Abstract
Background:
Evidence suggests a link between the presence of subjective memory complaints (SMC) and lower volume of the hippocampus, one of the first regions to show neuropathological lesions in Alzheimer’s disease. However, it remains unknown whether this pattern of hippocampal atrophy is regionally specific and whether SMC are also paralleled by changes in peripheral levels of amyloid-beta (Aβ).
Methods:
The volume of hippocampal subregions and plasma Aβ levels were cross-sectionally compared between elderly individuals with (SMC+; N = 47) and without SMC (SMC−; N = 48). Significant volume differences in hippocampal subregions were further correlated with plasma Aβ levels and with objective memory performance.
Results:
Individuals with SMC exhibited significantly higher Aβ1–42 concentrations and lower volumes of CA1, CA4, dentate gyrus, and molecular layer compared with SMC− participants. Regression analyses further showed significant associations between lower volume of the dentate gyrus and both poorer memory performance and higher plasma Aβ1–42 levels in SMC+ participants.
Conclusions:
The presence of SMC, lower volumes of specific hippocampal regions, and higher plasma Aβ1–42 levels could be conditions associated with aging vulnerability. If such associations are confirmed in longitudinal studies, the combination may be markers recommending clinical follow-up in nondemented older adults.
Keywords: Aging, Subjective memory complaints, Hippocampus, Plasma amyloid-beta, Alzheimer’s disease, Biomarkers
Subjective memory complaints (SMC) are common among elderly nondemented individuals, and they are generally perceived as a normal consequence of aging (1). However, numerous studies have found that SMC are associated with psychiatric symptoms (2), adverse health outcomes (3), and increased health care utilization (4). Evidence has also suggested that SMC may increase risk of progression to cognitive impairment and dementia (5). Consequently, individuals with SMC develop Alzheimer’s disease (AD) more frequently (6), have more functional deficits (7) and AD-type brain pathology than SMC− participants (8).
As misperception may play an important role in self-reported memory problems, surrogate AD biomarkers should preferably accompany SMC-based assessments. Cerebrospinal fluid (CSF) and amyloid PET are the most reliable in vivo biomarkers of prodromal AD, but they are not suitable for screening purposes due to the invasive CSF sampling procedure and the high cost and limited availability of amyloid PET imaging (9). Alternatively, MRI-based AD biomarkers have demonstrated high sensitivity to prodromal AD (10). However, few studies to date have investigated the relationship between SMC and changes in hippocampal volume (11–14), and none of them has focused on specific regions within the hippocampal formation.
Hippocampal subregions not only differ in gene expression, connectivity, and function (15,16) but are also affected by AD lesions with different chronologies (17). Indeed, postmortem studies have shown neurofibrillary degeneration in CA1 and subiculum as well as amyloid plaques in other hippocampal subregions during preclinical AD (18), suggesting that the hippocampus is unevenly affected during the continuum normal aging-AD. Because SMC appear early in this continuum, we hypothesize that CA1 could be one of the first hippocampal subregions affected. Moreover, altered neurogenesis in the hippocampus has been suggested as an early critical event in AD due to its relevance for neural plasticity and network maintenance (19). We would therefore expect lower volume of the dentate gyrus (DG) in SMC+ participants because, along with the olfactory bulb, the DG is the only site of neurogenesis in humans (20). If lower volume of CA1 and DG results from some degree of synaptic and/or neuronal loss, it should also be accompanied by poorer memory performance. To evaluate these predictions, we have cross-sectionally compared the volume of different hippocampal subregions between elderly participants with and without SMC. We have further evaluated whether volume differences of hippocampal subregions are associated with alterations in memory performance in each group, separately.
Accumulated evidence suggests that soluble amyloid-beta (Aβ) oligomers precede plaque formation and constitute the principal instigators of synapse loss and neuronal injury in AD (21), likely leading to synaptic deficits in asymptomatic elderly participants. Given that plasma enzyme-linked immunosorbent assays (ELISA) mainly detect soluble Aβ, another straightforward prediction of the present study is that SMC+ participants will show higher plasma Aβ concentrations than SMC− individuals, and that higher Aβ levels will be associated with lower volumes of hippocampal subregions affected during early stages of neurodegeneration.
Methods
Participants
The study sample consisted of 95 nondemented elderly participants (mean age: 68.8±3.8 years; range: 62–78 years) recruited from senior citizen’s associations, screening programs, and hospital outpatient services. Forty-seven of them reported SMC that were corroborated with the Memory Functioning Questionnaire and with a structured interview. The remaining 48 participants were clinically normal and did not report SMC as confirmed by the same instruments.
All participants showed normal memory performance relative to appropriate reference values for age and education and underwent neurological examination, neuropsychological memory testing (Free and Cued Selective Reminding Test, Rey-Osterrieth Complex Figure Test, verbal paired associates subtest of the Wechsler Memory Scale, and the Camel and Cactus Test), and MRI evaluation (T1-3D and FLAIR). Individuals with medical conditions and/or history of disorders that may affect the cerebral integrity (eg, stroke, coronary heart diseases, diabetes, head trauma, neurodegenerative diseases, depression, hydrocephalus, intracranial mass, MRI infarcts, and use of psychoactive medication) were not allowed to participate in the study. All participants showed a global score of 0 (no dementia) in the Clinical Dementia Rating, as well as normal independent functions. Depression was excluded with the shorter version of the Geriatric Depression Scale (scores ≤ 5) given the association between plasma Aβ levels and depression in older adults (22). Participants gave informed consent prior to their inclusion in the study, which was approved by the Ethical Committee for Human Research at Pablo de Olavide University.
Plasma Aβ Measurements
Plasma Aβ levels were determined by a double-antibody sandwich ELISA (human Aβ1–40 and high sensitive Aβ1–42, Wako Chemicals, Tokyo, Japan). Briefly, venous blood samples were collected after overnight fasting in 10mL K2-ethylenediaminetetraacetic acid (EDTA)-coated tubes (BD Diagnostics) and were immediately centrifuged (3,500rpm) at 4°C for 5 minutes. Supernatant plasma was collected and aliquoted into 250 μL polypropylene tubes containing 8.32 μL of a protease inhibitor cocktail (cOmplete Ultra Tablets mini, Roche). Plasma samples were stored at −80°C and thawed immediately before assay.
Samples and standards were incubated overnight at 8°C with antibodies specific for Aβ1–40 and Aβ1–42 peptides, and the wells were read for absorption at 450nm (Victor 3, PerkinElmer, Waltham, MA), according to the manufacturer’s instructions. Plasma Aβ levels were measured in duplicate (50 μL), and the average of the two measurements (pg/mL) was used for statistical analyses. Both inter-assay and intra-assay coefficients of variation were below 10%. The detection limit for these assays was 1.04 and 0.54 pg/mL for Aβ1–40 and Aβ1–42, respectively.
MRI Acquisition
MRI studies were acquired on a whole-body Philips Achieva 3T scanner equipped with an 8-channel head coil. One high-resolution MP-RAGE (magnetization-prepared rapid gradient echo) T1-weighted anatomical sequence was obtained from each participant (0.8mm3 isotropic voxel resolution, no gap between slices, TR = 11ms, TE = 4.5ms, flip angle = 8°, acquisition time = 9.1 minutes).
Segmentation of Hippocampal Subregions
MRI data were preprocessed using the analysis pipeline of Freesurfer v5.3 (http://surfer.nmr.mgh.harvard.edu/) involving intensity normalization, registration to Talairach space, skull stripping, segmentation of white matter, tesselation of the white matter boundary, and automatic correction of topological defects (23). Removal of non-brain tissues was manually performed on a slice-by-slice basis for each participant to increase segmentation reliability.
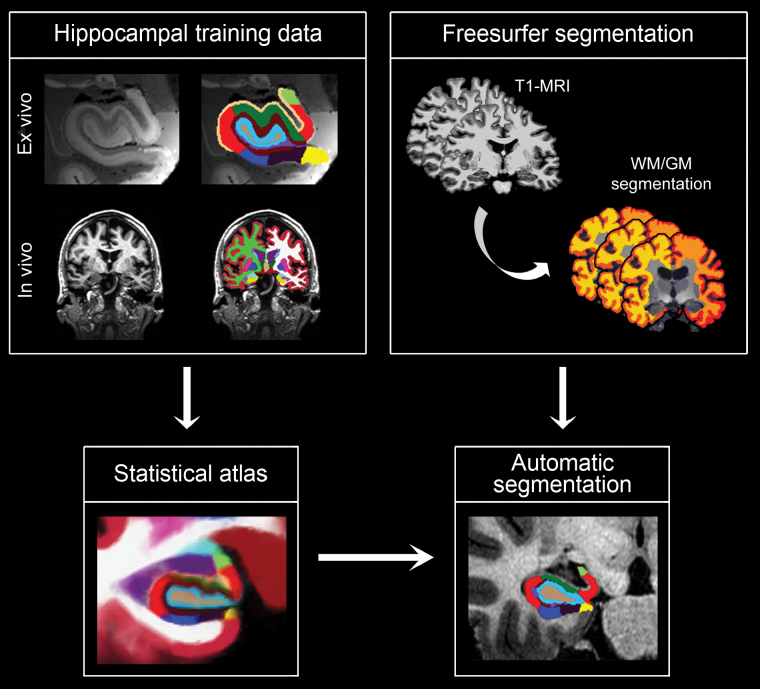
Hippocampal subregions were automatically segmented from the T1-weighted scans using a novel approach based on Bayesian inference and a probabilistic atlas of the hippocampal formation built upon ultra-high resolution (0.13mm isotropic, on average), ex vivo MRI data from 15 cases (24). The following subregions resulted from hippocampal segmentation: parasubiculum, presubiculum, subiculum, CA1, CA3, CA4 subfields, granule cell layer of DG, hippocampus-amygdala-transition-area, fimbria, molecular layer (ML), hippocampal fissure, and tail. Segmentation results were visually inspected for errors in each participant. Figure 1 shows the analysis pipeline to obtain volumes of hippocampal subregions.
Figure 1.
Analysis pipeline to obtain volumes of hippocampal subregions. Left column: Automatic segmentation of hippocampal subregions relied on a computational atlas of the hippocampal formation derived from (i) ultra-high resolution (0.13mm isotropic resolution, on average) ex vivo MRI scans of 15 autopsy samples that were manually segmented into 13 different hippocampal subregions and (ii) a separate data set of in vivo, T1-weighted MRI scans of the whole brain (1mm isotropic resolution) (24). Right column: The cerebral MRI of each participant was preprocessed and segmented using the analysis pipeline of Freesurfer; the output was used to initialize the hippocampal segmentation, from which the volume of hippocampal subregions was obtained.
The intracranial volume of each individual was also estimated using the Freesurfer analysis pipeline. Skull-stripped images were revised and corrected manually to ensure that extracranial and skull structures were successfully removed while preserving intracranial structures.
Statistical Analyses
Statistical analyses were performed with SPSS v21 (SPSS Inc., Chicago, IL). Parametric statistics were used because the sample size was above 40 participants and the null hypothesis of non-normal distribution was rejected with the Kolmogorov–Smirnov test for demographic variables, memory scores, plasma Aβ measurements (ie, Aβ1–40, Aβ1–42), and hippocampal volumes. Group differences in demographics were assessed with unpaired t tests, with the exception of gender, which was evaluated with the chi-square test. Group differences in plasma Aβ levels (Aβ1–40, Aβ1–42, Aβ1–42/Aβ1–40 ratio) and memory scores were assessed using a global multivariate analysis of covariance (MANCOVA) adjusted for age and gender.
Mixed ANCOVAs, with the group (SMC− vs SMC+) as the between-participants factor, the hemisphere (left vs right) as the within-participants factor, and age and intracranial volume as covariates, were performed to inspect group differences in the volume of each hippocampal subregion. Gender was not included as a covariate in the general linear model because this variable did not reach statistical significance for any hippocampal subregion.
Linear regression analyses were further conducted in each group separately to assess whether volume differences in any of the hippocampal subregions were related to alterations in memory performance obtained with different neuropsychological tests. A similar statistical approach was employed to test for significant associations between volume of hippocampal subregions and plasma Aβ levels. If correlations reached significance in at least one of the two groups, group differences between regression slopes were also computed. For the latter, the interaction between the group and either the MRI volume or the Aβ levels was introduced in the statistical model. All regression analyses were adjusted for age and intracranial volume. Permutation tests (N = 10,000 randomizations) were conducted to correct for multiple comparisons in all statistical analyses.
Results
Demographic Characteristics and Memory Functioning
As shown in Table 1, elderly participants with and without SMC were homogeneous in demographic characteristics and performed similarly in objective memory tests. As expected, self-perception of memory was significantly affected in SMC+ (p = 10–8 for everyday memory; p = .001 for memory of texts; p = 10–5 for memory of past events) compared with SMC− participants.
Table 1.
Demographic, Aβ Measurements, and Memory Functioning
| SMC− | SMC+ | |
|---|---|---|
| Age | 68.1±3.2 | 69.6±4.3 |
| Gender (m/f) | 26/21 | 22/26 |
| Education, y | 11.0±4.6 | 10.9±4.5 |
| MMSE | 29.1±1.2 | 29.2±1.4 |
| Plasma Aβ | ||
| Aβ1–40 (pg/mL) | 229.0±29.5 | 226.1±35.6 |
| Aβ1–42 (pg/mL) | 21.0±7.0 | 25.3±6.4* |
| Aβ1–42/1–40 (pg/mL) | 0.09±0.02 | 0.11±0.03*** |
| Subjective memory | ||
| Everyday (MFQ) | 29.6±7.1 | 41.4±9.8** |
| Texts (MFQ) | 6.6±3.8 | 9.6±4.3** |
| Past events (MFQ) | 16.9±3.7 | 19.9±2.4** |
| Strategies (MFQ) | 24.0±8.6 | 26.2±7.8 |
| Objective memory | ||
| Free recall (FCSRT) | 41.5±4.9 | 41.0±5.8 |
| Delayed recall (FCSRT) | 13.7±2.0 | 13.6±2.1 |
| Immediate memory (ROCFT) | 19.4±5.8 | 18.5±5.5 |
| Delayed memory (ROCFT) | 19.0±5.9 | 17.7±5.8 |
| Word pairs (WMS) | 5.3±2.1 | 5.2±2.2 |
| Camel and Cactus Test | 53.1±3.3 | 53.5±4.3 |
Notes: Results are expressed as mean ± standard deviation. m/f = male/female; Aβ = amyloid-beta; FCSRT = Free and Cued Selective Reminding Test; MFQ = Memory Functioning Questionnaire; MMSE = Mini-Mental State Examination; ROCFT = Rey-Osterrieth Complex Figure Test; SMC = subjective memory complaints; WMS = Wechsler Memory Scale, third edition.
*p < .01; **p < .001; ***p < .0001.
Plasma Aβ Levels
Plasma Aβ concentrations significantly differed between the two groups (F 3,89 = 6.67, p = .0004). The univariate ANCOVAs showed higher levels of Aβ1–42 (F 1,91 = 7.56, p = .007) and higher Aβ1–42/Aβ1–40 ratio (F 1,91 = 15.76, p = .0001) in SMC+ compared with SMC− participants (see Table 1).
Volume of Hippocampal Subregions
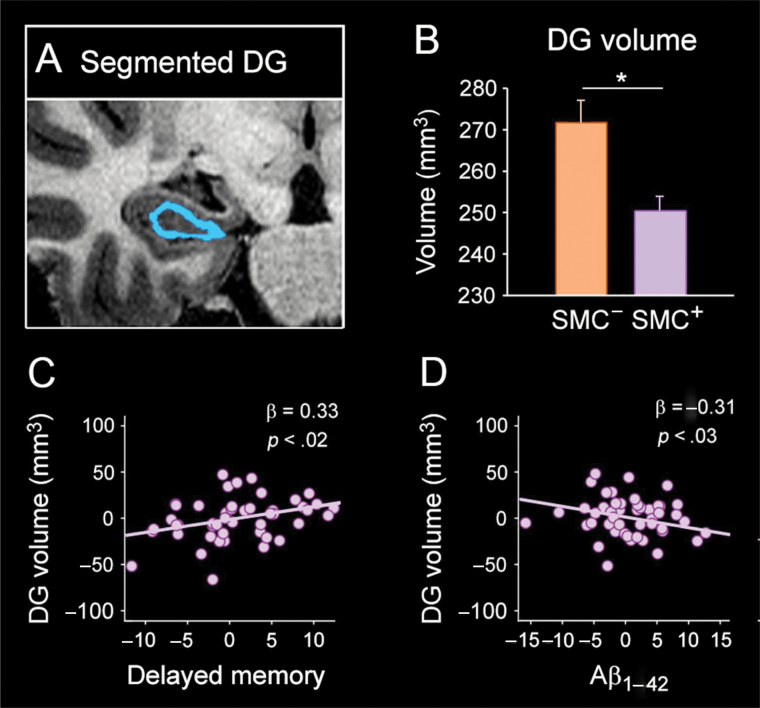
Mean volumes of hippocampal subregions for each group are reported in Table 2. The mixed MANCOVA revealed lower hippocampal volumes in SMC+ participants with a consistent trend for greater atrophy of the left hemisphere (Table 2, corrected p values). More specifically, SMC+ individuals showed lower volumes of the whole hippocampus (p = .04), CA1 (p = .02), and CA4 (p = .03) subfields, DG (p = .02), and ML (p = .03) than SMC− participants. No significant differences were observed in the opposite direction for any hippocampal subregion (ie, lower volumes in SMC− compared with SMC+ participants). Figure 2B displays group differences in DG volumes.
Table 2.
Volume of Hippocampal Subregions
| SMC− | SMC+ | |
|---|---|---|
| Left | ||
| Total hippocampus | 3159±367 | 2966±235* |
| CA1 | 583±70 | 539±44* |
| CA3 | 191±29 | 175±17 |
| CA4 | 235±32 | 219±19* |
| Parasubiculum | 59±12 | 59±10 |
| Presubiculum | 349±43 | 337±37 |
| Subiculum | 390±48 | 371±41 |
| DG | 271±37 | 250±23* |
| Fimbria | 76±15 | 67±15 |
| Tail | 497±73 | 476±58 |
| Fissure | 151±25 | 144±26 |
| ML | 449±55 | 417±33* |
| HATA | 57±10 | 51±7 |
| Right | ||
| Total hippocampus | 3256±384 | 3089±237 |
| CA1 | 608±80 | 576±50 |
| CA3 | 212±36 | 200±21 |
| CA4 | 251±35 | 235±20 |
| Parasubiculum | 58±10 | 56±11 |
| Presubiculum | 335±44 | 324±36 |
| Subiculum | 392±54 | 379±37 |
| DG | 288±41 | 269±24 |
| Fimbria | 68±17 | 64±16 |
| Tail | 513±69 | 482±63 |
| Fissure | 162±26 | 159±25 |
| ML | 470±61 | 445±35 |
| HATA | 58±10 | 55±7 |
Notes: Results (in mm3) are expressed as mean ± standard deviation. DG = dentate gyrus; HATA = hippocampus-amygdala-transition-area; ML = molecular layer; SMC = subjective memory complaints.
*p < .05.
Figure 2.
Effects of SMC on structural integrity of DG. (A) A sample coronal slice of the hippocampus highlighting (in blue) the segmented DG. (B) Group differences in DG volume (mm3). (C) Significant association between lower volume of the DG and poorer memory performance in SMC+ participants. (D) Significant association between lower volume of the DG and higher plasma Aβ1–42 levels in the SMC+ group. Statistical analyses were all adjusted by age and ICV, and p values were previously corrected with permutation-based tests. Aβ = amyloid-beta; DG = dentate gyrus; ICV = intracranial volume; SMC = subjective memory complaints.
Relationship Between Volume of Hippocampal Subregions and Memory Functioning
We next investigated whether volume differences of hippocampal subregions were associated with variations of memory performance in each group, separately. Regression analyses showed significant associations between regional hippocampal volume and performance of different memory tests in SMC+ participants. Thus, lower volumes of total hippocampus (β = 0.36, p = .009), DG (β = 0.33, p = .02), and ML (β = 0.31, p = .03) were related to poorer delayed memory in the Rey-Osterrieth Complex Figure Test. However, there was no interaction effect with group for any of these variables. Lower volumes of total hippocampus and ML were also associated with poorer semantic memory in the Camel and Cactus Test (β = 0.31, p = .02) and with poorer delayed memory in the Free and Cued Selective Reminding Test (β = 0.31, p = .03), respectively. Group differences in regression slopes only reached significance for the relationship between the volume of ML and delayed recall in the Free and Cued Selective Reminding Test (β = −0.9, p = .02), this relationship being stronger in SMC+ than in SMC− participants. Figure 2C shows significant associations between lower volume of DG and poorer delayed memory in SMC+ participants.
Relationship Between Volume of Hippocampal Subregions and Plasma Aβ Levels
Finally, we assessed whether lower volumes of hippocampal subregions were related to higher plasma Aβ1–42 levels. Regression analyses revealed that lower volumes of DG (β = −0.31, p = .03) and ML (β = −0.33, p = .01) were significantly associated with higher Aβ1–42 levels in SMC+ participants, but this relationship did not differ from the one shown by SMC− individuals. No other hippocampal subregions showed a significant relationship with plasma Aβ1–42 levels in any of the two groups. Figure 2D shows the significant relationship between lower volumes of DG and higher Aβ1–42 levels in SMC+ participants.
Discussion
The present study shows, for the first time, that nondemented elderly participants with self-reported memory complaints have lower volumes of specific hippocampal subregions (ie, CA1 and CA4 subfields, the granule cell layer of DG, and the ML) and higher concentrations of plasma Aβ1–42 than SMC− participants. These findings are in agreement with the high prevalence of pathological CSF values (25) and the high levels of AD-type neuropathological lesions (8) observed in SMC participants. Moreover, we have found that lower volumes of DG and the overlying ML in SMC+ participants not only are associated with poorer episodic and semantic memory, respectively, but also with higher plasma Aβ1–42 levels.
Interestingly, the lower volumes of hippocampal subregions were restricted to the left side in SMC+ participants, corroborating earlier findings (12). In fact, there is evidence that gray matter reduction in the left hippocampus precedes clinical AD, this being a particularly useful biomarker of cognitive decline during asymptomatic stages (26). It might happen that the vulnerability of the left hippocampus to AD pathology can be extended to SMC+ individuals who are prone to develop faster cognitive decline or eventually AD.
We found that SMC participants showed lower volume of the CA1 subfield. Previous evidence suggests that neuronal loss in CA1 is not an age-related phenomenon but rather characterizes an overt AD process (27). Contrary to our initial hypothesis, lower volume of CA1 was not associated with lower memory performance in SMC+ participants. One possible explanation for this negative result is that early CA1 dysfunctions are being compensated for by other mechanisms of neural plasticity elsewhere in the brain. Despite the lack of relationship, previous evidence has shown that CA1 atrophy predicts decline to mild cognitive impairment (MCI) in cognitively normal individuals (28) and subsequent conversion to AD (29), suggesting that lower CA1 volume associated with SMC may signal either incipient neurodegeneration or increased aging vulnerability.
To our knowledge, this is the first study revealing lower volumes of CA4, DG, and overlying ML in SMC participants using in vivo MRI. One of our predictions was that SMC participants would have lower volume of the DG due to the prominent role of this structure in neurogenesis, and consequently in synaptic plasticity, which makes this hippocampal subregion extremely vulnerable to AD pathological mechanisms. Although the functional significance of neurogenesis in the adult human hippocampus remains to be determined, evidence suggests that hippocampal neurogenesis remains throughout old age (30,31). Altered neurogenesis in the hippocampus may affect discriminative learning and memory mainly due to the role of these regions in pattern separation, which require transformation of similar inputs into more discordant outputs (32). It is therefore not surprising that SMC+ participants showed a significant relationship between lower volume of DG and poorer episodic memory in the present study.
Only one study to date has examined the relationship between objective memory and hippocampal volume in participants with SMC (12), although the influence of depressive symptoms could not be completely ruled out. We found that the lower the volume of DG, the poorer the memory performance in individuals with SMC. This result is consistent with previous evidence showing impaired memory performance together with structural and functional changes in CA3 and DG in MCI patients (33). Therefore, the relationship between DG volume and memory performance in individuals with either SMC or MCI provides strong support to the notion that neuroplasticity in the aging brain may be an important vulnerability factor for developing AD.
Our study also showed that higher plasma Aβ1–42 levels were significantly associated with lower volume of the DG in SMC+ participants. Plasma concentrations of Aβ1–42 are elevated in familial AD with mutations in the presenilin (PS) or amyloid precursor protein (APP) genes (34), as well as in Down’s syndrome patients (35), and in first-degree relatives of AD patients at increased risk of developing AD (36). Furthermore, higher plasma Aβ1–42 levels have been associated with faster cognitive decline (37), higher atrophy of the temporal lobe in cognitively intact elderly participants (38), and conversion to MCI (39). Evidence suggests that cerebral and peripheral Aβ levels form a dynamic equilibrium that seems to be altered during AD progression (40). Thus, previous research has shown that elevated peripheral Aβ levels may enter the brain, accelerating AD pathogenesis (41), or reducing its clearance from the brain (42). Although studies considering plasma Aβ as a potential biomarker of dementia have yielded contradictory results (9), our findings suggest that combining plasma Aβ measurements with volume differences of specific regions of the hippocampal formation may be helpful in assessing aging vulnerability in elderly participants with SMC.
We speculate that the association between DG atrophy and higher plasma Aβ1–42 concentration may signal incipient AD-related pathology in SMC+ participants. In line with this assumption, previous studies have shown that associations between CSF Aβ1–42 levels and patterns of cortical atrophy differ between asymptomatic elderly participants and MCI patients. Interestingly, significant correlations were restricted to the temporal lobe, medial parietal cortex, and posterior cingulate in MCI (43,44), whereas correlations with the temporal lobe were absent in asymptomatic elderly participants (45). All the above regions not only show high levels of amyloid PET retention in MCI and AD patients (46) but also are important common nodes of different hippocampal networks showing decreased functional connectivity in MCI (47). Future research should longitudinally assess whether the association between lower volume of DG and higher plasma Aβ1–42 levels in SMC+ participants is able to discriminate between older adults who experience faster decline from those who remain stable. If such association is confirmed, older adults with SMC may be a target population for applying novel pharmacological strategies aimed to modify AD pathological mechanisms.
This study has some limitations that should be mentioned. First, participants lacked biomarkers of AD pathology (ie, CSF Aβ/tau and/or amyloid PET) impeding the confirmation of preclinical AD in SMC+ participants. Second, the present study is cross-sectional, hindering the assessment of progression and therefore the validation of hippocampal volume changes and plasma Aβ1–42 results in this population. Furthermore, the hippocampal atlas was applied to 1mm resolution T1 data, where the internal boundaries of hippocampal subregions are not clearly distinguishable. Although automated segmentations were visually inspected for errors in each individual to guarantee the reliability of the volume estimates, it should be noted that the internal boundaries of the hippocampal segmentation mostly rely on the prior knowledge encoded in the atlas rather than on the image contrast. Therefore, future research conducted with SMC+ participants should combine in vivo biomarkers of AD pathology with MRI hippocampal acquisitions of higher resolution in a longitudinal framework. This approach would hopefully contribute to reducing the required sample sizes for preventive clinical trials in AD and offer follow-up to elderly participants at higher risk in developing preclinical AD.
Funding
This work was supported by research grants from the Spanish Ministry of Economy and Competitiveness (SAF2011-25463 to J.L.C., PSI2014-55747-R to M.A.), the Regional Ministry of Innovation, Science and Enterprise, Junta de Andalucia (P12-CTS-2327 to J.L.C.), CIBERNED (CB06/05/1111 to J.L.C.), the Fellows Gipuzkoa Program to J.E.I., the National Institutes of Health, National Center for Research Resources (P41-RR14075 to K.V.L.), the National Institutes of Health, National Institute of Biomedical Imaging and Bioengineering (R01EB013565 to K.V.L.), and the Lundbeck Foundation (R141-2013-13117 to K.V.L.).
References
- 1. Jonker C, Geerlings MI, Schmand B. Are memory complaints predictive for dementia? A review of clinical and population-based studies. Int J Geriatr Psychiatry. 2000;15:983–991. doi:10.1002/1099-1166(200011)15:11<983::AID-GPS238>3.0.CO;2-5 [DOI] [PubMed] [Google Scholar]
- 2. Jorm AF, Butterworth P, Anstey KJ, et al. Memory complaints in a community sample aged 60-64 years: associations with cognitive functioning, psychiatric symptoms, medical conditions, APOE genotype, hippocampus and amygdala volumes, and white-matter hyperintensities. Psychol Med. 2004;34:1495–1506. doi:10.1017/S0033291704003162 [DOI] [PubMed] [Google Scholar]
- 3. Glodzik-Sobanska L, Reisberg B, De Santi S, et al. Subjective memory complaints: presence, severity and future outcome in normal older subjects. Dement Geriatr Cogn Disord. 2007;24:177–184. doi:10.1159/000105604 [DOI] [PubMed] [Google Scholar]
- 4. Waldorff FB, Siersma V, Waldemar G. Association between subjective memory complaints and health care utilisation: a three-year follow up. BMC Geriatr. 2009;9:43. doi:10.1186/1471-2318-9-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reisberg B, Shulman MB, Torossian C, Leng L, Zhu W. Outcome over 7 years of healthy adults with and without SMC. Alzheimers Dement. 2010;6:11–24. doi:10.1016/j.jalz.2009.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reid LM, Maclullich AM. Subjective memory complaints and cognitive impairment in older people. Dement Geriatr Cogn Disord. 2006;22:471–485. doi:10.1159/000096295 [DOI] [PubMed] [Google Scholar]
- 7. Ogata S, Hayashi C, Sugiura K, Hayakawa K. Association between subjective memory complaints and impaired higher-level functional capacity in people aged 60 years or older. Arch Gerontol Geriatr. 2015;60:201–205. doi:10.1016/j.archger.2014.10.015 [DOI] [PubMed] [Google Scholar]
- 8. Kryscio RJ, Abner EL, Cooper GE, et al. Self-reported memory complaints: implications from a longitudinal cohort with autopsies. Neurology. 2014;83:1359–1365. doi:10.1212/WNL.0000000000000856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Toledo JB, Shaw LM, Trojanowski JQ. Plasma amyloid beta measurements - a desired but elusive Alzheimer’s disease biomarker. Alzheimers Res Ther. 2013;5:8. doi:10.1186/alzrt162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dickerson BC, Wolk DA; Alzheimer’s Disease Neuroimaging Initiative MRI cortical thickness biomarker predicts AD-like CSF and cognitive decline in normal adults. Neurology. 2012;78:84–90. doi:10.1212/WNL.0b013e31823efc6c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Leon MJ, Golomb J, George AE, et al. The radiologic prediction of Alzheimer disease: the atrophic hippocampal formation. AJNR Am J Neuroradiol. 1993;14:897–906. [PMC free article] [PubMed] [Google Scholar]
- 12. van der Flier WM, van Buchem MA, Weverling-Rijnsburger AW, et al. Memory complaints in patients with normal cognition are associated with smaller hippocampal volumes. J Neurol. 2004;251:671–675. doi:10.1007/s00415-004-0390-7 [DOI] [PubMed] [Google Scholar]
- 13. Saykin AJ, Wishart HA, Rabin LA, et al. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67:834–842. doi:10.1212/01.wnl.0000234032.77541.a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Striepens N, Scheef L, Wind A, et al. Volume loss of the medial temporal lobe structures in subjective memory impairment. Dement Geriatr Cogn Disord. 2010;29:75–81. doi:10.1159/000264630 [DOI] [PubMed] [Google Scholar]
- 15. Moser MB, Moser EI. Functional differentiation in the hippocampus. Hippocampus. 1998;8:608–619. doi:10.1002/(SICI)1098-1063(1998)8:6<608::AID-HIPO3>3.0.CO;2-7 [DOI] [PubMed] [Google Scholar]
- 16. Masser DR, Bixler GV, Brucklacher RM, et al. Hippocampal subregions exhibit both distinct and shared transcriptomic responses to aging and nonneurodegenerative cognitive decline. J Gerontol A Biol Sci Med Sci. 2014;69:1311–1324. doi:10.1093/gerona/glu091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schönheit B, Zarski R, Ohm TG. Spatial and temporal relationships between plaques and tangles in Alzheimer-pathology. Neurobiol Aging. 2004;25:697–711. doi:10.1016/j.neurobiolaging.2003.09.009 [DOI] [PubMed] [Google Scholar]
- 18. Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. [DOI] [PubMed] [Google Scholar]
- 19. Mu Y, Gage FH. Adult hippocampal neurogenesis and its role in Alzheimer’s disease. Mol Neurodegener. 2011;6:85. doi:10.1186/1750-1326-6-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eriksson PS, Perfilieva E, Björk-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi:10.1038/3305 [DOI] [PubMed] [Google Scholar]
- 21. Walsh DM, Selkoe DJ. Amyloid-beta oligomers - a decade of discovery. J Neurochem. 2007;101:1172–1184. doi:10.1111/j.1471-4159.2006.04426.x [DOI] [PubMed] [Google Scholar]
- 22. Metti AL, Cauley JA, Newman AB, et al. Plasma beta amyloid level and depression in older adults. J Gerontol A Biol Sci Med Sci. 2013;68:74–79. doi:10.1093/gerona/gls093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–11055. doi:10.1073/pnas.200033797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Iglesias JE, Augustinack JC, Nguyen K, et al. ; Alzheimer’s Disease Neuroimaging Initiative A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: application to adaptive segmentation of in vivo MRI. Neuroimage. 2015;115:117–137. doi:10.1016/j.neuroimage.2015.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Visser PJ, Verhey F, Knol DL, et al. Prevalence and prognostic value of CSF markers of Alzheimer’s disease pathology in patients with subjective cognitive impairment or mild cognitive impairment in the DESCRIPA study: a prospective cohort study. Lancet Neurol. 2009;8:619–627. doi:10.1016/S1474-4422(09)70139-5 [DOI] [PubMed] [Google Scholar]
- 26. Thompson PM, Hayashi KM, De Zubicaray GI, et al. Mapping hippocampal and ventricular change in Alzheimer disease. Neuroimage. 2004;22:1754–1766. doi:10.1016/j.neuroimage.2004.03.040 [DOI] [PubMed] [Google Scholar]
- 27. West MJ, Coleman PD, Flood DG, Troncoso JC. Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer’s disease. Lancet. 1994;344:769–772. doi:10.1016/S0140-6736(94)92338-8 [DOI] [PubMed] [Google Scholar]
- 28. Apostolova LG, Mosconi L, Thompson PM, et al. Subregional hippocampal atrophy predicts Alzheimer’s dementia in the cognitively normal. Neurobiol Aging. 2010;31:1077–1088. doi:10.1016/j.neurobiolaging.2008.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Apostolova LG, Dutton RA, Dinov ID, et al. Conversion of mild cognitive impairment to Alzheimer disease predicted by hippocampal atrophy maps. Arch Neurol. 2006;63:693–699. doi:10.1001/archneur.63.5.693 [DOI] [PubMed] [Google Scholar]
- 30. Spalding KL, Bergmann O, Alkass K, et al. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153:1219–1227. doi:10.1016/j.cell.2013.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sun LY, Bartke A. Adult neurogenesis in the hippocampus of long-lived mice during aging. J Gerontol A Biol Sci Med Sci. 2007;62:117–125. [DOI] [PubMed] [Google Scholar]
- 32. Drew LJ, Fusi S, Hen R. Adult neurogenesis in the mammalian hippocampus: why the dentate gyrus? Learn Mem. 2013;20:710–729. doi:10.1101/lm.026542.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yassa MA, Stark SM, Bakker A, Albert MS, Gallagher M, Stark CE. High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic mild cognitive impairment. Neuroimage. 2010;51:1242–1252. doi:10.1016/j.neuroimage.2010.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Scheuner D, Eckman C, Jensen M, et al. Secreted amyloid beta-protein similar to that in the senile plaques of AD is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat Med. 1996;2:864–870. doi:10.1038/nm0896-864 [DOI] [PubMed] [Google Scholar]
- 35. Schupf N, Patel B, Silverman W, et al. Elevated Aβ1–42 and onset of dementia in adults with Down syndrome. Neurosci Lett. 2001;301:199–203. doi:10.1016/S0304-3940(01)01657-3 [DOI] [PubMed] [Google Scholar]
- 36. Ertekin-Taner N, Younkin LH, Yager DM, et al. Plasma amyloid beta protein is elevated in late-onset Alzheimer disease families. Neurology. 2008;70:596–606. doi:10.1212/01.wnl.0000278386.00035.21 [DOI] [PubMed] [Google Scholar]
- 37. Cosentino SA, Stern Y, Sokolov E, et al. Plasma ß-amyloid and cognitive decline. Arch Neurol. 2010;67:1485–1490. doi:10.1001/archneurol.2010.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Llado-Saz S, Atienza M, Cantero JL. Increased levels of plasma amyloid-beta are related to cortical thinning and cognitive decline in cognitively normal elderly subjects. Neurobiol Aging. 2015;36:2791–2797. doi:10.1001/archneurol.2010.189 [DOI] [PubMed] [Google Scholar]
- 39. Cammarata S, Borghi R, Giliberto L, et al. Aβ42 plasma levels are elevated in amnestic mild cognitive impairment. J Alzheimers Dis. 2009;18:267–271. doi:10.3233/JAD-2009-1144 [DOI] [PubMed] [Google Scholar]
- 40. DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM. Peripheral anti-A beta antibody alters CNS and plasma A beta clearance and decreases brain A beta burden in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2001;98:8850–8855. doi:10.1073/pnas.151261398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Eisele YS, Obermüller U, Heilbronner G, et al. Peripherally applied Abeta-containing inoculates induce cerebral beta-amyloidosis. Science. 2010;330:980–982. doi:10.1126/science.1194516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Marques MA, Kulstad JJ, Savard CE, et al. Peripheral amyloid-beta levels regulate amyloid-beta clearance from the central nervous system. J Alzheimers Dis. 2009;16:325–329. doi:10.3233/JAD-2009-0964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fjell AM, Walhovd KB, Fennema-Notestine C, et al. ; Alzheimer’s Disease Neuroimaging Initiative CSF biomarkers in prediction of cerebral and clinical change in mild cognitive impairment and Alzheimer’s disease. J Neurosci. 2010;30:2088–2101. doi:10.1523/JNEUROSCI.3785-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jack CR Jr, Wiste HJ Vemuri P et al. ; Alzheimer’s Disease Neuroimaging Initiative Brain beta-amyloid measures and magnetic resonance imaging atrophy both predict time-to-progression from mild cognitive impairment to Alzheimer’s disease. Brain. 2010;133:3336–3348. doi:10.1093/brain/ awq277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fjell AM, Walhovd KB, Fennema-Notestine C, et al. ; Alzheimer’s Disease Neuroimaging Initiative Brain atrophy in healthy aging is related to CSF levels of Aβ1-42. Cereb Cortex. 2010;20:2069–2079. doi:10.1093/cercor/bhp279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Villain N, Chételat G, Grassiot B, et al. ; AIBL Research Group Regional dynamics of amyloid-β deposition in healthy elderly, mild cognitive impairment and Alzheimer’s disease: a voxelwise PiB-PET longitudinal study. Brain. 2012;135(Pt 7):2126–2139. doi:10.1093/brain/aws125 [DOI] [PubMed] [Google Scholar]
- 47. Bai F, Xie C, Watson DR, et al. Aberrant hippocampal subregion networks associated with the classifications of aMCI subjects: a longitudinal resting-state study. PLoS One. 2011;6:e29288. doi:10.1371/journal.pone. 0029288 [DOI] [PMC free article] [PubMed] [Google Scholar]