Abstract
Carcinoid tumors are rare, slow-growing neuroendocrine tumors that most frequently develop in the gastrointestinal tract or lungs and have high potential for metastasis. Metastasis to the brain is rare, but to another intracranial tumor is extremely rare. Of the intracranial tumors, meningiomas are the most common to host metastases, which may be related to its rich vascularity and E-cadherin expression. We describe the case of a 65-year-old female with active chemotherapy-treated neuroendocrine carcinoma who presented with left-sided facial numbness, headaches, and blurry vision. Initial imaging revealed a 1 cm irregular dural-based left petrous apex mass suggestive of a meningioma that was re-imaged four months later as a rapidly enlarging, extra-axial, mass extending into the cavernous sinus, effacing Meckel’s cave that resembled a trigeminal schwannoma. Pathology revealed a carcinoid tumor metastatic to meningioma. While the mass displayed characteristic imaging findings of a schwannoma, rapid growth in the setting of known active malignancy should prompt the clinician to consider mixed pathology from metastatic disease or a more aggressive meningioma.
Keywords: Meningioma, neuroendocrine, rectal cancer, metastasis, schwannoma
Case report
A 65-year-old female with medical history significant for stage 4 rectal neuroendocrine carcinoma actively treated with chemotherapy presented with numbness and tingling of the left face, intermittent headaches, and blurry vision for approximately three to four months. On physical exam she exhibited numbness in the left V1 territory with no other cranial nerve deficits.
Imaging
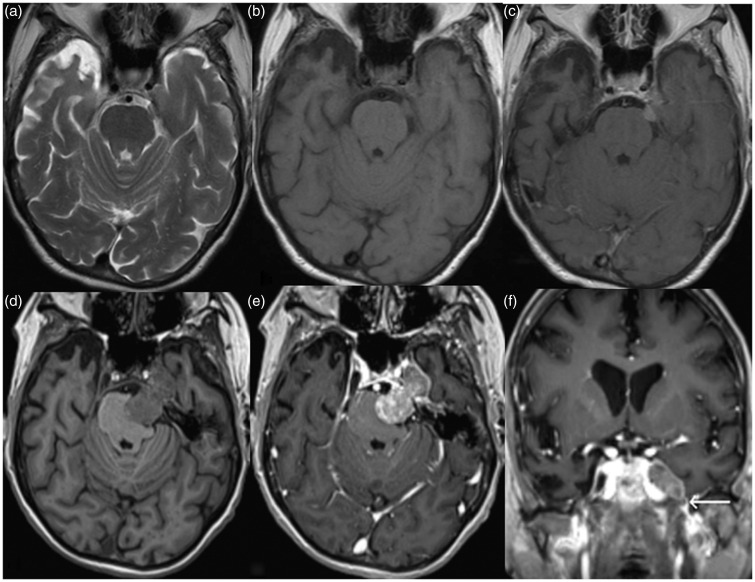
Initial contrast enhanced brain MRI (magnetic resonance imaging) was performed which demonstrated an irregularly shaped 1 cm dural based mass extending into the left prepontine cistern from the medial margin of the left petrous apex suggestive of a meningioma (Figure 1(a)). Four months later contrast enhanced brain MRI was performed due to worsening symptoms and preoperative evaluation. There was interval growth of the mass, measuring approximately 38 mm × 22 mm × 20 mm. The extra-axial heterogeneously enhancing mass located along the left petrous apex extended anteriorly into the left cavernous sinus, along the trigeminal nerve, and effacing Meckel’s cave. Inferolateral extension was present into the region of the left foramen ovale (Figure 1(f)). There was resulting mild mass effect upon adjacent medial left temporal lobe, left pons, and inferior left midbrain. Trigeminal schwannoma was the main consideration in the differential diagnosis.
Figure 1.
Axial T2, pre and post gadolinium T1-weighted magnetic resonance (MR) images (a), (b), and (c) demonstrate a small T2 hypointense homogeneously enhancing dural-based mass along the left petrous apex extending into the left prepontine cistern, and a diagnosis of meningioma was considered. Four months later, the symptoms progressed, and follow-up axial pre and post gadolinium T1-weighted MR images ((d) and (e)) reveal a large heterogeneously enhancing mass along the expected course of left trigeminal nerve resulting in effacement of the left ventral pons with extension into the left cavernous sinus and asymmetric effacement of left Meckel’s cave. Based on the imaging characteristics and minimal inferior extension into the foramen ovale (white arrow) on corresponding coronal post gadolinium T1-weighted image (f), a trigeminal schwannoma was suspected. A carcinoid tumor metastatic to meningioma was found on histology.
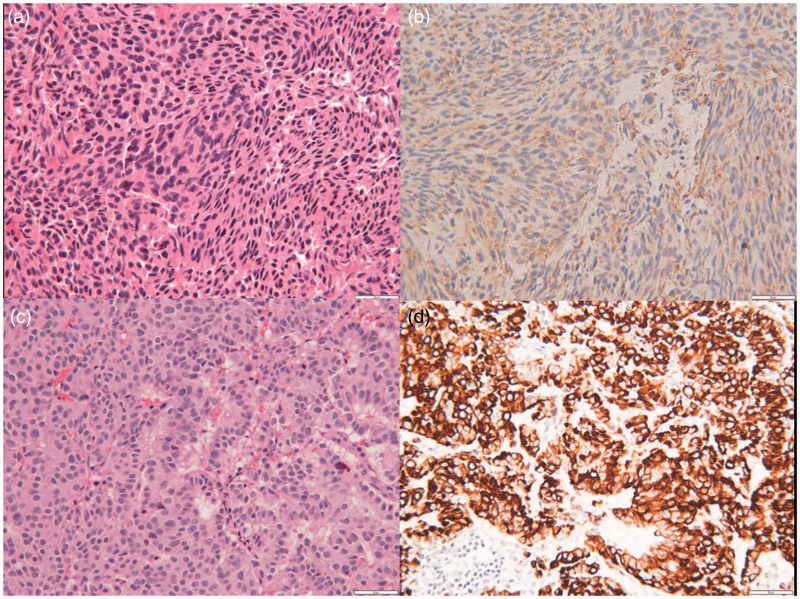
Neurosurgical stereotactic subtotal resection was performed with a left orbital zygomatic subtemporal approach to the middle cranial fossa. Pathologic diagnosis proved to be a conglomerate of carcinoid tumor and meningioma (Figure 2).
Figure 2.
(a) and (b) Meningioma component. Hematoxylin and eosin staining demonstrates fascicles of syncytial cells with monomorphic nuclei and indistinct cell borders. Immunohistochemical staining for epithelial membrane antigen demonstrates fine membranous staining. (c) and (d) Metastatic neuroendocrine carcinoma component. Hematoxylin and eosin staining demonstrates nests and cords of epithelioid cells with enlarged nuclei and prominent nucleoli. Cytokeratin CAM 5.2 immunohistochemical staining is strongly positive within the tumor cell cytoplasm (all pictures are 200× magnification with 50 µm internal measures).
Discussion
Carcinoid tumors arise from neuroendocrine cells, which are distributed throughout the human body with over 80% occurring in the gastrointestinal tract, lung, pancreas, ovary, uterus, urinary bladder, salivary glands, and testes.1 Most carcinoid tumors are slow growing; however, they are well known to have aggressive potential to metastasize. Metastases mainly occur in regional lymph nodes, lung, liver, bones, and very rarely the brain.1
Intracranial carcinoid metastasis is rare but to another tumor is extremely rare.1,2 The most common intracranial tumor to host any kind of metastasis is a meningioma followed by hemangioblastoma, astrocytoma, pituitary adenoma, schwannoma, oligodendroglioma, and ependymoma.3 The first reported case of a tumor metastasizing to an intracranial meningioma was a colon adenocarcinoma in 1930.4 Since then, an increasing number of tumor-to-tumor metastases have been described, with the majority of these metastases originating from breast and lung carcinomas.5,6 However, the only carcinoid tumor metastasis to a meningioma described before was of pulmonary origin in 1981, without the accompanying sophisticated high spatial and contrast resolution imaging available nowadays.7
Biologic factors that make meningiomas fertile soil for metastases are poorly understood, but there are multiple theories. Meningiomas contain a rich vascular network creating susceptible tissue for hematogenous metastases.8 Meningiomas are slow growing and indolent tumors, have low metabolic rates, and are high in collagen and lipid content, which may provide a less competitive metabolic environment.5
Cell adhesion molecules have been hypothesized to be the reason why some tumors metastasize to meningiomas. Specifically E-cadherin has been correlated. There is suggestion that downregulation of E-cadherin expression leads some tumor cells to enter the bloodstream directed to the secondary sites.9 E-cadherin immunoreactivity has been reported in a wide variety of meningiomas. The expression is considered the principal reason for the development of a cohesive chimeric mass of composite pathology.5
Beyond the fact that this is a rare tumor-to-tumor metastasis, also of interest is the morphology and geography of the tumor, which was deceiving for a trigeminal schwannoma on follow-up imaging. This tumor-to-tumor metastasis demonstrated a somewhat dumbbell shape, isointensity to gray matter, avid enhancement, and growth along the left trigeminal distribution – all characteristics similar to a trigeminal schwannoma.
Although an intracranial tumor may exhibit characteristic imaging findings, interval rapid growth in the setting of known active malignancy should prompt the clinician to consider a mixed pathology may be present from metastatic disease or a more aggressive meningioma. A study by Jääskeläinen et al. correlating intracranial meningioma growth with histology showed mean time of doubling volume of 415 days (ranges) (138–1045 days) in grade I (benign), 178 days (34–551) in grade II (atypical), and 205 days (30–472) in grade III (anaplastic).10 In this case the initially seen left prepontine cistern mass did not demonstrate aggressive features of an anaplastic meningioma. Therefore the growth rate exhibited by this mass is discordant with its imaging features.
Acknowledgements
The manuscript does not contain clinical studies or patient data.
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Maiuri F, Cappabianca P, Del Basso De Caro M, et al. Single brain metastases of carcinoid tumors. J Neurooncol 2004; 66: 327–332. [DOI] [PubMed] [Google Scholar]
- 2.Hood B, Bray E, Bregy A, et al. Primary carcinoid tumor of the cavernous sinus. World Neurosurg 2014; 81: 202.e9–e13. [DOI] [PubMed] [Google Scholar]
- 3.Takei H, Powell SZ. Tumor-to-tumor metastasis to the central nervous system. Neuropathology 2009; 29: 303–308. [DOI] [PubMed] [Google Scholar]
- 4.Fried BM. Metastatic inoculation of a meningioma by cancer cells from a bronchiogenic carcinoma. Am J Pathol 1930; 6: 47–52. [PMC free article] [PubMed] [Google Scholar]
- 5.Glass R, Hukku SR, Gershenhorn B, et al. Metastasis of lung adenosquamous carcinoma to meningioma: Case report with literature review. Int J Clin Exp Pathol 2013; 6: 2625–2630. [PMC free article] [PubMed] [Google Scholar]
- 6.Caroli E, Salvati M, Giangaspero F, et al. Intrameningioma metastasis as first clinical manifestation of occult primary breast carcinoma. Neurosurg Rev 2006; 29: 49–54. [DOI] [PubMed] [Google Scholar]
- 7.Smith TW, Wang SY, Schoene WC. Malignant carcinoid tumor metastatic to a meningioma. Cancer 1981; 47: 1872–1877. [DOI] [PubMed] [Google Scholar]
- 8.Hamperl M, Goehre F, Schwan S, et al. Tumor-to-tumor metastasis – bronchial carcinoma in meningioma. Clin Neuropathol 2015; 34: 302–306. [DOI] [PubMed] [Google Scholar]
- 9.Kafka A, Tomas D, Beros V, et al. Brain metastases from lung cancer show increased expression of DVL1, DVL3 and beta-catenin and down-regulation of E-cadherin. Int J Mol Sci 2014; 15: 10635–10651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jääskeläinen J, Haltia M, Laasonen E, et al. The growth rate of intracranial meningiomas and its relation to histology. An analysis of 43 patients. Surg Neurol 1985; 24: 165–172. [DOI] [PubMed] [Google Scholar]