1182. [DOI] [PubMed] [Google Scholar]
- 34.Di Costanzo A, Santoro L, de Cristofaro M, et al. Familial aggregation of white matter lesions in myotonic dystrophy type 1. Neuromuscul Disord 2008; 18: 299–305. [DOI] [PubMed] [Google Scholar]
- 35.Romeo V, Pegoraro E, Ferrati C, et al. Brain involvement in myotonic dystrophies: Neuroimaging and neuropsychological comparative study in DM1 and DM2. J Neurol 2010; 257: 1246–1255. [DOI] [PubMed] [Google Scholar]
- 36.Weber YG, Roebling R, Kassubek J, et al. Comparative analysis of brain structure, metabolism, and cognition in myotonic dystrophy 1 and 2. Neurology 2010; 74: 1108–1117. [DOI] [PubMed] [Google Scholar]
- 37.Minnerop M, Weber B, Schoene-Bake JC, et al. The brain in myotonic dystrophy 1 and 2: Evidence for a predominant white matter disease. Brain 2011; 134: 3530–3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Franc DT, Muetzel RL, Robinson PR, et al. Cerebral and muscle MRI abnormalities in myotonic dystrophy. Neuromuscul Disord 2012; 22: 483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Axford MM, Pearson CE. Illuminating CNS and cognitive issues in myotonic dystrophy: Workshop report. Neuromuscul Disord 2013; 23: 370–374. [DOI] [PubMed] [Google Scholar]
- 40.Mathieu J, Boivin H, Meunier D, et al. Assessment of a disease-specific muscular impairment rating scale in myotonic dystrophy. Neurology 2001; 56: 336–340. [DOI] [PubMed] [Google Scholar]
- 41.Scheltens P, Barkhof F, Leys D, et al. A semiquantative rating scale for the assessment of signal hyperintensities on magnetic resonance imaging. J Neurol Sci 1993; 114: 7–12. [DOI] [PubMed] [Google Scholar]
- 42.Victoroff J, Mack WJ, Grafton ST, et al. A method to improve interrater reliability of visual inspection of brain MRI scans in dementia. Neurology 1994; 44: 2267–2276. [DOI] [PubMed] [Google Scholar]
- 43.Goodkin DE, Vanderburg-Medendorp S, Ross J. The effect of repositioning error on serial magnetic resonance imaging scans. Arch Neurol 1993; 50: 569–571. [DOI] [PubMed] [Google Scholar]
- 44.Gouw AA, van der Flier WM, van Straaten EC, et al. LADIS study group. Reliability and sensitivity of visual scales versus volumetry for evaluating white matter hyperintensity progression. Cerebrovasc Dis 2008; 25: 247–253. [DOI] [PubMed] [Google Scholar]
- 45.Courchesne E, Yeung-Courchesne R, Egaas B. Methodology in neuroanatomic measurement. Neurology 1994; 44: 203–208. [DOI] [PubMed] [Google Scholar]
- 46.Refsum S, Lonnum A, Sjaastad O, et al. Dystrophia myotonica. Repeated pneumoencephalographic studies in ten patients. Neurology 1967; 17: 345–348. [DOI] [PubMed] [Google Scholar]
- 47.Hedman AM, van Haren NE, Schnack HG, et al. Human brain changes across the life span: A review of 56 longitudinal magnetic resonance imaging studies. Hum Brain Mapp 2012; 33: 1987–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mietelska-Porowska A, Wasik U, Goras M, et al. Tau protein modifications and interactions: Their role in function and dysfunction. Int J Mol Sci 2014; 15: 4671–4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vermersch P, Sergeant N, Ruchoux MM, et al. Specific tau variants in the brains of patients with myotonic dystrophy. Neurology 1996; 47: 711–717. [DOI] [PubMed] [Google Scholar]
- 50.Mizukami K, Sasaki M, Baba A, et al. An autopsy case of myotonic dystrophy with mental disorders and various neuropathologic features. Psychiatry Clin Neurosci 1999; 53: 51–55. [DOI] [PubMed] [Google Scholar]
- 51.Sergeant N, Sablonnière B, Schraen-Maschke S, et al. Dysregulation of human brain microtubule-associated tau mRNA maturation in myotonic dystrophy type 1. Hum Mol Genet 2001; 10: 2143–2155. [DOI] [PubMed] [Google Scholar]
- 52.Oyamada R, Hayashi M, Katoh Y, et al. Neurofibrillary tangles and deposition of oxidative products in the brain in cases of myotonic dystrophy. Neuropathology 2006; 26: 107–114. [DOI] [PubMed] [Google Scholar]
- 53.Leroy O, Wang J, Maurage CA, et al. Brain-specific change in alternative splicing of Tau exon 6 in myotonic dystrophy type 1. Biochim Biophys Acta 2006; 1762: 460–467. [DOI] [PubMed] [Google Scholar]
- 54.Itoh K, Mitani M, Kawamoto K, et al. Neuropathology does not correlate with regional differences in the extent of expansion of CTG repeats in the brain with Myotonic Dystrophy Type 1. Acta Histochem Cytochem 2010; 43: 149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jiang H, Mankodi A, Swanson MS, et al. Myotonic dystrophy type 1 is associated with nuclear foci of mutant RNA, sequestration of muscleblind proteins and deregulated alternative splicing in neurons. Hum Mol Genet 2004; 13: 3079–3088. [DOI] [PubMed] [Google Scholar]
- 56.Bauer NG, Richter-Landsberg C, Ffrench-Constant C. Role of the oligodendroglial cytoskeleton in differentiation and myelination. Glia 2009; 57: 1691–1705. [DOI] [PubMed] [Google Scholar]
- 57.Jinnai K, Mitani M, Futamura N. Somatic instability of CTG repeats in the cerebellum of myotonic dystrophy type 1. Muscle Nerve 2013; 48: 105–108. [DOI] [PubMed] [Google Scholar]
- 58.Conforti R, Cirillo M, Saturnino PP, et al. Dilated Virchow-Robin spaces and multiple sclerosis: 3 T magnetic resonance study. Radiol Med 2014; 119: 408–414. [DOI] [PubMed] [Google Scholar]
- 59.Giorgio A, De Stefano N. Clinical use of brain volumetry. J Magn Reson Imaging 2013; 37: 1–14. [DOI] [PubMed] [Google Scholar]
- 60.Kapeller P, Barber R, Vermeulen RJ, et al. European Task Force of Age Related White Matter Changes. Visual rating of age-related white matter changes on magnetic resonance imaging: Scale comparison, interrater agreement, and correlations with quantitative measurements. Stroke 2003; 34: 441–445. [DOI] [PubMed] [Google Scholar]
- 61.Prins ND, van Straaten EC, van Dijk EJ, et al. Measuring progression of cerebral white matter lesions on MRI: visual rating and volumetrics. Neurology 2004; 62: 1533–1539. [DOI] [PubMed] [Google Scholar]
- 62.Enzinger C, Fazekas F, Ropele S, et al. Progression of cerebral white matter lesions: Clinical and radiological considerations. J Neurol Sci 2007; 257: 5–10. [DOI] [PubMed] [Google Scholar]