Abstract
Background. Few studies have characterized the role of sex on the incidence of invasive pneumococcal disease (IPD). We examined sex differences in rates of IPD, and trends after the introduction of pneumococcal conjugate vaccines (PCVs).
Methods. We used active population and laboratory-based IPD surveillance data from the Centers for Disease Control and Prevention Active Bacterial Core surveillance program (1998–2013) in Tennessee. Population-based rates of IPD by sex, race, age group, and PCV era were calculated. Rates were compared using incidence rate ratios.
Results. Throughout the study years, rates of IPD were higher in male than in female subjects, particularly in children <2 years and adults 40–64 years of age, with male subjects having IPD rates 1.5–2 times higher than female subjects. The proportions of comorbid conditions were similar in male and female subjects . Sex rate differences persisted after stratification by race. Although the introductions of 7-valent PCV (PCV7) and 13-valent PCV (PCV13) were associated with declines in IPD rates in both sexes, rates of IPD after PCV13 were still significantly higher in male than in female subjects among children and adults 40–64 and >74 years of age.
Conclusions. Rates of IPD were generally higher in male than in female subjects. These sex differences were observed in different race groups and persisted after introduction of both PCVs.
Keywords: streptococcus pneumoniae, sex disparities, pneumococcal conjugate vaccine
Although commonly overlooked, sex has been reported as a factor influencing the susceptibility to certain infections, including malaria, human immunodeficiency virus (HIV), parasitic diseases, and influenza [1]. These sex differences are thought to be due to biological and behavioral differences between men and women, but the precise mechanisms are poorly understood. Furthermore, the effects of sex on infectious disease susceptibility seem to vary with age [2, 3]. For example, previous reports suggest that male subjects tend to have an increased susceptibility to infectious diseases during childhood, whereas female subjects are at greater risk of infection during pregnancy [2].
Invasive pneumococcal disease (IPD) is a significant cause of disease and death, particularly in young children and older adults [4]. Age, race, and the presence of comorbid conditions are well-recognized risk factors for IPD; with the very young and elderly, black individuals, and those with comorbid conditions having higher IPD rates than those without these characteristics [5, 6]. A few early studies have suggested that rates of IPD may be higher in male than in female subjects, but this has not been fully characterized [7–10]. Immunologic and environmental factors that contribute to IPD risk may also vary by sex and age. For instance, exposure to colonized or vaccinated children may vary by age group, race, and sex [11].
In 2000, the 7-valent pneumococcal conjugate vaccine (PCV7) was introduced in the routine US childhood immunization schedule [12]. PCV7 led to a substantial decrease in overall IPD and pneumonia rates in both vaccinated children and unvaccinated groups [13–15], as well as a decline in racial disparities in pneumococcal diseases [6, 16, 17]. Owing to subsequent increases in residual IPD caused by serotypes not covered by PCV7 [18], a 13-valent pneumococcal conjugate vaccine (PCV13) replaced PCV7 in 2010 [12, 19]. Studies in recent years have demonstrated a further decrease in IPD and pneumonia rates and racial disparities in these rates after PCV13 introduction [20, 21]. However, studies describing sex differences in IPD were conducted before the introduction of the PCV7 and PCV13 [7, 8, 10], and the impact of these vaccines on potential sex differences in IPD rates remains unknown.
Determining which groups remain at increased risk of IPD after widespread vaccination with PCV13 is important to enable effective targeting of new research and prevention strategies. Using Centers for Disease Control and Prevention (CDC) Active Bacterial Core surveillance (ABCs) [22] data for Tennessee, we sought to characterize sex differences in IPD rates and to determine the impact of PCV7 and PCV13 introduction on this disparity.
METHODS
Study Population
The ABCs conducts active population and laboratory-based surveillance of selected infections, including IPD, across multiple sites in the country. This study included data from the Tennessee ABCs site and the population maintained under surveillance from 1998 through 2013. During this period, >2.5 million persons (39% of the Tennessee population) represented the surveillance population, with 48% male, 52% female, 72% white, 25% black, and 3% other race.
Active Surveillance
We defined subjects of all ages, residing in ABCs surveillance catchment areas as having IPD if Streptococcus pneumoniae was isolated by culture from a sterile site (eg, blood, cerebrospinal fluid, or pleural fluid) [23]. Pneumococcal isolates were recovered and subsequently serotyped by Quellung reaction at the University of Texas Health Science Center at San Antonio or the CDC pneumococcal laboratory [23]. Surveillance officers were trained to systematically collect and abstract clinical information and laboratory data from the medical records of patients with IPD. We classified IPD by the following clinical syndromes: isolated bacteremia, meningitis, bacteremic pneumonia, or other IPD, (eg, septic arthritis, osteomyelitis, or endocarditis). The Vanderbilt University Institutional Review Board reviewed and approved the study.
Comorbid conditions were also identified by systematic chart review. We considered patients to have heart disease if they had heart failure, congenital heart disease, or atherosclerotic cardiovascular disease (including myocardial infarction and congenital heart disease), but high cholesterol and hypertension were excluded. Patients with immunodeficiency included those who had an immunoglobulin deficiency, were receiving chemotherapy, or had received an organ transplant. Other comorbid conditions included chronic lung disease, HIV/AIDS, solid tumor (excluding skin cancer), dialysis or chronic renal failure, sickle cell disease, and hematologic malignancy.
Statistical Analysis
We calculated annual IPD rates per 100 000 persons and stratified rates by age group (<2, 2–17, 18–39, 40–64, 65–74, and ≥75 years) using annual population estimates from the National Center for Health Statistics as denominators. We compared IPD rates by sex, stratified by race, because IPD rates have been historically higher among blacks relative to whites [24]. Thirteen persons (<1%) did not have a sex recorded and were excluded from the analysis. Race groups included blacks and whites; 318 individuals (<5%) had missing race data or belonged to other race groups and were excluded from analyses stratified by race. Furthermore, we grouped annualized IPD rates into 4 different vaccine eras: pre-PCV7 (1998–1999), early PCV7 (2001–2004), late PCV7 (2005–2009), and post-PCV13 (2011–2013). The period after PCV7 introduction was subdivided into 2 vaccine eras because IPD rates declined rapidly early after PCV7 introduction (2001–2004), whereas the decline slowed down during 2005–2009 owing in part to documented serotype replacement. Because PCV7 and PCV13 were introduced in 2000 and 2010, respectively, these transition years were excluded from the analyses.
We calculated incidence rate ratios (IRRs) and their 95% confidence intervals (CIs) to compare rates. We calculated the 95% CI as described elsewhere [25]. IRRs were considered to indicate significantly different rates if the 95% CI excluded 1. All analyses were conducted using Stata software (version 13.0; StataCorp) [26].
RESULTS
Characteristics of IPD Cases
From 1998 through 2013, we identified 8383 persons with IPD from the Tennessee ABCs surveillance system. The highest frequency occurred in adults 40–64 or ≥75 years of age (39% and 17%, respectively). Most presented with bacteremic pneumonia (66%). The most common comorbid conditions identified were chronic lung disease (19%) and history of smoking (20%), which were mainly present among adult age groups. Collectively, nearly half of all persons with IPD (49%) had ≥1 comorbid condition.
Of all persons with IPD, 53% were male. More male than female subjects were <65 years of age (76% vs 64%). Both sexes had similar distributions of IPD clinical syndromes. However, a greater proportion of male subjects had a history of smoking (22% vs 19% for female subjects) or HIV/AIDS (8% vs 5%). Female subjects were more likely than male subjects to have chronic lung disease (22% vs 17%) and chronic heart disease (10% vs 7%). The proportions of patients with ≥1 comorbid condition were similar in both sexes (Table 1 and Supplementary Table 1).
Table 1.
Demographic Characteristics of Patients With IPD in ABCs Data for Tennessee, 1998–2013
| Characteristic | Persons With IPD, No. (%)a |
||
|---|---|---|---|
| All (n = 8383) | Male (n = 4416) | Female (n = 3967) | |
| Age, mean (IQR), y | 48 (32–69) | 45 (28–64) | 51 (36–73) |
| Race | |||
| Black | 2945 (35) | 1599 (36) | 1346 (34) |
| White | 5121 (61) | 2638 (60) | 2483 (63) |
| Other/unknown | 317 (4) | 179 (4) | 138 (3) |
| Age group, y | |||
| <2 | 969 (12) | 579 (13) | 390 (10) |
| 2–17 | 653 (8) | 383 (9) | 270 (7) |
| 18–39 | 1053 (13) | 581 (13) | 472 (12) |
| 40–64 | 3229 (39) | 1809 (41) | 1420 (36) |
| 65–74 | 1029 (12) | 499 (11) | 530 (13) |
| ≥75 | 1450 (17) | 565 (13) | 885 (22) |
| Clinical presentationb | |||
| Isolated bacteremia | 1562 (19) | 847 (19) | 715 (18) |
| Bacteremic pneumonia | 5563 (66) | 2901 (66) | 2662 (67) |
| Meningitis | 491 (6) | 260 (6) | 231 (6) |
| Other | 1199 (14) | 639 (14) | 560 (14) |
| Comorbid conditions | |||
| Chronic lung disease | 1626 (19) | 764 (17) | 862 (22) |
| Cancer | 430 (5) | 245 (6) | 185 (5) |
| Chronic heart disease | 711 (8) | 306 (7) | 405 (10) |
| HIV/AIDS | 551 (7) | 342 (8) | 209 (5) |
| Other immunodeficiency | 446 (5) | 231 (5) | 215 (5) |
| History of smoking | 1698 (20) | 954 (22) | 744 (19) |
| Chronic renal disease | 532 (6) | 288 (7) | 244 (6) |
| ≥1 comorbid condition | 4099 (49) | 2143 (49) | 1966 (50) |
Abbreviations: ABCs, Active Bacterial Core surveillance; HIV, human immunodeficiency virus; IPD, invasive pneumococcal disease; IQR, interquartile range.
a Data represent No. (%) unless otherwise specified.
b Isolated bacteremia was defined as a positive blood culture and no other signs of focal infection. Other clinical syndrome categories were not considered mutually exclusive.
Comparison of IPD Rates by Sex, Age, and Period
Among children <2 years old, IPD rates declined substantially after introduction of both PCVs. In this age group, rates of IPD were significantly higher in boys than in girls during 3 vaccine eras, the pre-PCV7, early PCV7, and post-PCV13 eras (Table 2). Similar trends were observed among older children. Among children 2–17 years of age, boys had significantly higher IPD rates than girls during the early PCV7 and post-PCV13 eras (Table 2).
Table 2.
Annualized Rates of IPD by Sex in ABCs Data for Tennessee, 1998–2013
| Age Group and Era | Annualized Rate of IPD Cases per 100 000 population. |
IRR (95% CI) | |
|---|---|---|---|
| Male Subjects | Female Subjects | ||
| <2 y | |||
| Pre-PCV7 | 210 | 133 | 1.6 (1.2–2.0) |
| Early PCV7 | 81 | 52 | 1.6 (1.2–2.1) |
| Late PCV7 | 54 | 53 | 1.0 (.8–1.3) |
| Post-PCV13 | 28 | 14 | 2.0 (1.1–3.4) |
| 2–17 y | |||
| Pre-PCV7 | 10 | 8 | 1.3 (.9–1.9) |
| Early PCV7 | 8 | 5 | 1.7 (1.2–2.3) |
| Late PCV7 | 7 | 6 | 1.2 (.9–1.5) |
| Post-PCV13 | 4 | 2 | 1.7 (1.0–2.7)b |
| 18–39 y | |||
| Pre-PCV7 | 9 | 8 | 1.2 (.9–1.7) |
| Early PCV7 | 8 | 5 | 1.5 (1.2–1.9) |
| Late PCV7 | 8 | 7 | 1.2 (.9–1.4) |
| Post-PCV13 | 5 | 5 | 1.0 (.8–1.4) |
| 40–64 y | |||
| Pre-PCV7 | 23 | 14 | 1.6 (1.2–2.0) |
| Early PCV7 | 25 | 14 | 1.7 (1.5–2.0) |
| Late PCV7 | 27 | 22 | 1.2 (1.1–1.4) |
| Post-PCV13 | 21 | 16 | 1.3 (1.1–1.6) |
| 65–74 y | |||
| Pre-PCV7 | 48 | 35 | 1.4 (1.0–1.9) |
| Early PCV7 | 38 | 33 | 1.1 (.9–1.5) |
| Late PCV7 | 38 | 34 | 1.1 (.9–1.4) |
| Post-PCV13 | 33 | 25 | 1.3 (1.0–1.8) |
| ≥75 y | |||
| Pre-PCV7 | 89 | 78 | 1.1 (.9–1.5) |
| Early PCV7 | 59 | 62 | 1.0 (.8–1.2) |
| Late PCV7 | 66 | 56 | 1.2 (1.0–1.4) |
| Post-PCV13 | 61 | 40 | 1.5 (1.2–2.0) |
Abbreviations: ABCs, Active Bacterial Core surveillance; CI, confidence interval; IPD, invasive pneumococcal disease; IRR, incidence rate ratio; PCV7, 7-valent pneumococcal conjugate vaccine; PCV13, 13-valent pneumococcal conjugate vaccine.
a Vaccine eras were defined as follows: pre-PCV7 (1998–1999), early PCV7 (2001–2004), late PCV7 (2005–2009), and post-PCV13 (2011–2013); see Methods for further explanation.
b Lower bound of 95% CI was >1 before rounding (2-sided P = .04).
Among adults aged 18–39 years, men had significantly higher IPD rates than women during the early PCV7 era (Table 2). Men aged 40–64 years had significantly higher rates than women in this age group during all 4 vaccine eras. Among adults 65–74 years of age, men did not have significantly higher rates than women during the study period. Adults age ≥75 years had the highest IPD rates among adult age groups, and men had significantly higher IPD rates than women only in the post-PCV13 era (IRR, 1.5; 95% CI, 1.2–2.0).
Comparison of IPD Rates by Sex, Age, Race and Period
Sex differences in IPD rates by age groups remained after stratification by race (Supplementary Table 2). The numbers of cases excluded from this analysis because of unknown race or other race were as follows, by age group: <2 years, 75 subjects (8%); 2–17 years, 39 (6%); 18–39 years, 46 (4%); 40–64 years, 103 (3%); 65–74 years, 15 (1%); and ≥75 years, 39 (3%).
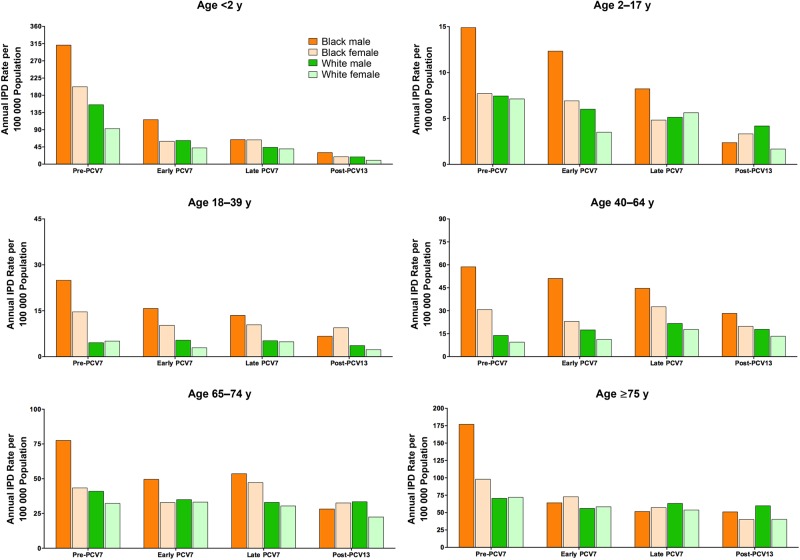
Among children <2 years old, white boys had significantly higher rates than white girls during the pre-PCV7 era (Figure 1). Black boys in this age group had the highest IPD rates among all sex-race groups and had significantly higher rates than black girls during the pre-PCV7 and early PCV7 eras (IRR, 1.5 [95%, CI, 1.1–2.2] and 1.9 [1.3–3.0], respectively; Figure 1 and Supplementary Table 2). Post-PCV13 rates showed similar trends but were based on small numbers of IPD cases, and comparisons did not reach statistical significance.
Figure 1.
Invasive pneumococcal disease (IPD) rates in Tennessee per 100 000 population in black male, black female, white male, and white female subjects, stratified by age group. Note differences in the scales of the y-axes. Abbreviations: PCV7, 7-valent pneumococcal conjugate vaccine; PCV13, 13-valent pneumococcal conjugate vaccine.
IPD rates among children 2–17 years old were substantially lower among both blacks and whites than the rates described for younger children. During the early PCV7 and post-PCV13 eras, white boys in this group had IPD rates 1.7 and 2.5 times those of white girls, respectively, but there were no significant differences noted during other eras. The highest IPD rates were among black boys who had IPD rates of 15 per 100 000 person-years during the pre-PCV7 era. Black boys had significantly higher IPD rates than black girls during the pre-PCV7, early PCV7, and late PCV7 vaccine eras (IRR, 1.9 [95% CI, 1.1–3.5], 1.8 [1.1–2.8], and 1.7 [1.1–2.7], respectively). However, the rates during the post-PCV13 era were based on very few cases and lacked precision, complicating rate comparisons (Figure 1 and Supplementary Table 2).
Among adults age 18–39 years, white men had significantly higher IPD rates than white women during the early PCV7 era (IRR, 1.8; 95% CI, 1.2–2.7). Black men had significantly higher IPD rates than black women during the pre-PCV7 and early PCV7 eras (IRR, 1.7 [95% CI, 1.1–2.5] and 1.5 [1.1–2.2], respectively; Figure 1). Rates during the post-PCV13 period were low and based on few IPD cases. White men 40–64 years of age had significantly higher IPD rates than white women in this age group during all 4 vaccine eras (Figure 1 and Supplementary Table 2). Similarly, black men had significantly higher rates than black women during all 4 vaccine eras in the 40–64 year age group.
Among adults 65–74 years of age, white men had significantly higher IPD rates than white women during the post-PCV13 study period only (IRR, 1.5; 95% CI, 1.1–2.1; Figure 1 and Supplementary Table 2). Black men did not have significantly higher rates than black women. In adults ≥75 years of age, white men had higher IPD rates than white women during the post-PCV13 era only (IRR, 1.5; 95% CI, 1.1–2.0). In contrast, black men had higher IPD rates than black women during the pre-PCV7 era only (IRR, 1.8; 95% CI, 1.1–3.0; Figure 1 and Supplementary Table 2). Separate analyses that focused on IPD due to serotypes covered by PCV7 or exclusively covered by PCV13 or not covered by PCVs showed similar patterns to the data reported for all IPD (information available in Supplementary Materials).
DISCUSSION
We sought to determine whether sex differences in IPD rates existed and assess whether these disparities changed after introduction of the PCVs. We found that male subjects had generally higher rates of IPD than female subjects. In spite of the substantial reductions in the incidence in IPD after introduction of PCV7 and PCV13 and marked decreases in racial disparities in IPD, sex differences in IPD rates persist in some age groups.
Previous studies have shown marked racial disparities in the occurrence of pneumococcal diseases, with blacks experiencing higher rates of IPD than whites [24]. After PCV7 and PCV13 introduction in the United States, this racial disparity was drastically reduced or eliminated, especially in young children who directly receive pneumococcal vaccination [6, 16, 17]. Acknowledging historical racial disparities, our study examined sex disparities, stratifying the comparisons by race groups. We observed that in many age groups, IPD rates were higher among black male than among black female subjects. Similarly, white male subjects had higher IPD rates than white female subjects. These patterns were seen among those <65 years old, especially evident among young children and middle-age adults, but were less consistent among older adults. The observed sex disparities in IPD incidence have persisted after widespread use of PCVs in children.
The exact mechanism for these sex disparities in IPD rates and the effect of age on these differences is poorly understood. Male and female subjects in our study had similar proportions of cases with ≥1 comorbid condition, suggesting that these cannot fully explain the sex differences we observed. We found that male subjects had higher IPD rates across all age groups, but these differences were less pronounced among adults ≥65 years of age. Sex hormone concentrations and changes in those concentrations during the aging process may influence susceptibility to infectious diseases. Estrogen receptors are found on a variety of immune cells, including T cells, B cells, dendritic cells, and macrophages [27]. Some studies suggest that females tend to resemble males immunologically as they age [28], which may explain why sex disparities were less pronounced than in younger adults. In addition, sex-related behaviors, such as smoking and exposure to young and school-aged children who are likely to be colonized with pneumococcus, are probably important drivers of IPD risk, especially among adults, but these are difficult to measure and account for. Importantly, these concerns are probably less relevant among young children, among whom we noted strong sex differences. Nevertheless, further studies are needed to better understand the role of sex hormones on the immune system, and other behaviors or environmental exposures that may determine susceptibility to IPD.
Although our study used systematically collected clinical data and relied on laboratory confirmation to accurately examine sex differences in IPD incidence, we acknowledge several limitations. First, ABCs active surveillance efforts may have missed IPD episodes, which then would be excluded from our analysis. However, it would be unlikely to have missed IPD cases differentially by sex. Second, although we collected information on a number of comorbid conditions through systematic chart abstraction and review, our data collection relies on information available in medical records, which may be incomplete. Third, other behaviors or environmental risk factors may be incompletely recorded or missing from medical records. Fourth, the limited data and small number of IPD cases precluded additional stratifications that could explore some relevant biological transitions, such as puberty or menopause. Additional studies will be needed to address these processes. Finally, our study was restricted to the Tennessee population, and studies in other populations would be useful to supplement our findings.
IPD rates among very young and middle-aged male subjects were higher than among female subjects in this age group. These sex differences were observed in different race groups and were not eliminated with the introduction of PCVs. These observations highlight the need for more epidemiologic and basic science research to further investigate behavioral and biological explanations for sex disparities in susceptibility to infectious diseases.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank Brenda G. Barnes, RN, and all the members of the Tennessee Active Bacterial Core surveillance network.
Financial support. This work was supported by Pfizer (ASPIRE award), the Agency for Healthcare Research and Quality, (grant 1R03HS022342), the Centers for Disease Control and Prevention (grant 5-U50-CK000198-3), and National Institutes of Health (grant T32 AI095202).
Potential conflicts of interest. A. d. S. M. and N. H. received funding from Pfizer, which manufactures 13-valent pneumococcal conjugate vaccine; W. S. is a member of data safety monitoring boards for both Merck and Pfizer and has consulted for GlaxoSmithKline, Noravax, and Genentech; M. R. G. has received grant funding from Medimmune; N. H. receives or has received grant funding within the past 2 years from Baxter, AstraZeneca, and Gilead; and C. G. G. has served as a consultant for Pfizer. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.van Lunzen J, Altfeld M. Sex differences in infectious diseases-common but neglected. J Infect Dis 2014; 209(suppl 3):S79–80. [DOI] [PubMed] [Google Scholar]
- 2.Anker M. Addressing sex and gender in epidemic-prone infectious diseases. Geneva, Switzerland: World Health Organization, 2007. [Google Scholar]
- 3.Muenchhoff M, Goulder PJ. Sex differences in pediatric infectious diseases. J Infect Dis 2014; 209(suppl 3):S120–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang SS, Johnson KM, Ray GT et al. . Healthcare utilization and cost of pneumococcal disease in the United States. Vaccine 2011; 29:3398–412. [DOI] [PubMed] [Google Scholar]
- 5.Pelton SI, Weycker D, Farkouh RA, Strutton DR, Shea KM, Edelsberg J. Risk of pneumococcal disease in children with chronic medical conditions in the era of pneumococcal conjugate vaccine. Clin Infect Dis 2014; 59:615–23. [DOI] [PubMed] [Google Scholar]
- 6.Talbot TR, Poehling KA, Hartert TV et al. . Elimination of racial differences in invasive pneumococcal disease in young children after introduction of the conjugate pneumococcal vaccine. Pediatr Infect Dis J 2004; 23:726–31. [DOI] [PubMed] [Google Scholar]
- 7.Sankilampi U, Herva E, Haikala R, Liimatainen O, Renkonen OV, Leinonen M. Epidemiology of invasive Streptococcus pneumoniae infections in adults in Finland. Epidemiol and Infect 1997; 118:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eskola J, Takala AK, Kela E, Pekkanen E, Kalliokoski R, Leinonen M. Epidemiology of invasive pneumococcal infections in children in Finland. JAMA 1992; 268:3323–7. [PubMed] [Google Scholar]
- 9.Burman LA, Norrby R, Trollfors B. Invasive pneumococcal infections: incidence, predisposing factors, and prognosis. Rev Infect Dis 1985; 7:133–42. [DOI] [PubMed] [Google Scholar]
- 10.Scott JA, Hall AJ, Dagan R et al. . Serogroup-specific epidemiology of Streptococcus pneumoniae: associations with age, sex, and geography in 7,000 episodes of invasive disease. Clin Infect Dis 1996; 22:973–81. [DOI] [PubMed] [Google Scholar]
- 11.Walter ND, Taylor TH Jr, Dowell SF, Mathis S, Moore MR; Active Bacterial Core Surveillance System Team. Holiday spikes in pneumococcal disease among older adults. New Engl J Med 2009; 361:2584–5. [DOI] [PubMed] [Google Scholar]
- 12.Nuorti JP, Whitney CG. Prevention of pneumococcal disease among infants and children—use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2010; 59:1–18. [PubMed] [Google Scholar]
- 13.Pilishvili T, Lexau C, Farley MM et al. . Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis 2010; 201:32–41. [DOI] [PubMed] [Google Scholar]
- 14.Griffin MR, Zhu Y, Moore MR, Whitney CG, Grijalva CG. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. New Engl J Med 2013; 369:155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grijalva CG, Nuorti JP, Arbogast PG, Martin SW, Edwards KM, Griffin MR. Decline in pneumonia admissions after routine childhood immunisation with pneumococcal conjugate vaccine in the USA: a time-series analysis. Lancet 2007; 369:1179–86. [DOI] [PubMed] [Google Scholar]
- 16.Flannery B, Schrag S, Bennett N et al. . Impact of childhood vaccination on racial disparities in invasive Streptococcus pneumoniae infections. JAMA 2004; 291:2197–203. [DOI] [PubMed] [Google Scholar]
- 17.de St Maurice A, Grijalva CG, Fonnesbeck C, Schaffner W, Halasa NB. Racial and regional differences in rates of invasive pneumococcal disease. Pediatrics 2015; 136:e1186–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore MR, Gertz RE Jr, Woodbury RL et al. . Population snapshot of emergent Streptococcus pneumoniae serotype 19A in the United States, 2005. J Infect Dis 2008; 197:1016–27. [DOI] [PubMed] [Google Scholar]
- 19.Pelton SI, Huot H, Finkelstein JA et al. . Emergence of 19A as virulent and multidrug resistant pneumococcus in Massachusetts following universal immunization of infants with pneumococcal conjugate vaccine. Pediatr Infect Dis J 2007; 26:468–72. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan SL, Barson WJ, Lin PL et al. . Early trends for invasive pneumococcal infections in children after the introduction of the 13-valent pneumococcal conjugate vaccine. Pediatr Infect Dis J 2013; 32:203–7. [DOI] [PubMed] [Google Scholar]
- 21.Moore MR, Link-Gelles R, Schaffner W et al. . Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis 2015; 15:301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Active Bacterial Core surveillance. Surveillance population. http://www.cdc.gov/abcs/methodology/surv-pop.html Accessed 30 May 2014.
- 23.Schuchat A, Hilger T, Zell E et al. . Active Bacterial Core surveillance of the Emerging Infections Program Network. Emerg Infect Dis 2001; 7:92–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wortham JM, Zell ER, Pondo T et al. . Racial disparities in invasive Streptococcus pneumoniae infections, 1998–2009. Clin Infect Dis 2014; 58:1250–7. [DOI] [PubMed] [Google Scholar]
- 25.Rothman KJ. Modern epidemiology. 1st ed. Boston, MA: Little, Brown & Co, 1986. [Google Scholar]
- 26.StataCorp. Stata 13 base reference manual. College Station, TX: Stata Press, 2015. [Google Scholar]
- 27.Gubbels Bupp MR. Sex, the aging immune system, and chronic disease. Cell Immunol 2015; 294:102–10. [DOI] [PubMed] [Google Scholar]
- 28.Simell B, Lahdenkari M, Reunanen A, Kayhty H, Vakevainen M. Effects of ageing and gender on naturally acquired antibodies to pneumococcal capsular polysaccharides and virulence-associated proteins. Clin Vaccine Immunol 2008; 15:1391–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.