Abstract
Background:
The association between glucose levels and incident frailty in older persons remains unclear. We examined the extent to which higher glucose levels in older adults with and without diabetes are related to risk of frailty.
Methods:
The data are from the Adult Changes in Thought study. We identified 1,848 individuals aged 65+ without dementia for whom glucose levels from laboratory measurements of glucose and glycated hemoglobin were available. Physical frailty using modified Fried’s criteria was determined from biennial assessments. Frailty hazard was modeled as a function of time-varying measures of diabetes and average glucose levels using Cox regression.
Results:
A total of 578 incident frailty cases (94 with diabetes, 484 without) occurred during a median follow-up of 4.8 years. The adjusted hazard ratio for frailty comparing those with and without diabetes was 1.52 (95% confidence interval = 1.19–1.94). In participants without diabetes, modeling suggested elevated frailty risk with greater average glucose levels (p = .019); for example, a glucose level of 110mg/dL compared with 100mg/dL yielded a hazard ratio of 1.32 (95% confidence interval = 1.09–1.59). In participants with diabetes, glucose levels less than 160mg/dL and greater than 180mg/dL were related to increased risk of frailty (p = .001).
Conclusion:
Higher glucose levels may be a risk factor for frailty in older adults without diabetes. The apparent U-shape association between glucose levels and frailty in people with diabetes is consistent with the literature on glycemia and mortality and deserves further examination.
Keywords: Frailty, Diabetes, Glucose levels, Older adults, Prospective
With the aging of the global population physical frailty has become an important concern in public health worldwide (1,2). The most widely used operational definition of frailty includes indicators of muscle loss, body-composition change, and energy-level impairment (2). Frail older adults are at increased risks for falls, disability, and mortality (1–4). The prevalence of frailty increases with age, affecting about a quarter of persons aged 85 and older (5). Mounting evidence suggests that diabetes is associated with increased risk of frailty (4,6–8). However, some evidence also indicates that glucose levels within the higher end of the normal range in persons without diabetes might increase risks for morbidity and mortality (9–11). As glycemia and diabetes are rising nationally (12) and globally (13), it is important to understand the potential consequences of the diabetes epidemics for the incidence of late-life decline and increased vulnerability as characterized by frailty (1,2,14). We evaluated longitudinal clinical data from a prospective cohort with previously published frailty ascertainment (15) to test the hypothesis that diabetes and higher glucose levels are associated with the risk of frailty. Of secondary interest, we additionally examined whether glucose and frailty associations were stronger or weaker depending on the recency of the glucose measures.
Methods
Participants
The Adult Changes in Thought (ACT) study is an ongoing population-based prospective cohort study of incident dementia in older adults. Enrollment of initial participants without dementia began in 1994–1996, with additional participants enrolled between 2000 and 2003, and then continuous enrollment in 2005 to replenish those who developed dementia, died, or withdrew. Study participants aged 65 and older were randomly sampled from the Seattle-area Group Health Cooperative (hereafter Group Health [GH]), a health care system in Washington State. Participants (total N = 4,723 enrolled in ACT as of September 30, 2012) returned at 2-year intervals for clinical examinations and screening for incident cases of dementia. Information collected during these clinical assessments can also be used to ascertain frailty. Because the current analyses were to investigate the association between glucose levels and risk of incident frailty, only participants with at least two biennial visits after the initial ACT enrollment visit were eligible for these analyses; the first biennial follow-up visit was needed to define the weight-loss component of frailty (defined later) and to establish a “baseline” visit at which the participant was known not to have prevalent frailty. We limited the sample to these participants who were known not to be frail at the “baseline” visit and who were free of stroke and Parkinson’s disease at that time (N = 2,598). Further, to ensure adequate glucose ascertainment history taken as part of clinical care at GH, we restricted analyses to those participants who had been enrolled in GH for at least 5 years before baseline and for whom there were at least five measurements of glucose or glycated hemoglobin over the course of 2 or more years before baseline (750 were excluded). The demographic and comorbid characteristics of the 1,848 participants who were eligible for this study after applying inclusion criteria were mostly similar to those for all ACT participants, with the exception of coronary artery disease, and the expected differences with regard to study cohort, age, and frailty criteria (see Supplementary Table S1). The study procedures were approved by the institutional review boards of GH and the University of Washington, and participants provided written informed consent.
Outcome Ascertainment
We defined frailty using a modified version of Fried and colleagues’ (2) operational definition. The components of frailty included weakness (grip strength), slowness (walking speed), low physical activity, weight loss, and self-reported exhaustion. Participants were classified as having each component using thresholds defined by Fried and colleagues (2) whenever possible. Details of the thresholds used are described elsewhere (15). Briefly, weakness was indicated by low grip strength stratified by sex and body mass index (BMI; see Supplementary Table S2; Gray et al. (15)); slowness by walking speed of less than 0.6 m/s in a timed 10-foot walk test; low physical activity by self-reported exercise of fewer than three times per week; exhaustion by self-reported positive response to at least one of two questions from the Center for Epidemiological Studies Depression (CESD) scale (“I felt that everything I did was an effort”; “ I could not get going”); and, finally, weight loss as a loss of more than 7.5% of body weight since the previous ACT visit (over 2 years). Participants were classified as frail if they had three or more positive components, prefrail if one or two were present, and not frail if none were present. The date of onset of incident frailty for each participant was assigned as the midpoint between the ACT study visit that triggered frailty classification and the preceding study visit, per convention used for other outcomes in ACT studies.
Diabetes and Glucose Levels
We defined participants as having treated diabetes if they filled at least two prescriptions for diabetes-related medications within a year using automated GH pharmacy dispensing data as previously published (see Supplementary Table S3; Crane et al. (9)). The onset date of treated diabetes was defined as the date of the second fill. Measures of fasting or random glucose and glycated hemoglobin (HbA1c or total glycated hemoglobin) were ascertained from computerized laboratory databases, which capture measurements taken as part of participants’ clinical care at GH.
Other Covariates
Demographic characteristics and measured or self-reported health information were collected at the ACT enrollment visit and at each biennial follow-up visit. Information included age, sex, self-reported race, level of education, depression level, smoking status, self-rated health, BMI, cognitive functioning, and a history of congestive heart failure, coronary artery disease, or chronic obstructive pulmonary disease. Depression level was measured using the CESD scale (16), but with the two exhaustion components (used for the frailty outcome) removed. Cognitive functioning was measured using the Cognitive Abilities Screening Instrument (CASI (17)). BMI was calculated using measured weight and height.
Statistical Analysis
We combined glucose and glycated hemoglobin measures using a hierarchical Bayesian framework, as in a prior ACT study (9), to compute a time-varying estimate of each study participant’s average glucose level over a 5-year rolling window beginning during the 5 years prior to baseline and continuing until each participant’s end of follow-up. We then modeled the associations between diabetes, average glucose levels, and the cause-specific hazards for frailty using Cox regression with age as the time scale. Participants were followed from baseline until either frailty onset or a censoring event whichever occurred first. Censoring events included GH disenrollment, dementia onset, death, or a participant’s last ACT biennial study visit before September 30, 2012. Some of these censoring events act as competing risks; the Cox proportional hazards model provides unbiased estimates of cause-specific hazard ratios (HRs) in the presence of competing risks (18). We used separate Cox models for the analysis of diabetes and frailty and the analysis of average glucose and frailty. For the former, the primary exposure was modeled using a time-varying binary indicator for ever (treated) diabetes versus no diabetes. For the latter, the baseline hazards were stratified by diabetes status, and then we used natural cubic splines (19) to allow for estimation of a smooth, nonlinear association between average glucose levels in the preceding 5 years and the frailty hazard. We estimated separate spline parameters for those with and without diabetes to allow for potentially different relationships in those groups.
We adjusted regression models for ACT study cohort, age, and CASI at baseline; sex, education, and self-reported race; and time-varying measures of coronary artery disease, congestive heart failure, chronic obstructive pulmonary disease, stroke, smoking, depression, self-rated health, and BMI. To account for missing values of these adjustment covariates or the individual components of the frailty outcome, we used multiple imputation via chained equations (20) to generate five imputed data sets with missing values filled in. We then performed the Cox regression analyses on each of the imputed data sets, with estimates pooled according to Rubin’s rules (21) to generate final results reported here.
To explore the secondary question of whether glucose and frailty associations were stronger or weaker depending on the recency of the glucose measures, we repeated all glucose analyses for average glucose levels in the preceding 5–10 years (rather than the preceding 5 years). We also tested for interactions between sex and baseline age and our exposure measures. We additionally adjusted our regression models for baseline prefrailty. Because weight loss constituted a component of the frailty outcome, we also evaluated models in which we did not adjust for BMI or in which we only adjusted for baseline BMI. Finally, we performed assessments of model diagnostics and influential observations using scaled Schoenfeld residuals and standardized delta-beta plots of the exposure parameters. Statistical analyses, summarization, and management were performed using SAS software, version 9.2 (SAS Institute, Inc., Cary, NC); Stata 12.1 (Stat Corp., College Station, TX); and R, version 3.1.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline Characteristics
Baseline characteristics of the 1,848 study participants are presented in Table 1. Of these, 200 had diabetes and 1,648 had no diabetes initially; their median age at study baseline was 76 years (interquartile range [IQR] = 72–81). Glucose measures from laboratory records included 32,586 clinical measurements of random or fasting glucose and 8,662 measurements of glycated hemoglobin. During the 5 years preceding the baseline visit, the median glucose level for participants without diabetes was 100mg/dL (IQR = 96–107; estimated HbA1c = 5.1%; IQR = 5.0–5.4), and the median glucose level for participants with diabetes was 168mg/dL (IQR = 153–187; estimated HbA1c = 7.5%; IQR = 7.0–8.1). The distribution of participants’ average glucose levels during the 5 years prior to beginning and end of study follow-up according to diabetes status, along with the number of incident frailty events within groups defined by glucose level, are summarized in Supplementary Table S4.
Table 1.
Participant Characteristics at Beginning of Study Follow-Up (Baseline)
| Characteristic | Without Diabetes, n = 1,648 | With Diabetes, n = 200 | ||
|---|---|---|---|---|
| n | %* | n | %* | |
| Average glucose level in 5 years prior to baseline | ||||
| Measured in mg/dL, median (25th, 75th) | 100 (96, 107) | 168 (153, 187) | ||
| Measured as HbA1c, median (25th, 75th) | 5.1 (5.0, 5.4) | 7.5 (7.0, 8.1) | ||
| Age, median (25th, 75th) | 76 (72, 81) | 75 (72, 80) | ||
| Cohort | ||||
| Original | 1,104 | 67.0 | 123 | 61.5 |
| Expansion | 313 | 19.0 | 45 | 22.5 |
| Replacement | 231 | 14.0 | 32 | 16.0 |
| Female | 966 | 58.6 | 104 | 52.0 |
| Nonwhite | 118 | 7.2 | 28 | 14.1 |
| Missing | 2 | 0.1 | 1 | 0.5 |
| At least some college | 1,081 | 65.6 | 116 | 58.0 |
| Congestive heart failure | 79 | 4.8 | 14 | 7.2 |
| Missing | 5 | 0.3 | 5 | 2.5 |
| Coronary artery disease | 405 | 24.7 | 68 | 34.2 |
| Missing | 7 | 0.4 | 1 | 0.5 |
| Body mass index | ||||
| Underweight | 18 | 1.1 | 1 | 0.5 |
| Normal | 578 | 35.1 | 28 | 14.0 |
| Overweight | 683 | 41.4 | 91 | 45.5 |
| Obese | 369 | 22.4 | 80 | 40.0 |
| Chronic obstructive pulmonary disease | 204 | 12.4 | 29 | 14.6 |
| Missing | 6 | 0.4 | 1 | 0.5 |
| Smoking status | ||||
| Never | 791 | 48.1 | 87 | 43.9 |
| Former | 781 | 47.5 | 109 | 55.1 |
| Current | 71 | 4.3 | 2 | 1.0 |
| Missing | 5 | 0.3 | 2 | 1.0 |
| Self-rated health | ||||
| Excellent or very good | 827 | 50.2 | 76 | 38.0 |
| Good | 626 | 38.0 | 78 | 39.0 |
| Fair or poor | 194 | 11.8 | 46 | 23.0 |
| Missing | 1 | 0.1 | 0 | 0.0 |
| CASI, median (25th, 75th) | 95 (92, 97) | 94 (91, 96) | ||
| Missing or invalid | 14 | 0.8 | 3 | 1.5 |
| CESD score for depression, median (25th, 75th) | 2 (0, 4) | 2 (1, 4) | ||
| Missing | 3 | 0.2 | 0 | 0.0 |
| Prefrail (1–2 frailly components) | 967 | 58.7 | 140 | 70.0 |
| Frail according to each component | ||||
| Grip strength | 468 | 28.4 | 62 | 31.0 |
| Walking speed | 82 | 5.0 | 13 | 6.5 |
| Physical activity | 443 | 26.9 | 68 | 34.0 |
| Weight loss | 90 | 5.5 | 18 | 9.0 |
| Exhaustion | 187 | 11.3 | 39 | 19.5 |
Notes: CASI = Cognitive Abilities Screening Instrument; CESD = Center for Epidemiological Studies Depression scale.
*Column percentages based on nonmissing data. In analyses, missing data are filled in via multiple imputation.
Diabetes and Frailty
Across imputed data sets, we observed an average of 578 incident frailty events during a median follow-up of 4.8 years including 484 cases in 1,595 participants (30.4%) without diabetes at the end of follow-up, and 94 cases in 253 older adults (37.2%) with diabetes at the end of follow-up. In adjusted Cox proportional hazard models, the HR for incident frailty comparing those with and without diabetes was 1.52 (95% confidence interval [CI] = 1.19–1.94).
Glucose Levels and Frailty
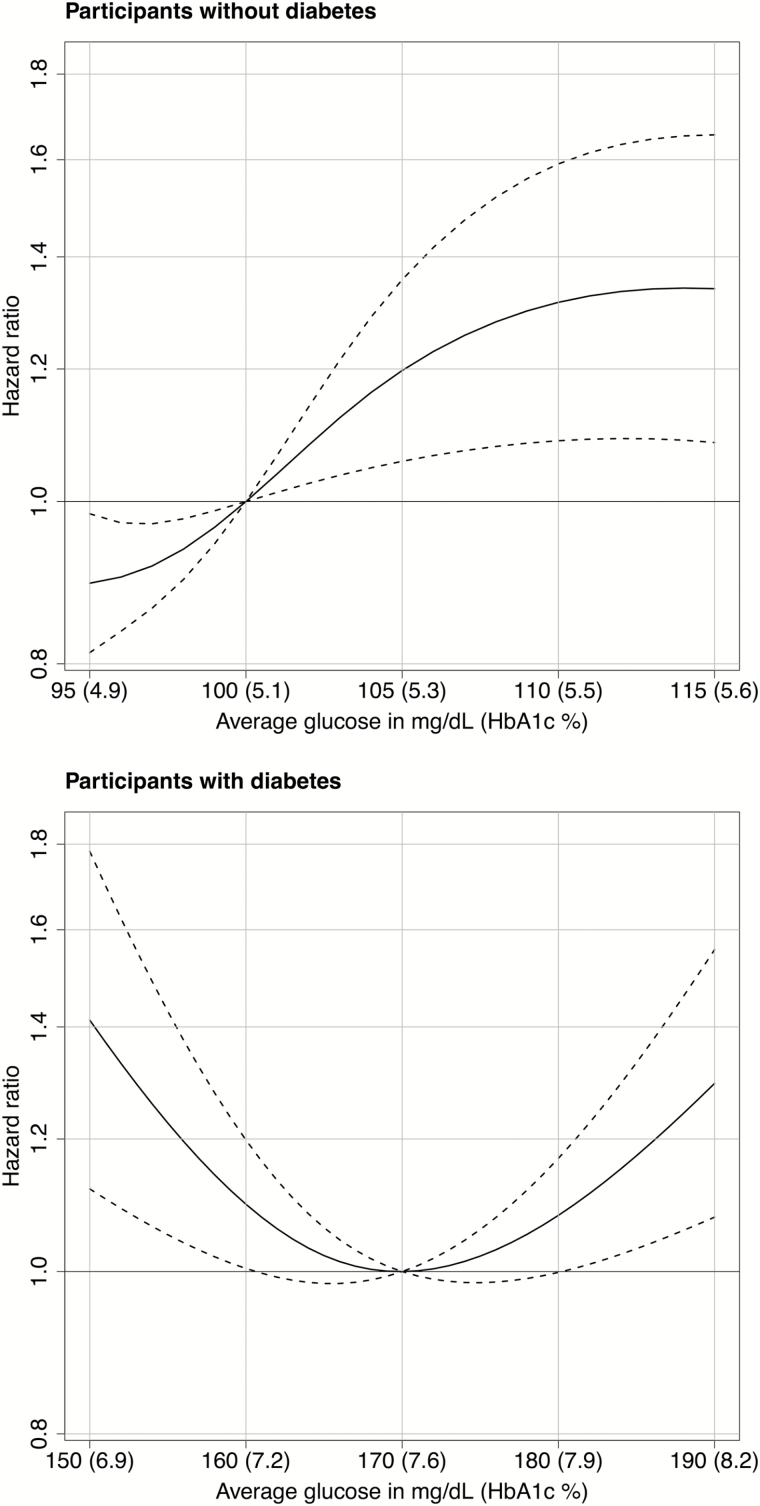
Estimated associations between average glucose levels in the preceding 5 years and development of frailty are shown in Table 2 and Figure 1. The solid curve on the graphs illustrates the estimated HR for incident frailty when comparing different levels of average glucose relative to a reference level. The references for the figures were selected to approximate the median average glucose level in those with and without diabetes at baseline. For those without diabetes, the reference is 100mg/dL (HbA1c = 5.1%); for those with diabetes, the reference is 170mg/dL (HbA1c = 7.6%). Dotted curves are the 95% CIs for the HR estimates. The table lists a few selected estimates from those graphs for purposes of clarification and interpretation. In participants without diabetes, modeling suggested an elevated frailty risk with greater average glucose levels (P = 0.019); for example, a glucose level of 110mg/dL compared with 100mg/dL yielded an HR of 1.32 (95% CI = 1.09–1.59). In participants with diabetes, average glucose levels of less than 160mg/dL and greater than 180mg/dL were associated with increased risk of frailty relative to levels around 170mg/dL (p = .001), such that an average glucose level of 150 and 190mg/dL yielded incident frailty HRs of 1.41 (95% CI = 1.12–1.78) and 1.30 (95% CI = 1.08–1.56), respectively, compared with an average level of 170mg/dL.
Table 2.
Cause-Specific HR for Frailty Associated With Diabetes and Average Glucose Levels Over the Preceding 5 Years
| Characteristic | Frailty | |
|---|---|---|
| HR 95% CI | p Value | |
| With diabetes vs without diabetes* | 1.52 (1.19, 1.94) | .001 |
| Glucose levels in those without diabetes† | ||
| 95mg/dL (HbA1c = 4.9) | 0.89 (0.81, 0.98) | .019 |
| 100mg/dL (HbA1c = 5.1) | 1.00 (REF) | |
| 105mg/dL (HbA1c = 5.3) | 1.20 (1.06, 1.36) | |
| 110mg/dL (HbA1c = 5.5) | 1.32 (1.09, 1.59) | |
| 115mg/dL (HbA1c = 5.6) | 1.34 (1.08, 1.66) | |
| Glucose levels in those with diabetes† | ||
| 150mg/dL (HbA1c = 6.9) | 1.41 (1.12, 1.78) | .001 |
| 160mg/dL (HbA1c = 7.2) | 1.10 (1.00, 1.20) | |
| 170mg/dL (HbA1c = 7.6) | 1.00 (REF) | |
| 180mg/dL (HbA1c = 7.9) | 1.08 (1.00, 1.17) | |
| 190mg/dL (HbA1c = 8.2) | 1.30 (1.08, 1.56) | |
Notes: CI = confidence interval; HR = hazard ratio; REF = reference category.
*Adjusted for Adult Changes in Thought study cohort, age (at baseline and via the time axis), Cognitive Abilities Screening Instrument score at baseline, gender, education, race/ethnicity, and time-varying measures of stroke, coronary artery disease, congestive heart failure, chronic obstructive pulmonary disease, smoking, Center for Epidemiological Studies Depression score, self-rated health, and body mass index.
†Stratified the baseline hazard by diabetes status and modeled glucose levels using splines.
Figure 1.
Risk of incident frailty associated with the average glucose level or hemoglobin A1c (HbA1c) level during the preceding 5 years according to the presence or absence of diabetes. Solid curves represent estimates of the hazard ratios for the risk of incident frailty when comparing different average glucose levels relative to a reference level of 100mg/dL (HbA1c = 5.1%) for participants without diabetes (upper panel) and 170mg/dL (HbA1c = 7.6%) for participants with diabetes (lower panel). The dashed lines represent pointwise 95% confidence intervals.
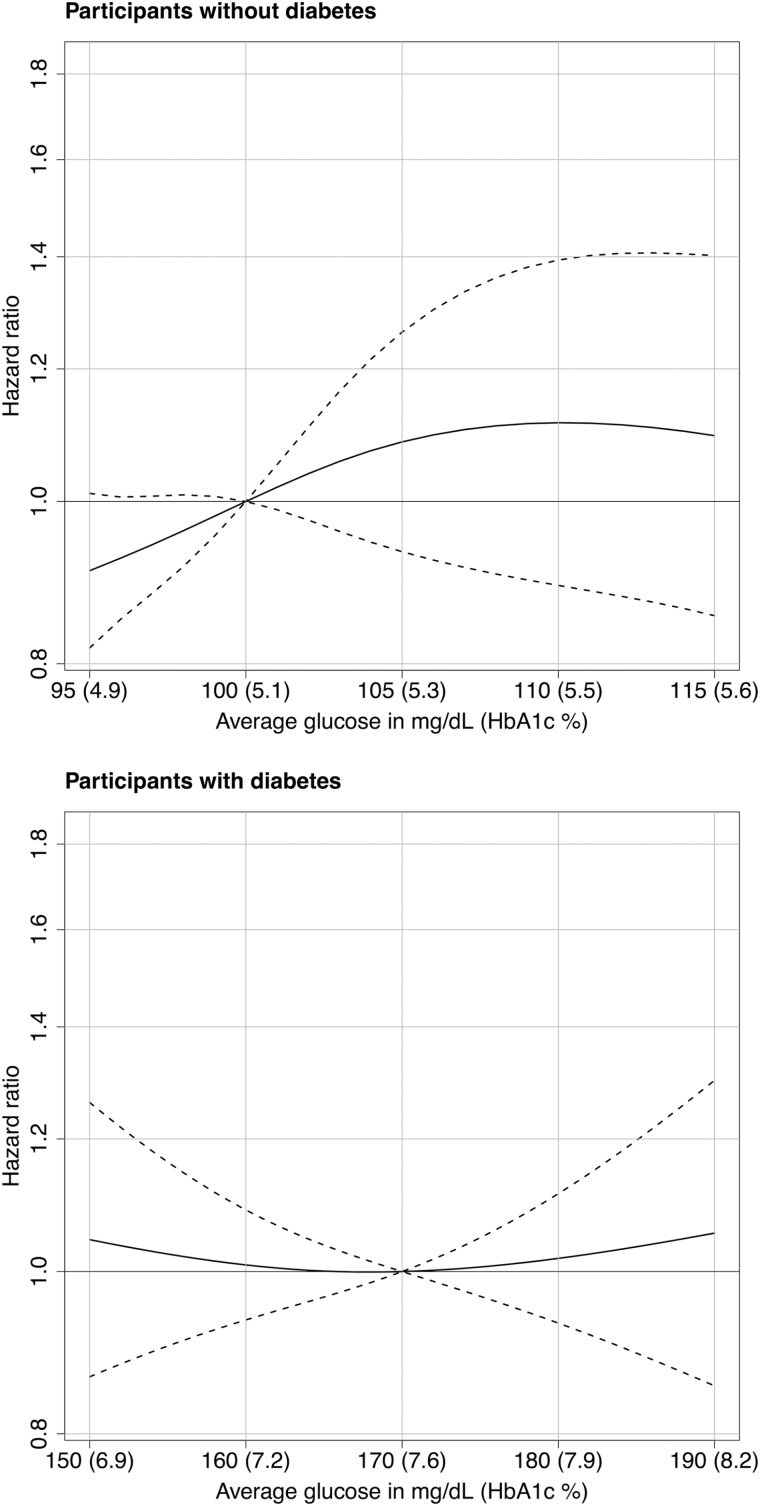
Figure 2 shows the results of analyses of the risk of frailty associated with glucose levels averaged over the preceding 5–10 years (i.e., distal exposure) rather than over the preceding 0–5 years (i.e., proximal exposure). Unlike with proximal measures, we found no evidence of an association between the frailty hazard and glucose levels in the prior 5–10 years (see Supplementary Table S5).
Figure 2.
Risk of incident frailty associated with the average glucose level or hemoglobin A1c (HbA1c) level during the preceding 5–10 years according to the presence or absence of diabetes. Solid curves represent estimates of the hazard ratios for the risk of incident frailty when comparing different average glucose levels relative to a reference level of 100mg/dL (HbA1c = 5.1%) for participants without diabetes (upper panel) and 170mg/dL (HbA1c = 7.6%) for participants with diabetes. The dashed lines represent pointwise 95% confidence intervals.
Sensitivity Analyses
We did not find significant interactions between age or sex and diabetes or glucose levels in those with or without diabetes (p > .05 for all). Our additional adjustment for baseline prefrailty, baseline BMI, and excluding BMI showed no major differences between primary and sensitivity analysis results (see Supplementary Table S6). Delta-beta investigations did not produce any concerns that our analysis estimates were unduly influenced by a few participants.
Discussion
In this prospective community-based cohort study, we confirmed that diabetes is a frailty risk factor. We also found that higher glucose levels, based on a recent 5-year average, were associated with an increased risk of frailty in older adults free of diabetes. This association between glucose levels and incident frailty remained after adjustment for potential confounders, with risk estimated to increase (nonlinearly) across the glucose levels of 95–115mg/dL, suggesting that elevated glucose levels even in ranges far lower than those seen with diabetes may be associated with risk for the onset of frailty. In older adults with diabetes, we observed a U-shape relationship such that the lowest and highest glucose levels were associated with increased risk of frailty, with the lowest hazards at glucose levels of about 170mg/dL. We also found that proximal rather than distal glucose levels were associated with risk of incident frailty. The findings were consistent across a variety of sensitivity analyses.
Most studies that have investigated the association between glucose metabolism and the risk of frailty have focused on diabetes itself (4), measured levels of glycated hemoglobin (7), or measured insulin resistance (22). These studies have shown that diabetes and elevated glucose levels are associated with increased risk of frailty, but their use of single time-point measures of glucose is a limitation. Most of them categorized glucose exposures. The single time-point exposure approach does not enable longitudinal evolution of glucose levels over time, and categorizing glucose exposure could preclude insights such as the U-shape relationship we found in people with diabetes. To our knowledge, no prior study has evaluated risk of frailty in the context of a time-varying and continuous glucose exposure.
We used a hierarchical Bayesian model to develop a time-varying estimate of each participant’s glucose levels as used in a prior study (9). This approach enabled us to incorporate clinically obtained measurements of random and fasting blood glucose and glycated hemoglobin in a single composite estimate of average glucose level. We found evidence of a monotonically increasing relationship between higher glucose levels and higher frailty risk in people without diabetes. We also found that in older adults with diabetes in our population, glucose levels lower than 160mg/dL and higher than 180mg/dL were associated with higher risk of frailty. These latter results among the group with diabetes suggest the possibility that in addition to the biologically plausible relationship of high glucose levels being associated with increased risk of frailty, “too tight” glycemic control that results in low glucose levels, at least relative to level that may be typical for persons with diabetes, could also be tied to greater frailty risk.
Diabetes and higher glucose levels may contribute to an increased risk of frailty through several potential mechanisms, including chronic inflammation (23), chronic hyperglycemia that increases microvascular damage (24), and skeletal muscle mitochondrial dysfunction (25). The associations between glucose control and frailty in people with diabetes appear to be similar to those described in the studies that investigated glycemic indexes and mortality (26,27). Elevated risks of frailty with relatively lower glucose levels in people with diabetes might represent glucose variability that contributes to endothelial damage (28) or may be due to other factors associated with relatively lower glucose levels. The finding of an association with proximal rather than distal glucose levels could suggest that the development of frailty is actually driving the disturbed glucose dynamics and, in particular, the U-shape relationship observed in those with diabetes. Perhaps as individuals become more frail, diabetes management becomes altered in important ways. For example, increased frailty and its accompanying reduction in ability to self-manage medications and eating regimens might lead to increased risk of hypoglycemia. Conversely, fear of the potential for hypoglycemia in such individuals might lead to looser or more permissive glucose control and thus more hyperglycemia. These assertions, however, should be further elucidated by conducting randomized controlled trials of diabetes treatments in older population. Animal models that approximate human frailty might be another exciting venue to investigate the link between glycemia and frailty. The identification of animal models suitable for frailty research is under development and should greatly facilitate the identification of etiological underpinning of physical vulnerability in humans (29).
The strengths of this study include the prospective community-based design, the large sample with relatively low attrition, access to extended clinical laboratory and pharmacy data, use of a previously published model for glucose exposures, prospective ascertainment of cases of frailty, and extensive sensitivity analyses. We also acknowledge some limitations of our study. As with any observational study, there exists the possibility of residual and unmeasured confounding. Given the ethnic makeup of our study population, our results may not be widely generalizable. Many of our covariates were obtained by self-report. Further, while we based our glucose exposure on numerous measurements of glucose and glycated hemoglobin, with an average of 18 measurements of blood glucose and 5 measurements of glycated hemoglobin available per person, these clinical laboratory measurements were obtained at irregular intervals. Next, although glycated hemoglobin measures might be somewhat inaccurate in anemic states, and we did not control for hemoglobin levels, such a bias would only impact a small proportion of HbA1c values and would have no bearing on glucose measures, which, in turn, constituted almost 80% of the lab measures used in our analyses. Our sensitive analysis also revealed no differences in HbA1c values between those who had hematocrit less than 35 versus normal hematocrit group. Our operationalization of diabetes might raise a concern that some people treated for prediabetes with oral medications such as metformin were also included. This concern is not valid in GH setting due to its strict formulary control. We reviewed dozens of medical records of ACT participants who were treated with medications for diabetes and uniformly glucose levels had been documented to have been high (and to meet the hyperglycemia classification) for some time (typically months but sometimes longer) before initiating medications. Finally, because many participants likely had diabetes for many years before they were treated with antidiabetes medications, for our analysis, this could result in some of the higher glucose values observed in participants classified as not having diabetes reflecting in reality untreated diabetes. Still, in those participants classified as not having diabetes, the monotonically increasing relationship between higher glucose levels and frailty risk is unlikely to have been entirely driven by untreated diabetes
Conclusion
In conclusion, our analyses provide the first evidence to our knowledge that higher average glucose levels even within the higher end of the normal range may be associated with increased risk of frailty in older adults without diabetes. Further, the apparent U-shape association between average glucose levels and frailty in people with diabetes is consistent with the literature on glycemia and mortality and supports the need for studies of optimal control levels in older persons with diabetes.
Ethics
The study procedures were approved by the institutional review boards of GH and the University of Washington, and participants provided written informed consent.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
This work was supported by National Institutes of Health Grants U01 AG006781 (PI: E.B.L.).
Conflict of Interest
O.Z., P.K.C., S.L.G., and E.B.L. declared no conflict of interests. R.L.W. has received funding as a biostatistician from an unrelated research grant awarded to Group Health Research Institute from Pfizer.
Supplementary Material
References
- 1. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. doi:S0140-6736(12)62167-9 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 3. Gill TM, Gahbauer EA, Han L, Allore HG. Trajectories of disability in the last year of life. N Engl J Med. 2010;362:1173–1180. doi:10.1056/NEJMoa0909087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Woods NF, LaCroix AZ, Gray SL, et al. ; Women’s Health Initiative Frailty: emergence and consequences in women aged 65 and older in the Women’s Health Initiative Observational Study. J Am Geriatr Soc. 2005;53:1321–1330. doi:10.1111/j.1532-5415.2005.53405.x [DOI] [PubMed] [Google Scholar]
- 5. Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60:1487–1492. doi:10.1111/j.1532-5415.2012.04054.x [DOI] [PubMed] [Google Scholar]
- 6. Blaum CS, Xue QL, Tian J, Semba RD, Fried LP, Walston J. Is hyperglycemia associated with frailty status in older women? J Am Geriatr Soc. 2009;57:840–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kalyani RR, Tian J, Xue QL, et al. Hyperglycemia and incidence of frailty and lower extremity mobility limitations in older women. J Am Geriatr Soc. 2012;60:1701–1707. doi:10.1111/j.1532-5415.2012.04099.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stenholm S, Strandberg TE, Pitkälä K, Sainio P, Heliövaara M, Koskinen S. Midlife obesity and risk of frailty in old age during a 22-year follow-up in men and women: the Mini-Finland Follow-up Survey. J Gerontol A Biol Sci Med Sci. 2014;69:73–78. doi:10.1093/gerona/glt052 [DOI] [PubMed] [Google Scholar]
- 9. Crane PK, Walker R, Hubbard RA, et al. Glucose levels and risk of dementia. N Engl J Med. 2013;369:540–548. doi:10.1056/NEJMoa1215740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jee SH, Ohrr H, Sull JW, Yun JE, Ji M, Samet JM. Fasting serum glucose level and cancer risk in Korean men and women. JAMA. 2005;293:194–202. [DOI] [PubMed] [Google Scholar]
- 11. Selvin E, Steffes MW, Zhu H, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med. 2010;362:800–811. doi:10.1056/NEJMoa0908359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Selvin E, Parrinello CM, Sacks DB, Coresh J. Trends in prevalence and control of diabetes in the United States, 1988-1994 and 1999-2010. Ann Intern Med. 2014;160:517–525. doi:10.7326/M13-2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Danaei G, Finucane MM, Lu Y, et al. ; Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Glucose) National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet. 2011;378:31–40. doi:10.1016/S0140-6736(11)60679-X [DOI] [PubMed] [Google Scholar]
- 14. Arterburn DE, Crane PK, Sullivan SD. The coming epidemic of obesity in elderly Americans. J Am Geriatr Soc. 2004;52:1907–1912. [DOI] [PubMed] [Google Scholar]
- 15. Gray SL, Anderson ML, Hubbard RA, et al. Frailty and incident dementia. J Gerontol A Biol Sci Med Sci. 2013;68:1083–1090. doi:10.1093/gerona/glt013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). Am J Prev Med. 1994;10:77–84.8037935 [Google Scholar]
- 17. Teng EL, Hasegawa K, Homma A, et al. The Cognitive Abilities Screening Instrument (CASI): a practical test for cross-cultural epidemiological studies of dementia. Int Psychogeriatr. 1994;6:45–58; discussion 62. [DOI] [PubMed] [Google Scholar]
- 18. Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York, NY: Wiley; 2002. [Google Scholar]
- 19. Harrell FE. Regression Modeling Strategies. New York, NY: Springer; 2001. [Google Scholar]
- 20. van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16:219–242. [DOI] [PubMed] [Google Scholar]
- 21. Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: Wiley; 1987. [Google Scholar]
- 22. Barzilay JI, Blaum C, Moore T, et al. Insulin resistance and inflammation as precursors of frailty: the Cardiovascular Health Study. Arch Intern Med. 2007;167:635–641. [DOI] [PubMed] [Google Scholar]
- 23. Ferrucci L, Penninx BW, Volpato S, et al. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc. 2002;50:1947–1954. [DOI] [PubMed] [Google Scholar]
- 24. Sheetz MJ, King GL. Molecular understanding of hyperglycemia’s adverse effects for diabetic complications. JAMA. 2002;288:2579–2588. [DOI] [PubMed] [Google Scholar]
- 25. Phielix E, Schrauwen-Hinderling VB, Mensink M, et al. Lower intrinsic ADP-stimulated mitochondrial respiration underlies in vivo mitochondrial dysfunction in muscle of male type 2 diabetic patients. Diabetes. 2008;57:2943–2949. doi:10.2337/db08-0391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Currie CJ, Peters JR, Tynan A, et al. Survival as a function of HbA(1c) in people with type 2 diabetes: a retrospective cohort study. Lancet. 2010;375:481–489. doi:10.1016/S0140-6736(09)61969-3 [DOI] [PubMed] [Google Scholar]
- 27. Huang ES, Liu JY, Moffet HH, John PM, Karter AJ. Glycemic control, complications, and death in older diabetic patients: the diabetes and aging study. Diabetes Care. 2011;34:1329–1336. doi:10.2337/dc10-2377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rogers SC, Zhang X, Azhar G, Luo S, Wei JY. Exposure to high or low glucose levels accelerates the appearance of markers of endothelial cell senescence and induces dysregulation of nitric oxide synthase. J Gerontol A Biol Sci Med Sci. 2013;68:1469–1481. doi:10.1093/gerona/glt033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Walston J, Hadley EC, Ferrucci L, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54:991–1001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.