Abstract
β-glucans, which can activate innate immune responses, are a major component in the cell wall of the cyst form of Pneumocystis. In the current study, we examined whether β-1,3-glucans are masked by surface proteins in Pneumocystis and what role β-glucans play in Pneumocystis-associated inflammation. For 3 species, including Pneumocystis jirovecii, which causes Pneumocystis pneumonia in humans, Pneumocystis carinii, and Pneumocystis murina, β-1,3-glucans were masked in most organisms, as demonstrated by increased exposure following trypsin treatment. Using quantitative polymerase chain reaction and microarray techniques, we demonstrated in a mouse model of Pneumocystis pneumonia that treatment with caspofungin, an inhibitor of β-1,3-glucan synthesis, for 21 days decreased expression of a broad panel of inflammatory markers, including interferon γ, tumor necrosis factor α, interleukin 1β, interleukin 6, and multiple chemokines/chemokine ligands. Thus, β-glucans in Pneumocystis cysts are largely masked, which likely decreases innate immune activation; this mechanism presumably was developed for interactions with immunocompetent hosts, in whom organism loads are substantially lower. In immunosuppressed hosts with a high organism burden, organism death and release of glucans appears to be an important contributor to deleterious host inflammatory responses.
Keywords: Pneumocystis, glucan, cell wall, Msg
Pneumocystis jirovecii is a pathogen that causes life-threatening pneumonia (Pneumocystis pneumonia [PCP]) in patients with AIDS and other immunocompromised patients [1, 2]. Members of the genus Pneumocystis can uniquely infect different mammalian hosts, with Pneumocystis jirovecii infecting humans, Pneumocystis carinii infecting rats, and Pneumocystis murina infecting mice [3–5]. There are 2 major forms, trophic forms and cysts, in the Pneumocystis life cycle. β-glucans are major components of the Pneumocystis cyst cell wall but are absent in the trophic form [6, 7].
β-Glucans can activate host innate immune responses through interactions with dectin-1, a β-1,3-glucan receptor found on dendritic cells, macrophages, and other cells [8–11], which induces phagocytosis and proinflammatory cytokine release [9, 12]. Given our recent demonstration that Pneumocystis lacks enzymes needed for chitin synthesis or high mannosylation of glycoproteins, β-glucans may be primary activators of innate immunity following infection [13]. Pneumocystis β-glucans have been shown to induce release of proinflammatory cytokines and chemokines in vitro [14–18], and recent studies suggest that β-glucans are exposed on the surface of cysts in vivo [6, 19, 20]. Echinocandins such as caspofungin are antifungal drugs that inhibit the synthesis of β-1,3 glucan. Echinocandin treatment depletes cysts in rodent models of PCP [6, 7, 21] and can lead to decreased inflammation in Pneumocystis-infected animals during immune reconstitution [22].
Some pathogens, such as Candida and Aspergillus, mask β-glucan by a dense layer of mannoproteins to evade activation of host innate immune responses [23–25]. The most abundant surface protein of Pneumocystis is the major surface glycoprotein (Msg), which is encoded by a multicopy gene family and may play an important role in evading adaptive immune responses [26, 27]. Msg is present on both the cyst and trophic forms of Pneumocystis; while its life cycle is unknown, trophic forms have been postulated to undergo binary fission or to conjugate and undergo meiosis, leading to the development of cysts with up to 8 intracystic bodies, which subsequently are released as new trophic forms. Given the potentially important role of β-glucans in activating host immunity, we undertook the current investigation to determine whether Pneumocystis β-glucans are masked by Msg and other surface proteins and, further, to determine the effects of caspofungin treatment on modulating host inflammatory responses in immunodeficient animals with PCP, specifically in the absence of immune reconstitution, as a model of human disease in persistently immunodeficient hosts.
MATERIALS AND METHODS
Pneumocystis and Yeast Preparations
P. carinii or P. murina organisms were isolated from the lungs of immunosuppressed rats or from scid or CD40L-KO mice, respectively, by Ficoll-Hypaque density gradient centrifugation [28]. P. jirovecii–infected lung was obtained at autopsy from a patient with Pneumocystis pneumonia. Animal and human subject experimentation guidelines of the National Institutes of Health were followed in the conduct of these studies.
Saccharomyces cerevisiae strain YPH 499 was obtained from Stratagene (Santa Clara, California) and was grown in YPDA medium (0.0075% L-adenine hemisulfate salt, 1% yeast extract, 2% Bacto peptone, and 2% dextrose) at 30°C.
Expression of Recombinant Factor G and Dectin-Fc
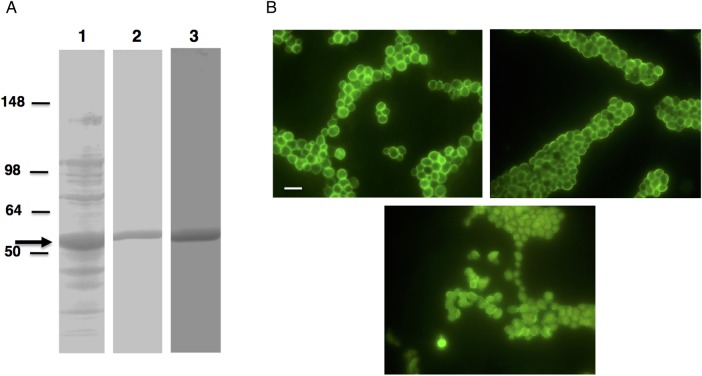
Partial complementary DNA (cDNA) sequence (base pairs 754–2004; GB# AB547712.1) encoding the clotting factor G α subunit of Limulus polyphemus [29], which binds to β-1,3-glucan [30], was optimized for bacterial expression, synthesized, and cloned into pET28 vector (GenScript USA, Piscataway, New Jersey); 4 amino acids (Met, Ala, Leu, and Cys) were added to the N-terminus, and 3 human influenza virus hemagglutinin tags were added to the C-terminus. The construct was transformed into Escherichia coli strain BL21-CodonPlus(DE3)-RIL (Agilent Technologies, Santa Clara, California) and expressed as a histidine-tagged fusion protein; sodium dodecyl sulfate polyacrylamide gel electrophoresis (Figure 1A) showed a prominent band denoting an approximately 52-kDa protein. Recombinant protein was purified using HisPur Cobalt Purification Kit (Thermo Scientific, Rockford, Illinois; Figure 1A) and biotinylated using the Lightning-Link Biotin conjugation kit (type A; Innova Biosciences, Cambridge, United Kingdom) or labeled with Alexa Fluor 594 by using the Lightning-Link Rapid FluoProbes 594 kit (Innova Biosciences).
Figure 1.
A, Expression of recombinant factor G. Partial complementary DNA sequence encoding the clotting factor G α subunit of Limulus polyphemus was cloned into Pet 28 b vector and was expressed as histidine (His)–tagged fusion protein. Sodium dodecyl sulfate polyacrylamide gel electrophoresis analysis of the bacterial cells expressing recombinant protein showed a band of the expected size (approximately 52 kDa; arrow) when stained with Coomassie Blue (lane 1, crude preparation; lane 2, purified protein), and Western blotting with His-tagged antibody revealed immunoreactivity (lane 3). B, Immunofluorescence microscopy of β-glucan. Methods to detect β-glucan were developed using Saccharomyces cerevisiae. Yeast cells showed immunofluorescence when labeled with biotinylated recombinant factor G and Alexa Fluor 488–conjugated streptavidin (top left), dectin-Fc recombinant protein and Alexa Fluor 488–labeled donkey anti-mouse immunoglobulin G (IgG; top right), or mouse β-1,3-glucan monoclonal antibody and FITC-conjugated goat anti-mouse IgG (bottom). All images are 1000× original magnification. Bar, 10 µm.
Dectin-Fc construct encoding a chimeric protein containing the extracellular domain of the murine dectin-1 receptor [8] and the Fc fragment of murine immunoglobulin G1 (IgG1) in pSecTag 2 vector (provided by Dr Chad Steele) [19] were transfected into Cos1 cells, using Fugene 6 (Roche Applied Bioscience, Indianapolis, Indiana). After 48 hours, the culture medium was collected and centrifuged to remove cells before immunofluorescence analysis.
Immunofluorescence Analysis
Pneumocystis-infected lung homogenate or partially purified organisms in phosphate-buffered saline (PBS) were added to individual wells of 8-well slides, heat fixed, and blocked with 3% bovine serum albumin (BSA; Jackson Immunoresearch laboratories, West Grove, Pennsylvania) or 10% donkey serum (Sigma-Aldrich, St. Louis, Missouri) at 37°C for 30 minutes. A mouse anti-β-1,3-glucan monoclonal antibody (Biosupplies Australia, Bundoora, Australia) or, alternately, recombinant dectin-Fc fusion protein or Alexa Fluor 594–conjugated or biotinylated recombinant factor G were used for detection of β-1,3-glucan (demonstrated with S. cerevisiae in Figure 1B). For Msg detection, monoclonal antibody RA-E7, which recognizes P. carinii Msg (provided by Drs Peter Walzer and Michael Linke) [31], a mouse antiserum produced against recombinant P. murina Msg protein [32], and monoclonal antibody 6B8, which reacts with P. jirovecii Msg [33], were used. FITC-conjugated goat anti-mouse IgG (Santa Cruz Biotechnology, Santa Cruz, California) or Alexa Fluor 488– or 594–conjugated donkey anti-mouse IgG (Invitrogen) were used as secondary antibodies. Alexa Fluor 488–conjugated streptavidin (Invitrogen) was used for the detection of biotinylated factor G. All detection reagents were incubated in 1% BSA in Tris-buffered saline at 37°C for 1 hour. The images were collected using a fluorescence microscope.
To quantitate the proportion of cysts with exposed β-1,3-glucan, parallel slides were prepared from lung homogenates. One slide was treated with trypsin (10 ng/µL, Promega) for 1.5 hours, and the other was incubated with 40 mM ammonium bicarbonate buffer alone, followed by staining as described above to detect cysts. The total number of cysts in 10 random fields at 400× original magnification were counted, and the ratio of the untreated to trypsin-treated cyst counts was determined.
Animal Treatment Studies
CD40L knockout mice (B6;129S2-Tnfsf5tm1Imx/J), which are highly susceptible to Pneumocystis infection [34], were obtained from Jackson Laboratory (Bar Harbor, Maine) and bred at the National Institutes of Health. In a series of studies summarized in Supplementary Table 1, mice with PCP were treated with low (30 µg/kg/d) or high (10 mg/kg/d) doses of caspofungin administered intraperitoneally for varying periods, to examine the effect of Pneumocystis β-glucans on the host inflammatory response. For high-dose (10 mg/kg/d) studies, caspofungin was administered 5 days per week. Untreated mice with PCP and uninfected mice served as controls. Pneumocystis infection was transmitted via the respiratory route by cohousing study mice with a P. murina–infected seeder mouse, to mimic the natural mode of transmission; mice in each study were cohoused in a single cage. After animals were euthanized, the lungs were removed, and lung sections were placed in PBS, for quantitation of the Pneumocystis burden; in RNALater (Qiagen, Valencia, California), for gene expression studies; and in HistoChoice fixative with 20% ethanol (Amresco, Solon, Ohio), for immunofluorescence and immunohistochemical studies. In some studies, Pneumocystis organisms were partially purified by Ficoll-Hypaque density gradient centrifugation [28].
Microarray Hybridization and Analysis
Processing of samples for microarray analysis was performed as previously described [34, 35]. Detailed information is provided in Supplementary Methods. The microarray data used in the analysis, including CEL files, have been deposited in the Gene Expression Omnibus Database (available at: http://www.ncbi.nlm.nih.gov/gds) and are available under accession number GSE79079.
Quantitative Polymerase Chain Reaction (qPCR) Analysis
cDNA was reverse transcribed from total RNA extracted from lung samples, using the High Capacity RNA to cDNA kit (Applied Biosystems). Differential expression of 3 panels of genes was examined by using predesigned Taqman probes and primers from Applied Biosystems. Panel 1 included Ccl2, Ccl5, Ccr2, CD19, CD3e, CD68, CD86, Ctla4, Cxcr3, Gzmb, Icos, IFNγ, IL-10, IL-12b, IL-13, IL-17a, IL-1β, IL-4, IL-6, Tgfβ1, TNFα, and Tnfrsf18. Panel 2 included Ccl5, IL-6, IFNγ, TNFα, CD4, CD7, CD14, CD83, CD180, Cxcl2, Ccl17, Osm, CD80, Trem1, IL-1α, Tnfsf15, Ccl4, Ccl22, IL-1β, Tnfrsf9, Nlrp3, Tbx21, C7, Gzma, and Tlr11. Panel 3 included Clec7a, Emr1, IL-17a, Cxcl16, Ccr6, Cxcl15, Cxcl9, IL-21, IFNar1, IL-23a, Clec4n, IL-10, IL-4, Cxcr6, and IL-2. Real-time PCR was performed using Taqman gene expression master mix and the ViiA 7 Real-Time PCR system (Applied Biosystems) according to the manufacturer's instructions. Change in gene expression was calculated by the relative quantification (ΔΔCT) method, standardized to mouse 18S ribosomal RNA levels [36].
Quantitation of Pneumocystis Organisms
Organism load was determined by qPCR as previously described, using the single-copy dhfr gene of P. murina as the target [37]; results are expressed as dhfr copies per milligram of lung tissue.
Immunohistochemical Staining
P. murina–infected and uninfected lung tissue sections were labeled for macrophage analysis with rat anti-mouse F4/80 as described in the Supplementary Methods.
Statistical Analysis
For cyst and macrophage counts and qPCR studies, groups were compared using unpaired Student t tests. P values of ≤.05 were considered significant. Although this study was exploratory, for the qPCR studies we also report results with Bonferroni adjustment for multiple comparisons [38]. For the microarray study, selection criteria for the comparison of infected animals to uninfected controls included an absolute log fold change (base 2) of ≥1.0, and for comparison of caspofungin-treated to untreated Pneumocystis-infected animals an absolute log fold change (base 2) of ≥0.5 was used, with P values of ≤ .05 for both. Gene Ontology (GO) term enrichment analysis was performed using DAVID (available at: http://david.abcc.ncifcrf.gov/) [39, 40] with default parameters, default mouse background, and the functional annotation chart report tool.
RESULTS
β-Glucans in the Cell Wall of Pneumocystis Are Masked by Proteins
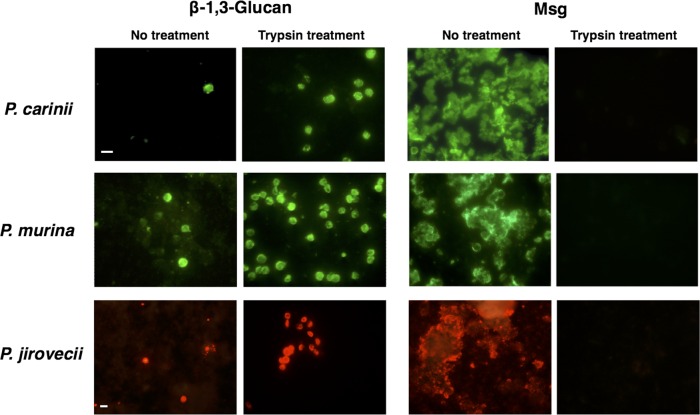
To determine whether the β-glucans in the cell wall of Pneumocystis are exposed or masked, we examined partially purified P. carinii organisms untreated or treated with trypsin for glucan exposure, using a mouse anti-β-1,3-glucan monoclonal antibody. As shown in Figure 2, only a small number of cysts in untreated samples were labeled with a β-1,3-glucan antibody, while staining with an anti-Msg antibody labeled a large number of organisms. In contrast, trypsin treatment exposed β-1,3-glucans on a large number of cysts. The absence of immunoreactivity with the anti-Msg antibody after trypsin digestion demonstrates that this treatment completely removed Msg and presumably other proteins from the surface of the organisms. Similar results were seen when using P. murina– and P. jirovecii–infected lung tissue (Figure 2). We quantitated the number of cysts in 10 microscopic fields for each sample; for all 3 species, the cyst numbers after trypsin treatment were significantly higher than in untreated samples (Table 1). The percentage of exposed cysts in untreated samples were 0.9% for P. carinii, 7.2% for P. murina, and 17.2% for P. jirovecii. For P. jirovecii, the infected lung was an autopsy sample obtained after a patient did not respond to prolonged anti-Pneumocystis therapy, and thus greater glucan exposure may be a consequence of prior treatment.
Figure 2.
Immunofluorescence microscopy of β-1,3-glucan and Msg in Pneumocystis organisms before and after trypsin treatment. Partially purified Pneumocystis carinii organisms were labeled with mouse β-1,3-glucan monoclonal antibody or Msg monoclonal antibody RA-E7 and FITC-conjugated goat anti-mouse immunoglobulin G (IgG; top row). Partially purified Pneumocystis murina organisms were labeled with Dectin-Fc recombinant protein or Msg antibody and FITC-conjugated goat anti-mouse IgG (middle row). Pneumocystis jirovecii organisms in human lung homogenate were labeled with Alexa Fluor 594–conjugated factor G recombinant protein or Msg monoclonal antibody 6B8 and Alexa Fluor 594–labeled donkey anti-mouse IgG (bottom row). The left 2 panels show labeling for β-1,3-glucan, and the right 2 panels show labeling for Msg. For all 3 Pneumocystis species, the number of cysts with detectable β-1,3-glucan increased after trypsinization, which also removed all detectable Msg. All images for P. carinii and P. murina are 1000× original magnification, and all images for P. jirovecii are 600× original magnification. Bars, 10 µm.
Table 1.
Percentage of Pneumocystis Organisms in Which the Glucans Are Masked, Based on Unmasking Following Trypsin Treatment
| Pneumocystis Species, Treatment | Cysts, No./HPF, Mean ± SDa | Percentage Exposedb | P Value |
|---|---|---|---|
| P. carinii | |||
| None | 3.5 ± 2.3 | 0.9 | <.0001 |
| Trypsin | 403.5 ± 93.1 | ||
| P. murina | |||
| None | 1.1 ± 1.1 | 7.2 | <.0001 |
| Trypsin | 15.3 ± 3.2 | ||
| P. jirovecii | |||
| None | 4.5 ± 2.2 | 17.2 | .0006 |
| Trypsin | 26.2 ± 13.5 |
a Data are from 10 random high-power fields (HPFs; 400× original magnification) that were examined for each organism following labeling for β-1,3-glucans, as described in “Materials and Methods” section.
b Data represent the ratio of counts for untreated samples to those for trypsin-treated samples.
Effect of Caspofungin on Inflammatory Gene Expression
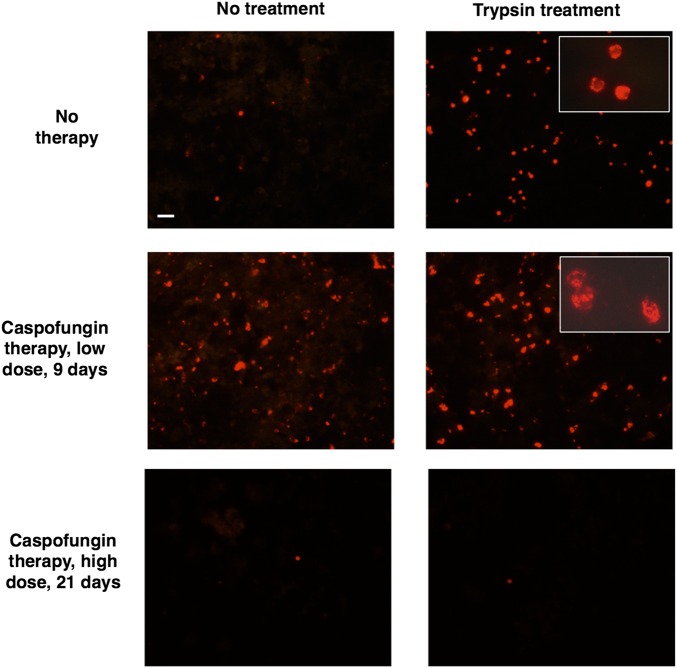
We used caspofungin, a potent inhibitor of β-1,3-glucan synthase, to examine the role that β-1,3-glucans play in inducing an inflammatory response during PCP. We initially treated infected mice with a low dose of caspofungin (30 µg/kg) once daily for 3 days in 2 studies (experiments 1 and 2); infected untreated mice served as controls. This low dose was previously used in studies to unmask β-glucans in Aspergillus and Candida [25]. Caspofungin treatment appeared to increase β-1,3-glucan exposure and to change the cyst structure as previously reported (Figure 3) [6]. Using a proinflammatory qPCR kit targeting 22 genes (qPCR; panel 1), we found significant upregulation of CD68, IFN-γ, and Tnfrsf18 (Gitr) and significant downregulation of IL-6 in caspofungin-treated mice as compared to untreated mice, suggesting that this low dose had led to T-cell activation but decreased inflammation (Figure 4).
Figure 3.
Immunofluorescence microscopy of β-1,3-glucan in Pneumocystis murina organisms following caspofungin treatment. Mice infected with P. murina were untreated (top), treated with caspofungin 30 µg/kg for 9 days (middle), or treated with caspofungin 10 mg/kg for 21 days (5 days per week; bottom). Partially purified P. murina organisms were evaluated for β-1,3-glucan exposure, using Alexa Fluor 594–conjugated factor G. Untreated controls showed only rare labeling of cysts without trypsin treatment, while the group treated with caspofungin for 9 days showed labeling of many cysts prior to trypsin treatment. Both groups had large numbers of labeled cysts after trypsinization. Following 21 days of caspofungin treatment, very few cysts were detected either prior to or following trypsin treatment. All large panels, 400× original magnification. Insets show high-power (1000× original magnification) magnification of cysts to demonstrate the altered cyst structure associated with caspofungin treatment. Bar, 20 µm.
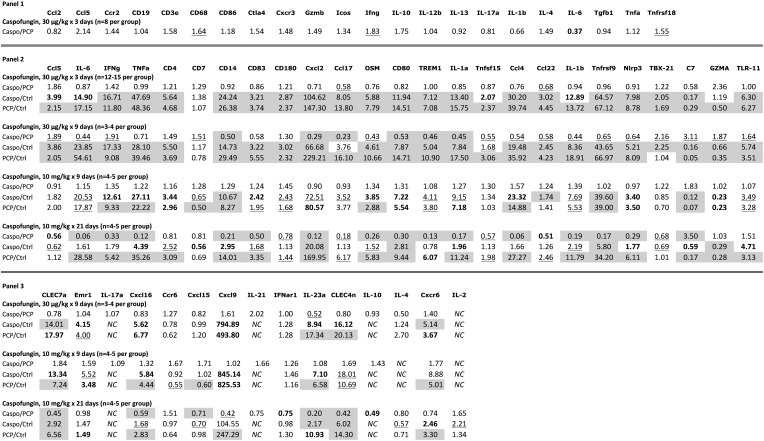
Figure 4.
Relative expression of select immune response genes as determined by quantitative polymerase chain reaction. Three panels of genes were examined. Panel 1 was used to evaluate the lungs of animals treated with a low dose of caspofungin (30 µg/kg once daily for 3 days); panel 2 examined animals treated with low or high (10 mg/kg once daily) doses for 3–21 days; panel 3 examined animals treated with low or high doses treated for 9–21 days. Specific dose and duration are indicated in the figure for each group of animals. For animals receiving high-dose caspofungin, the drug was administered 5 days per week. Data are shown as the fold change comparing caspofungin-treated animals to untreated infected animals (Caspo/PCP), caspofungin-treated animals to uninfected animals (Caspo/Ctrl), and untreated infected animals to uninfected animals (PCP/Ctrl). The following coding is used to represent significant P values, as follows: underlined, .05 ≥ P ≥ .01; bolded, .01 > P ≥ .001; and shaded, P < .001. All the shaded values, as well as IL-23a for Caspo/Ctrl, remained significant after applying Bonferroni correction for multiple comparisons (corrected P < .002–.003). Standard abbreviations are used for the individual genes. Abbreviation: NC, not calculable.
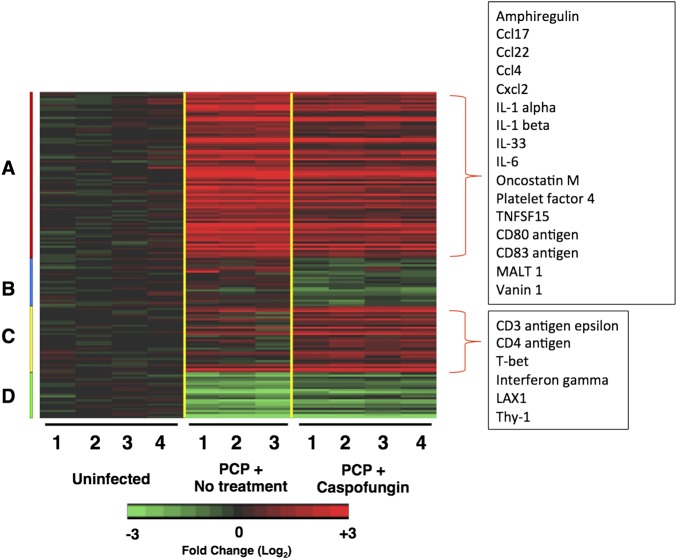
To explore this further, we used microarray techniques to analyze changes in gene expression following 9 days of once-daily treatment with caspofungin (30 µg/kg) in mice with PCP, compared with untreated infected mice and uninfected mice (experiment 3). As previously reported, there were robust differences between uninfected and infected animals, with 1621 and 1485 genes in the untreated and caspofungin-treated groups, respectively, that were differentially expressed, many of which are related to host inflammatory and immune responses (data not shown) [34, 35]. In comparing mice treated with caspofungin for 9 days to infected untreated mice, 151 differentially expressed genes were identified by use of less stringent criteria (Figure 5 and Supplementary Table 2). Among the significantly downregulated genes were markers of neutrophil, lymphocyte, and macrophage activation, including the neutrophil chemoattractants Cxcl1 and Cxcl2; Ccl4, a ligand of the T-helper type 1 (Th1) chemokine receptor Ccr5; Ccl17 and Ccl22, which are ligands for the Th2 chemokine receptor Ccr4; IL-33, which drives Th2 cytokine production; CD14, a macrophage marker; and the inflammatory cytokines IL-1α, IL-1β, and IL-6. Consistent with the initial qPCR findings, Th1 cell markers IFN-γ and Tbx21 were upregulated. Th17-associated genes (eg, Ccr6, IL-17a, and IL-17Ra) were not differentially expressed between treated and untreated animals.
Figure 5.
Differentially expressed immune response genes in caspofungin-treated mice. A, Heat map of microarray analysis showing genes that were selected on the basis of differential expression in the lungs of caspofungin-treated Pneumocystis murina–infected mice (30 µg/kg once daily for 9 days), compared with untreated infected mice. Red indicates increased expressed as compared to that for uninfected controls, while green indicates decreased expression. Numbers under the columns represent individual animals. Color coding on the left indicates 4 clusters of selected genes with similar expression patterns. Examples of immune-related genes identified in cluster A (downregulated in caspofungin-treated animals relative to animals with Pneumocystis pneumonia [PCP]) and cluster C (upregulated in caspofungin-treated animals relative to those with PCP) are listed in the boxes.
Based on findings of the microarray data and those from earlier studies, we developed a custom qPCR panel (panel 2) to further explore the effects of caspofungin in this setting. As shown in Figure 4, qPCR results for the same samples largely confirmed the microarray findings, with concordance between the two for 21 of 25 genes in the panel, again suggesting an increase in Th1 responses but a decrease in other inflammatory markers. These studies thus suggest that even low-dose caspofungin can lead to a decrease in the inflammatory response to Pneumocystis in heavily infected animals.
We also reevaluated the initial studies by using a 3-day regimen of low-dose caspofungin, combined with a third study that used the same dose and duration (experiment 4). Among the selected genes, C7 and Gzma were downregulated in infected animals as compared to uninfected controls, while all the remaining genes, with the exception of CD7, were significantly upregulated in infected animals, in some cases to extremely high levels (for some genes, >30–100-fold; Figure 4). In comparing caspofungin-treated to untreated infected animals, no genes showed significantly increased expression; Ccl17 and Ccl22, both ligands of Ccr4, a Th2 marker, were significantly downregulated. Expression of Ccl5, IFN-γ, and Tbx21 was increased, although not significantly.
We next determined whether a higher dose of caspofungin (10 mg/kg), which can markedly decrease the number of cysts in an infected lung, would influence the inflammatory response in heavily infected animals even in the absence of immune reconstitution. While we found no significant differences between caspofungin-treated and untreated Pneumocystis-infected animals in any of the genes examined by qPCR after 9 days of treatment, other than an increase toward normal levels of C7 (experiment 5), by day 21 there were significant declines in levels of all of the genes examined other than C7, which significantly increased toward normal levels, and Gzma and Tlr11, which showed nonsignificant changes (experiment 6). At that time point, 8 genes, including IFN-γ, IL-6, Ccl17, and Ccl22, were expressed at levels comparable to those in uninfected control animals, with a substantial reduction in TNF-α, IL-1β, Cxcl2, and CD14 expression (Figure 4). The Pneumocystis organism load, as determined by qPCR, had concomitantly decreased by approximately 1.5 logs in caspofungin-treated mice, compared with untreated mice, although they were still heavily infected (Supplementary Table 1). Similar downregulation of most genes was seen in a replicate experiment, although all 5 control animals and 2 of 5 caspofungin-treated animals were euthanized before completion of the 21-day study because of progressive PCP (data not shown).
We used 1 additional panel (qPCR panel 3) to examine the changes in additional immune-related genes for a subset of the studies (Figure 4). In the 9-day studies (involving both low and high doses of caspofungin), many of these genes were again upregulated when either caspofungin-treated or untreated infected animals were compared to uninfected controls. Especially prominent were increases in Cxcl9 expression, as previously shown [35], and smaller but still substantial increases in dectin-1 (CLEC7a) and dectin-2 (CLEC4n) expression. As previously shown in healthy animals, there was also an increase in expression of Cxcr6 and its ligand, Cxcl16 [35]. Again, most of the upregulated genes were significantly downregulated following 3 weeks of high-dose caspofungin treatment, compared with findings for infected controls.
Immunohistochemical Staining
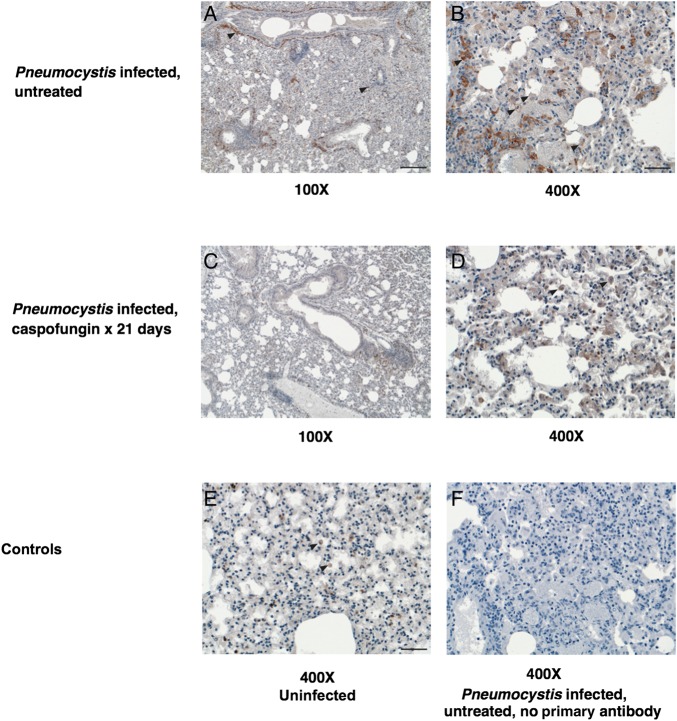
To see whether the decreased expression of inflammatory genes seen after caspofungin treatment was associated with a decrease in macrophages, we labeled lung samples with an anti-F4/80 antibody (Figure 6). The number of F4/80+ macrophages was significantly greater in untreated infected lungs (n = 3) than in caspofungin-treated lungs (n = 3; 242 vs 71 cells/high-power field; P = .0001) and uninfected lungs (n = 2; 242 vs 39 cells/high-power field; P = .0006) but was not significantly different between caspofungin-treated and uninfected lungs (71 vs 39 cells/high-power field; P = .09).
Figure 6.
Immunohistochemical labeling of mouse lungs for macrophages. To examine the differences in macrophage presence and distribution in lungs from untreated infected mice and mice treated with caspofungin, Pneumocystis-infected and control mouse lungs were labeled with murine macrophage marker anti-F4/80; representative sections are shown here. A–D, Left panels are low-power images (100× original magnification), and right panels are high-power images (400× original magnification) of untreated infected control lung (top row) and caspofungin-treated infected lung (middle row). Macrophages, labeled reddish brown with variable intensity in their membranes and cytoplasm, are denoted with black arrowheads. Caspofungin-treated mouse lungs contained lesser numbers of macrophages overall and in particular an absence of aggregates of macrophages around larger airways. E and F, The bottom row shows controls, including uninfected lung (left) and Pneumocystis-infected lung exposed to all reagents except the primary antibody (right), with both images at high power. Uninfected mouse tissue (E) shows low numbers of macrophages that are normally resident in mouse lung. Bars, 200 µm (A) and 50 µm (B and E).
DISCUSSION
In the current study, we have demonstrated that, in the vast majority of organisms, β-1,3-glucans present in the Pneumocystis cyst cell wall are not exposed on the organism cell surface but rather are masked by Msg and/or other surface proteins. We have also shown that treatment of P. murina–infected mice with high doses of caspofungin, an inhibitor of β-1,3-glucan synthesis, results in significant decreases in the expression of multiple genes related to immune and inflammatory responses, suggesting that β-glucans play a critical role in inflammation during PCP.
Our findings provide important insights into the inflammatory responses induced by Pneumocystis. Immune responses to microbes can be initiated by recognition of microbial pathogen-associated molecular patterns by host pattern-recognition receptors such as dectin-1, dectin-2, and macrophage mannose receptor [24]. Characteristic fungal pathogen-associated molecular patterns include mannans, glucans, and chitin. We have recently demonstrated that Pneumocystis cannot synthesize either chitin, or mannans beyond their core structure, thus eliminating 2 potential triggers of pattern-recognition receptors [13]. The masking of β-glucans by trypsin-sensitive molecules, presumably surface proteins such as Msg, potentially further minimizes activation of immune responses. Previous studies have found that β-glucans are exposed on the surface of cysts, but they did not investigate what proportion of cysts had β-glucans exposed [6, 19, 20]. We also found that β-glucans were exposed, but only in a minority of cysts. The highest levels were in human Pneumocystis, which we hypothesize is a consequence of high levels of infection and of treatment of PCP, which can result in the death of organisms and the loss of protein synthesis, resulting in exposure of β-glucans. Presumably β-glucan masking evolved for the benefit of organisms infecting immunocompetent hosts, in whom the level of infection is typically 3–4 logs lower than in immunocompromised hosts [37]. We hypothesize that the increased inflammation and associated hypoxia typically seen in patients with PCP 3–4 days after initiating therapy [41] may be a result of an abrupt increase in β-glucan exposure following treatment-induced death of organisms.
Our results further suggest that β-glucans are major contributors to the host inflammatory response seen in heavily infected immunodeficient animals, even without immune reconstitution. Linke et al recently demonstrated that echinocandin treatment in animals undergoing immune reconstitution results in decreases in pulmonary lymphocyte counts (both CD4+ and CD8+ T cells) and neutrophil counts, as well as decreases in G-CSF, TNF-α, Cxcl2, IL-6, and IL-17 levels [22]. Of note they found no differences in IFN-γ, IL-2, IL-4, IL-5, or IL-10 levels. Our microarray analysis suggested that low-dose treatment with caspofungin for 9 days leads to a decrease in expression of genes for some inflammatory cytokines, such as IL-6, but an increase in Th1-specific genes, including Tbx21 (T-bet), a primary transcription factor driving Th1 development, as well as interferon γ. Using 2 qPCR panels, we demonstrated decreased expression, following 3 weeks of high-dose caspofungin treatment, of a broad range of inflammation-associated genes, with normalization of expression levels for a subset of those genes, including IFN-γ and IL-6. These data support the in vitro studies and extend the in vivo studies highlighting the role that β-glucans present in the wall of cysts play in driving inflammation during PCP. The decrease in organism load of approximately 1.5 logs, as evaluated by qPCR, during 3 weeks of caspofungin treatment may have contributed to the decreased inflammation through mechanisms in addition to reduction in cysts. Decreased Pneumocystis trophic form organism load has been reported in some but not all prior studies with echinocandins [6, 22, 42]. However, the previously reported decreased inflammation, without an associated decrease in trophic forms, in mice treated with anidulafungin further supports that β-glucans are an important contributor to Pneumocystis-associated inflammation [6, 22, 42].
We used 3 immunofluorescence methods to detect β-1,3-glucan in Pneumocystis cyst walls. Initially, we used a highly specific monoclonal antibody (Biosupplies, Australia); we verified these results by means of 2 other methods, using a recombinant dectin-Fc fusion protein or a recombinant factor G protein. Dectin-Fc fusion protein has previously been shown to bind to β-1,3-glucan present in Pneumocystis cyst walls [19]. All 3 methods gave similar results. Use of recombinant dectin-Fc or factor G thus provides a cost-effective way to detect β-1,3-glucan.
Understanding the defense mechanisms by which Pneumocystis organisms evade and block the host immune system is important for developing effective therapeutic interventions. Currently corticosteroids are recommended for human immunodeficiency virus–infected patients with hypoxia, which can be exacerbated following initiation of anti-Pneumocystis therapy [43, 44]. Our data and those from other studies suggest that β-glucans are major contributors to this inflammation. While echinocandins alone should not be used for treatment of PCP, since they treat only 1 stage of the life cycle, it is possible that combining echinocandins with standard anti-Pneumocystis agents such as trimethoprim-sulfamethoxazole may lead to diminished inflammation, compared with standard therapy alone [42].
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank Rene Costello and Howard Mostowski, for providing animal care; the staff of the Histopathology Unit at the Veterinary Diagnostic Laboratory and Monica Gamez at Kansas State University, for technical assistance; Chad Steele, for providing the dectin-Fc construct; and Peter Walzer and Michael Linke, for providing antibody RA-E7.
Disclaimer. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Financial support. This work was supported by the Intramural Research Program, National Institutes of Health Clinical Center; and the National Cancer Institute, National Institutes of Health (contract HHSN261200800001E).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Kovacs JA, Masur H. Evolving health effects of Pneumocystis: one hundred years of progress in diagnosis and treatment. JAMA 2009; 301:2578–85. [DOI] [PubMed] [Google Scholar]
- 2.Thomas CF Jr, Limper AH. Current insights into the biology and pathogenesis of Pneumocystis pneumonia. Nat Rev Microbiol 2007; 5:298–308. [DOI] [PubMed] [Google Scholar]
- 3.Ma L, Imamichi H, Sukura A, Kovacs JA. Genetic divergence of the dihydrofolate reductase and dihydropteroate synthase genes in Pneumocystis carinii from 7 different host species. J Infect Dis 2001; 184:1358–62. [DOI] [PubMed] [Google Scholar]
- 4.Sinclair K, Wakefield AE, Banerji S, Hopkin JM. Pneumocystis carinii organisms derived from rat and human hosts are genetically distinct. Mol Biochem Parasitol 1991; 45:183–4. [DOI] [PubMed] [Google Scholar]
- 5.Stringer JR, Beard CB, Miller RF, Wakefield AE. A new name (Pneumocystis jiroveci) for Pneumocystis from humans. Emerg Infect Dis 2002; 8:891–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cushion MT, Linke MJ, Ashbaugh A et al. Echinocandin treatment of Pneumocystis pneumonia in rodent models depletes cysts leaving trophic burdens that cannot transmit the infection. PLoS One 2010; 5:e8524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmatz DM, Romancheck MA, Pittarelli LA et al. Treatment of Pneumocystis carinii pneumonia with 1,3-beta glucan synthesis inhibitors. Proc Natl Acad Sci U S A 1990; 87:5950–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown GD, Gordon S. Immune recognition. A new receptor for beta-glucans. Nature 2001; 413:36–7. [DOI] [PubMed] [Google Scholar]
- 9.Drummond RA, Brown GD. The role of Dectin-1 in the host defence against fungal infections. Curr Opin Microbiol 2011; 14:392–9. [DOI] [PubMed] [Google Scholar]
- 10.Kimberg M, Brown GD. Dectin-1 and its role in antifungal immunity. Med Mycol 2008; 46:631–6. [DOI] [PubMed] [Google Scholar]
- 11.Taylor PR, Tsoni SV, Willment JA et al. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol 2007; 8:31–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dennehy KM, Brown GD. The role of the beta-glucan receptor Dectin-1 in control of fungal infection. J Leukoc Biol 2007; 82:253–8. [DOI] [PubMed] [Google Scholar]
- 13.Ma L, Chen Z, Huang da W et al. Genome analysis of three Pneumocystis species reveals adaptation mechanisms to life exclusively in mammalian hosts. Nat Commun 2016; 7:10740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carmona EM, Lamont JD, Xue A, Wylam M, Limper AH. Pneumocystis cell wall beta-glucan stimulates calcium-dependent signaling of IL-8 secretion by human airway epithelial cells. Respir Res 2010; 11:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans SE, Hahn PY, McCann F, Kottom TJ, Pavlovic ZV, Limper AH. Pneumocystis cell wall beta-glucans stimulate alveolar epithelial cell chemokine generation through nuclear factor-kappaB-dependent mechanisms. Am J Respir Cell Mol Biol 2005; 32:490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffman OA, Standing JE, Limper AH. Pneumocystis carinii stimulates tumor necrosis factor-α release from alveolar macrophages through a β-glucan-mediated mechanism. J Immunol 1993; 150:3932–40. [PubMed] [Google Scholar]
- 17.Saijo S, Fujikado N, Furuta T et al. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat Immunol 2007; 8:39–46. [DOI] [PubMed] [Google Scholar]
- 18.Vassallo R, Standing JE, Limper AH. Isolated Pneumocystis carinii cell wall glucan provokes lower respiratory tract inflammatory responses. J Immunol 2000; 164:3755–63. [DOI] [PubMed] [Google Scholar]
- 19.Rapaka RR, Goetzman ES, Zheng M et al. Enhanced defense against Pneumocystis carinii mediated by a novel dectin-1 receptor Fc fusion protein. J Immunol 2007; 178:3702–12. [DOI] [PubMed] [Google Scholar]
- 20.Ricks D, Chen K, Zheng M, Steele C, Kolls JK. Dectin immunoadhesins and Pneumocystis pneumonia. Infect Immun 2013; 81:3451–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmatz DM, Powles M, McFadden DC, Pittarelli LA, Liberator PA, Anderson JW. Treatment and prevention of Pneumocystis carinii pneumonia and further elucidation of the P. carinii life cycle with 1,3-b-glucan synthesis inhibitor L-671,329. J Protozool 1991; 38:151S–3. [PubMed] [Google Scholar]
- 22.Linke MJ, Ashbaugh A, Collins MS, Lynch K, Cushion MT. Characterization of a distinct host response profile to Pneumocystis murina asci during clearance of Pneumocystis pneumonia. Infect Immun 2013; 81:984–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hohl TM, Feldmesser M, Perlin DS, Pamer EG. Caspofungin modulates inflammatory responses to Aspergillus fumigatus through stage-specific effects on fungal beta-glucan exposure. J Infect Dis 2008; 198:176–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levitz SM. Innate recognition of fungal cell walls. PLoS Pathog 2010; 6:e1000758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wheeler RT, Fink GR. A drug-sensitive genetic network masks fungi from the immune system. PLoS Pathog 2006; 2:e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kovacs JA, Powell F, Edman JC et al. Multiple genes encode the major surface glycoprotein of Pneumocystis carinii. J Biol Chem 1993; 268:6034–40. [PubMed] [Google Scholar]
- 27.Lundgren B, Lipschik GY, Kovacs JA. Purification and characterization of a major human Pneumocystis carinii surface antigen. J Clin Invest 1991; 87:163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kovacs JA, Halpern JL, Swan JC, Moss J, Parrillo JE, Masur H. Identification of antigens and antibodies specific for Pneumocystis carinii. J Immunol 1988; 140:2023–31. [PubMed] [Google Scholar]
- 29.Yoneda A, Kurokawa T. A sensitive sandwich ELISA to measure (1-->3)-beta-d-glucan levels in blood. J Immunol Methods 2011; 365:158–65. [DOI] [PubMed] [Google Scholar]
- 30.Takaki Y, Seki N, Kawabata Si S, Iwanaga S, Muta T. Duplicated binding sites for (1-->3)-beta-D-glucan in the horseshoe crab coagulation factor G: implications for a molecular basis of the pattern recognition in innate immunity. J Biol Chem 2002; 277:14281–7. [DOI] [PubMed] [Google Scholar]
- 31.Linke MJ, Sunkin SM, Andrews RP, Stringer JR, Walzer PD. Expression, structure, and location of epitopes of the major surface glycoprotein of Pneumocystis carinii f. sp. carinii. Clin Diagn Lab Immunol 1998; 5:50–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bishop LR, Helman D, Kovacs JA. Discordant antibody and cellular responses to Pneumocystis major surface glycoprotein variants in mice. BMC Immunol 2012; 13:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kovacs JA, Halpern JL, Lundgren B, Swan JC, Parrillo JE, Masur H. Monoclonal antibodies to Pneumocystis carinii: identification of specific antigens and characterization of antigenic differences between rat and human isolates. J Infect Dis 1989; 159:60–70. [DOI] [PubMed] [Google Scholar]
- 34.Hernandez-Novoa B, Bishop L, Logun C et al. Immune responses to Pneumocystis murina are robust in healthy mice but largely absent in CD40 ligand-deficient mice. J Leukoc Biol 2008; 84:420–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bishop LR, Lionakis MS, Sassi M et al. Characterization of chemokine and chemokine receptor expression during Pneumocystis infection in healthy and immunodeficient mice. Microbes Infect 2015; 17:638–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25:402–8. [DOI] [PubMed] [Google Scholar]
- 37.Vestereng VH, Bishop LR, Hernandez B, Kutty G, Larsen HH, Kovacs JA. Quantitative real-time polymerase chain-reaction assay allows characterization of Pneumocystis infection in immunocompetent mice. J Infect Dis 2004; 189:1540–4. [DOI] [PubMed] [Google Scholar]
- 38.Dunn OJ. Multiple comparisons among means. J Am Stat Assoc 1961; 56:52–64. [Google Scholar]
- 39.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 2009; 4:44–57. [DOI] [PubMed] [Google Scholar]
- 40.Huang DW, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 2009; 37:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montaner JS, Lawson LM, Levitt N, Belzberg A, Schechter MT, Ruedy J. Corticosteroids prevent early deterioration in patients with moderately severe Pneumocystis carinii pneumonia and the acquired immunodeficiency syndrome (AIDS). Ann Intern Med 1990; 113:14–20. [DOI] [PubMed] [Google Scholar]
- 42.Lobo ML, Esteves F, de Sousa B et al. Therapeutic potential of caspofungin combined with trimethoprim-sulfamethoxazole for Pneumocystis pneumonia: a pilot study in mice. PLoS One 2013; 8:e70619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bozzette SA, Sattler FR, Chiu J et al. A controlled trial of early adjunctive treatment with corticosteroids for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. N Engl J Med 1990; 323:1451–7. [DOI] [PubMed] [Google Scholar]
- 44.Masur H, Brooks JT, Benson CA, Holmes KK, Pau AK, Kaplan JE. Prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: Updated Guidelines from the Centers for Disease Control and Prevention, National Institutes of Health, and HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014; 58:1308–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.