Abstract
Background:
Age-related changes in testosterone levels in older persons and especially in women have not been fully explored. The objective of this study was to describe age-related trajectories of total testosterone (TT), ammonium sulfate precipitation–measured bioavailable testosterone (mBT), and sex hormone–binding glycoprotein (SHBG) in men and women from the Baltimore Longitudinal Study of Aging, with special focus on the oldest adults.
Methods:
Participants included 788 White men and women aged 30–96 years with excellent representation of old and oldest old, who reported not taking medications known to interfere with testosterone. Longitudinal data were included when available. TT, mBT, and SHBG were assayed. Age-related trajectories of mBT were compared with those obtained using calculated bioavailable testosterone (cBT). Generalized least square models were performed to describe age-related trajectories of TT, mBT, and SHBG in men and women.
Results:
mBT linearly declines over the life span and even at older ages in both sexes. In men, TT remains quite stable until the age of 70 years and then declines at older ages, whereas in women TT progressively declines in premenopausal years and slightly increases at older ages. Differences in age-related trajectories between total and bioavailable testosterone are only partially explained by age changes in SHBG, whose levels increases at accelerated rates in old persons. Noteworthy, although mBT and cBT highly correlated with one another, mBT is a much stronger correlate of chronological age than cBT.
Conclusion:
In both men and women, mBT linearly declines over the life span and even at old ages. Its relationship with age-related phenotypes should be further investigated.
Keywords: Aging, Testosterone, Trajectories, Men, Women
Male aging has long been associated with progressive decline in circulating testosterone at population level (1). It is well established that concomitant pathology may cause or accelerate such decline, although it is often observed even in healthy men (2,3). A number of studies have shown that older men with lower testosterone are more likely to suffer and/or to develop poor health conditions, including central adiposity, sarcopenia, insulin resistance, diabetes, cardiovascular diseases, depression, impotence, osteoporosis, disability, frailty, and even premature death (4,5). Noteworthy, adverse age-related changes or conditions in men may be either the cause or the effect of the changes in testosterone levels. Conversely, evidence on age-associated changes in testosterone levels in women and their consequences is scant.
Circulating testosterone concentrations are approximately 20–25 times higher in adult men compared with those in women. In both men and women, the majority of the circulating testosterone is protein bound, with only approximately 0.5%–3% as free testosterone (FT) (5). In men, an average of 40% of the protein-bound testosterone circulates linked to sex hormone–binding glycoprotein (SHBG), whereas the remainder binds to albumin with an association constant much weaker than for SHBG. Because women have higher SHBG levels and lower testosterone than men, in women more than 80% of the protein-bound testosterone is associated with SHBG. The albumin-bound testosterone, also called “weakly bound” testosterone, rapidly dissociates, and is promptly bioavailable as the concentration of FT is reduced. Therefore, the sum of albumin-bound and free testosterone is commonly referred to as “bioavailable” testosterone (BT), which represents the fraction of testosterone that is bioavailable to cells for signaling (6,7).
The exact contribution and role of BT compared with total testosterone (TT) or FT in aging men and women has been only partially studied and is not yet understood. Most of the existing literature on the clinical consequences of age-related changes in testosterone levels focused on the total one or free fraction, while little is known on the relationship between BT levels and aging-associated adverse outcomes. What has been driving the interest on BT (ie, the sum of FT plus albumin-bound testosterone) was the consideration that BT represents the fraction of circulating testosterone that readily enters into the cells, interacts with intracellular androgen receptors, and regulates gene expression and cellular function (8). In fact, although historically only the free fraction of testosterone in the circulation was thought to be taken up by tissues and therefore be biologically active (the “free hormone hypothesis”), it has been then demonstrated that testosterone is weakly bound to serum albumin and freely dissociates in the capillary bed, thereby becoming readily available for tissue uptake (9). As a consequence, all non-SHBG–bound testosterone is commonly considered biologically available for tissues, and therefore BT may be a better biomarker of testosterone bioactivity than TT (10). Recent evidence from animal studies, suggesting that megalin, a receptor in reproductive tissues, can promote the cellular uptake of testosterone bound to SHBG through a process of endocytosis, has fueled new energy on the debate on this topic. Indeed, the hypothesis that multiple entry mechanisms (“free” and “protein bound”) of testosterone may coexist in the same cell has been proposed, but not yet demonstrated (11).
Because SHBG levels can be greatly affected by numerous health conditions and diseases (eg, obesity, insulin resistance, cirrhosis of the liver, malnutrition, or renal impairment), the proportion of testosterone that is not bioavailable is highly variable, especially at old ages, making the interpretation of TT very difficult. Hence, levels of BT that factor out effect of SHBG may be a more appropriate representation of tissue activity (12).
A number of previous cross-sectional and longitudinal studies performed in men reported that after the age of 30 years, TT levels decline whereas SHBG levels increase with age. Because of the increased levels of SHBG, the fraction of testosterone bound to SHBG increases with aging and, therefore, BT levels decline more rapidly than TT levels (13–27). However, a controversy exists about hormonal trends at advanced ages. For instance, a cross-sectional study reported that in men older than 70 years, TT levels remained stable, SHBG levels increased, and FT levels declined with aging (24). In contrast, a 4-year longitudinal study in men aged 71–86 years showed that TT levels declined with age with BT levels declining at a faster rate (25). Moreover, changes of circulating testosterone levels across life span in women have not been exhaustively characterized yet. Although a few studies reported a steep decline in TT and BT in early premenopausal years (28–30), knowledge about changes in testosterone in women at older ages remains limited and controversial (31–33).
Of remarkable note, most of the literature on “bio available “ testosterone is rarely based on direct assay measurements (“measured” BT, mBT) but usually calculated by formulae (“calculated” BT, cBT). However, a number of previous studies reported the inaccuracy of conventional methods to estimate BT compared with assay measurements, such as the direct measure of BT by selective ammonium sulfate precipitation of SHBG-bound testosterone, which is usually referred to as the gold standard method (10,34,35). In particular, the use of cBT is problematic because it does not account for potential age-related changes in affinity of SHBG to testosterone. For example, it has been shown in men that cBT significantly overestimates mBT, and this discrepancy tends to increase for increasing age (36). Therefore, due to the uncertainty of the meaning and measurement precision of cBT, use of mBT is becoming more popular and advisable (37).
Using 10 years of observational data (2004–2014) from the Baltimore Longitudinal Study of Aging (BLSA), including direct measurements of BT (introduced in BLSA in 2004), we described age-related trajectories of TT, BT, and SHBG in White men and women aged 30–96 years, with special focus on those older than 65 years.
Methods
Study Population
The BLSA is a study of human aging established and conducted by the National Institute on Aging (NIA) Intramural Research Program since 1958 (38). BLSA is an ongoing longitudinal study that continuously enrolls participants with different ages and follows them for life with visits conducted every 1–4 years, with more frequent visits at older ages. Visits typically take place over 3 days at the NIA Clinical Research Unit in Baltimore, Maryland. Detailed medical history, blood samples, anthropomorphic, demographic, and medication data are collected at every visit. Informed consent is obtained from each participant at every visit. The BLSA protocol is approved by the Intramural Research Program of NIA and the Institutional Review Board of the National Institute of Environmental Health Sciences, Research Triangle Park, North Carolina.
mBT was introduced in BLSA in 2004. There were 3,050 visits with available TT, mBT, and SHBG from October 2004 to June 2014. In this study, we only included White participants (2,112 visits), because we do not yet have enough data to characterize trajectories of testosterone in men and women of other races, and other studies had reported significant race differences in testosterone levels (31,39,40). In addition, 399 visits were excluded from the analysis because of self-reported hormonal treatment or other medications that may interfere with hormonal levels (including sex hormones and their antagonists, contraceptive agents, modulators of genital system, prostaglandins, prolactin inhibitors, testosterone-5-alpha reductase inhibitors and other drugs used in benign prostatic hypertrophy, corticosteroids). Additional 25 observations were excluded because the testosterone levels were overtly abnormal, most likely due to unreported iatrogenic intervention, even if not self-reported. Only five visits were collected in participants aged 29 or younger, and they were not included in the final analysis. A total of 1,683 observations, collected in 788 participants (444 men and 344 women) aged 30 or older, were used for the present analysis. Of these, 1,263 observations (75%) were collected after the age of 65 years, with almost half in oldest old (age 80 years and older). People were aged up to 98 years at follow-up. The average follow-up time was 2 years (range 0–10). Number of participants by number of visits as well as number of participants by years of follow-up are reported in Supplemental Material (Supplementary Table 1a and b).
Laboratory Assessment
Blood samples were collected in the morning after an overnight fast. Serum TT levels were measured in a commercial laboratory using high-performance liquid chromatography-tandem mass spectometry (Esoterix part of LabCorp, Calabasas Hills, CA, certified by the Centers for Disease Control and Prevention’s Hormone Standardization Project [CDC-HoSt Program]). Serum mBT levels were measured using technique described by Nankin and colleagues in which SHBG-bound steroids were separated from albumin-bound and free steroids using ammonium sulfate (Esoterix part of LabCorp) (41). The interassay coefficient of variation (CV) was 14.7% at 9.7ng/dL, 6.6% at 42.6ng/dL, and 2.2% at 77.0ng/dL, respectively. cBT values have also been computed using the formula available online at the website for the International Society for the Study of the Aging Male (www.issam.ch).
Finally, SHBG levels were measured using immunoradiometric assay (Esoterix part of LabCorp). The interassay CV was 8.2% at 64.8 nmol/L, 7.2% at 116 nmol/L, and 5.9% at 184 nmol/L.
Baseline Information
With participants in a gown, body weight was measured in kilograms with a calibrated scale to the nearest 0.1kg. Body height was measured in centimeters by a stadiometer to the nearest 0.1cm. Body mass index was calculated by dividing body weight in kilograms by the square of height in meters (kg/m2). Obesity was defined as body mass index (BMI) ≥ 30kg/m2. Current and former smokers were ascertained by a questionnaire. Physical activity (PA) levels were ascertained in BLSA participants by a self-reported questionnaire and then quantified assigning to each activity a corresponding value in metabolic units (METS, or metabolic equivalents of resting oxygen consumption). The MET unit assigned to the activity was multiplied by the average number of minutes performing each activity in a 24-hour period, providing a value for PA in MET-minutes/day (42). PA levels were then categorized according to MET intensity as follows: (i) very low (0–49 MET-minutes/day); (ii) low (50–249 MET-minutes/day); (iii) medium (250–499 MET-minutes/day); and (iv) high (≥500 MET-minutes/day) (42). Multimorbidity was defined as presence of two or more chronic diseases from a predefined list of 15 conditions (including hypertension, diabetes, coronary artery disease, congestive heart failure, stroke, peripheral arterial disease, chronic kidney disease, chronic obstructive pulmonary disease, anemia, cancer, cognitive impairment, Parkinson’s disease, depression, history of hip fracture, and lower extremities joint disease) (43).
Statistical Analyses
Baseline characteristics of the population were calculated as means ± SD or percentages. A simple correlative analysis (Person coefficients) was first performed to explore the correlation between mBT and cBT and their respective correlations with chronological age, in men and women separately. Due to the longitudinal nature of the data, we used generalized least square (GLS) models to characterize age-related trajectories of TT, mBT, and SHBG, while accounting for the within-subject correlation by directly modeling the correlation structure (44). Of note, for the purpose of our analyses, we chose to use GLS method over other different methods, for example mixed-effects models, because it allows to use a very flexible parametric natural spline to model the average trajectory of testosterone over life span, while accounting for the within-subjects correlations, which is done through directly modeling the within-subjects dependence using various variance–covariance structures for the outcome. Moreover, GLS model also has the advantage to accommodate different follow-ups for different subjects. Therefore, for each hormone, we started building the model by first selecting the correlation structure for the dependence among observations. Several GLS models were fitted with different correlation structures, and Akaike information criterion (AIC) was used to choose the correlation structure that best fit the data. Once the correlation structure was determined, a series of GLS models with increasing complexities and flexibilities in age function were fitted to determine the longitudinal age-related trajectory that best fit the data: (i) stationary model (intercept only); (ii) linear model (intercept and age); (iii) quadratic model (intercept, age, and age square); and (iv) natural cubic spline model (age). We compared the models based on AIC and log likelihood ratio test (LRT). AIC offers a relative estimate of the information lost when a given model is used to represent the “true” model. Although the true relationship is impossible to determine, the model with the lowest AIC score is then the model that best represents the true relationship with the given data. To discriminate among models with different shape of trajectories (stationary, linear, quadratic, or natural cubic spline model) and to compare their goodness of fit (two by two), we also used the LRT. All the models we compared were estimated with the maximum likelihood method, and the final models were estimated with restricted maximum likelihood model. Results from LRT are presented in Supplemental Material.
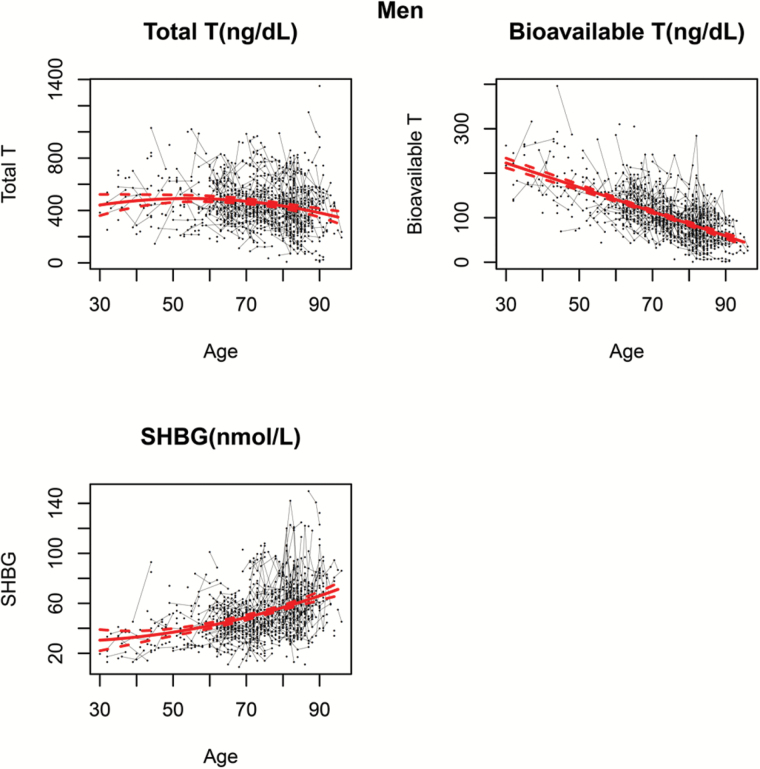
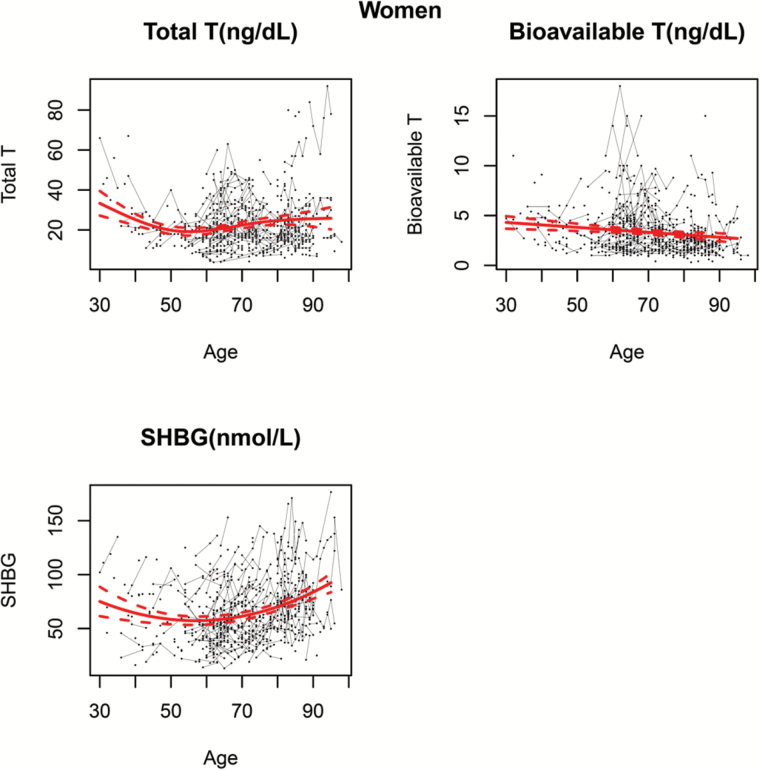
Furthermore, Figures 1 and 2 represent levels of TT, BT, and SHBG over the life span in BLSA men and women. The black dots correspond to the sample raw data. For participants with repeated measures, connecting lines are used to show the observed longitudinal trends in hormonal levels within the same person. The thick red line represents the population average trajectory as a function of age which is estimated from the final generalized model using all the available data, while 95% confidence intervals, depicted as dashed red lines, show the precision of this estimate.
Figure 1.
Age-related trajectories of (A) total testosterone, (B) measured bioavailable testosterone, and (C) sex hormone–biding glycoprotein over the life span in men. The black dots correspond to the sample raw data. For participants with repeated measures, connecting lines are used to show the observed longitudinal trends in hormonal levels within the same person. The thick red line represents the population average trajectory as a function of age which is estimated from the final generalized model using all the available data, whereas 95% confidence intervals, depicted as dashed red lines, show the precision of this estimate.
Figure 2.
Age-related trajectories of (A) total testosterone, (B) measured bioavailable testosterone, and (C) sex hormone–biding glycoprotein over the life span in women. The black dots correspond to the sample raw data. For participants with repeated measures, connecting lines are used to show the observed longitudinal trends in hormonal levels within the same person. The thick red line represents the population average trajectory as a function of age which is estimated from the final generalized model using all the available data, whereas 95% confidence intervals, depicted as dashed red lines, show the precision of this estimate.
Finally, we conducted sensitivity analysis to check whether participants with longer follow-up were younger or had different levels of testosterone. Moreover, we ran additional analyses adjusting for calendar year of the first visit to verify whether results could be biased by secular trend or birth cohort. All the analyses were conducted in R 3.1.2. We used gls function from nlme package (Pinheiro & Bates) to fit the generalized least square models. We used ns function from splines package to fit the natural splines.
Results
Characteristics of Baseline Population
Baseline population included 788 BLSA participants (444 men and 344 women) aged 30–96 years (average age: 68.3±13.9 years). The average values of TT, mBT, cBT, and SHBG in men and women, together with additional baseline information, are summarized in Table 1.
Table 1.
Characteristics of Baseline Population Presented as Means (±SD) or Percentage, According to Men and Women
| Men (n = 444) | Women (n = 344) | |
|---|---|---|
| Age (y) | 68.9±13.7 | 67.4±14.2 |
| Total testosterone (ng/dL) | 457.6±192.2 | 21.8±12.8 |
| Measured bioavailable testosterone (ng/dL) | 114.9±57.7 | 3.3±2.7 |
| Calculated bioavailable testosterone (ng/dL) | 176.3±67.3 | 6.2±3.6 |
| Sex hormone–binding glycoprotein (nmol/L) | 48.7±20.5 | 63.5±26.9 |
| Height (cm) | 175.6±7.2 | 162.2±6.5 |
| Weight (kg) | 84.7±14.9 | 69.5±14.3 |
| Body mass index (kg/m2) | 27.4±4.3 | 26.4±5.1 |
| Smokers (current or former) | 241 (52.3%) | 121 (35.2%) |
| Physical activity levels (%) | ||
| Very low | 6.8% | 11.3% |
| Low | 33.3% | 39.5% |
| Multimorbidity (≥2 chronic diseases) | 159 (35.8%) | 107 (31.1%) |
Preliminary Correlative Analysis
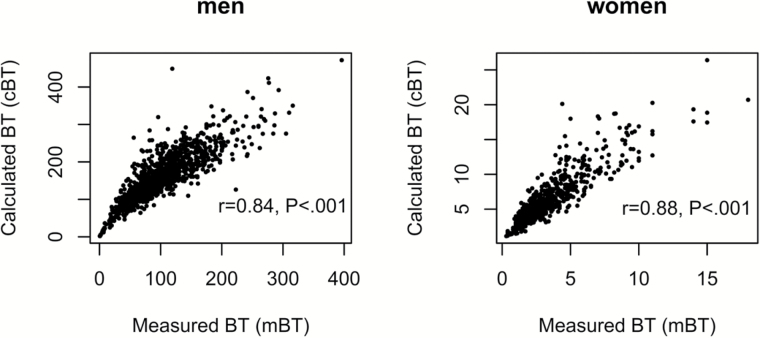
The correlation between mBT and cBT and their respective correlations with chronological age were preliminary explored. Although mBT and cBT were highly correlated (r = .84, p < .001 in men and r = .88, p < .001 in women, Figure 3), mBT was a much stronger correlate of chronological age than cBT in both men and women (r = −.65 vs r = −.47 in men and r = −.23 vs r = −.11 in women, Table 2), and, therefore, mBT was used for describing age-related trajectories.
Figure 3.
Correlations between measured bioavailable testosterone (BT) and calculated BT in (A) men and (B) women.
Table 2.
Correlations of Measured and Calculated BT With Chronological Age in Men and Women
| Age (y) | ||
|---|---|---|
| Men | Women | |
| Measured BT | r = −.65, p < .001 | r = −.23, p < .001 |
| Calculated BT | r = −.47, p < .001 | r = −.11, p = .004 |
Note: BT = bioavailable testosterone.
Trajectories Over the Life Span in Men
In men, the correlation structure compound symmetry best fits the data and was selected in the GLS models for TT, mBT, and SHBG. Figure 1 shows estimated population average trajectories of TT, mBT, and SHBG over the life span in men. Average TT remains quite stable until the age of 70 years; then it progressively declines, with accelerated rate for increasing age (quadratic model fits the best for TT, as reported in Supplementary Table 2). At the same time, mBT presents a linear decline with aging with a rate of decline that is steeper than for TT (linear model fits the data best). SHBG increases with age with rate that accelerates after the age of 70 years (quadratic model fits the best for SHBG).
Trajectories Over the Life Span in Women
In women, the correlation structure compound symmetry best fits the data and was selected in the GLS models for TT and mBT, while autoregressive correlation of order 1 best fits the data and was selected for SHBG. Figure 2 shows estimated population average trajectories of TT, mBT, and SHBG over the life span in women. A cubic spline model provides the best fit for TT data, which progressively declines before menopause and tends to remain stable or slightly increases afterwards. In addition, similarly to men, mBT in women declines linearly over the observed portion of the life span (linear model fits the data best, as reported in Supplementary Table 2). Moreover, SHBG describes a U-shaped trajectory that is best described by a quadratic model, with slight decrease before menopause and accelerated increase with age afterwards.
Analysis Restricted to Older Men and Women (age 65 years or older)
After exploring the age-related changes over the whole life span, we performed similar analyses restricting the sample population to men and women aged 65 or older (Supplementary Table 2). In men aged 65 or older, TT presents an accelerated decline with increasing age, whereas mBT continues to decline linearly (Supplementary Figure 1A and B). In women aged 65 or older, TT increases slightly with age, whereas mBT decreases linearly (Supplementary Figure 2A and B). In both older men and women, SHBG increases linearly (Supplementary Figures 1C and 2C).
Sensitivity Analysis
Sensitivity analyses were performed to test whether participants with longer follow-up were younger or had different levels of testosterone compared with those with shorter follow-up. As length of follow-up resulted no significantly correlated to either baseline age or levels of testosterone in both men and women (not shown), we concluded that differential lengths of follow-up did not bias our findings. Moreover, we ran additional analyses adjusting for calendar year of the first visit in order to account for the fact that people of the same age visited at different years may have different hormonal levels due to secular trend or birth cohort. As the age-related hormonal trend did not significantly change after the adjustment, we concluded that our findings were not biased by secular trend or birth cohort (data not shown).
Discussion
Using almost 10 years of observational data (2004–2014) from men and women enrolled in the BLSA with an excellent representation of older adults (75%) and oldest old (aged up to 98 years at follow-up), we described age-related trajectories of TT, mBT, and SHBG in White individuals. In men, we found that the TT remains quite stable until the age of 70 years; then it declines progressively, with accelerated rate for increasing age, whereas the decline in mBT presents a linear trend over the life span. In addition, in men SHBG increases with age over the life span, and the rate of increase is steeper after the age of 70 years. In women, we found that TT progressively declines before menopause and tends to remain stable or slightly increases afterwards, whereas mBT declines linearly and progressively over the whole life span. Moreover, in women SHBG describes a U-shaped trajectory, with slight decrease before menopause and accelerated increase with age afterwards. Of note, although mBT and cBT are highly correlated each other’s, mBT is a much stronger correlate of chronological age than cBT in both sexes.
Theoretically, the ideal study design to delineate the longitudinal trajectory of testosterone over the adult and old life would be to enroll men and women in their 30s and follow them up for at least 40–50 years, measuring testosterone serially always with the same method. Because this approach is hardly feasible, we instead took the approach of “accelerated longitudinal design” also known as “cross-sequential design.” In the accelerated longitudinal design, differently aged cohorts are followed longitudinally over limited time periods, and the data collected are collated to estimate much longer longitudinal trajectories with relatively good precision (45).
Following this approach, despite a large amount of existent literature describing age-related changes in testosterone circulating levels, our study provides novel, important information and insights that were never addressed in previous studies.
In men, we demonstrated that testosterone levels progressively decline, whereas SHBG increases with age, with a consequent steeper decrease in BT compared with TT. These findings substantially confirm previous cross-sectional and longitudinal studies (13–27). However, a unique strength of our study is showing that the age-related decline in testosterone levels extends to older persons, especially the oldest old, which have been rarely included in previous studies. In fact, only two previous studies specifically focused on changes in testosterone levels at old age, but their results were inconsistent. In particular, while Yeap and colleagues reported that, at age of 70 years and older, TT levels remained stable whereas calculated FT continued to decline (24), a subsequent 4-year observational study in men aged 71–86 years, consistent with our findings, found that TT levels still decrease with age, although the decline in calculated BT was steeper (25). Moreover, in men we found that, while BT linearly declines over the life span, TT remains quite stable until the age of 70 years in our sample. Interestingly, the evident flatness of TT in middle-aged male BLSA participants contrasts with the traditional belief that TT starts to progressively decline after the age of 30–40 years. We certainly acknowledge that the number of individuals younger than 65 years was relatively small in our population sample and, therefore, our ability to estimate with precision the age-related trajectory of TT in this age range is somewhat limited. Nevertheless, consistently with our findings, a recent report including data from 61,131 men aged 0–99 years observed that TT, after the pubertal increase peaking at the age of 20 years, remains stable until the eighth decade (46). Moreover, a recent meta-analysis of 13 studies showed that in healthy men, TT peaks at 15.4 nmol/L at an average age of 19 years and falls to 13.0 nmol/L by an age of 40 years, with no evidence for a further fall in mean TT up to old age, although, after 40 years, an increasing variance in TT levels was observed with aging (47).
A second important strength of our study is that we described age-related trajectories of TT, mBT, and SHBG also in women, where testosterone trajectories over the life span and especially at older ages have not been studied rigorously. The few studies that explored the age-related changes in testosterone levels in women were mostly cross-sectional and were not focused on the oldest old (28–33). In a cross-sectional analysis of women aged 18–75 years, Davison and colleagues reported a steep decline in testosterone levels over the early reproductive age, flatten out in midlife, and increase slightly in the later years (29). Partially in keeping with this evidence, we found that TT declines with age before menopause and increase slightly afterwards. Our data also showed that in women, mBT linearly declines over the life span, even at very old ages. Furthermore, consistently with what previously reported by Maruyama and colleagues (48) and Maggio and colleagues (49), we found that in women, SHBG increases at an accelerated rate after the age of 65 years, whereas other authors reported decline or no significant change with aging in older women (29–31).
Third, the ammonium sulfate precipitation method, which is the method currently used in BLSA for assessing BT, is more accurate than methods that estimate BT through formulas that use TT and SHBG concentrations used in previous studies, including a study in BLSA (17). By using this direct assessment method, we were able to better characterize age-related changes occurring in BT levels with aging, without relying on general assumption about the fraction of TT that is linked to SHBG. Although the exact explanation is still not completely understood, a discrepancy between cBT and mBT (ie, cBT significantly higher than mBT) has been previously described in men (36) and confirmed by our data in both sexes. Therefore, this discrepancy needs to be taken into consideration when calculating BT rather than when measuring BT (37). Also, our data demonstrated for the first time that mBT is a much stronger linear correlate of measure of chronological aging than cBT. Whether mBT may also be a stronger correlate of accelerated biological aging remains unclear, and future researches are needed to determine whether mBT may also have a better functional, cognitive, and health predictive power than cBT in older adults, with potential implications for clinical translation.
In brief, we have shown that, for both sexes, mBT shows a linear decline with age over the life span, whereas different shape and rate of decline are shown by TT. It is known from previous studies that SHBG plays a key role in modulating the biological activity of the sex hormones. Therefore, the different shape in life-span trajectories between TT and mBT is most likely mediated by age-related changes in SHBG levels. Evidence supports that SHBG levels are strongly affected by changes in body composition that occur with aging. In particular, SHBG is inversely related to adiposity and especially visceral adiposity, and individuals who experience decline in BMI with aging tend to develop high levels of SHBG (15,26,50–58). Whether changes in body composition that occur with aging are causes or the consequence of changes in hormonal levels is not clear at this time. Interestingly, in men after the age of 70 years, the linear decline we found for mBT cannot be solely explained by changes in SHBG, which shows a quadratic increase with age. Analogously, the accelerated decline of TT at very old age cannot be simply justified by the concurrent accelerated increase in absolute SHBG, but rather are consistent with the idea that SHBG-binding characteristics changes with aging. Although no in vitro evidence exists, a fascinating hypothesis to explain these trends may be the idea of age-related changes in SHBG-binding affinity. Previous literature, in fact, demonstrated that the proportion of occupied versus unoccupied SHBG-binding sites differ over different stages of life in men and women. Particularly in men, the number of unoccupied sites had been demonstrated to increase at old ages compared with that at middle ages (59). Therefore, in aging men, despite the absolute increase in SHBG, less SHBG-binding sites could be occupied by testosterone. Indeed, further investigations are required to understand whether and how aging may affect SHBG affinity for testosterone. In fact, if the hypothesis about a possible effect of aging on SHBG affinity will be confirmed, modulating it could turn out a powerful and largely unexplored therapeutic target for future research aimed at preventing some of the burdensome effects of aging that has been linked to declining testosterone.
Of note, consistently with a number of studies observing a secular decline in male testosterone levels (22,60–63), we observed that levels of TT in men included in the present analysis were a bit lower compared with those presented by Harman and colleagues in BLSA more than a decade ago (17).
Our study has limitations, which need to be acknowledged. First, the relatively modest sample size of our sample population may affect the generalizability of our results. In particular, we acknowledge that there are relatively few observations for men and women under a certain age (50 or 60 years). Because of the small number of observations in the younger age group, estimates are not as precise as those at older ages as witnessed by wider confidence intervals. Second, we acknowledge that time trend analyses cannot fully separate the effects of chronological age, period of observation, and birth cohort on longitudinal trajectories, namely the age–period–cohort identification problem (64). However, we addressed this issue by running additional sensitivity analyses adjusting for calendar year of the first visit, and we excluded a bias in our findings due to secular trend or birth cohort. In addition, due to previous evidence reporting race differences in levels of testosterone in men and women, we restricted our analysis to White men and women. New studies in populations with a greater representation of other races are required to compare the age-related trajectories of serum testosterone concentrations among different ethnic groups. Moreover, due to the exploratory and descriptive nature of our analysis, we focused on the crude relationship with chronological age and we did not investigate potential predictors or confounders that may affect testosterone levels and age-related trajectories in men and women. Certainly, further studies in larger and more diverse populations, followed for a longer time, are necessary to confirm and validate our findings and to understand how different behavioral and environmental factors may affect age-related trajectories of testosterone and SHBG and their mutual interactions.
In conclusion, although further studies in independent longitudinal samples are required to validate and provide generalizability to our results and to fully understand the underlying mechanisms, we found that ammonium sulfate precipitation–measured bioavailable testosterone linearly declines over the life span and even at old ages in both men and women, representing a strong correlate of chronological aging. Contrariwise, in men, TT remains quite stable until the age of 70 years and then declines at older ages, whereas in women, TT progressively declines in premenopausal years and slightly increases at older ages. Differences in age-related trajectories between total and bioavailable testosterone are only partially explained by age-related changes in SHBG, whose levels increases at accelerated rates in both older men and women.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
This research was supported by the Intramural Research Program of NIH, National Institute on Aging. Data for these analyses were obtained from the Baltimore Longitudinal Study of Aging, a study performed by the National Institute on Aging.
Conflict of Interest
L.F. and S.A.S. declare they are Associate Editors of the Journals of Gerontology: Medical Sciences. All the other authors declare to have no conflict of interests.
Supplementary Material
References
- 1. Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005;26:833–876. [DOI] [PubMed] [Google Scholar]
- 2. Shi Z, Araujo AB, Martin S, O’Loughlin P, Wittert GA. Longitudinal changes in testosterone over five years in community-dwelling men. J Clin Endocrinol Metab. 2013;98(8):3289–3297. doi:10.1210/jc.2012–3842 [DOI] [PubMed] [Google Scholar]
- 3. Sartorius G, Spasevska S, Idan A, et al. Serum testosterone, dihydrotestosterone and estradiol concentrations in older men self-reporting very good health: the healthy man study. Clin Endocrinol. 2012;77(5):755–763. doi:10.1111/j.1365-2265.2012.04432.x [DOI] [PubMed] [Google Scholar]
- 4. Matsumoto AM. Andropause: clinical implications of the decline in serum testosterone levels with aging in men. J Gerontol A Biol Sci Med Sci. 2002;57:M76–M99. [DOI] [PubMed] [Google Scholar]
- 5. Yeap BB, Araujo AB, Wittert GA. Do low testosterone levels contribute to ill-health during male ageing? Crit Rev Clin Lab Sci. 2012;49(5–6):168–823. [DOI] [PubMed] [Google Scholar]
- 6. Wheeler MJ. The determination of bio-available testosterone. Ann Clin Biochem. 1995;32:345–357. [DOI] [PubMed] [Google Scholar]
- 7. Bhasin S, Cunningham GR, Hayes FJ, et al. ; Task Force, Endocrine Society Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(6):2536–2559. doi:10.1210/jc.2009–2354 [DOI] [PubMed] [Google Scholar]
- 8. Asthana S, Bhasin S, Butler RN, et al. Masculine vitality: pros and cons of testosterone in treating the andropause. J Gerontol A Biol Sci Med Sci. 2004;59(5):461–465. [DOI] [PubMed] [Google Scholar]
- 9. Manni A, Pardridge WM, Cefalu W, Nisula BC, Bardin CW, Santner SJ, Santen RJ. Bioavailability of albumin-bound testosterone. J Clin Endocrinol Metab. 1985;61(4):705–710. [DOI] [PubMed] [Google Scholar]
- 10. Giton F, Guéchot J, Fiet J. Comparative determinations of non SHBG-bound serum testosterone, using ammonium sulfate precipitation, Concanavalin A binding or calculation in men. Steroids. 2012;77(12):1306–1311. doi:10.1016/j.steroids.2012.04.009 [DOI] [PubMed] [Google Scholar]
- 11. Hammes A, Andreassen TK, Spoelgen R, et al. Role of endocytosis in cellular uptake of sex steroids. Cell. 2005;122(5):751–762. [DOI] [PubMed] [Google Scholar]
- 12. Cooper LA, Page ST, Amory JK, Anawalt BD, Matsumoto AM. The association of obesity with sex hormone-binding globulin is stronger than the association with ageing—implications for the interpretation of total testosterone measurements. Clin Endocrinol (Oxf). 2015;83(6):828–833. doi:10.1111/cen.12768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Horstman AM, Dillon EL, Urban RJ, Sheffield-Moore M. The role of androgens and estrogens on healthy aging and longevity. J Gerontol A Biol Sci Med Sci. 2012;67:1140–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ferrini RL, Barrett-Connor E. Sex hormones and age: a cross-sectional study of testosterone and estradiol and their bioavailable fractions in community-dwelling men. Am J Epidemiol. 1998;147:750–754. [DOI] [PubMed] [Google Scholar]
- 15. van den Beld AW, de Jong FH, Grobbee DE, Pols HA, Lamberts SW. Measures of bioavailable serum testosterone and estradiol and their relationships with muscle strength, bone density, and body composition in elderly men. J Clin Endocrinol Metab. 2000;85:3276–3282. [DOI] [PubMed] [Google Scholar]
- 16. Leifke E, Gorenoi V, Wichers C, Von Zur Mühlen A, Von Büren E, Brabant G. Age-related changes of serum sex hormones, insulin-like growth factor-1 and sex-hormone binding globulin levels in men: cross-sectional data from a healthy male cohort. Clin Endocrinol (Oxf). 2000;53:689–695. [DOI] [PubMed] [Google Scholar]
- 17. Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR; Baltimore Longitudinal Study of Aging Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86:724–731. [DOI] [PubMed] [Google Scholar]
- 18. Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87(2):589–598. [DOI] [PubMed] [Google Scholar]
- 19. Muller M, den Tonkelaar I, Thijssen JH, Grobbee DE, van der Schouw YT. Endogenous sex hormones in men aged 40-80 years. Eur J Endocrinol. 2003;149:583–589. [DOI] [PubMed] [Google Scholar]
- 20. Mohr BA, Guay AT, O’Donnell AB, McKinlay JB. Normal, bound and nonbound testosterone levels in normally ageing men: results from the Massachusetts Male Ageing Study. Clin Endocrinol (Oxf). 2005;62:64–73. [DOI] [PubMed] [Google Scholar]
- 21. Orwoll E, Lambert LC, Marshall LM, et al. Testosterone and estradiol among older men. J Clin Endocrinol Metab. 2006;91:1336–1344. [DOI] [PubMed] [Google Scholar]
- 22. Travison TG, Araujo AB, O’Donnell AB, Kupelian V, McKinlay JB. A population-level decline in serum testosterone levels in American men. J Clin Endocrinol Metab. 2007;92:196–202. [DOI] [PubMed] [Google Scholar]
- 23. Liu PY, Beilin J, Meier C, et al. Age-related changes in serum testosterone and sex hormone binding globulin in Australian men: longitudinal analyses of two geographically separate regional cohorts. J Clin Endocrinol Metab. 2007;92(9):3599–3603. [DOI] [PubMed] [Google Scholar]
- 24. Yeap BB, Almeida OP, Hyde Z, Norman PE, Chubb SA, Jamrozik K, Flicker L. In men older than 70 years, total testosterone remains stable while free testosterone declines with age. The Health in Men Study. Eur J Endocrinol. 2007;156:585–594. [DOI] [PubMed] [Google Scholar]
- 25. Lapauw B, Goemaere S, Zmierczak H, et al. The decline of serum testosterone levels in community-dwelling men over 70 years of age: descriptive data and predictors of longitudinal changes. Eur J Endocrinol. 2008;159(4):459–468. [DOI] [PubMed] [Google Scholar]
- 26. Camacho EM, Huhtaniemi IT, O’Neill TW, et al. ; EMAS Group Age-associated changes in hypothalamic-pituitary-testicular function in middle-aged and older men are modified by weight change and lifestyle factors: longitudinal results from the European Male Ageing Study. Eur J Endocrinol. 2013;168(3):445–455. [DOI] [PubMed] [Google Scholar]
- 27. Shi Z, Araujo AB, Martin S, O’Loughlin P, Wittert GA. Longitudinal changes in testosterone over five years in community-dwelling men. J Clin Endocrinol Metab. 2013;98:3289–3297. [DOI] [PubMed] [Google Scholar]
- 28. Khosla S, Melton LJ, 3rd, Atkinson EJ, O’Fallon WM, Klee GG, Riggs BL. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J Clin Endocrinol Metab. 1998;83:2266–2274. [DOI] [PubMed] [Google Scholar]
- 29. Davison SL, Bell R, Donath S, Montalto JG, Davis SR. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab. 2005;90:3847–3853. [DOI] [PubMed] [Google Scholar]
- 30. Burger HG, Dudley EC, Cui J, Dennerstein L, Hopper JL. A prospective longitudinal study of serum testosterone, dehydroepiandrosterone sulfate, and sex hormone-binding globulin levels through the menopause transition. J Clin Endocrinol Metab. 2000;85:2832–2838. [DOI] [PubMed] [Google Scholar]
- 31. Spencer JB, Klein M, Kumar A, Azziz R. The age-associated decline of androgens in reproductive age and menopausal Black and White women. J Clin Endocrinol Metab. 2007;92:4730–4733. [DOI] [PubMed] [Google Scholar]
- 32. van Geel TA, Geusens PP, Winkens B, Sels JP, Dinant GJ. Measures of bioavailable serum testosterone and estradiol and their relationships with muscle mass, muscle strength and bone mineral density in postmenopausal women: a cross-sectional study. Eur J Endocrinol. 2009;160:681–687. [DOI] [PubMed] [Google Scholar]
- 33. Sowers MF, Zheng H, McConnell D, Nan B, Karvonen-Gutierrez CA, Randolph JF., Jr Testosterone, sex hormone-binding globulin and free androgen index among adult women: chronological and ovarian aging. Hum Reprod. 2009;24:2276–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Giton F, Urien S, Born C, et al. Determination of bioavailable testosterone [non sex hormone binding globulin (SHBG)-bound testosterone] in a population of healthy French men: influence of androstenediol on testosterone binding to SHBG. Clin Chem. 2007;53(12):2160–2168. [DOI] [PubMed] [Google Scholar]
- 35. Egleston BL, Chandler DW, Dorgan JF. Validity of estimating non-sex hormone-binding globulin bound testosterone and oestradiol from total hormone measurements in boys and girls. Ann Clin Biochem. 2010;47:233–241. doi:10.1258/acb.2010.009112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Déchaud H, Denuzière A, Rinaldi S, Bocquet J, Lejeune H, Pugeat M. Age-associated discrepancy between measured and calculated bioavailable testosterone in men. Clin Chem. 2007;53:723–728. [DOI] [PubMed] [Google Scholar]
- 37. Giton F, Fiet J, Guéchot J, Ibrahim F, Bronsard F, Chopin D, Raynaud JP. Serum bioavailable testosterone: assayed or calculated? Clin Chem. 2006;52:474–481. [DOI] [PubMed] [Google Scholar]
- 38. Stone JL, Norris AH. Activities and attitudes of participants in the Baltimore longitudinal study. J Gerontol. 1966;21:575–580. [DOI] [PubMed] [Google Scholar]
- 39. Mazur A. The age-testosterone relationship in Black, White, and Mexican-American men, and reasons for ethnic differences. Aging Male. 2009;12(2–3):66–76. [DOI] [PubMed] [Google Scholar]
- 40. Richard A, Rohrmann S, Zhang L, et al. Racial variation in sex steroid hormone concentration in black and white men: a meta-analysis. Andrology. 2014;2(3):428–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nankin HR, Pinto R, Fan DF, Troen P. Daytime titers of testosterone, LH, estrone, estradiol, and testosterone binding protein: acute effects of LH and LH-releasing hormone in men. J Clin Endo Metab. 1975;41:271–281. [DOI] [PubMed] [Google Scholar]
- 42. Talbot LA, Morrell CH, Fleg JL, Metter EJ. Changes in leisure time physical activity and risk of all-cause mortality in men and women: the Baltimore Longitudinal Study of Aging. Prev Med. 2007;45:169–176. [DOI] [PubMed] [Google Scholar]
- 43. Fabbri E, An Y, Schrack JA, et al. Energy metabolism and the burden of multimorbidity in older adults: results from the Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci. 2015;70(11):1297–1303. doi:10.1093/gerona/glu209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Harrell FE. Regression Modeling Strategies. 2nd ed New York: Springer; 2015. [Google Scholar]
- 45. Nesselroade JR, Baltes PB. Longitudinal Research in the Study of Behavior and Development. New York: Academic Press; 1979. [Google Scholar]
- 46. Handelsman DJ, Sikaris K, Ly LP. Estimating age-specific trends in circulating testosterone and sex hormone-binding globulin in males and females across the lifespan. Ann Clin Biochem. In Press. pii: 0004563215610589. [DOI] [PubMed] [Google Scholar]
- 47. Kelsey TW, Li LQ, Mitchell RT, Whelan A, Anderson RA, Wallace WH. A validated age-related normative model for male total testosterone shows increasing variance but no decline after age 40 years. PLoS One. 2014;9(10):e109346 doi:10.1371/journal.pone.0109346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Maruyama Y, Aoki N, Suzuki Y, Sinohara H, Yamamoto T. Variation with age in the levels of sex-steroid-binding plasma protein as determined by radioimmunoassay. Acta Endocrinol (Copenh). 1984;106:428–432. [DOI] [PubMed] [Google Scholar]
- 49. Maggio M, Lauretani F, Basaria S, et al. Sex hormone binding globulin levels across the adult lifespan in women—the role of body mass index and fasting insulin. J Endocrinol Invest. 2008;31:597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gambera A, Scagliola P, Falsetti L, Sartori E, Bianchi U. Androgens, insulin-like growth factor-I (IGF-I), and carrier proteins (SHBG, IGFBP-3) in postmenopause. Menopause. 2004;11:159–166. [DOI] [PubMed] [Google Scholar]
- 51. Haffner SM, Valdez RA, Stern MP, Katz MS. Obesity, body fat distribution and sex hormones in men. Int J Obes Relat Metab Disorders. 1993;17:643–649. [PubMed] [Google Scholar]
- 52. Stefanick ML, Williams PT, Krauss RM, Terry RB, Vranizan KM, Wood PD. Relationship of plasma estradiol, testosterone, and sex hormone-binding globulin with lipoproteins, apolipoproteins, and high-density lipoprotein subfractions in men. J Clin Endocrinol Metabol. 1987;64:723–729. [DOI] [PubMed] [Google Scholar]
- 53. Seidell JC, Björntorp P, Sjöström L, Kvist H, Sannerstedt R. Visceral fat accumulation in men is positively associated with insulin, glucose, and C-peptide levels, but negatively with testosterone levels. Metabolism. 1990;39:897–901. [DOI] [PubMed] [Google Scholar]
- 54. Pasquali R, Casimirri F, Cantobelli S, et al. Effect of obesity and body fat distribution on sex hormones and insulin in men. Metabolism. 1991;40:101–104. [DOI] [PubMed] [Google Scholar]
- 55. Svartberg J, von Muhlen D, Sundsfjord J, Jorde R. Waist circumference and testosterone levels in community dwelling men. Eur J Epidemiol. 2004;19:657–663. [DOI] [PubMed] [Google Scholar]
- 56. Derby CA, Zilber S, Brambilla D, Morales KH, McKinlay JB. Body mass index, waist circumference and waist to hip ratio and change in sex steroid hormones: the Massachusetts Male Ageing Study. Clin Endocrinol (Oxf). 2006;65:125–131. [DOI] [PubMed] [Google Scholar]
- 57. Mohr BA, Bhasin S, Link CL, O’Donnell AB, McKinlay JB. The effect of changes in adiposity on testosterone levels in older men: longitudinal results from the Massachusetts Male Aging Study. Eur J Endocrinol. 2006;155:443–452. [DOI] [PubMed] [Google Scholar]
- 58. Travison TG, Araujo AB, Kupelian V, O’Donnell AB, McKinlay JB. The relative contributions of aging, health, and lifestyle factors to serum testosterone decline in men. J Clin Endocrinol Metab. 2007;92:549–555. [DOI] [PubMed] [Google Scholar]
- 59. Hammond GL, Wu TS, Simard M. Evolving utility of sex hormone-binding globulin measurements in clinical medicine. Curr Opin Endocrinol Diabetes Obes. 2012;19:183–189. [DOI] [PubMed] [Google Scholar]
- 60. Andersson AM, Jensen TK, Juul A, Petersen JH, Jørgensen T, Skakkebaek NE. Secular decline in male testosterone and sex hormone binding globulin serum levels in Danish population surveys. J Clin Endocrinol Metab. 2007;92:4696–4705. [DOI] [PubMed] [Google Scholar]
- 61. Travison TG, Araujo AB, Hall SA, McKinlay JB. Temporal trends in testosterone levels and treatment in older men. Curr Opin Endocrinol Diabetes Obes. 2009;16(3):211–217. [DOI] [PubMed] [Google Scholar]
- 62. Perheentupa A, Mäkinen J, Laatikainen T, Vierula M, Skakkebaek NE, Andersson AM, Toppari J. A cohort effect on serum testosterone levels in Finnish men. Eur J Endocrinol. 2013;168(2):227–233. [DOI] [PubMed] [Google Scholar]
- 63. Mazur A, Westerman R, Mueller U. Is rising obesity causing a secular (age-independent) decline in testosterone among American men? PLoS One. 2013;8(10):e76178 doi:10.1371/journal.pone.0076178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tu YK, Krämer N, Lee WC. Addressing the identification problem in age-period-cohort analysis: a tutorial on the use of partial least squares and principal components analysis. Epidemiology. 2012;23(4):583–593. doi:10.1097/EDE.0b013e31824d57a9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.