Abstract
Hyperintense perilesional edema in brain masses on T1-weighted images (T1WI) is an unusual radiological finding. We report three cases showing this particular type of edema, one representing cerebral hemorrhagic cavernous malformation (CCM, cavernoma) and the other two, metastases of melanoma. The association between this sign and cavernoma was recently recognized. On the other hand, in melanotic lesions, the relationship with T1WI-hyperintense perilesional edema has not yet been described. Despite being an infrequent sign, it can considerably narrow the differential diagnosis, which gives it a high value for clinical practice. Moreover, given the high prevalence of the entities that manifest this imaging feature, it can be occasionally noticed.
Keywords: Cerebral cavernous malformation, cavernoma, melanoma, hyperintense edema on T1
Case reports
Case 1
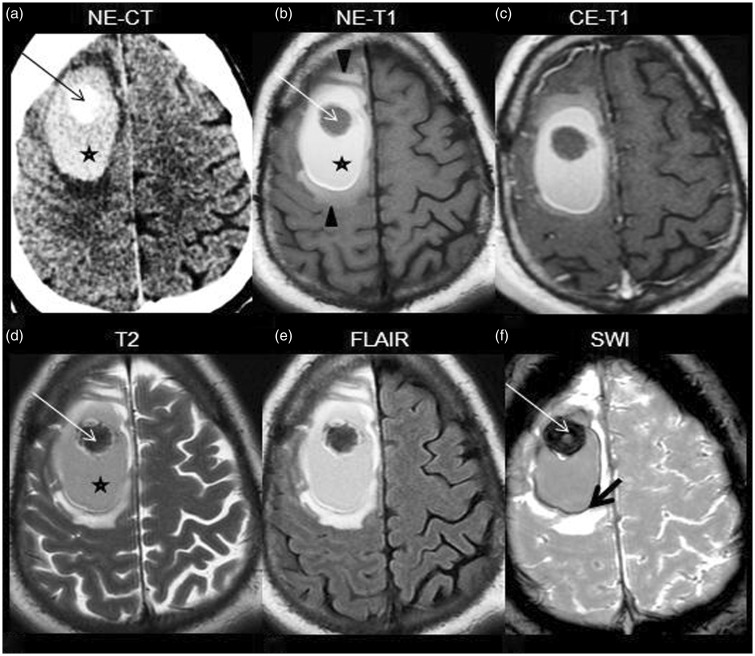
The patient was a 33-year-old man with headache, left hemiparesis, hemihypesthesia and partial seizures of the left arm for a week. No personal or familial significant history was present. Computed tomography (CT) followed by magnetic resonance (MR) (Figure 1(a)–(f)) demonstrated a nodular lesion in the right frontal subcortical region, surrounded by an area suggestive of hematoma in the late subacute stage and by hyperintense perilesional edema on T1-weighted imaging (T1WI). The differential diagnosis was between cavernoma and hemorrhagic metastasis. The patient underwent excision of the lesion and evacuation of the hematoma. Pathology revealed cerebral hemorrhagic cavernous malformation (CCM).
Figure 1.
NE-CT shows a hyperdense nodular lesion (arrow in (a)), surrounded by a dense area (star in (a)), involving the subcortical region of the right precentral gyrus and right superior and middle frontal gyri. On MR, the lesion is hypointense on both T1WI and T2WI (arrow in b, d), has high susceptibility effect on SWI (thin arrow in (f)), and it is surrounded by a hyperintense area on T1WI and T2WI suggestive of subacute hematoma (star in (b) and (d)) and more peripherally, by a thin hypointense hemosiderin ring best visualized on SWI (thick arrow in (f)). Hyperintense edema on T1WI is depicted (arrowheads in (b)). No contrast-enhancement is noticed (c). NE-CT: nonenhanced computed tomography; NE-T1: nonenhanced T1-weighted imaging; CE-T1 contrast-enhanced T1-weighted imaging; MR: magnetic resonance; SWI: susceptibility-weighted imaging.
Case 2
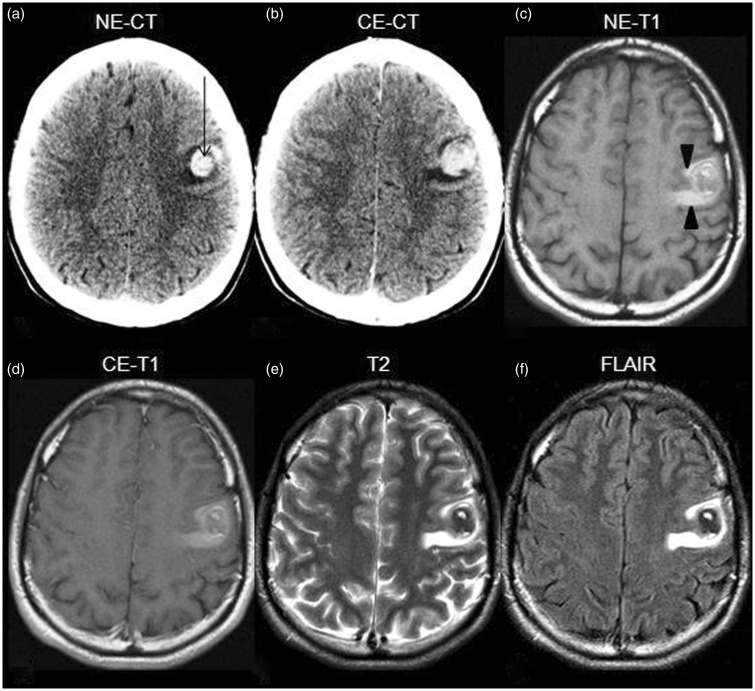
The patient was a 25-year-old man with a history of treated cutaneous melanoma, without known metastases or recurrence for two years of follow-up. He presented with dysarthria and right arm paresthesias. CT and MR demonstrated a focal lesion in the left frontal subcortical region surrounded by hyperintense signal on T1WI (Figure 2(a)–(f)). Based on the medical history, it was assumed to be a metastatic melanoma and was subsequently excised. No hemorrhagic landmarks were present on the pathologic examination.
Figure 2.
NE-CT demonstrates a hyperdense focal lesion (arrow in (a)), non-enhancing and surrounded by edema (b) located in the cortico-subcortical junction of the left frontal lobe. On MR it is heterogeneous and does not present contrast enhancement ((c)–(f)). Remarkably, hyperintense perilesional edema on T1WI can be seen (arrowheads in (c)). NE-CT: nonenhanced computed tomography; CE-CT: contrast-enhanced computed tomography; MR: magnetic resonance; NE-T1: nonenhanced T1-weighted imaging; CE-T1: contrast-enhanced T1-weighted imaging.
Case 3
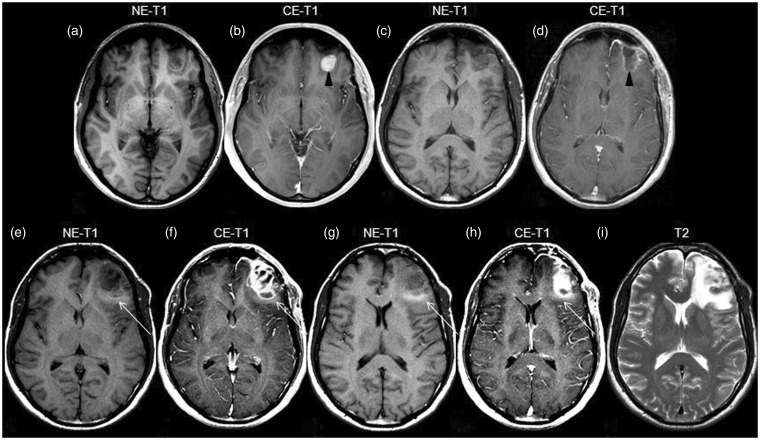
The patient was a 45-year-old man with a five-year previous history of cutaneous melanoma and pulmonary metastasis. On screening MR a small cerebral metastasis in the left frontal lobe was observed and the patient underwent surgery, without neurological sequela. MR carried out six months later showed recurrence. Peripheral hyperintense edema on T1WI can be seen (Figure 3(a)–(i)).
Figure 3.
Initial, preoperative MR showing a left frontal lesion, hypointense on T1WI (a) and presenting contrast enhancement (arrowhead in b). Follow-up MR, three months after surgery ((c), (d)), shows linear enhancement in the periphery of the excised area (arrowhead in (d)) and lack of edema. Next MR, six months after surgery ((e)–(i)), demonstrates recurrence with significant contrast enhancement ((f), (h)). Notably, hyperintense edema on T1WI is now present (arrow in (e)–(h)). T2WI demonstrates greater extension of the edema (i). T1WI: T1-weighted imaging; NE-T1: nonenhanced T1-weighted imaging; CE-T1 contrast-enhanced T1-weighted imaging; MR: magnetic resonance.
Discussion
The association between hyperintense perilesional edema and cavernoma was recently recognized in an elegant study including cerebral hemorrhagic lesions of different causes: primary hemorrhages, primary malignancies and metastases of breast, lung, liver and thyroid neoplasms.1 On the other hand, in melanotic lesions, the relationship with T1WI-hyperintense perilesional edema has not yet been described, while, given the melanin component and the high propensity to hemorrhage, central areas of high signal on T1WI, focally or diffusely present within the lesion, are frequently observed.2,3 CCM is considered the third most prevalent vascular malformation of the brain, after developmental venous anomaly (DVA) and capillary telangiectasia, affecting 0.5% to 0.7% of the population.4 Histologically, CCM is formed by dilated capillaries without elastic and muscular tunics, which makes it susceptible to multiple bleedings and explains the MR appearance of hemorrhage in different phases of evolution. In opposition to capillary telangiectasia and DVA, there is no cerebral tissue between its vascular channels. It is limited at the periphery by a pseudocapsule of gliotic brain infiltrated by hemosiderin, resulting in a hypointense peripheral ring on T2WI. When associated with DVA, the main hypothesis of the pathogenesis of CCM sustains that the first step is a stenosis of a branch of the DVA. This produces high pressure in the capillary bed and chronic microbleeds that stimulate fibroblastic and endothelial proliferation with the final result of CCM. As well, cavernoma can also occur as a result of radiation injury, through a similar mechanism of proliferation and dilation of the vascular endothelium and capillaries.5
CCM can affect all cerebral regions, though it is more frequent in the subcortical areas of frontal and temporal lobes. When situated in the posterior fossa, it has a tendency to involve the pons and the cerebellar hemispheres. The involvement of the medulla is uncommon, as opposed to capillary telangiectasia.5
One-third to one-half of CCM are clinically silent. The symptomatology is usually associated with hemorrhagic events. The risk factors for bleeding are: greater than 1 cm, multiple cavernomas, infratentorial location, association with DVA, CCM3 genotype of the familial form, feminine gender and clinical manifestation before age 35 years.5,6
There are different forms to classify CCM, the most used being the sporadic versus familial forms7 and the Zabramski classification.4 The sporadic form usually presents as a unique lesion and it has a strong association with DVA (up to 44%), which is commonly adjacent to the CCM and involved in its pathogenesis. On the other hand, the familial form is represented by multiple lesions, has no association with DVA and it does have higher risk of complications.7 The subtype of giant cavernoma is a rare entity,8 defined as greater than 4 cm, heterogeneous, multicystic (“bubbles of blood”) and without association with DVA. If atypical, a cavernoma can be extremely difficult to differentiate from a primary neoplasia.9 The Zabramski classification is based on the hemorrhagic aspect of CCM and includes: type 1, subacute hemorrhage (hyperintense on T1WI, hyper- or hypointense on T2WI); type 2, “popcorn ball” appearance (mixed-signal intensity on T1- and T2WI, hypointense ring on T2WI); type 3, chronic hemorrhage (iso- or hypointense on T1- and T2WI, also hypointense ring) and type 4, patchy microbleeds (punctate hyperintense lesions on T2*, “black dots”).4
On CT, up to half of CCM cannot be identified, the others being slightly hyperdense and well defined, sometimes with calcifications. The MR appearance is heterogeneous, a consequence of different stages of hemorrhage. A hypointense peripheral ring frequently appears on T2WI, explained by the presence of hemosiderin. On T2*-gradient recalled echo (GRE) and susceptibility-weighted imaging (SWI) it manifests high susceptibility magnetic properties, presenting a pronounced hypointense signal.4,7,8 When a CCM is studied, the administration of contrast is recommended in order to search for a DVA. The reasons are the high association between the two malformations and, more important, when dealing with a surgical CCM, being aware of the presence of a DVA is essential in order to avoid a venous infarct that would result from cutting the DVA. CCM usually does not enhance.4,5,8 Sometimes it is difficult to differentiate a complicated cavernoma from a hemorrhagic neoplasm. Of note, it has been shown that hyperintense perilesional signal on T1WI can be seen in more than half of the patients with CCM, this being of particular value when the diagnosis is uncertain and a prompt therapeutic decision is required.1
Melanotic brain lesions correspond in most cases to a secondary location, representing the third most frequent type of brain metastases, after lung and breast. Nevertheless, they sometimes occur in patients with occult primary melanoma or even as primary solitary cerebral or meningeal tumor, these being the groups in which the differentiation from other hemorrhagic lesions represents a challenge.2
The underlying pathologic bases for the radiological appearance of metastatic brain melanoma are still discussed.10,11 The classically described MR findings were based on the grade of melanocytic component: The more melanin, the more T1WI hyperintensity and T2WI hypointensity is expected. Although typical MR patterns for melanin-containing lesions were characterized, their specificity continues to be unknown. The explanation resides in the great propensity to hemorrhage of the metastatic melanoma, which can significantly modify the classically described patterns of the nonhemorrhagic metastases.2 Moreover, the mechanism responsible for the features of the peripheral edema in metastatic melanoma is still unknown. Perhaps it consists of a slow diapedesis phenomenon of the pigments of melanin through fenestrated or altered walls of small vessels toward the periphery of the lesion. Or maybe repeated hemorrhages could lead to extravasations of blood products in the peripheral edema. However, in order to be feasible, both hypotheses would need to allow sufficient evolution time of the metastasis, which give the possibility for alteration of the peripheral vessel walls to occur.2,10,11
Lastly, the accurate differentiation between metastasis of melanoma and CCM is of critical importance. Metastasis of melanoma generally benefits from more aggressive treatment compared with CCM. Also, the outcome is favorable for the latter, even when surgery in eloquent brain regions or brainstem is performed, whereby the prognosis is frequently dismal in metastatic melanoma.4,11 Both entities are commonly found in young patients, the neurologic manifestations are usually unspecific and the imaging presentation is not always characteristic. Factors that can suggest metastasis of melanoma are the history of skin involvement, other brain or body metastases, the predilection for the cortico-subcortical junction, higher grade of perilesional edema, a short time since the diagnosis of the primary tumor and the aggressiveness of melanoma according to Breslow thickness and Clark level.2,11 Even though not always present, the enhancement after contrast administration can be helpful, indicating metastasis.2 Conversely, the lack of enhancement, the effect of susceptibility on T2* sequences and the presence of adjacent DVA suggest CCM.5
To summarize, while the association between hyperintense peripheral edema on T1WI and cavernoma was previously described, this relationship has not yet been recognized in melanotic lesions, this being the novel insight that the present report brings. Despite being an infrequent sign, perilesional edema with high signal on T1WI can narrow considerably the differential diagnosis, which gives it a high value for clinical practice. Moreover, given the high prevalence of the entities that manifest this imaging feature, it can be occasionally noticed. Finally, its recognition is simple, reproducible and does not need further special training. A way forward could be comparative studies for a better characterization of this sign in these two entities.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Yun TJ, Na DG, Kwon BJ, et al. A T1 hyperintense perilesional signal aids in the differentiation of a cavernous angioma from other hemorrhagic masses. AJNR Am J Neuroradiol 2008; 29: 494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Escott EJ. A variety of appearances of malignant melanoma in the head: A review. Radiographics 2001; 21: 625–639. [DOI] [PubMed] [Google Scholar]
- 3.Ginat DT, Meyers SP. Intracranial lesions with high signal intensity on T1-weighted MR images: Differential diagnosis. Radiographics 2012; 32: 499–516. [DOI] [PubMed] [Google Scholar]
- 4.Zabramski JM, Wascher TM, Spetzler RF, et al. The natural history of familial cavernous malformations: Results of an ongoing study. J Neurosurg 1994; 80: 422–432. [DOI] [PubMed] [Google Scholar]
- 5.Cortés Vela JJ, Concepción Aramendía L, Ballenilla Marco F, et al. Cerebral cavernous malformations: Spectrum of neuroradiological findings [article in English and Spanish]. Radiologia 2012; 54: 401–409. [DOI] [PubMed] [Google Scholar]
- 6.Al-Shahi Salman R, Hall JM, Horne MA, et al. Untreated clinical course of cerebral cavernous malformations: A prospective, population-based cohort study. Lancet Neurol 2012; 11: 217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petersen T, Morrison L, Schrader RM, et al. Familial versus sporadic cavernous malformations: Differences in developmental venous anomaly association and lesion phenotype. AJNR Am J Neuroradiol 2010; 31: 377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kan P, Tubay M, Osborn A, et al. Radiographic features of tumefactive giant cavernous angiomas. Acta Neurochir (Wien) 2008; 150: 49–55. discussion 55. [DOI] [PubMed] [Google Scholar]
- 9.Wilson DM, Cohen B, Keshari K, et al. Case report: Glioblastoma multiforme complicating familial cavernous malformations. Clin Neuroradiol 2014; 24: 293–296. [DOI] [PubMed] [Google Scholar]
- 10.Hung T, Morin J, Munday WR, et al. Angiotropism in primary cutaneous melanoma with brain metastasis: A study of 20 cases. Am J Dermatopathol 2013; 35: 650–654. [DOI] [PubMed] [Google Scholar]
- 11.Izraely S, Sagi-Assif O, Klein A, et al. The metastatic microenvironment: Brain-residing melanoma metastasis and dormant micrometastasis. Int J Cancer 2012; 131: 1071–1082. [DOI] [PubMed] [Google Scholar]