Abstract
It is unclear whether differential roles of CD4+ versus CD8+ T-cell senescence/exhaustion and effects of antiretroviral therapy (ART) on these processes may contribute to morbidity in treated human immunodeficiency virus type 1 (HIV) infection. In a prospective 96-week trial, 328 HIV–infected ART-naive participants were randomly assigned to receive tenofovir-emtricitabine plus either atazanavir/ritonavir, darunavir/ritonavir, or raltegravir. Markers of CD4+ T-cell senescence (ie, the percentage of CD28−CD57+ cells among CD4+ T cells ) and CD4+/CD8+ T-cell exhaustion (ie, the percentage of PD-1+ cells among CD4+/CD8+ T cells) decreased after ART. There were no changes in markers of CD8+ T-cell senescence after ART and no differential changes in all markers in ART groups. Senescent CD4+ and CD8+ T cells may have differential roles in HIV pathogenesis.
Keywords: antiretroviral therapy, human immunodeficiency virus, inflammation, immune activation, biomarkers
Human immunodeficiency virus type 1 (HIV) infection is characterized by a state of inflammation and immune activation that persists despite suppressive antiretroviral therapy (ART) and may contribute to the development of end-organ diseases such as cardiovascular disease (CVD) [1]. Chronic HIV infection is characterized by T-cell senescence, a terminal state with dysregulated immune function and production of proinflammatory cytokines [2]. Senescent T cells may increase the risk of morbidity, such as CVD [3], but data from individuals with HIV infection are limited and controversial [4, 5].
The mechanisms driving T-cell exhaustion, immunosenescence, activation, and chronic comorbidities during chronic HIV infection are not completely understood. In addition, although we and others have found that effective ART only partially decreases immune activation and inflammation [1], limited data exist on the effect of ART initiation in the setting of a randomized trial. Furthermore, it is unclear whether changes in these markers would differ in the setting of an integrase inhibitor–based regimen, compared with boosted protease inhibitor (PI) therapy.
AIDS Clinical Trials Group (ACTG) A5260s was a prospective cardiovascular substudy of ACTG A5257, in which participants were randomly assigned equally to receive one of the following 3 regimens: tenofovir disoproxil fumarate (TDF)/emtricitabine (FTC) plus either an integrase-based regimen containing raltegravir (RAL), a PI-based regimen containing atazanavir (ATV)/ritonavir (r), or TDF/FTC plus darunavir (DRV)/r [1]. In this study, we found that ATV/r was associated with slower progression of carotid intima media thickness, compared with DRV/r or RAL, and that levels of markers of inflammation and T-cell activation inconsistently and partially decreased after these regimens [1], without a consistent advantage for any of the regimens.
The objective of this analysis was to characterize the changes in biomarkers of T-cell senescence longitudinally among treatment-naive individuals initiating these randomly assigned ART regimens. In this article, we describe for the first time the effects of different successful ART regimens on T-cell senescence and exhaustion in the setting of a large randomized ART initiation trial. In addition, associations between key biomarkers of T-cell exhaustion, immune senescence and activation, and inflammation were explored in ART-naive HIV-infected participants at study entry and through week 96 of a successful ART regimen.
METHODS
Study Design and Participants
A5260s included 328 HIV-infected, ART-naive adults with no CVD or diabetes mellitus or use of lipid-lowering medications. The design of this substudy has been previously reported [1], as well as key results related to cardiovascular and bone end points [6, 7]. The parent study and substudy (ClinicalTrials.gov identifiers NCT00811954 and NCT00851799) were approved by the local institutional review boards. For this preplanned analysis, to avoid the confounding effect of viremia on markers of immunosenescence and exhaustion, the A5260s population was restricted to a subset of participants with virological suppression, no ART interruptions lasting >7 days, and an HIV RNA level of < 50 copies/mL by study week 24 and thereafter.
Biomarker and Laboratory Assessment
Blood samples were collected at study entry, prior to ART initiation, and after 24 and 96 weeks of treatment. Peripheral blood mononuclear cells (PBMCs) were isolated and cryopreserved and then underwent immunophenotyping by multicolor flow cytometry as described previously [1, 6]. The fluorochrome-conjugated antibodies used were anti-CD3 PE-Cy7 (clone SK7), anti-CD4 V450 (clone RPA-T4), anti-CD8 APC (clone RPA-T8), anti-CD8 APC-Cy7 (clone SK1), anti-CD28 PE-Cy 5 (clone CD28.2), anti-CD57 PE (clone NK-1), and anti- CD279 (PD-1) APC (clone MIH4), all from BD Biosciences. A representative gating strategy is shown in Supplementary Figure 1.
Statistical Analyses
Changes in biomarker values over time were estimated by determining the mean difference between levels during treatment and the level at baseline on the log10 scale and then back transforming the values to represent mean fold changes from baseline. For all pair-wise treatment group comparisons, shifts in the distribution of change from baseline were evaluated using Wilcoxon rank sum tests and are described as relative fold changes. While nominal P values are presented, adjusted P values, determined using Benjamini-Hochberg methods to control the false discovery rate, are also provided to acknowledge comparisons across the multiple biomarkers at each study week. Exploratory bivariate associations, quantified with Spearman rank correlations, were examined at concurrent study weeks between levels and changes in biomarkers. All analyses were performed with SAS, version 9.4 (SAS Institute, Cary, North Carolina).
RESULTS
Baseline Characteristics
Baseline demographic characteristics of the 328 participants from the A5260s study were previously described [1]. The 234 participants (71%) included in the virologically suppressed population for this analysis had similar baseline demographic characteristics across treatment groups, as previously described [1]. Briefly, 90% were men, 48% were white, the median age was 36 years, the median CD4+ T-cell count was 338 cells/mm3, and the median HIV RNA level was 4.6 log10 copies/mL. Baseline levels of all biomarker parameters are presented in Table 1. No notable differences in distributions were apparent between treatment groups.
Table 1.
Baseline Values and Fold Changes From Baseline Over Time for 212 Study Participants, by Treatment Group
| Biomarkers | Baseline, Mean (95% CIa) (n = 58) | ATV/r (n = 64), Mean (95% CIa) |
RAL (n = 72), Mean (95% CIa) |
DRV/r (n = 76), Mean (95% CIa) |
|||
|---|---|---|---|---|---|---|---|
| Week 24 (n = 58) | Week 96 (n = 61) | Week 24 (n = 65) | Week 96 (n = 70) | Week 24 (n = 73) | Week 96 (n = 72) | ||
| Immunosenescence, CD28−CD57+ cells, % | |||||||
| Among CD8+ T cells | 24.35 (17.8,30.75) | 1.04 (0.91,1.19) | 0.96 (0.85,1.09) | 1.15 (1.07,1.23) | 1.07 (0.98,1.17) | 1.13 (1.07,1.20) | 0.99 (0.89,1.10) |
| Among CD4+ T cells | 5.01 (2.24,9.97) | 0.73 (0.60,0.90) | 0.66 (0.52,0.85) | 0.85 (0.72,1.00) | 0.76 (0.62,0.93) | 0.85 (0.74,0.97) | 0.65 (0.54,0.79) |
| Cell exhaustion, PD-1+ cells, % | |||||||
| Among CD8+ T cells | 2.33 (1.48,3.87) | 0.43 (0.34,0.55) | 0.42 (0.33,0.54) | 0.42 (0.36,0.49) | 0.33 (0.27,0.40) | 0.35 (0.30,0.42) | 0.32 (0.24,0.43) |
| Among CD4+ T cells | 4.37 (2.57,7.62) | 0.58 (0.48,0.70) | 0.38 (0.30,0.47) | 0.60 (0.50,0.70) | 0.44 (0.37,0.52) | 0.50 (0.44,0.58) | 0.39 (0.32,0.48) |
Data are for participants with information on biomarkers available at the specified time points for calculations of changes.
Abbreviations: ATV/r, atazanavir/ritonavir; DRV/r, darunavir/ritonavir; RAL, raltegravir.
a 95% confidence intervals (CIs) excluding 1.0 are considered statistically significant.
Changes in Markers of Immunosenescence and Exhaustion
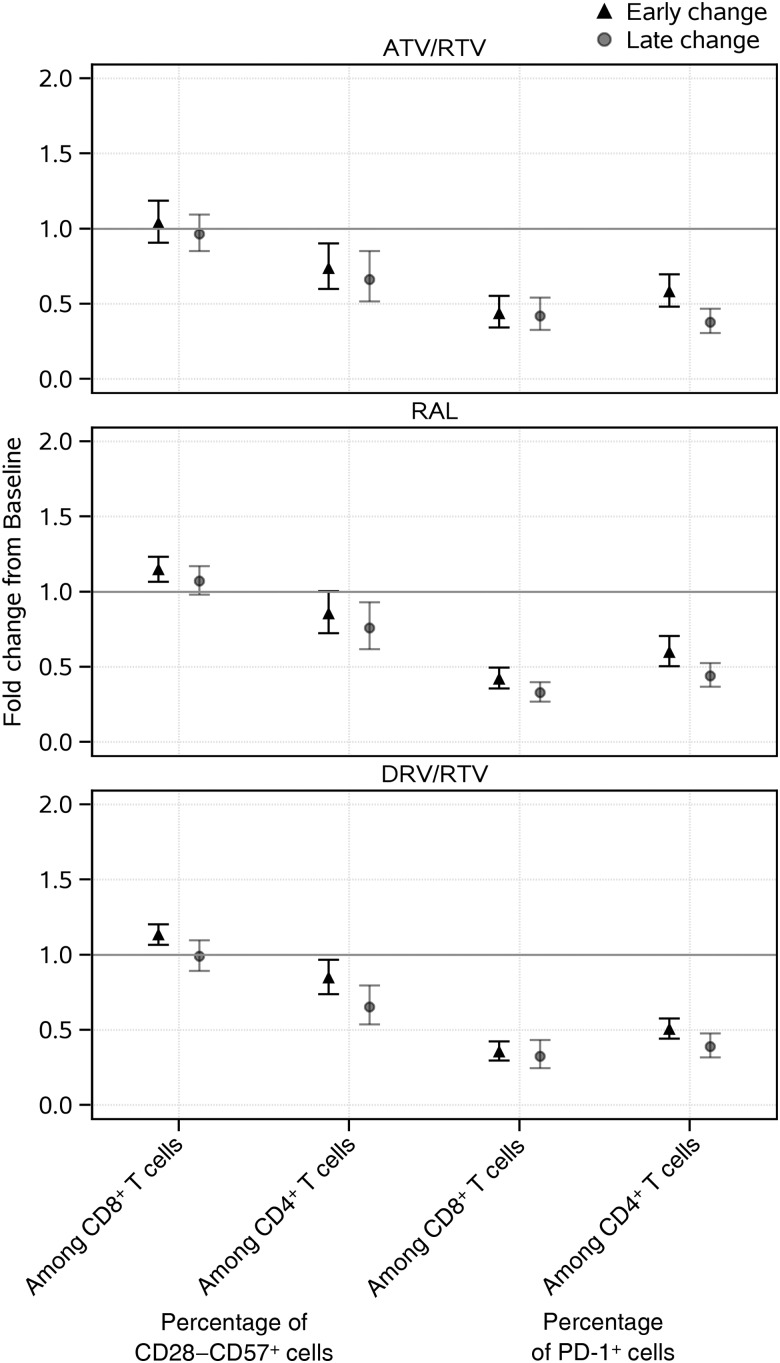
Sustained decreases (of about 31% or more) from baseline in the percentage of CD28−CD57+ cells among CD4+ T cells were evident among all treatment groups (Figure 1 and Table 1). In contrast, there was no apparent change from baseline to 96 weeks in the percentage of CD28−CD57+ cells among CD8+ T cells. For exhaustion markers, declines from baseline were evident for the percentage of PD-1+ cells among both CD4+ and CD8+ T cells across all treatment groups, by week 24, with further apparent declines for CD4+ T-cell exhaustion by week 96; week 96 levels were on average at least 56% lower than baseline levels. Differences by treatment group were not apparent for any immunosenescence or exhaustion marker (P > .1; Supplementary Table 1). Overall, sustained declines over time were evident in all treatment groups for CD4+ T-cell markers of senescence and exhaustion.
Figure 1.
Early and late changes in immunosenescence markers, by treatment arm. Point estimates and error bars reflect mean and 95% confidence intervals, respectively. Early change represents change from baseline to week 24; late change represents change from baseline to week 96. Abbreviations: ATV/r, atazanavir/ritonavir; DRV/r, darunavir/ritonavir; RAL, raltegravir; RTV, ritonavir.
Correlations Between Biomarkers of T-Cell Senescence, Exhaustion, Inflammation, and Immune Activation
At baseline, plasma viral load was correlated with markers of exhaustion (PD-1+; r = 0.24–0.31; P < .001) but not senescence (|r|<0.15, P > .05). With regard to associations of baseline markers of immunosenescence with markers of T-cell and monocyte activation or of inflammation at baseline (Supplementary Table 2), the strongest associations were apparent between the percentage of CD38+DR+ cells among CD4+ T cells and the percentages of CD28−CD57+ cells (r = 0.42) and PD-1+ cells (r = 0.40) among CD4+ T cells (Supplementary Figure 2). There was also a moderate association at baseline between monocyte activation (sCD163) and CD4+ T-cell exhaustion (ie, the percentage of PD-1+ cells among CD4+ T cells; r = 0.35). These moderate associations were also apparent when examining the concurrent changes over 96 weeks between these set of markers, with correlations ranging from 0.33 to 0.55 (Supplementary Table 3); in contrast, a moderate association between the levels of these markers at week 96 was only apparent for CD4+ T-cell activation and senescence (r = 0.44). All remaining associations between biomarkers were not apparent or were weak (|r|<0.3; Supplementary Tables 2, 3).
DISCUSSION
In this prospective study of ART-naive participants who achieved virological suppression after initiation of TDF/FTC along with RAL, ATV/r, or DRV/r, RAL did not have a more favorable effect on decreasing immunosenescence or exhaustion as compared to the PIs. To our knowledge, this is the largest prospective study describing changes in markers of immune senescence and exhaustion after initiation of successful ART. We also describe associations with markers of immune activation and inflammation that have been associated with serious clinical events in HIV–infected persons, including CVD and mortality [8]. Of note, although sustained and similar declines over time from baseline were evident in all treatment groups for all CD4+ T-cell markers of senescence and exhaustion, consistent reductions in markers of CD8+ T-cell senescence were not apparent. Overall, these results add to the current literature outlining the incomplete reversal of inflammation, senescence, and immune activation in the setting of effective treatment [9].
Understanding how different initial ART regimens reduce chronic immune dysfunction in successfully treated HIV infection is an ongoing research priority. Other studies have suggested that integrase inhibitors may reduce inflammation and viral load more effectively than other antiretroviral agents [10, 11]. Intensification with the integrase inhibitor RAL has shown variable effects on immune activation [10]. Despite this limited evidence, the differential effects of integrase inhibitors as compared to other ART agents on T-cell senescence remain unknown. We did not find any benefit of RAL over PIs on reducing immunosenescence or exhaustion during the first 96 weeks of successful treatment. This is consistent with a study by Kaplan et al, in which no differential effect of PIs were seen as compared to nonnucleoside reverse-transcriptase inhibitors on T-cell senescence [4]. A strength of our study is that, in contrast to prior RAL switch or intensification-related studies, our data are not confounded by prior ART.
The age-associated remodeling of the immune system through accumulation of senescent T cells may contribute to numerous aging-related pathologies [2]. Senescent T cells have been associated with CVD [3], but the differential role of CD4+ T-cell senescence as compared to CD8+ T-cell senescence in the development of chronic comorbidities such as CVD and bone disease remain unclear. CD8+ T-cell senescence has been associated with progression of numerous diseases [2], including CVD, in a study of HIV-infected patients [4], but this was not confirmed by others [5]. Senescent CD4+ T cells are rare, long-lived senescent T cells with proatherogenic properties [12] that may result from persistent antigenic stimulation [12] and play a critical role in the development of age-related pathologies [2]. HIV-infected patients with a low CD4+ T-cell count during ART have a greater number of senescent CD4+ T cells than those with increased CD4+ T-cell levels [13]. The lack of change in markers of CD8+ T-cell senescence in our study may be due to our cohort having a relatively high overall pre-ART CD4+ T-cell count and, perhaps, a lower overall pre-ART level of senescent CD8+ T cells, compared with cohorts in other studies [4, 5, 9]. Together, these prior studies highlight possible differential roles of senescent CD4+ T cells and senescent CD8+ T cells [14] in the pathogenesis of HIV and its comorbidities and are consistent with our results that effective ART differentially reduced CD4+ (and not CD8+) T-cell senescence.
In view of the limited data regarding the interplay between T-cell exhaustion, senescence, inflammation, and immune activation in chronic HIV infection, we found modest bivariate associations only between biomarkers of CD4+ and CD8+ T-cell activation and exhaustion at baseline and changes over 96 weeks. At week 96, these associations were apparent for CD4+ but not CD8+ T-cell activation and exhaustion biomarkers. The only other moderate association detected was between baseline biomarkers of CD8+ T-cell exhaustion and sCD163, a biomarker of monocyte activation that has been associated with CVD [15]. This finding may be explained by the fact that normal CD4+ T cells orchestrate multiple signals from T cells, B cells, and antigen-presenting cells and that senescent CD4+ T cells contribute to the pathogenesis of states of immune activation, such as autoimmunity [2]. Further studies are needed to elucidate the cross-talk between HIV infection, T-cell activation, T-cell senescence, and T-cell exhaustion and chronic comorbidities, such as CVD and bone disease.
Our study had several limitations that have previously been described. Briefly these include limited power to detect effect sizes with adjustment for multiple biomarker comparisons, selection bias of A5260s participants when restricting analysis to the cohort of virologically suppressed individuals who continued to receive ART, and inclusion of mostly men, which may limit generalizability of our findings. Finally, the relatively younger age of our study population with no CVD may have limited our ability to study the cross-talk between T-cell senescence, exhaustion, and immune activation.
In conclusion, in this prospective study we did not find differential changes in T-cell senescence and exhaustion after 96 weeks among treatment-naive individuals initiating and continuing to receive successful ART regimens of TDF/FTC with RAL, ATV/r, or DRV/r. Despite successful ART therapy, markers of CD8+ T-cell senescence did not decline; increased CD4+ T-cell activation was associated with increased CD4+ T-cell exhaustion and senescence. Overall, these data suggest differential roles of senescent CD4+ and CD8+ T cells in HIV immunopathogenesis in the setting of effective ART. Furthermore, these results also highlight the need to further understand the role of T-cell senescence and exhaustion in chronic HIV infection that may lead to adjunct ART regimens with more-potent antiinflammatory effects as a strategy to prevent chronic comorbidities associated with HIV infection.
STUDY GROUP MEMBERS
ACTG 5260s team members are as follows: H. Hodis, C. Godfrey, B. Jarocki, A. Benns, and K. Braun.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank the staff, and patients from the following hospitals who participate in the AIDS Clinical Trials Group (ACTG) (in alphabetical order): Beth Israel Deaconess Medical Center, Brigham and Womens Hospital, Case University Clinical Research site (CRS), Duke University Medical Center, Harbor–University of California, Los Angeles (UCLA) Medical Center, Houston AIDS Research Team CRS, John Hopkins Adult AIDS CRS, Metrohealth, New Jersey Medical School, New York University Human immunodeficiency Virus/AIDS CRS, Northwestern University, Rush University Medical Center ACTG, The Ohio State University, The Ponce De Leon Center CRS, UCLA Care Center, University of California, San Francisco AIDS CRS, University of Cincinnati, University of Colorado, University of North Carolina AIDS CRS, University of Pittsburg CRS, University of Rochester ACTG AIDS Care, University of Southern California, University of Washington, Vanderbilt Therapeutics CRS, and Washington University.
J. S. C., J. H. S., G. A. M., T. T. B., and T. K. were responsible for the study concept and design. T. T. T. T., C. M., and H. J. R. performed the statistical analyses. T. K., T. T. T. T., C. M., and G. A. M. drafted the manuscript. T. K., O. O. Y., M. P. D., J. H. S., J. S. C., G. A. M., and T. T. B. collected the data. All coauthors participated in discussions about the design of the study and interpretation of the findings and critically reviewed the manuscript.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH) or any of the funders.
Financial support. This research was supported by the NIH (grants HL095132, HL095126, AI 068636, AI068634, AI69471, AI069501, and AI56933), Gilead, Merck, Bristol Myers Squibb (BMS), Janssen, and the National Institute of Allergy and Infectious Diseases (awards UM1 AI068634, UM1 AI068636, and UM1 AI106701).
Potential conflicts of interest. T. T. B. has served as a consultant for BMS, GSK, Merck, Abbott, Gilead, and ViiV Healthcare and has received research funding from Merck and GSK. J. S. C. has served as a consultant for Gilead and has received research funding from Merck. J. H. S. served on a data safety monitoring board for Lilly. G. A. M. has served as consultant or received research grants from BMS, Pfizer, Gilead, ICON, and GSK/ViiV. M. P. D. has served as a consultant for Gilead and Astra Zeneca and receives research funding from Gilead, ViiV, and Merck. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Kelesidis T, Tran TT, Stein JH et al. . Changes in inflammation and immune activation with atazanavir-, raltegravir-, darunavir-based initial antiviral therapy: ACTG 5260s. Clin Infect Dis 2015; 61:651–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chou JP, Effros RB. T cell replicative senescence in human aging. Curr Pharm Des 2013; 19:1680–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samani NJ, Boultby R, Butler R, Thompson JR, Goodall AH. Telomere shortening in atherosclerosis. Lancet 2001; 358:472–3. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan RC, Sinclair E, Landay AL et al. . T cell activation and senescence predict subclinical carotid artery disease in HIV-infected women. J Infect Dis 2011; 203:452–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tenorio AR, Zheng Y, Bosch RJ et al. . Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis 2014; 210:1248–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown TT, Moser C, Currier JS et al. . Changes in bone mineral density after initiation of antiretroviral treatment with tenofovir disoproxil fumarate/emtricitabine plus atazanavir/ritonavir, darunavir/ritonavir, or raltegravir. J Infect Dis 2015; 212:1241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stein JH, Ribaudo HJ, Hodis HN et al. . A prospective, randomized clinical trial of antiretroviral therapies on carotid wall thickness. AIDS 2015; 29:1775–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duprez DA, Neuhaus J, Kuller LH et al. . Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS One 2012; 7:e44454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tassiopoulos K, Landay A, Collier AC et al. . CD28-negative CD4+ and CD8+ T cells in antiretroviral therapy-naive HIV-infected adults enrolled in adult clinical trials group studies. J Infect Dis 2012; 205:1730–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hatano H, Hayes TL, Dahl V et al. . A randomized, controlled trial of raltegravir intensification in antiretroviral-treated, HIV-infected patients with a suboptimal CD4+ T cell response. J Infect Dis 2011; 203:960–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scopelliti F, Pollicita M, Ceccherini-Silberstein F et al. . Comparative antiviral activity of integrase inhibitors in human monocyte-derived macrophages and lymphocytes. Antiviral Res 2011; 92:255–61. [DOI] [PubMed] [Google Scholar]
- 12.Liuzzo G, Biasucci LM, Trotta G et al. . Unusual CD4+CD28null T lymphocytes and recurrence of acute coronary events. J Am Coll Cardiol 2007; 50:1450–8. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez S, French MA, Price P. Immunosenescent CD57+CD4+ T-cells accumulate and contribute to interferon-gamma responses in HIV patients responding stably to ART. Dis Markers 2011; 31:337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valenzuela HF, Effros RB. Divergent telomerase and CD28 expression patterns in human CD4 and CD8T cells following repeated encounters with the same antigenic stimulus. Clin Immunol 2002; 105:117–25. [DOI] [PubMed] [Google Scholar]
- 15.Burdo TH, Lo J, Abbara S et al. . Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis 2011; 204:1227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.