Abstract
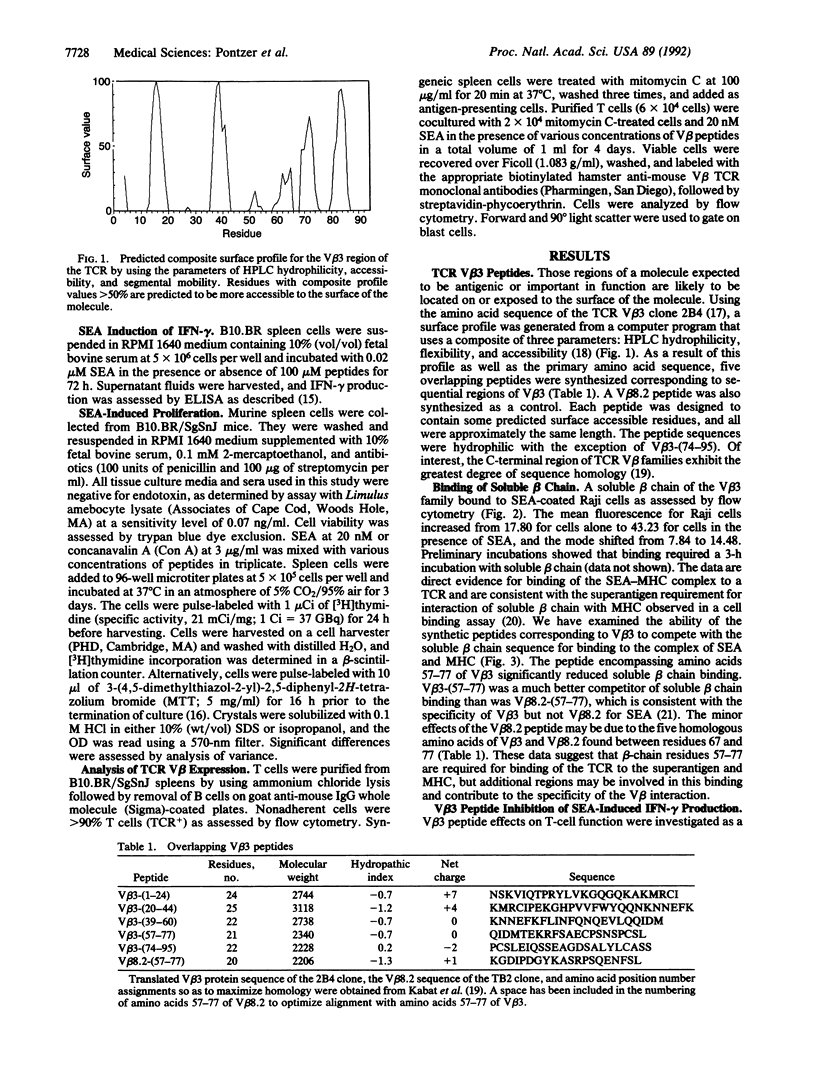
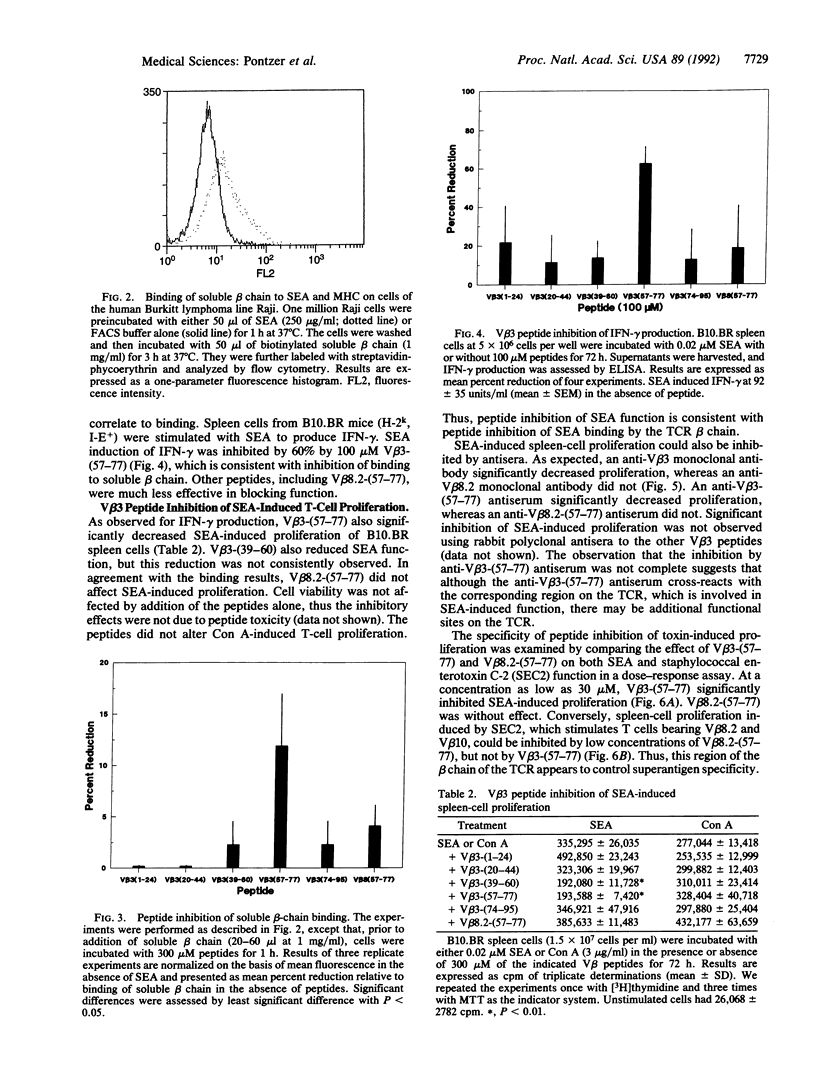
We have examined the interaction of the microbial superantigen staphylococcal enterotoxin A (SEA) with peptides corresponding to overlapping regions of the T-cell antigen receptor beta chain variable region V beta 3. SEA is known to stimulate murine T cells bearing certain V beta elements, among them V beta 3. Five peptides were synthesized representing amino acids 1-24, 20-44, 39-60, 57-77, and 74-95 of V beta 3. We demonstrate here that soluble V beta 3-bearing beta chains can bind to a complex of SEA and major histocompatibility complex class II and that the synthetic peptide V beta 3-(57-77) blocked this interaction. The peptide V beta 3-(57-77) also inhibited SEA-induced interferon-gamma production and SEA-induced proliferation of B10.BR spleen cells. Conversely, the peptide corresponding to amino acids 57-77 of V beta 8.2, a V beta element that is not recognized by SEA, decreased staphylococcal enterotoxin C-2-induced proliferation but did not affect SEA-induced proliferation. The peptide inhibition of SEA-induced function was due at least in part to inhibition of V beta 3-bearing T-cell activity, since the percentage of T cells reactive with an anti-V beta 3 monoclonal antibody was significantly reduced by V beta 3-(57-77). These data suggest that the region of V beta 3 encompassing amino acids 57-77 is an area that displays the appropriate sequence and conformation for binding of the SEA molecule and blocking of the resultant interaction with the T-cell antigen receptor.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Callahan J. E., Herman A., Kappler J. W., Marrack P. Stimulation of B10.BR T cells with superantigenic staphylococcal toxins. J Immunol. 1990 Apr 1;144(7):2473–2479. [PubMed] [Google Scholar]
- Carlsson R., Sjögren H. O. Kinetics of IL-2 and interferon-gamma production, expression of IL-2 receptors, and cell proliferation in human mononuclear cells exposed to staphylococcal enterotoxin A. Cell Immunol. 1985 Nov;96(1):175–183. doi: 10.1016/0008-8749(85)90349-1. [DOI] [PubMed] [Google Scholar]
- Cazenave P. A., Marche P. N., Jouvin-Marche E., Voegtlé D., Bonhomme F., Bandeira A., Coutinho A. V beta 17 gene polymorphism in wild-derived mouse strains: two amino acid substitutions in the V beta 17 region greatly alter T cell receptor specificity. Cell. 1990 Nov 16;63(4):717–728. doi: 10.1016/0092-8674(90)90138-5. [DOI] [PubMed] [Google Scholar]
- Chang C. D., Meienhofer J. Solid-phase peptide synthesis using mild base cleavage of N alpha-fluorenylmethyloxycarbonylamino acids, exemplified by a synthesis of dihydrosomatostatin. Int J Pept Protein Res. 1978 Mar;11(3):246–249. doi: 10.1111/j.1399-3011.1978.tb02845.x. [DOI] [PubMed] [Google Scholar]
- Chien Y. H., Gascoigne N. R., Kavaler J., Lee N. E., Davis M. M. Somatic recombination in a murine T-cell receptor gene. Nature. 1984 May 24;309(5966):322–326. doi: 10.1038/309322a0. [DOI] [PubMed] [Google Scholar]
- Choi Y. W., Herman A., DiGiusto D., Wade T., Marrack P., Kappler J. Residues of the variable region of the T-cell-receptor beta-chain that interact with S. aureus toxin superantigens. Nature. 1990 Aug 2;346(6283):471–473. doi: 10.1038/346471a0. [DOI] [PubMed] [Google Scholar]
- Chothia C., Boswell D. R., Lesk A. M. The outline structure of the T-cell alpha beta receptor. EMBO J. 1988 Dec 1;7(12):3745–3755. doi: 10.1002/j.1460-2075.1988.tb03258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claverie J. M., Prochnicka-Chalufour A., Bougueleret L. Implications of a Fab-like structure for the T-cell receptor. Immunol Today. 1989 Jan;10(1):10–14. doi: 10.1016/0167-5699(89)90058-3. [DOI] [PubMed] [Google Scholar]
- Davis M. M., Bjorkman P. J. T-cell antigen receptor genes and T-cell recognition. Nature. 1988 Aug 4;334(6181):395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- Fleischer B., Gerardy-Schahn R., Metzroth B., Carrel S., Gerlach D., Köhler W. An evolutionary conserved mechanism of T cell activation by microbial toxins. Evidence for different affinities of T cell receptor-toxin interaction. J Immunol. 1991 Jan 1;146(1):11–17. [PubMed] [Google Scholar]
- Gascoigne N. R., Ames K. T. Direct binding of secreted T-cell receptor beta chain to superantigen associated with class II major histocompatibility complex protein. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):613–616. doi: 10.1073/pnas.88.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascoigne N. R. Transport and secretion of truncated T cell receptor beta-chain occurs in the absence of association with CD3. J Biol Chem. 1990 Jun 5;265(16):9296–9301. [PubMed] [Google Scholar]
- Gerlier D., Thomasset N. Use of MTT colorimetric assay to measure cell activation. J Immunol Methods. 1986 Nov 20;94(1-2):57–63. doi: 10.1016/0022-1759(86)90215-2. [DOI] [PubMed] [Google Scholar]
- Grossman D., Van M., Mollick J. A., Highlander S. K., Rich R. R. Mutation of the disulfide loop in staphylococcal enterotoxin A. Consequences for T cell recognition. J Immunol. 1991 Nov 15;147(10):3274–3281. [PubMed] [Google Scholar]
- Janeway C. A., Jr, Yagi J., Conrad P. J., Katz M. E., Jones B., Vroegop S., Buxser S. T-cell responses to Mls and to bacterial proteins that mimic its behavior. Immunol Rev. 1989 Feb;107:61–88. doi: 10.1111/j.1600-065x.1989.tb00003.x. [DOI] [PubMed] [Google Scholar]
- Jarpe M. A., Hayes M. P., Russell J. K., Johnson H. M., Russell S. W. Causal association of interferon-gamma with tumor regression. J Interferon Res. 1989 Apr;9(2):239–244. doi: 10.1089/jir.1989.9.239. [DOI] [PubMed] [Google Scholar]
- Johnson H. M., Magazine H. I. Potent mitogenic activity of staphylococcal enterotoxin A requires induction of interleukin 2. Int Arch Allergy Appl Immunol. 1988;87(1):87–90. doi: 10.1159/000234654. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Roehm N., Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987 Apr 24;49(2):273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- Langford M. P., Stanton G. J., Johnson H. M. Biological effects of staphylococcal enterotoxin A on human peripheral lymphocytes. Infect Immun. 1978 Oct;22(1):62–68. doi: 10.1128/iai.22.1.62-68.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker J. M., Guo D., Hodges R. S. New hydrophilicity scale derived from high-performance liquid chromatography peptide retention data: correlation of predicted surface residues with antigenicity and X-ray-derived accessible sites. Biochemistry. 1986 Sep 23;25(19):5425–5432. doi: 10.1021/bi00367a013. [DOI] [PubMed] [Google Scholar]
- Pontzer C. H., Russell J. K., Johnson H. M. Localization of an immune functional site on staphylococcal enterotoxin A using the synthetic peptide approach. J Immunol. 1989 Jul 1;143(1):280–284. [PubMed] [Google Scholar]
- Pullen A. M., Bill J., Kubo R. T., Marrack P., Kappler J. W. Analysis of the interaction site for the self superantigen Mls-1a on T cell receptor V beta. J Exp Med. 1991 May 1;173(5):1183–1192. doi: 10.1084/jem.173.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullen A. M., Marrack P., Kappler J. W. The T-cell repertoire is heavily influenced by tolerance to polymorphic self-antigens. Nature. 1988 Oct 27;335(6193):796–801. doi: 10.1038/335796a0. [DOI] [PubMed] [Google Scholar]
- Pullen A. M., Wade T., Marrack P., Kappler J. W. Identification of the region of T cell receptor beta chain that interacts with the self-superantigen MIs-1a. Cell. 1990 Jun 29;61(7):1365–1374. doi: 10.1016/0092-8674(90)90700-o. [DOI] [PubMed] [Google Scholar]
- Russell J. K., Jarpe M. A., Johnson H. M. Evidence for the alpha-helicity of class II MHC molecular binding sites for the superantigen, staphylococcal enterotoxin A. Biochem Biophys Res Commun. 1992 Feb 14;182(3):1016–1024. doi: 10.1016/0006-291x(92)91833-c. [DOI] [PubMed] [Google Scholar]
- Russell J. K., Pontzer C. H., Johnson H. M. The I-A beta b region (65-85) is a binding site for the superantigen, staphylococcal enterotoxin A. Biochem Biophys Res Commun. 1990 Apr 30;168(2):696–701. doi: 10.1016/0006-291x(90)92377-c. [DOI] [PubMed] [Google Scholar]
- White J., Herman A., Pullen A. M., Kubo R., Kappler J. W., Marrack P. The V beta-specific superantigen staphylococcal enterotoxin B: stimulation of mature T cells and clonal deletion in neonatal mice. Cell. 1989 Jan 13;56(1):27–35. doi: 10.1016/0092-8674(89)90980-x. [DOI] [PubMed] [Google Scholar]



