Summary
Sympathovagal imbalance contributes to progressive worsening of heart failure (HF) and is associated with untoward clinical outcomes. Based on compelling pre-clinical studies that supported the role of autonomic modulation in HF models, a series of clinical studies were initiated using spinal cord stimulation, vagus nerve stimulation, and baroreceptor activation therapy in patients with HF with a reduced ejection fraction. Whereas the phase II studies with baroreceptor activation therapy remain encouraging, the larger clinical studies with spinal cord stimulation and vagus nerve stimulation have yielded disappointing results. Here we will focus on the pre-clinical studies that supported the role of neuromodulation in the failing heart, as well provide a critical review of the recent clinical trials that have sought to modulate autonomic tone in HF patients. This review will conclude with an analysis of some of the difficulties in translating device-based modulation of the autonomic nervous system from pre-clinical models into successful clinical trials, as well as provide suggestions for how to move the field of neuromodulation forward.
Key Words: autonomic nervous system, baroreceptor activation therapy, heart failure, neuromodulation, spinal cord stimulation, vagus nerve stimulation
Abbreviations and Acronyms: ANS, autonomic nervous system; BAT, baroreceptor activation therapy; HF, heart failure; HFrEF, heart failure with a reduced ejection fraction; LV, left ventricular; LVEF, left ventricular ejection fraction; MSNA, muscle sympathetic nervous activity; MI, myocardial infarction; NE, norepinephrine; NT-proBNP, N-terminal pro–B-type natriuretic peptide; NYHA, New York Heart Association; OMT, optimal medical therapy; SCS, spinal cord stimulation; SNS, sympathetic nervous system; VF, ventricular fibrillation; VNS, vagus nerve stimulation; VT, ventricular tachycardia
Overview of the Cardiac Autonomic Nervous System
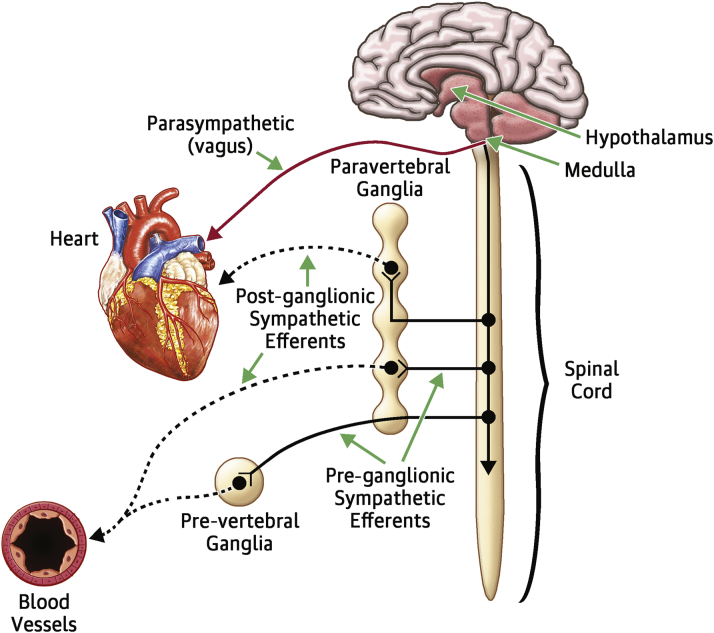
Details of the complex regulation of the autonomic nervous system (ANS) have been provided in several recent reviews and will be discussed here only briefly in order to provide the proper context for the discussion of the clinical studies of device-based modulation of ANS (“neuromodulation”) in heart failure (HF) 1, 2. ANS consists of the parasympathetic nervous system and the sympathetic nervous system (SNS). Physiologically, these 2 systems are diametrically opposed, yet work together synergistically in a reciprocal manner, in order to provide the cardiovascular system with the ability to respond quickly to both internal and external stimuli (3). Both the SNS and the ANS are reflex circuits composed of “motor” (efferent) fibers that convey information from the central nervous system to the heart (Figure 1) and “sensory” (afferent) sympathetic and parasympathetic fibers that convey information from the heart to the central nervous system. The heart also receives afferent parasympathetic input from a series of mechanosensitive nerve endings in large arteries and the carotid sinuses, collectively referred to as baroreceptors, because they are sensitive to changes in blood pressure and blood volume. The baroreceptors from the carotid arteries have axons in the glossopharyngeal nerve, and those from the aorta have axons that travel in the vagus nerve. The baroreflex is a major homeostatic mechanism for maintaining blood pressure and is responsible for controlling the afterload of the heart. Baroreceptors are activated by the opening of mechanosensitive ion channels within the sensory terminals, which in turn activate afferent fibers that terminate in the nucleus tractus solitarius in the medulla oblongata. Increased baroreflex activity (e.g., in hypertension) results in a reflex increase in parasympathetic activity that triggers a reflex inhibition of sympathetic tone, thus restoring autonomic balance. Conversely, decreased baroreflex activity (e.g., in hypotension) results in withdrawal of parasympathetic tone that results in a reflex increase in sympathetic tone.
Figure 1.
The Autonomic Nervous System
Diagram of pre-ganglionic and post-ganglionic sympathetic and parasympathetic fibers.
Reproduced with permission from Klauber RE. Cardiovascular Pharmacology Concepts: Autonomic Ganglia. January 27, 2012. Available at: http://cvpharmacology.com/autonomic_ganglia. Accessed March 10, 2016.
Sympathovagal Imbalance in Heart Failure
The clinical syndrome of heart failure with a reduced ejection fraction (HFrEF) is associated with sustained activation of the sympathetic nervous system that is accompanied by a withdrawal of parasympathetic tone 2, 4, 5. Although these disturbances in autonomic control were initially attributed to loss of the inhibitory input from arterial or cardiopulmonary baroreceptor reflexes, there is increasing evidence that excitatory reflexes may also participate in the autonomic imbalance that occurs in HF (2). Under normal conditions, inhibitory inputs from “high pressure” carotid sinus and aortic arch baroreceptors and the “low pressure” cardiopulmonary mechanoreceptors are the principal inhibitors of sympathetic outflow, whereas discharge from the nonbaroreflex peripheral chemoreceptors and muscle “metaboreceptors” are the major excitatory inputs to sympathetic outflow. The parasympathetic limb of the baroreceptor heart rate reflex is also responsive to arterial baroreceptor afferent inhibitory input. At rest, healthy individuals display low sympathetic discharge and high rate variability. In HF patients, the peripheral baroreflex responses become suppressed (“blunted”) as HF worsens (6). Blunting of the peripheral arterial and cardiopulmonary baroreceptors results in a derepression of the sympathetic outflow from the central nervous systems and a net increase in efferent sympathetic nerve activity that is accompanied by decreased efferent parasympathetic tone. Consequently, patients with HF have a loss of heart rate variability and increased peripheral vascular resistance (2).
Dysregulation of the ANS in HF has received considerable attention over the past 3 decades, because of the well-recognized association between increased sympathetic activity and “neurohormonal” activation. Although increased sympathetic stimulation provides short-term support for the cardiovascular system, the sustained activation of the SNS is maladaptive in the long term because it is directly toxic to the heart and circulation and also leads to activation of the renin-angiotensin system, which can also be deleterious to the heart and circulation (reviewed in [7]). However, the role of the parasympathetic nervous system in the pathophysiology of HF is less well understood. In isolated organ preparations, human in vitro data, and in animal models, local muscarinic receptor stimulation results in inhibition of norepinephrine (NE) release from sympathetic nerve terminals 8, 9. In vivo, it has been shown that cardiac NE spillover was greater in patients with HF than those with normal LV function, and that infusion with acetylcholine attenuates the amount of NE release in these patients. This effect was not seen in the presence of atropine, suggesting that it is mediated via muscarinic receptor activation 10, 11. The ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) trial was the first large multicenter clinical study to examine impairment in vagal activity as a prognostic marker following myocardial infarction (MI). ATRAMI enrolled 1,284 post-MI patients and followed them over a 2-year period and showed that patients with depressed baroreflex sensitivity (a marker of decreased vagal activity) had decreased survival (5). The depressed baroreflex sensitivity was also shown to be associated with a worse New York Heart Association (NYHA) functional class and higher mortality in HF patients. The prognostic value of the depressed baroreflex sensitivity among patients with HFrEF was also observed in the presence of beta-blocker therapy 12, 13. These observations have led to the development of various device-based therapies that are designed to restore the sympathovagal imbalance in patients with HF.
Therapeutic Modulation of the Autonomic Nervous System in Heart Failure
It bears emphasis that many of the current therapies for HFrEF patients reverse the sympathovagal imbalance that develops in HF, including pharmacologic therapy with beta-blockers and angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers, exercise training, and cardiac resynchronization therapy (reviewed in [14]). Despite the tremendous progress in treating patients with HF, the great majority of patients with HF will eventually develop worsening HF (15). Thus, there continues to be an unmet need for new therapies for treating patients with HF. To this end, there has been growing interest in directly modulating the ANS as a means of counteracting the sympathovagal imbalance that develops in HF. In the following review, we will focus on the pre-clinical and clinical studies that have employed spinal cord stimulation (SCS), vagus nerve stimulation (VNS), and baroreceptor activation therapy (BAT) in HF, with the goal of deconstructing these studies in order to better understand why it has been so difficult to translate the encouraging pre-clinical studies into successful phase II/III clinical trials. The important therapeutic areas of renal nerve denervation and left cardiac sympathetic denervation have been the subject of several recent reviews and will not be discussed herein (1).
Vagus nerve stimulation
VNS has been used in humans and has been approved by the U.S. Food and Drug Administration for the treatment of epilepsy (1997) and refractory depression (2005). The device that is used for the treatment of epilepsy and depression is composed of a pulse generator, a bipolar lead that is implanted in the mid-cervical portion of the left vagus nerve, and delivers a biphasic current that continuously cycles between on and off periods to stimulate afferent vagus nerve fibers. Importantly, 2 of 3 VNS devices used in patients with HFrEF were developed for use in patients with epilepsy and/or depression.
Pre-clinical studies of VNS
The salutary role VNS in the heart was first shown by a series of experimental studies by Vanoli et al. (16), who demonstrated that VNS prevented ventricular fibrillation (VF) induced by acute myocardial ischemia in the setting of a healed MI. In animal models of HF, VNS resulted in increased survival (17), improved ventricular function 17, 18, as well as decreased inflammation (18). Indeed, the anti-inflammatory effects of VNS following ischemia and reperfusion injury are accompanied by a reduction in the number of macrophages and apoptotic cells that is paralleled by decreased levels of circulating pro-inflammatory cytokines (19), which has been referred to as the “cholinergic anti-inflammatory reflex” (20).
Clinical studies of VNS
In the clinical setting VNS is performed by placing an electrode cuff around the right or left cervical vagus 21, 22, thereby stimulating both the efferent and afferent vagus nerve fibers. It should be recognized that stimulation of afferent vagus nerve fibers experimentally can have profound effects on the activity of the contralateral efferent parasympathetic tone (increased activity) and efferent sympathetic tone (inhibition of activity). However, it is unclear at the time of this writing whether it is preferable to stimulate afferent of efferent vagus nerve fibers.
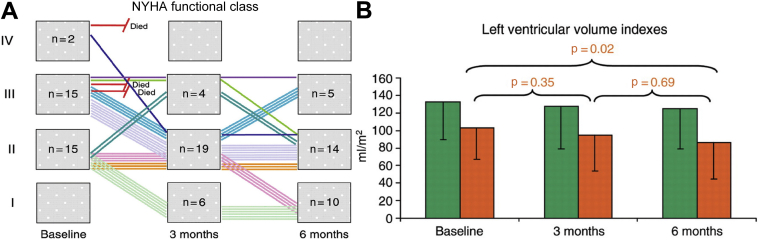
Four clinical studies of VNS in humans have been completed and published thus far (Table 1). The first VNS study in humans was the CardioFit pilot, which enrolled 32 patients with a history of chronic NYHA functional class II to IV HF and a left ventricular ejection fraction (LVEF) <35% (22). The patients were already receiving optimal medical treatment (OMT) with beta-blockers, angiotensin-converting enzyme inhibitors/angiotensin-receptor blockers and loop diuretics. Additionally, 19 of the 32 patients had an implantable cardioverter-defibrillator. The CardioFit (Bio Control Medical, Yehud, Israel) system is a “closed loop” device system with an intracardiac right ventricular sensing lead and a bipolar cuff placed around the right cervical vagus nerve (Figure 2A). The stimulation intensity of VNS, which was limited by patient symptoms of hoarseness and/or referred jaw pain, was uptitrated to 4.1 ± 1.2 mA. A clear demonstration of efferent vagus nerve stimulation in the CardioFit pilot trial was demonstrated by the change in resting heart rate during the trial, which decreased significantly during from 82 ± 13 beats/min to 76 ± 13 beats/min during the study. At 6 months ∼60% of patients improved by at least 1 NYHA functional class (Figure 3) and the Minnesota Living with Heart Failure Questionnaire quality of life score improved significantly, as did the distance on the 6-min walk test. An analysis (blinded) of the 2-dimensional echocardiograms showed that there was a significant reduction in LV end-systolic volume, and a significant increase in LVEF (from 22 ± 7% to 29 ± 8%), whereas there was a nonsignificant decrease in LV end-diastolic volume. A pre-specified follow-up of a group of patients at 1 (n = 23) and 2 years (n = 19) showed that many of the beneficial effects of VNS were maintained.
Table 1.
Vagal Nerve Stimulation
| Study Design | Patient Characteristics | N | Outcomes | Results | |
|---|---|---|---|---|---|
| CardioFit (NCT00461019) | Nonrandomized Open label |
NYHA functional classes II and III EF <35% |
32 | 1. Occurrence of all system and/or procedure-related adverse events (6 months) 2. NYHA functional class, 6MWD, LVESV, MLHFQ QoL scores |
No significant adverse events Significant improvement in NYHA functional class, 6MWD, LVESV, and QoL scores |
| NECTAR-HF (NCT01385176) | Randomized Double blind |
NYHA functional classes II and III EF ≤35% LVESD >5.5 cm QRS interval <130 ms |
96 | 1. LVESD (6 months) 2. NYHA functional class, Vo2 max, SF-36 and MLHFQ QoL scores, pro-BNP |
No sig change in LVESD Significant improvement in NYHA functional class and QoL scores |
| ANTHEM-HF (NCT01823887) | Randomized Open label |
NYHA functional classes II and III EF ≤40% QRS interval <150 ms |
60 | 1. Change in EF and LVESV (6 months) 2. NYHA functional class, 6MWD, MLHFQ QoL scores, LVESD, HRV, BNP |
Significant increase in EF (4.5%); no change in LVESV Significant improvement in NYHA functional class and QoL score |
| INOVATE-HF (NCT01303718) | Randomized Open label |
NYHA functional class III EF ≤40% LVESD 5–8 cm |
730 | 1. Composite all-cause mortality/HF hospitalizations (end of study); freedom from procedure-/system-related complications (90 days); all-cause death or complications (12 months) 2. LVESV index, 6MWD, KCCQ QoL scores, hospitalization-free days |
No significant difference in all-cause mortality and HF hospitalizations; significant improvement in 6MWD, KCCQ QoL; no safety issues identified |
6MWD = 6-min walk distance; ANTHEM-HF = Autonomic Regulation Therapy via Left or Right Cervical Vagus Nerve Stimulation in Patients With Chronic Heart Failure; BNP = B-type natriuretic peptide; CardioFit = CardioFit for the Treatment of Heart Failure; EF = ejection fraction; HF = heart failure; HRV = heart rate variability; INOVATE-HF = INcrease of VAgal TonE in Heart Failure; KCCQ = Kansas City Cardiomyopathy Questionnaire; LVESD = left ventricular end-systolic diameter; LVESV = left ventricular end-systolic volume; MLHFQ = Minnesota Living With Heart Failure Questionnaire; NECTAR-HF = Neural Cardiac Therapy for Heart Failure; NYHA = New York Heart Association; QoL = quality of life; SF-36 Short Form 36 Questionnaire; Vo2 max = maximum volume of oxygen consumed.
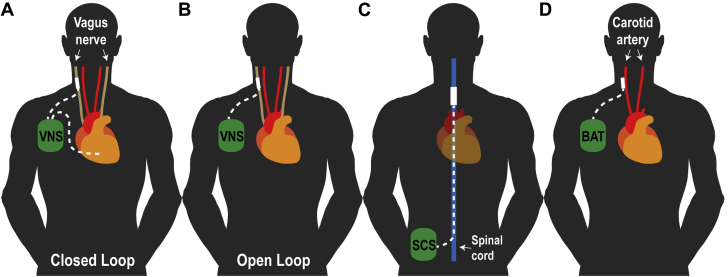
Figure 2.
Schematic Demonstrating the Location and Stimulation Sites for Device-Based Neuromodulation Modality
(A) Vagus nerve stimulator placed in right subpectoral region with standard transvenous pacing/sensing lead placed in right ventricular (closed loop) and vagus nerve stimulating lead (dotted white lines) tunneled to cervical vagus region. (B) Vagus nerve stimulator placed in right subpectoral region with vagus nerve stimulating lead (dotted white line) tunneled to cervical vagus region (open loop). (C) The spinal cord stimulation (SCS) generator is implanted in abdomen or paraspinous region with stimulation lead (blue line) placed in dorsal epidural space at thoracic level 4. (D) Baroreflex stimulation generator placed in right subpectoral region with bilateral stimulation leads tunneled to the carotid baroreceptor region. Modified and adapted with permission from Lopshire and Zipes (14). BAT = baroreceptor activation therapy; SCS = spinal cord stimulation; VNS = vagus nerve stimulation.
Figure 3.
Results of the CardioFit System Pilot Trial
(A) Change in New York Heart Association (NYHA) classification at 3 and 6 months after vagus nerve stimulation. (B) Change in left ventricular end-systolic volume index at 3 and 6 months.
Reproduced with permission from De Ferrari et al. (22).
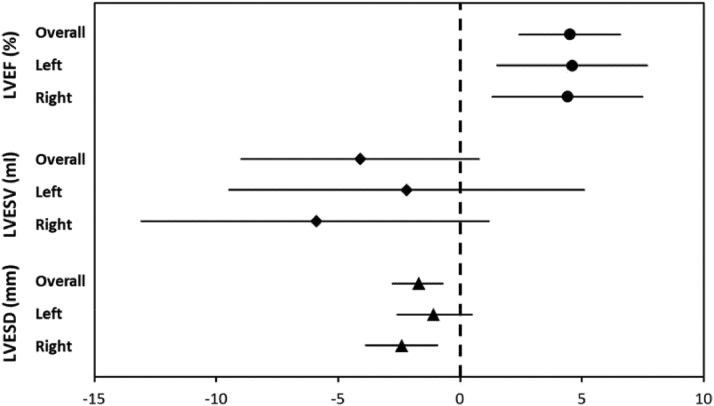
The ANTHEM-HF (Autonomic Regulation Therapy via Left or Right Cervical Vagus Nerve Stimulation in Patients With Chronic Heart Failure) study (23) enrolled 60 patients with NYHA functional classes II and III HF, LVEF <40%, and QRS <150 ms. Patients were randomized to either left or right cervical VNS using a proprietary “open loop” VNS system (Figure 2B) that did not incorporate a right ventricular sensing lead. The stimulation amplitude of VNS was uptitrated over a 10-week period, with an average stimulation amplitude of 2.0 ± 0.6 mA. The primary efficacy endpoint was the change in LV end-systolic volume. The LVEF increased by 4.5% (p < 0.05) in the pooled analysis of right and left VNS, whereas there was a nonsignificant change in LV end-systolic volume compared with baseline values. Overall, 77% of patients improved by at least 1 NYHA functional class at 6 months, with a significant improvement in the Minnesota Living with Heart Failure score. Although there was a trend toward greater improvement with right-sided VNS, these differences were not significant statistically (Figure 4).
Figure 4.
Primary Clinical Endpoint of the ANTHEM-HF Trial
Mean and 95% confidence intervals of echocardiographic changes after 6 months of autonomic regulation therapy (overall, left-side treatment, and right-side treatment). Reproduced with permission from Premchand RK et al. (23). ANTHEM-HF = Autonomic Regulation Therapy via Left or Right Cervical Vagus Nerve Stimulation in Patients With Chronic Heart Failure; LVEF = left ventricular ejection fraction; LVESV = left ventricular end-systolic volume; LVESD = left ventricular end-systolic diameter.
The NECTAR-HF (Neural Cardiac Therapy for Heart Failure) study enrolled 96 patients with NYHA functional classes II and III HF, LVEF <35%, a QRS interval <130 ms, and LV end-diastolic diameter >55 mm (24). All of the patients enrolled received a device implant and were then randomized 2:1 to active treatment or sham treatment for the first 6 months, followed by active treatment for all patients from 6 to 12 months. The device used in NECTAR-HF was also an open loop system (Figure 2B), similar to the one used in ANTHEM-HF, that employed a helical bipolar electrode. The stimulation amplitude of VNS was uptitrated and attained an average stimulation amplitude of 1.42 ± 0.8 mA, which was less than that which was achieved in the CardioFit pilot trial or the ANTHEM-HF trial. The primary efficacy endpoint, which was the change in LV end-systolic diameter at 6-month follow-up, was not significantly different (p = 0.60) in the treatment and control groups. Secondary endpoints, including LV end-diastolic dimension, LV end-systolic volume, LVEF, peak V02, and N-terminal pro–B-type natriuretic peptide (NT-proBNP) were not different between groups. However, there were statistically significant improvements in quality of life scores for the Minnesota Living with Heart Failure Questionnaire and the NYHA functional class in the group receiving treatment. Interestingly, an assessment of blinding, which was performed at 6 months revealed that 70% of the patients assigned to active treatment correctly guessed their randomization group, which was likely secondary to side effects of VNS with this device.
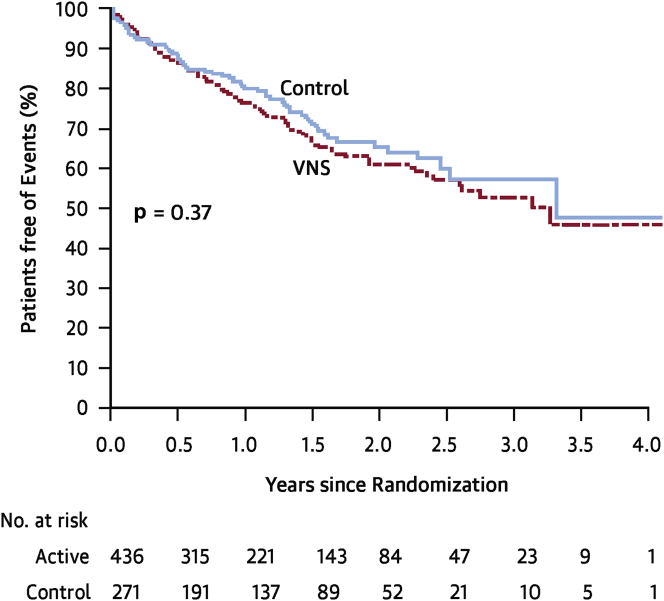
The INOVATE-HF (Increase of Vagal Tone in Heart Failure) was a pivotal phase III multicenter randomized clinical trial designed to assess the effects of VNS using the CardioFit closed loop system (Figure 2A) in patients with symptomatic HF despite OMT 25, 26. A total of 707 enrolled patients with NYHA functional class III symptoms, LVEF <40% and LV end-diastolic size 50 to 80 mm were randomized 3:2 to either active treatment with device implantation or no implantation. One month after implantation, patients in the treatment arm underwent multiple scheduled visits over 4 weeks, during which time the stimulation output was gradually increased with a goal of achieving current of 3.5 to 5.5 mA. Importantly, the stimulation protocol for INOVATE-HF differed slightly from the protocol that was used in the CardioFit pilot, in that the on time for stimulation was 5.1 ± 0.8 s in INOVATE-HF and was 7.1 ± 4.8 s in the pilot trial. The primary endpoint of this study was a composite of all-cause mortality or unplanned HF hospitalizations. There were 2 coprimary safety endpoints: freedom from procedure and system-related complication events at 90 days and number of patients with all-cause death or complications at 12 months. On December 15, 2015, INOVATE-HF was stopped by the Steering Committee on the recommendation of the independent Data and Safety Monitoring Board after the second planned interim analysis showed that the trial was unlikely to show a statistically significant benefit in the treatment arm. Patients were followed for up to 4.3 years with a mean follow-up of 16 months. The primary efficacy outcome occurred in 30.3% in the VNS group compared with 25.8% in the control group (hazard ratio: 1.14; 95% confidence interval: 0.86 to 1.53; p = 0.37) (Figure 5). Quality of life, NYHA functional class and 6-min walking distance were favorably affected by VNS (p < 0.05 for all); however, the LV end-systolic volume index was not different between groups (p = 0.36). The effects of treatment on 6 pre-specified subgroups for the primary efficacy composite outcome showed that the only significant treatment by subgroup interaction was for sex with worse outcomes with VNS among female subjects (p = 0.03); however, a multivariate analysis of the primary efficacy endpoint showed that sex was not an independent predictor of outcome. No statistically significant differences in heart rates were observed between control and VNS therapy arms. Of note, the mean stimulation current in INOVATE-HF was 3.9 ± 1.0 mA at the 6-month follow-up visit, with 73% of patients achieving the goal of ≥3.5 mA.
Figure 5.
Primary Efficacy Endpoint of the INOVATE-HF Trial
There was no significant difference in the primary composite outcome of death from any cause or a worsening heart failure event in the vagus nerve stimulation (VNS) treatment arm when compared with the control group (hazard ratio: 1.14; 95% confidence interval: 0.86 to 1.53; p = 0.37). Reproduced with permission from Gold et al. (26). INOVATE-HF = INcrease of VAgal TonE in Heart Failure.
Spinal cord stimulation
Spinal cord stimulation (SCS) has been used for over 40 years to treat chronic intractable pain. The concept for SCS originated following the revolutionary gate theory for the origin of pain, which raised the possibility of suppressing pain by “closing the gate” by activation of large diameter afferent fibers (27). The benefits of SCS have been reported in patients with refractory angina both due to end-stage coronary artery disease and cardiac syndrome X (28). Interestingly, SCS fared similarly to surgical and laser endomyocardial revascularization in severe refractory angina, without an increase in ischemic events, suggesting that the improvement in this condition was more complex than the suppression of the nociceptive input associated with myocardial ischemia. SCS applied at the C7 to C8 or T1 to T6 levels (Figure 2C) theoretically exerts its effects through activation of ANS, with a resultant overall increase in parasympathetic tone.
Pre-clinical studies of SCS
In an animal model of MI, preemptive SCS resulted in marked infarct size reduction, which was attenuated by alpha- and beta-receptor blockade, suggesting an SNS inhibition by SCS (29). SCS also reduced the occurrence of ventricular tachycardia (VT)/VF from 59% to 23% in a canine model in which ventricular arrhythmias were elicited by transient myocardial ischemia (30). In a subsequent study (31), 28 dogs with HF induced by anterior MI and rapid pacing were assigned for 5 weeks to no therapy, carvedilol or SCS (delivered at T4/T5 region for 2 h, 3× a day). LVEF that had declined to 18% after the induction of HF, recovered to 28%, 34%, and 47%, respectively, in the control, carvedilol, and SCS groups. Subsequent studies using the same animal model showed that SCS was superior to carvedilol + ramipril. Olgin et al. (32) raised the interesting possibility that SCS at the T1 to T2 level enhanced parasympathetic activity based on the observation that SCS resulted in a significant increase in sinus cycle length and the AH interval, which could be abolished by bilateral vagal transection. Other pre-clinical studies have shown that SCS results in reduced burden of VT/VF and greater improvements in EF in MI animal models than in control subjects, possibly via SNS inhibition and/or parasympathetic nervous system stimulation 30, 33, 34.
Clinical studies of SCS
Based on pre-clinical models, several clinical studies with SCS have been conducted in patients with HF (Table 2). The SCS HEART (Spinal Cord Stimulation for Heart Failure) study (35) evaluated the safety and efficacy of an implanted a SCS device in 17 patients with NYHA functional class III or ambulatory NYHA functional class IV HF. The primary efficacy endpoint was based on a composite score of changes in NYHA functional class, peak maximum O2 consumption, LV end-systolic volume, and LVEF. Analysis at 6 months showed that 73% of patients had improvement in ≥4 of 6 efficacy parameters and that there were no reported deaths or device-device interactions. As of the 18-month follow-up, 2 patients had died, 2 were hospitalized for HF, and there were no device-device interactions. Four patients with VT/VF before receiving the SCS therapy continued with VT/VF, requiring implantable cardioverter-defibrillator intervention.
Table 2.
Spinal Cord Stimulation
| Study Design | Patient Characteristics | N | Outcomes | Results | |
|---|---|---|---|---|---|
| SCS HEART (NCT01362725) | Nonrandomized Open label |
NYHA functional classes III and IV EF 20% to 35% |
17 | 1. Safety and efficacy (composite of change in NYHA functional class, Vo2 max, LVESV, EF) (6 months) 2. Long-term safety (24 months) |
Significant improvement in >4 of 6 efficacy parameters, without significant adverse events No significant long-term complications |
| DEFEAT-HF (NCT01112579) | Randomized Single blind |
NYHA functional class III EF ≤35% LVESD 5.5–8 cm QRS interval <120 ms |
66 | 1. LVESV index (6 months) 2. Vo2 max, pro-BNP |
No significant change in LVESV index No significant change in Vo2 max or pro-BNP level |
| Methodist SCS (NCT01124136) | Randomized Double blind |
NYHA functional classes III and IV EF ≤30% |
9 | Safety (composite of worsening HF, hospitalizations, arrhythmia, device-device interaction) and efficacy (change in EF, Vo2 max, BNP, QoL scores) (2 yrs) | No adverse events and no interference with ICD No significant change in EF or BNP |
| TAME-HF (NCT01820130) | Nonrandomized Open label |
NYHA functional class III EF ≤40% LVESD 5–8 cm |
0 | 1. Change in LVEDV, NYHA functional class, and 6MWD (6 months) 2. Safety, QoL scores, Vo2 max, LV systolic and diastolic function |
Withdrawn |
DEFEAT-HF = Determining the Feasibility of Spinal Cord Neuromodulation for the Treatment of Chronic Heart Failure; ICD = implantable cardioverter-defibrillator; LVEDV = left ventricular end-diastolic volume; Methodist SCS = Neurostimulation of Spinal Nerves That Affect the Heart; SCS = spinal cord stimulation; SCS HEART = Spinal Cord Stimulation for Heart Failure; TAME-HF = Trial of Autonomic Neuromodulation for Treatment of Chronic Heart Failure; other abbreviations as in Table 1.
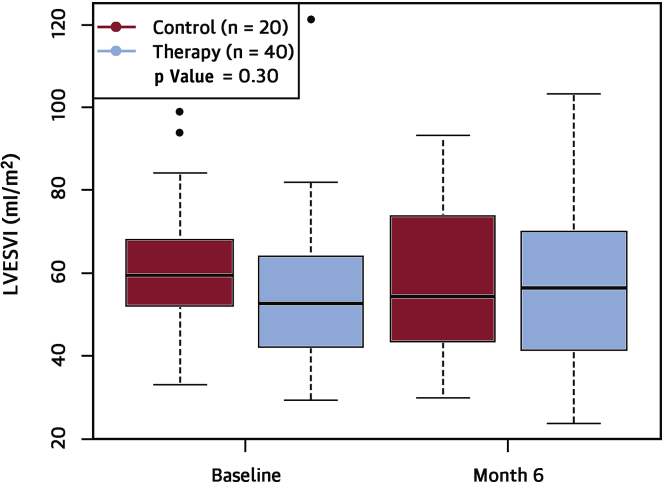
The largest randomized clinical trial of SCS in HF, the DEFEAT-HF (Determining the Feasibility of Spinal Cord Neuromodulation for the Treatment of Chronic Heart Failure) randomized controlled study was completed recently (36). DEFEAT-HF enrolled 81 patients with NYHA functional class III HF and a mean LVEF of 29 ± 5%, with 66 successfully randomized and implanted with the SCS device system. All of the patients were implanted with a SCS device that consisted of a single 8-electrode lead in the epidural space. The electrode was connected to an SCS stimulator, which was placed subcutaneously in the lateral abdominal wall. Stimulation electrodes were placed to encompass the T2 to T4 levels. Patients were randomized 3:2 to SCS or OMT (control) for 6 months; after 6 months, the control patients were crossed over to the active therapy arm and 12-month data were collected in both randomization arms. The stimulation in the treatment group was programmed on for 12 h a day, on the basis of individual sleep/wake cycles, at a stimulation frequency of 50 Hz, 200 ms pulse duration, and output set at 90% maximum tolerated voltage determined while sitting. The primary study endpoint was a reduction in the LV end-systolic volume index after 6 months of SCS therapy in the treatment arm versus the control arm. Secondary outcomes included change in peak O2 consumption and change in NT-proBNP at 6 months. The results of the DEFEAT-HF trial show that, compared with guideline-directed medical therapy alone, thoracic (T2 to T4) SCS in patients with NYHA functional class III HFrEF, did not lead to changes in LV structural remodeling (LV end-systolic volume index) at 6 months (Figure 6). Moreover, thoracic SCS did not lead to significant improvements in peak Vo2 nor circulating levels of NT-proBNP at 6 months. There were no differences between the groups in freedom from death or hospitalization for HF at 6 months, change on Minnesota Living with Heart Failure Score, change in NYHA functional class or change in 6-min walk distance. SCS appeared to be safe and well tolerated in patients with NYHA functional class III HF, which is consistent with the observation in patients without HF.
Figure 6.
Primary Efficacy Endpoint of the DEFEAT-HF Trial
There was no significant difference in the change in left ventricular end-systolic volume index (LVESVi) over 6 months between the spinal cord stimulation and control groups (p = 0.30). The bottom line of the box equals the 25th percentile, the top line equals the 75th percentile and the line within the box equals the median. The dots represent patient values that exceed the 75th percentile. Reproduced with permission from Zipes et al. (36). DEFEAT-HF = Determining the Feasibility of Spinal Cord Neuromodulation for the Treatment of Chronic Systolic Heart Failure.
Baroreflex activation therapy
BAT was initially developed for the treatment of resistant hypertension. Electrical stimulation of the baroreceptor fibers located in the carotid sinus (Figure 2D), leads to centrally mediated reduction of sympathetic outflow and increased parasympathetic tone, resulting in reduced systemic vascular resistance (37). BAT using a proprietary first-generation implantable carotid sinus stimulator (Rheos Baroreflex Hypertension Therapy System,CVRx, Minneapolis, Minnesota) was studied in patients with severe hypertension refractory to medical therapy. Implantation of the device involves exposure of the carotid sinuses and positioning of the electrodes on the carotid surface. The leads are than tunneled subcutaneously and connected to the stimulation device placed on the chest. Data from the DEBUT (Device Based Therapy in Hypertension) trial with BAT showed substantial reductions in patients with refractory hypertension (38). A second-generation Barostim neo system (CVRx), consists of a single lead that requires less dissection of the carotid artery for implantation and has a battery life of 3 years.
Pre-clinical studies of BAT
Several pre-clinical studies in large animal HF models have shown that monotherapy with BAT improves global LV systolic and diastolic function and partially reverses LV remodeling both globally and at cellular and molecular levels. When evaluated in dogs using a coronary artery microembolization model, BAT resulted in a significant decrease in LV end-diastolic pressure and circulating plasma NE. BAT also normalized the expression of cardiac beta1-adrenergic receptors, beta-adrenergic receptor kinase, and reduced interstitial fibrosis and cardiac myocyte hypertrophy (39). In a pacing-induced tachycardia model of HF, BAT was shown to improve survival, although arterial pressure, resting heart rate, and LV pressure were not different over time in baroreflex-activated versus control dogs (40).
Clinical studies of BAT
The first clinical experience with BAT in HF was a single-center, open-label evaluation in patients (n = 11) with NYHA functional class III HF and an LVEF <40% (41), wherein patients were treated with BAT for 6 months (Table 3). This study showed that there was a significant and sustained 30% reduction in SNS activity, as measured by microneurography of the peroneal nerve, that was accompanied by an overall improvement in NYHA functional class, quality of life score, and 6-min hall walk distance. Cardiac structure and function, assessed by 3-dimensional echocardiography, also improved. The rate of HF hospitalization was also substantially decreased compared with the 12 months before implantation of the BAT system. More recently, the Barostim neo system was evaluated in 146 patients with NYHA functional class III HF and a LVEF ≤35%, who were randomized to guideline-directed medical therapy + BAT (n = 76) or to guideline-directed medical therapy alone (n = 70). BAT is uptitrated over a series of follow-up visits, with a focus on achieving therapeutic stimulation without side effects, such as excessive reductions in heart rate or blood pressure. When compared with control subjects, patients assigned to BAT had an improvement in the 6-min walk test, in a quality of life scores, in NYHA functional class ranking, and in NT-pro-BNP values at 6 months. There was a trend toward a decrease in HF hospitalizations in BAT-treated patients; however, there was no significant difference in LVEF or other echocardiographic parameters between the BAT and the control group. Importantly, there was no difference between the BAT and the control group with respect to major adverse and neurological and cardiovascular events (42). In a subsequent analysis of the same patients, the most pronounced effect of BAT was observed in patients not treated with cardiac resynchronization patients, possibly because there is less sympathovagal imbalance in this subgroup of HF patients (43). Based on the positive results of these earlier trials, a large pivotal trial is planned: BeAT-HF (Barostim Therapy for Heart Failure; NCT02627196). The trial will randomize 480 patients with NYHA functional class III HF (LVEF ≤35%) in a 1:1 fashion to receive OMT or OMT + BAT. The primary outcome measures for BeAT-HF will be the rate of cardiovascular mortality and HF mortality at study completion (efficacy endpoint) and major adverse neurological and cardiovascular events at 6 months (safety endpoint).
Table 3.
Baroreflex Activation Therapy
| Study Design | Patient Characteristics | N | Outcomes | Results | |
|---|---|---|---|---|---|
| Rheos DHF (NCT00718939) | Randomized Double blind |
EF >45% Elevated BNP or pro-BNP |
6 | 1. LVMI; safety (occurrence of all adverse events) (6 months) 2. Change in blood pressure, BNP or pro-BNP, QoL scores |
Pending |
| Barostim neo HF (NCT01471860) and Barostim HOPE4HF (NCT01720160) | Randomized Open label |
NYHA functional class III EF ≤35% |
146 | 1. Safety (system and procedure-related adverse event) 2. Efficacy (change in NYHA functional class, QoL scores, 6MWD) (6 months) |
No significant adverse events Significant improvement in 6MWD, NYHA functional class, QoL scores, pro-BNP level |
| BeAT-HF (NCT02627196) | Randomized Open label |
NYHA functional class III EF ≤35% |
800 | 1. Cardiovascular mortality and HF morbidity (5 yrs); MANCE (6 months) | Pending (estimated 2021) |
BeAT-HF = Barostim Therapy for Heart Failure; DHF = Rheos Diastolic Heart Failure Trial; HOPE4HF = Barostim Hope for Heart Failure Study; LVMI = left ventricle mass index; MANCE = major adverse neurological and cardiovascular events; other abbreviations as in Table 1.
Neuromodulation of the Failing Heart: Lost in Translation?
The recent disappointing results of the DEFEAT-HF and INOVATE-HF trials raise the important question of whether device-based modulation of the ANS is a viable therapeutic strategy for patients with HF. The precise reason(s) why the pre-clinical and early clinical studies that supported the concept of neuromodulation have failed to translate into clinically meaningful endpoints in randomized clinical trials is not known; however, it may relate, at least in part, to the well-recognized problems associated with replicating the results of open-label trials that lack a proper randomized control group, or to the phenomenon of “regression to the mean” that plagues the reproducibility of small clinical trials. These statements notwithstanding, there are several issues that are unique to device-based strategies designed to modulate the ANS that warrant further discussion.
Dose matters
One of the consistent lessons learned from pharmacologic trials in HF trials is that choosing the proper dose is critical (44). Although choosing the proper dose in HF trials remains as much an art as a science, choosing the proper stimulation parameters for devices to achieve clinically meaningful modulation of the ANS is even more challenging, in large measure because of the lack of clear method for establishing the correct excitation parameters and/or duty cycles. For example, choosing the proper “dose” for VNS is critical, insofar as the vagus nerve is composed of bundles of small unmyelinated (C-fibers) and larger myelinated (A-fibers and B-fibers) nerves, whose activation properties are distinctly different. Because large diameter fibers reach activation threshold at lower stimulation intensities than smaller diameter fibers do, VNS results in the recruitment of A-fibers at lower stimulation thresholds and recruitment of B-fibers at higher thresholds and then ends with recruitment of C-fibers at higher stimulation thresholds (45). When uptitrating the stimulus strength of the VNS devices in the setting of clinical trials, the amplitude of the stimulus current is often limited by patient symptoms (e.g., cough, dysphonia) and/or untoward hemodynamic effects (e.g., bradycardia, hypotension). Thus, the populations of afferent and efferent vagus nerve fibers that are activated by VNS will vary depending on the stimulus strength employed and may therefore differ from patient to patient even though these patients are receiving the “same” treatment. Germane to this discussion, it is important to recognize that the stimulation amplitudes used for VNS in NECTAR-HF (1.2 mA), ANTHEM-HF (2.0 mA), the CardioFit pilot trial (4.2 mA), and INOVATE-HF (3.9 mA) were all quite different. What is unclear from these studies is that with the exception of the CardioFit pilot trial, in which VNS resulted in a decrease in heart rate and increased heart rate variability, it is unclear whether the various VNS stimulation protocols used in clinical trials were sufficient to stimulate the vagus nerve and/or inhibit the SNS. Beyond stimulus strength, choosing the proper duty cycle is largely empiric and is again selected to minimize the side effects of VNS. Importantly, the duty cycle for VNS for the pivotal INOVATE-HF trial, wherein there was no change in heart rate, was different from the duty cycle used for the CardioFit pilot trial, wherein therein there was a decrease in heart rate. Whether this change in the duty cycle was clinically important and/or explains the disparate outcomes in these 2 trials is not known.
Choosing the proper site of stimulation, strength of stimulation, and duty cycle for SCS is also challenging from the standpoint of designing clinical trials. Pertinent to this discussion, previous work in dogs has shown that SCS delivered at the T4 level demonstrates a greater antiarrhythmic effect (33), whereas that at the T1 level is associated with a heightened parasympathetic tone (32). In the SCS HEART study (35), continuous SCS was performed at the mid-line and left of the mid-line at T1 and T3 levels, whereas intermittent SCS was conducted at mid-line of T2 for T4 levels in the DEFEAT-HF trial (36). The stimulators in the SCS Heart study were programmed to deliver continuous therapy 24 h/day at 50 Hz, whereas SCS in the DEFEAT-HF trial was for 12 h/day at 50 Hz and was based on individual sleep/wake cycles. Whether the differences in clinical outcomes in these 2 trials is attributable to the site of stimulation, strength of stimulation, and duty cycle is unknown. Moreover, similar to the problem with VNS in the preceding discussed, it is unclear whether the various protocols that were used in the SCS HEART study and DEFEAT-HF trial were sufficient to restore the proper sympathovagal balance in patients with HF. Viewed together, the observations with regard to the difficulties with VNS and SCS suggest that there is a critical need to be able to perform “dose” response studies that will allow investigators to have a better understanding of the types of stimulation protocols that will be most efficacious.
Afferent versus efferent stimulation
As noted, VNS is accomplished by placing an electrode cuff around the right or left cervical vagus, resulting in stimulation of both efferent and afferent fibers of the vagus nerve. The device and stimulation protocols used in the NECTAR-HF and ANTHEM-HF trials were designed to stimulate afferent vagus nerve fibers, whereas the device and stimulation protocol uses in the CardioFit pilot trial and INOVATE-HF trial were designed, in theory, to stimulate efferent vagus nerve fibers. From a conceptual standpoint, it may be more advantageous to stimulate afferent vagus nerve fibers, insofar as stimulation of the afferent vagus nerve fibers has been shown experimentally to decrease sympathetic efferent nerve fiber activity to the heart (46), which is believed to be beneficial based on a wealth of experimental and clinical observations. Moreover, the majority of the preganglionic vagal fibers terminate in small ganglia located on the posterior surfaces of the atrium, whereas far fewer ganglia reside in ventricular tissue, raising the question of whether direct stimulation of efferent vagus nerve fibers is beneficial. However, it bears emphasizing that it is currently unknown whether stimulating afferent vagus nerves, efferent vagus nerves, or a combination of afferent and efferent vagus nerves is more beneficial in the setting of HF. Similar types of difficult questions can be raised about SCS, where electrode placement can lead to inhibition of SNS trafficking to the heart and/or in increased parasympathetic tone in the heart. Thus, there are a number of important questions about how to target device-based autonomic modulatory strategies.
The need to identify reliable “physiologic biomarkers” of autonomic tone
The results of the DEFEAT-HF and INOVATE-HF trials raise a number of important questions about how one might design future device-based clinical trials of autonomic modulation in HF. Based on the foregoing discussion, there is a need to identify physiological measurements that will allow investigators in future trials to determine the proper site of stimulation, the proper strength of stimulation, and the proper duty cycle for device-based therapies. Although there are no direct physiological measurements that reflect the restoration of normal sympathovagal tone in HF, there are a number “physiological biomarkers” that reflect excessive SNS activation in HF. Conceptually, normalization of markers of excessive SNS activation could be used as a surrogate for the restoration of “normal” autonomic tone, assuming that one could ascertain how much normalization was required. The measurements of excessive SNS activation that have been studied in HF include plasma or urinary NE levels, assessment of local tissue NE spillover, muscle sympathetic nerve activity (MSNA), uptake of the iodine 123I-metaiodobenzylguanidine in the heart, baroreflex sensitivity, and heart rate variability (2). Currently, MSNA and iodine 123I-metaiodobenzylguanidine imaging are considered to be the most accurate direct measurements of SNS activity in HF patients. As noted, the early studies with BAT showed that there was a sustained decrease in MSNA following BAT, suggesting that MSNA might be used to assess the normalization of autonomic tone in future studies.
Conclusions
HF progresses, at least in part, because of increased activity of the SNS that is accompanied by concomitant withdrawal of parasympathetic activity. Despite the use of guideline-directed medical therapy, most patients will ultimately develop worsening HF that is accompanied by increased morbidity and mortality. In the foregoing review, we have discussed the rationale for neuromodulation of the failing heart, as well as summarized the recent clinical experience with VNS, BAT, and SCS. Although the pre-clinical studies and early clinical studies with VNS and SCS appeared promising, the larger clinical trials have been neutral with respect to the primary clinical endpoints. The pivotal trial with BAT should start enrolling patients shortly, but it will not report results for several years. Thus, for the short term, we will be left to question whether neuromodulation is a viable therapeutic strategy for treating patients with HF.
Although the tendency for investigators and investors is to walk away from therapeutic areas that do not yield immediate positive results, particularly with regard to cardiac devices that are invasive and hence entail some procedural risk, it is instructive to recall that cardiac resynchronization therapy, which is now a class I indication in HF patients, took over 2 decades to evolve from a concept in animal models to widespread clinical application. Although the initial results of the early large clinical trials with device-based autonomic modulation of the failing heart have been disappointing, given how little we currently understand about how to modulate the ANS in HF, these initial results are perhaps not at all surprising. This statement notwithstanding, the progress in this field over the past 5 years has been astounding, and it clear that we have now entered an exciting new therapeutic era that may one day allow clinicians to use both devices and drugs to restore the proper sympathovagal balance in HF.
Footnotes
Dr. Byku has reported that she has no relationships relevant to the contents of this paper to disclose. Dr. Mann is a consultant for Bio Control Medical and Medtronic.
References
- 1.Schwartz P.J., La Rovere M.T., De Ferrari G.M., Mann D.L. Autonomic modulation for the management of patients with chronic heart failure. Circ Heart Fail. 2015;8:619–628. doi: 10.1161/CIRCHEARTFAILURE.114.001964. [DOI] [PubMed] [Google Scholar]
- 2.Floras J.S. Sympathetic nervous system activation in human heart failure: clinical implications of an updated model. J Am Coll Cardiol. 2009;54:375–385. doi: 10.1016/j.jacc.2009.03.061. [DOI] [PubMed] [Google Scholar]
- 3.Armour J.A. Cardiac neuronal hierarchy in health and disease. Am J Physiol Regul Integr Comp Physiol. 2004;287:R262–R271. doi: 10.1152/ajpregu.00183.2004. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz P.J., Vanoli E., Stramba-Badiale M., De Ferrari G.M., Billman G.E., Foreman R.D. Autonomic mechanisms and sudden death: new insights from analysis of baroreceptor reflexes in conscious dogs with and without a myocardial infarction. Circulation. 1988;78:969–979. doi: 10.1161/01.cir.78.4.969. [DOI] [PubMed] [Google Scholar]
- 5.La Rovere M.T., Bigger J.T., Jr., Marcus F.I., ATRAMI Investigators Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. Lancet. 1998;351:478–484. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 6.Marin-Neto J.A., Pintya A.O., Gallo Júnior L., Maciel B.C. Abnormal baroreflex control of heart rate in decompensated congestive heart failure and reversal after compensation. Am J Cardiol. 1991;67:604–610. doi: 10.1016/0002-9149(91)90899-v. [DOI] [PubMed] [Google Scholar]
- 7.Mann D.L., Bristow M.R. Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation. 2005;111:2837–2849. doi: 10.1161/CIRCULATIONAHA.104.500546. [DOI] [PubMed] [Google Scholar]
- 8.Vanhoutte P.M., Levy M.N. Prejunctional cholinergic modulation of adrenergic neurotransmission in the cardiovascular system. Am J Physiol. 1980;238:H275–H281. doi: 10.1152/ajpheart.1980.238.3.H275. [DOI] [PubMed] [Google Scholar]
- 9.Matkó J., Matyus L., Szollosi J. Analysis of cell surface molecular distributions and cellular signaling by flow cytometry. J Fluoresc. 1994;4:303–314. doi: 10.1007/BF01881445. [DOI] [PubMed] [Google Scholar]
- 10.Azevedo E.R., Parker J.D. Parasympathetic control of cardiac sympathetic activity: normal ventricular function versus congestive heart failure. Circulation. 1999;100:274–279. doi: 10.1161/01.cir.100.3.274. [DOI] [PubMed] [Google Scholar]
- 11.Newton G.E., Parker A.B., Landzberg J.S., Colucci W.S., Parker J.D. Muscarinic receptor modulation of basal and beta-adrenergic stimulated function of the failing human left ventricle. J Clin Invest. 1996;98:2756–2763. doi: 10.1172/JCI119101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.La Rovere M.T., Pinna G.D., Maestri R. Prognostic implications of baroreflex sensitivity in heart failure patients in the beta-blocking era. J Am Coll Cardiol. 2009;53:193–199. doi: 10.1016/j.jacc.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 13.De Ferrari G.M., Schwartz P.J. Vagus nerve stimulation: from pre-clinical to clinical application: challenges and future directions. Heart Fail Rev. 2011;16:195–203. doi: 10.1007/s10741-010-9216-0. [DOI] [PubMed] [Google Scholar]
- 14.Lopshire J.C., Zipes D.P. Device therapy to modulate the autonomic nervous system to treat heart failure. Curr Cardiol Rep. 2012;14:593–600. doi: 10.1007/s11886-012-0292-8. [DOI] [PubMed] [Google Scholar]
- 15.Merlo M., Stolfo D., Anzini M. Persistent recovery of normal left ventricular function and dimension in idiopathic dilated cardiomyopathy during long-term follow-up: does real healing exist? J Am Heart Assoc. 2015;4:e001504. doi: 10.1161/JAHA.114.001504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vanoli E., De Ferrari G.M., Stramba-Badiale M., Hull S.S., Jr., Foreman R.D., Schwartz P.J. Vagal stimulation and prevention of sudden death in conscious dogs with a healed myocardial infarction. Circ Res. 1991;68:1471–1481. doi: 10.1161/01.res.68.5.1471. [DOI] [PubMed] [Google Scholar]
- 17.Li M., Zheng C., Sato T., Kawada T., Sugimachi M., Sunagawa K. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation. 2004;109:120–124. doi: 10.1161/01.CIR.0000105721.71640.DA. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y., Popovic Z.B., Bibevski S. Chronic vagus nerve stimulation improves autonomic control and attenuates systemic inflammation and heart failure progression in a canine high-rate pacing model. Circ Heart Fail. 2009;2:692–699. doi: 10.1161/CIRCHEARTFAILURE.109.873968. [DOI] [PubMed] [Google Scholar]
- 19.Calvillo L., Vanoli E., Andreoli E. Vagal stimulation, through its nicotinic action, limits infarct size and the inflammatory response to myocardial ischemia and reperfusion. J Cardiovasc Pharmacol. 2011;58:500–507. doi: 10.1097/FJC.0b013e31822b7204. [DOI] [PubMed] [Google Scholar]
- 20.Tracey K.J. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz P.J., De Ferrari G.M., Sanzo A. Long term vagal stimulation in patients with advanced heart failure: first experience in man. Eur J Heart Fail. 2008;10:884–891. doi: 10.1016/j.ejheart.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 22.De Ferrari G.M., Crijns H.J., Borggrefe M., for the CardioFit Multicenter Trial Investigators Chronic vagus nerve stimulation: a new and promising therapeutic approach for chronic heart failure. Eur Heart J. 2011;32:847–855. doi: 10.1093/eurheartj/ehq391. [DOI] [PubMed] [Google Scholar]
- 23.Premchand R.K., Sharma K., Mittal S. Autonomic regulation therapy via left or right cervical vagus nerve stimulation in patients with chronic heart failure: results of the ANTHEM-HF Trial. J Card Fail. 2014;20:808–816. doi: 10.1016/j.cardfail.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 24.Zannad F., De Ferrari G.M., Tuinenburg A.E. Chronic vagal stimulation for the treatment of low ejection fraction heart failure: results of the Neural Cardiac Therapy for Heart Failure (NECTAR-HF) randomized controlled trial. Eur Heart J. 2015;36:425–433. doi: 10.1093/eurheartj/ehu345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hauptman P.J., Schwartz P.J., Gold M.R. Rationale and study design of the increase of vagal tone in heart failure study: INOVATE-HF. Am Heart J. 2012;163:954–962.e1. doi: 10.1016/j.ahj.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 26.Gold M.R., van Veldhuisen D.J., Hauptman P.J. Vagus nerve stimulation for the treatment of chronic systolic heart failure: the INOVATE-HF trial. J Am Coll Cardiol. 2016 Apr 4 doi: 10.1016/j.jacc.2016.03.525. [E-pub ahead of print]; [DOI] [PubMed] [Google Scholar]
- 27.Melzack R., Wall P.D. Pain mechanisms: a new theory. Science. 1965;150:971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 28.Wu M., Linderoth B., Foreman R.D. Putative mechanisms behind effects of spinal cord stimulation on vascular diseases: a review of experimental studies. Auton Neurosci. 2008;138:9–23. doi: 10.1016/j.autneu.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Southerland E.M., Milhorn D.M., Foreman R.D. Preemptive, but not reactive, spinal cord stimulation mitigates transient ischemia-induced myocardial infarction via cardiac adrenergic neurons. Am J Physiol Heart Circ Physiol. 2007;292:H311–H317. doi: 10.1152/ajpheart.00087.2006. [DOI] [PubMed] [Google Scholar]
- 30.Issa Z.F., Zhou X., Ujhelyi M.R. Thoracic spinal cord stimulation reduces the risk of ischemic ventricular arrhythmias in a postinfarction heart failure canine model. Circulation. 2005;111:3217–3220. doi: 10.1161/CIRCULATIONAHA.104.507897. [DOI] [PubMed] [Google Scholar]
- 31.Rosas-Ballina M., Olofsson P.S., Ochani M. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334:98–101. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olgin J.E., Takahashi T., Wilson E., Vereckei A., Steinberg H., Zipes D.P. Effects of thoracic spinal cord stimulation on cardiac autonomic regulation of the sinus and atrioventricular nodes. J Cardiovasc Electrophysiol. 2002;13:475–481. doi: 10.1046/j.1540-8167.2002.00475.x. [DOI] [PubMed] [Google Scholar]
- 33.Lopshire J.C., Zhou X., Dusa C. Spinal cord stimulation improves ventricular function and reduces ventricular arrhythmias in a canine postinfarction heart failure model. Circulation. 2009;120:286–294. doi: 10.1161/CIRCULATIONAHA.108.812412. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y., Yue W.S., Liao S.Y. Thoracic spinal cord stimulation improves cardiac contractile function and myocardial oxygen consumption in a porcine model of ischemic heart failure. J Cardiovasc Electrophysiol. 2012;23:534–540. doi: 10.1111/j.1540-8167.2011.02230.x. [DOI] [PubMed] [Google Scholar]
- 35.Tse H.F., Turner S., Sanders P. Thoracic Spinal Cord Stimulation for Heart Failure as a Restorative Treatment (SCS HEART study): first-in-man experience. Heart Rhythm. 2015;12:588–595. doi: 10.1016/j.hrthm.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 36.Zipes D.P., Neuzil P., Theres H., for the DEFEAT-HF Trial Investigators Determining the Feasibility of Spinal Cord Neuromodulation for the Treatment of Chronic Systolic Heart Failure: the DEFEAT-HF study. J Am Coll Cardiol HF. 2016;4:129–136. doi: 10.1016/j.jchf.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 37.Grassi G., Seravalle G., Quarti-Trevano F. Sympathetic and baroreflex cardiovascular control in hypertension-related left ventricular dysfunction. Hypertension. 2009;53:205–209. doi: 10.1161/HYPERTENSIONAHA.108.121467. [DOI] [PubMed] [Google Scholar]
- 38.Krum H., Sobotka P., Mahfoud F., Bohm M., Esler M., Schlaich M. Device-based antihypertensive therapy: therapeutic modulation of the autonomic nervous system. Circulation. 2011;123:209–215. doi: 10.1161/CIRCULATIONAHA.110.971580. [DOI] [PubMed] [Google Scholar]
- 39.Sabbah H.N., Gupta R.C., Imai M. Chronic electrical stimulation of the carotid sinus baroreflex improves left ventricular function and promotes reversal of ventricular remodeling in dogs with advanced heart failure. Circ Heart Fail. 2011;4:65–70. doi: 10.1161/CIRCHEARTFAILURE.110.955013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zucker I.H., Hackley J.F., Cornish K.G. Chronic baroreceptor activation enhances survival in dogs with pacing-induced heart failure. Hypertension. 2007;50:904–910. doi: 10.1161/HYPERTENSIONAHA.107.095216. [DOI] [PubMed] [Google Scholar]
- 41.Gronda E., Seravalle G., Brambilla G. Chronic baroreflex activation effects on sympathetic nerve traffic, baroreflex function, and cardiac haemodynamics in heart failure: a proof-of-concept study. Eur J Heart Fail. 2014;16:977–983. doi: 10.1002/ejhf.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abraham W.T., Zile M.R., Weaver F.A. Baroreflex activation therapy for the treatment of heart failure with a reduced ejection fraction. J Am Coll Cardiol HF. 2015;3:487–496. doi: 10.1016/j.jchf.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 43.Zile M.R., Abraham W.T., Weaver F.A. Baroreflex activation therapy for the treatment of heart failure with a reduced ejection fraction: safety and efficacy in patients with and without cardiac resynchronization therapy. Eur J Heart Fail. 2015;17:1066–1074. doi: 10.1002/ejhf.299. [DOI] [PubMed] [Google Scholar]
- 44.Mann D.L., Deswal A. Angiotensin-receptor blockade in acute myocardial infarction—a matter of dose. N Engl J Med. 2003;349:1963–1965. doi: 10.1056/NEJMe038163. [DOI] [PubMed] [Google Scholar]
- 45.Castoro M.A., Yoo P.B., Hincapie J.G. Excitation properties of the right cervical vagus nerve in adult dogs. Exp Neurol. 2011;227:62–68. doi: 10.1016/j.expneurol.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 46.Schwartz P.J., Pagani M., Lombardi F., Malliani A., Brown A.M. A cardiocardiac sympathovagal reflex in the cat. Circ Res. 1973;32:215–220. doi: 10.1161/01.res.32.2.215. [DOI] [PubMed] [Google Scholar]