Abstract
Upper limb amputees receive no proprioceptive or visual sensory feedback about their absent hand. In this study, we asked whether chronic amputees nevertheless retain the ability to accurately plan gripping movements. Fourteen patients and matched controls performed two grip selection tasks: overt grip selection (OGS), in which they used their intact hand to grasp an object that appeared in different orientations using the most natural (under- or overhand) precision grip, and prospective grip selection (PGS), in which they selected the most natural grip for either hand without moving. We evaluated planning accuracy by comparing concordance between grip preferences expressed in PGS vs. OGS for the intact hand and PGS vs. the inverse of OGS responses for the affected hand. Overall, amputees showed no deficits in the accuracy of grip selection planning based on either hand and a consistent preference for less awkward hand postures. We found no evidence for a speed-accuracy tradeoff. Furthermore, selection accuracy did not depend on phantom mobility, phantom limb pain, time since amputation, or the residual limb’s shoulder posture. Our findings demonstrate that unilateral upper limb amputees retain the ability to plan movements based on the biomechanics of their affected hand even many years after limb loss. This unimpaired representation may stem from persistent higher-level activity-independent internal representations or may be sustained by sensory feedback from the intact hand.
Keywords: Amputation, Deafferentation, Grip selection, Movement planning, Human, Upper limb
Introduction
While the last three decades have shown substantial progress toward understanding the central neurophysiological changes arising from peripheral nerve injuries including limb amputation, the links between these central changes and behavioral consequences remain opaque. Cortical reorganization of primary sensory and motor maps after forelimb amputation has long been recognized (e.g., Merzenich et al. 1983; Donoghue and Sanes 1988; Sanes et al. 1988; Kaas 2000), including expanded representations of somatotopically adjacent body representations. However, behavioral evidence for accompanying changes in performance remains scant and difficult to replicate (Haber 1958; Moore and Price 1999; Moore and Schady 2000). In apparent contrast to the evidence for map reorganization, there are reasons to believe that cortical representations of the amputated or deafferented limb may persist even long after injury (see Reilly and Sirigu 2008 for review), as revealed by transcranial magnetic stimulation over primary motor cortex in amputees who experience phantom limb phenomena (Pascual-Leone et al. 1996; Mercier et al. 2006) and single-unit recordings from primary motor cortex in tetraplegic patients Hochberg et al. (2006).
These findings of functionally persistent representations seem especially interesting when considered from the perspective of internal models for movement control. Specifically, internal forward models are hypothesized to play a major role in predicting sensory consequences slightly in advance of ongoing movements (e.g., Wolpert et al. 1998) and, perhaps, also may take part in long-range forecasting and predicting of movement outcomes (Frey 2010). Importantly, these internal models require regular updates from sensory and motor signals (e.g., Desmurget et al. 1999), something that would seem impossible for a missing limb. By investigating human amputees’ ability to internally represent and predict movements of their absent hand, we can improve our understanding of the behavioral consequences of amputation.
Because of the lack of afferent feedback from an absent hand, we expect impaired performance in tasks that require amputees to internally represent movements of their absent hand. A study by Nico et al. (2004) demonstrated that unilateral upper limb amputees have a relatively preserved ability to solve a hand laterality (left–right) judgment task that presumably involves motor imagery. Amputees showed small accuracy deficits for decisions involving their amputated hand, but still successfully performed the task. While this provides some evidence for preserved motor representations for an amputated hand, 31% of the participants reported using non-motor strategies. Other studies have also demonstrated that participants can and do solve mental object rotation tasks using non-motor strategies (Tomasino and Rumiati 2004; Daprati et al. 2010).
In order to investigate changes in motor planning in chronic unilateral upper limb amputees, we used a prospective grip selection task (PGS; e.g., Johnson 2000a; Johnson et al. 2002a). In this task, participants decided whether they would prefer an under- or overhand grasp to engage a stimulus object appearing in numerous orientations. Notably, the PGS task requires selection of a movement outcome without requiring use of explicit motor imagery. In order to evaluate accuracy, these prospective grip selection judgments (made while remaining still) are then compared with actual grip preferences exhibited in a comparable task involving overt movements. This procedure has proven useful in evaluating the accuracy of internal representations of the limbs’ biomechanical constraints in healthy (Johnson 1998) and hemiparetic adults (Johnson 2000b; Johnson et al. 2002a; Jenkinson et al. 2009) and shows consistent engagement of posterior parietal, premotor, and cerebellar mechanisms implicated in movement planning and control (Johnson et al. 2002b; Jacobs et al. 2010; Marangon et al., in press).
In addition, we took advantage of the consistent finding that changes in real or imagined body posture affect the performance of imagined movements in healthy adults (e.g., Parsons 1994; Shenton et al. 2004; De Lange et al. 2006; Ionta and Blanke 2009), by manipulating the posture of the residual limb in a way that would have also affected the position of the missing hand (were it still present). We reasoned that if mechanisms involved in prospective grip selection rely on current estimates of the involved effector’s state (even when distal segments are physically absent), then this manipulation should affect the accuracy and/or timing of grip selection judgments. The inclusion of a current state estimate in prospective movement planning would also be consistent with the hypothesis that motor planning (e.g., Jeannerod 1994; Wolpert and Miall 1996; for review, see Wolpert and Flanagan 2001), and possibly motor imagery (Grush 2004; Frey 2010), involve forward internal models.
Here, amputees performed a grip selection task in multiple shoulder postures. First and foremost, we hypothesized that amputees would show reduced grip selection accuracy for the affected hand, compared to the intact hand and to matched controls. We reasoned that this effect might arise from experience-dependent changes in the internal representation of their affected hand due to chronic absence of sensory feedback and possible reorganization of neural mechanisms. Second, we hypothesized that changes in shoulder posture would entail reduced effects on movement planning in amputees compared to controls. This outcome would suggest a reduced reliance on current sensory input for planning movements based on the now-missing limb. Either hypothesis, if supported, would provide functional evidence for altered motor representations in human amputees.
Materials and methods
Participants
Fourteen unilateral upper limb amputees (3 women, ages 49 ± 16 yrs) gave informed consent to participate in this study, as well as 14 healthy controls matched for age and handedness. All participants had normal or corrected-to-normal visual acuity. Amputees were 28 ± 16 years post-injury. See Table 1 for details on the amputee sample. Amputees rated their current level of phantom limb pain (PLP) and of phantom vividness on a visual analog scale (VAS), immediately before performing the experiments. An amputees’ prosthesis usage was considered “none” if they had not used any prosthesis in the last 5 years. All participants performed both tasks in counterbalanced order.
Table 1.
Demographic data on amputee participants
| Amputee participant characteristics
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Age | Sex | Above/below elbow | Affected side (left, right) | Dominant hand prior to limb loss | Years since amputee | Prosthesis | Current pain (0–1) | Current phantom (0–1) |
| 62 | M | BE | Right | Right | 42 | Mechanical | 0 | 0 |
| 53 | M | BE | Right | Right | 38 | None | 0 | 0.43 |
| 24 | M | AE | Right | Right | 15 | Cosmetic | 0.58 | 0.59 |
| 52 | F | AE | Right | Right | 31 | None | 1 | 1 |
| 50 | F | BE | Left | Right | 48 | None | 0 | 0.94 |
| 56 | M | AE | Right | Right | 34 | None | 0.19 | 0 |
| 27 | F | AE | Left | Right | 18 | None | 0.21 | 0.75 |
| 66 | M | BE | Right | Right | 45 | Mechanical | 0 | 0 |
| 62 | M | AE | Right | Right | 26 | None | 0.05 | 0 |
| 73 | M | AE | Right | Right | 8 | None | 0.22 | 0.09 |
| 61 | M | BE | Left | Right | 40 | Mechanical | 0 | 0.77 |
| 42 | M | BE | Left | Right | 38 | Mechanical | 0 | 0 |
| 28 | M | BE | Left | Right | 6 | Cosmetic | 0.35 | 0 |
| 35 | M | BE | Right | Right | 1 | None | 0 | 0.75 |
Current pain level and phantom (vividness of phantom mobility) measured by analog visual scale between experiments, from 0 (no pain/immobile) to 1 (vivid sensations of movement/worst pain imaginable). Prosthesis type measured by usage in the past 5 years
When referring to controls, the terms “affected” and “intact” refer to the hands that respectively match the affected (amputated) and intact sides of their yoked amputee participant.
All participants gave informed consent, and all procedures were approved by the local IRB and in accordance with the Declaration of Helsinki.
Apparatus and stimuli
Stimuli consisted of a graphically rendered “widget” [see Fig. 1a for example stimuli, and see Jacobs et al. (2010) for additional detail]. The widgets were 68-mm diameter (approximately 1.16° visual angle) shapes comprising a circle with indentations on opposite sides for finger positioning, allowing for only two precision grip orientations. Half of each stimulus was colored pink, and the other half was colored tan. Hand orientation during grip was identified by whether the thumb ended up on the pink side or tan side of the widget.
Fig. 1.
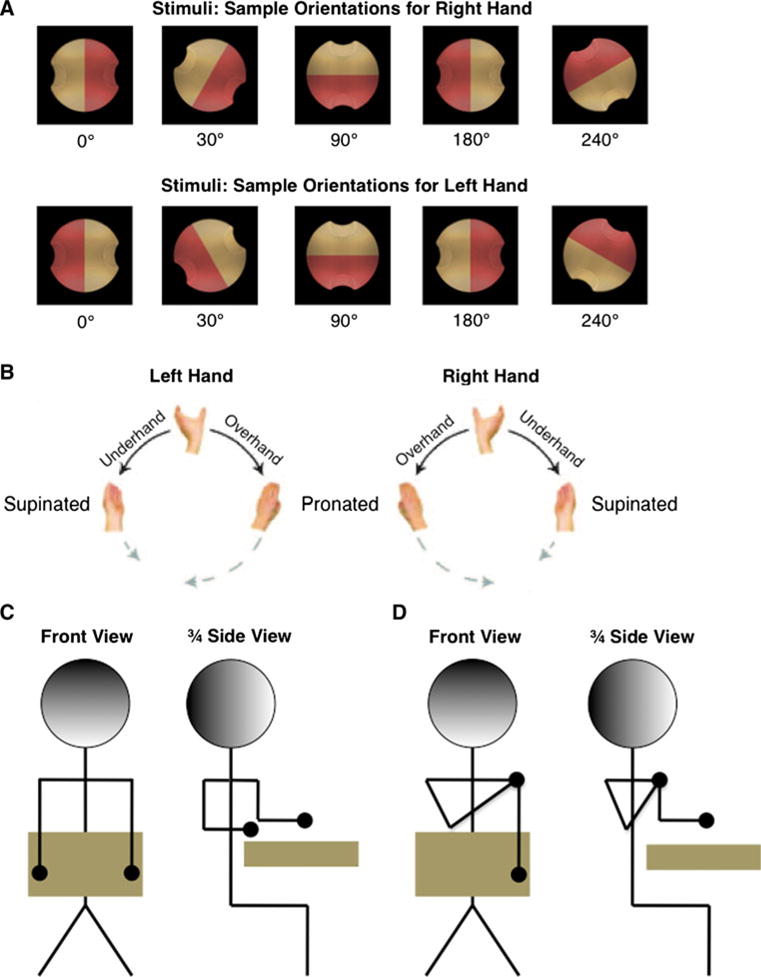
Experimental components. a Stimulus object at 4 sample orientations for each hand. b Possible hand postures. Adapted from Jacobs et al. (2010). c Illustration of “neutral” posture, both arms down. d “Crossed” postures, with one arm (here, right) crossed over chest
To create 3D objects for grasping, a transparent plastic (lexan) overlay was fitted over the surface of the computer monitor. The center of the overlay was a 3D transparent plastic disk of 32-mm radius, extending 25 mm from the surface of the screen. The plastic disk was equivalent in size to the stimulus, creating the appearance of a graspable object extending from the surface of the screen. A touch sensor wire (E112 Capacitive Touch Sensor, Quantum Research Group, Pittsburgh PA) was wrapped around the disk’s circumference.
A central fixation cross remained visible throughout the experiment, and participants were instructed to maintain fixation. Because the stimuli were also presented at the center of the screen, the fixation cross appeared in the middle of the stimulus object.
Orientations
Stimuli appeared against a black background in 12 orientations (30°) increments rotated around the line-of-sight (“z”) axis. Stimulus orientations were defined in terms of relative hand orientation (henceforth referred to as orientation, for brevity). Orientation accounts for the specific biomechanics of each hand; in that, a 30° orientation refers to a stimulus rotation equivalent to a 30° external rotation of the hand, compared to a 0° orientation. For example, a 0° stimulus for the right-hand would be the same image as a 180° stimulus for the left-hand (as shown in Fig. 1a); in either case, the thumb would be on the tan indentation at a neutral (overhand) posture. This enables direct comparison of grip preferences between left and right-hands (Johnson 2000a).
For each orientation, there were two possible grips, under- or overhand. These were determined by the color of the stimulus’ indentation on which the participant chose to place their thumb. Thumb placement represents this choice in terms of the final hand position. For example, a 0° stimulus orientation allows thumb placements of 0° (on the tan indentation) or 180° (on the pink indentation).
Posture manipulation
During the experiments, participants placed their arms in positions that entailed one of three shoulder postures (Fig. 1c, d). For arm-down (neutral) posture, participants rested their forearm on the table in front of them in a neutral position, palm down. For arm-crossed postures, participants crossed one arm (left or right, depending on posture condition) over their chest, with fingers touching the outside their opposite shoulder and their upper arm at an angle approximately 15° from vertical (Fig. 1d). For all postures, hand positioning was controlled by requiring participants to keep their index finger on a rest key. To ensure that the rest key remained in its intended position, the rest key was attached by Velcro to the table or to a vest worn by the participant.
For amputees, rest key positioning was adjusted to account for the individual participant’s residual limb. The arm-down posture always entailed the upper arm hanging vertically by the participant’s side, and the arm-crossed posture always entailed the upper arm crossing the body at approximately 15° from vertical. If an amputee was unable to hold down a rest key due to limited length or mobility of their residual limb, the position of that limb was monitored by experimenter.
During Experiment 1 (OGS), participants returned to the appropriate posture during each ITI; during Experiment 2 (PGS), participants remained in the appropriate posture throughout the session.
Experiment 1 procedure: overt grip selection (OGS) task
During OGS, participants were required to reach and grasp a stimulus object presented in various orientations in the most comfortable (precision grip) manner by placing the pads of the thumb (digit 1) and forefinger (digit 2) on the small indentations (see Fig. 1a). Participants could use either an over- or underhand grip (Fig. 1b).
The OGS experiment comprised a single session of 144 trials, divided into 6 blocks of 24 trials each. Posture alternated between blocks, with the posture during the first block counterbalanced across participants. All participants used the intact hand throughout. Thus, Experiment 1 entailed 4 trials of each combination of 12 stimulus orientations * 3 postures.
Each session began with a single 10-trial practice block to familiarize participants with the OGS task, with the participant in a neutral posture (both arms down). Each trial comprised the following epochs, as shown in Fig. 2a: (1) a variable delay of 0, 500, 1000, or 1500 ms; (2) a stimulus object, lasting 3000 ms; (3) a posture-return delay, lasting until the participant returned their hand to the rest key; and (4) an inter-trial interval (ITI), 1500-ms duration. Trial epochs 1–3 included a blue dot (7 mm diameter, 0.13° visual angle) on the same side of the screen as their hand, to warn the participant of an upcoming stimulus and remind them which hand to use.
Fig. 2.
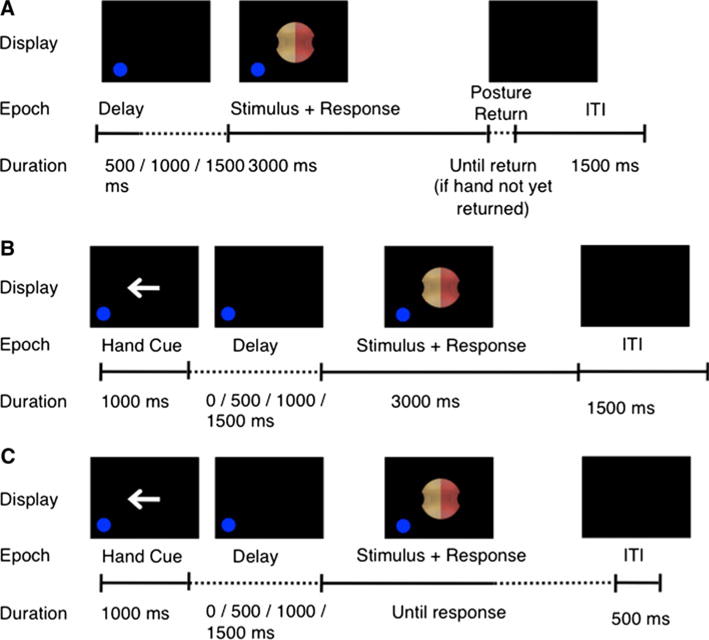
Trial time course. a Overt grip selection (OGS) task. b Prospective grip selection (PGS) task. c Awkwardness rating test
When the stimulus object appeared, participants were instructed to immediately reach to and grasp it, using the specified hand, in an under- or overhand precision grip. The experimenter used a keyboard to record on which color the participant placed his or her thumb. Trials were aborted if the participant moved either hand off the rest key during any trial stage other than the stimulus presentation stage or if the participant moved the wrong hand off the rest key during the stimulus presentation phase. Note that these rules resulted in an aborted trial if the participant was not in the correct posture, since such an error would entail a failure to press the correct rest key. If a trial was aborted, presentation paused until the participant reacquired the rest keys correctly; subsequently, an ITI was presented, followed by the next trial in the sequence.
Experiment 2 procedure: prospective grip selection (PGS) task
During PGS, participants were presented with the same stimuli as described earlier and instructed to remain still while reporting which side (“pink” or “tan”) of the stimulus their thumb would contact if they had grasped the object using the specified hand in a precision grip. Participants reported this response by speaking the appropriate color name into a microphone, and the experimenter recorded the chosen color. Microphone input was used only to determine onset time of vocal response (response time, RT). Participants were instructed to respond as quickly and accurately as possible; instructions did not include any mention of imagery or imagination. All participants performed PGS with both hands, since no overt movements were required.
For participants who performed Experiment 2 (PGS) first, they were presented with 5 OGS trials to familiarize themselves with the precision grasping movements and with the stimuli. During this familiarization session, the stimulus appeared at orientations not used during the main task. In addition, all participants performed 10 practice PGS trials before beginning data collection. Practice and familiarization blocks were performed with the arms in a neutral posture (arms down).
Similar to Experiment 1, the PGS experiment comprised a single session of 288 trials, divided into 6 blocks of 48 trials each. Posture alternated between blocks, and the posture during the first block was counterbalanced across participants. Hand varied between trials and counterbalanced within each block. Thus, Experiment 2 entailed 4 trials of each combination of 12 stimulus orientations * 2 hands * 3 postures.
Each PGS trial followed the structure of OGS trials, with two exceptions (Fig. 2b). First, each trial began with a hand choice cue of 1000-ms duration, in the form of an arrow pointing either left or right to indicate the hand used in the current trial. Second, there was no posture-return delay.
Trials were aborted if the participant moved either hand off the rest key at any time. As during Experiment 1, this rule resulted in an aborted trial if the participant was not in the correct posture.
Data collection and analysis
For Experiment 1 (OGS), onset time (OT) was defined as the time from start from stimulus appearance to release of the rest key, and movement time (MT) was defined as the time between release of the rest key and contact with the on the 3D plastic overlay, as detected by the touch sensor detailed above. For Experiment 2 (PGS), response time (RT) was defined as the time from stimulus appearance to start of vocal response. Trials with OT, MT, or RT more than two standard deviations from the mean were eliminated from the analyses; this eliminated 7.8 ± 2.1% of OGS trials and 4.8 ± 1.2% of PGS trials. Outlier rate did not differ between controls and amputees in either task (t test P > 0.6). Repeated-measures ANOVAs were performed on group means as described later in the text, with all post hoc comparisons carried out via Tukey’s HSD test. Correlations were calculated using Pearson’s r when the assumptions were met. The non-parametric measure of Kendall’s τ was also used because of its robustness in the presence of small sample sizes and outliers.
For a simple measure of grip preferences, we computed “choice likelihood” (CL) for each thumb placement. The CL was the probability of choosing to place the thumb in one versus the opposite indentation (that is, the probability of choosing a given thumb placement over the opposite option). For example, participants could choose to grasp a 0° orientation stimulus with thumb placements of 0° (pink) or 180° (tan). Thus, CL for a 0° thumb placement equaled the count of grasps with 0° thumb placement, divided by the total count of 0° and 180° placements. CL was computed independently for each hand and task.
PGS accuracy was determined by comparing the similarity between grip preferences during OGS execution, and grip preferences revealed during PGS selection, following the procedure detailed in Johnson (2000a). Briefly, we estimate an amputee’s OGS preferences with their affected hand as the inverse of their preferences for the intact hand, such that
| (1) |
In this equation, OGleft and OGright refer to the probabilities of selecting an overhand grip for a given stimulus orientation using the left- or right-hand, respectively. This takes advantage of how opposite arms and hands obey joint constraints 180° out of phase (MacKenzie and Iberall 1994) and prior evidence showing that, in healthy adults, grip preferences for one hand are virtually identical to the inverse of those from the other hand (R = 0.99, Experiment 3, Johnson 2000a). Using formula (1), PGS preferences can then be compared with estimated (for the affected hand) and actual (for the intact hand) OGS choices for amputees. For judgments with hand k and a stimulus in a particular orientation,
| (2) |
Accuracy scores were computed separately for each participant, hand, arm posture, and orientation.
Awkwardness ratings
Ten naïve right-handed participants (6 female, ages 23 ± 3 yrs) performed a variant of the OGS task to determine subjective awkwardness ratings for each hand. The awkwardness task comprised 92 trials, divided into 2 blocks of 48 trials each. Each block contained 2 trials for each combination of 12 stimulus orientations and 2 hands. Participants used a neutral posture (arms down, as described earlier) throughout.
The procedure followed Experiment 1, with two exceptions. First, the stimulus object lasted until the participant responded (Fig. 2c). Second, participants were instructed to grip the stimulus between thumb and index finger in a precision grip, using a grip that would put their thumb in contact with the pink indentation.
After adopting the grip, participants rated the movement from 1 (not awkward at all) to 6 (impossible). Awkwardness ratings were z-scored within each participant, to eliminate any between-participant differences in offset or scaling.
Results
Our results characterize the performance of healthy controls and unilateral upper limb amputees during overt and prospective grip selection tasks. After a brief overview of task performance and awkwardness ratings, we examine the effects of arm posture on both kinds of grip selection movements. Informed by our results from the analysis of arm posture effects, we then describe the differences in grip selection behavior between healthy participants and unilateral upper limb amputees. Finally, we conclude by examining the influence of participant characteristics on grip selection.
Task performance
On average, control participants successfully performed the OGS task on 95 ± 5% of trials, as measured by the frequency of trials on which they moved directly to a correct position (i.e., either supinated or pronated with a precision grip aligned at the indentations on the stimulus) within 3 s of stimulus presentation. Controls performed the PGS task successfully on 91 ± 5% of trials, as measured by the frequency of trials on which the participant gave a single clear verbal response within 3 s. For amputees, the mean success rate was 97 ± 3% for OGS and 93 ± 5% for PGS. For either task, success rate did not differ significantly between groups (OGS P = 0.212, PGS P = 0.164). Given the near-ceiling performance on both tasks, we did not analyze errors in further detail.
Awkwardness ratings
As shown in Fig. 3 (black lines), a separate group of naïve participants reported minimal awkwardness for thumb placements of 90–120° and maximum awkwardness for thumb placements of 240–300°. Figure 3 also shows the likelihood of control participants (gray lines) and amputees (dotted lines) selecting each grip (i.e., the choice likelihood) at each orientation. As expected, control and amputee participants were much more likely to choose non-awkward grips in OGS. The correlation between OGS choice likelihood and awkwardness for controls was −0.948 for the left-hand and −0.922 for the right-hand; for amputees, these correlations reached −0.908 and −0.935, respectively (P < 0.0001 for all four). Note that this strong inverse relationship between grip preferences and awkwardness arises even though we calculated the two measures from different populations (choice likelihoods from amputees and matched controls and awkwardness ratings from naïve young participants).
Fig. 3.
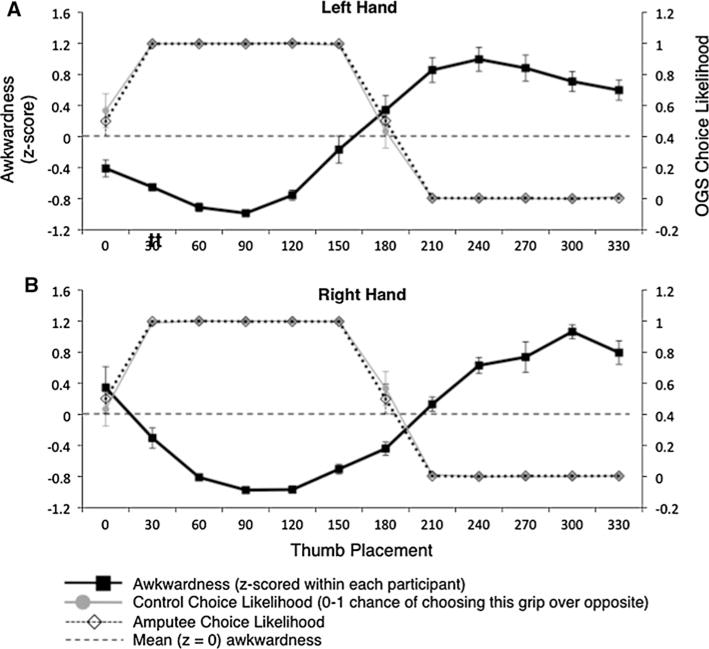
Awkwardness ratings and OGS choice likelihood, for each thumb placement, group mean ± SEM. All OGS data from intact hand, mirror reversed to produce affected (left) hand data; see text for details. Congruence between high choice likelihood and low awkwardness demonstrates selection of non-awkward grips. a Left-hand. b Right-hand
More interestingly, despite the absence of movements, grip preferences in PGS also showed a strong negative correlation with rated awkwardness, as shown by previous work with healthy controls (Johnson 2000a). When calculating PGS choice likelihood from either group (amputee or control) or hand (intact or affected), the correlation between choice likelihood and awkwardness rating ranged from −0.896 to −0.970 (P < 0.0001 in all cases). Figure 4 illustrates this by demonstrating the relationship between awkwardness, control choice likelihoods, and amputee choice likelihoods, for the affected hand in the PGS task. Recall that a control participant’s “affected” hand was determined by matching with their yoked amputee participant. Notably, Fig. 4 also illustrates the match between grip selections in amputees and controls. Group mean choice likelihoods were nearly identical between the two groups (left-hand r = 0.989, P < 0.0001; right-hand r = 0.987, P < 0.0001). This demonstrates that amputees and controls show similar patterns of orientation sensitivity in their grip selections.
Fig. 4.
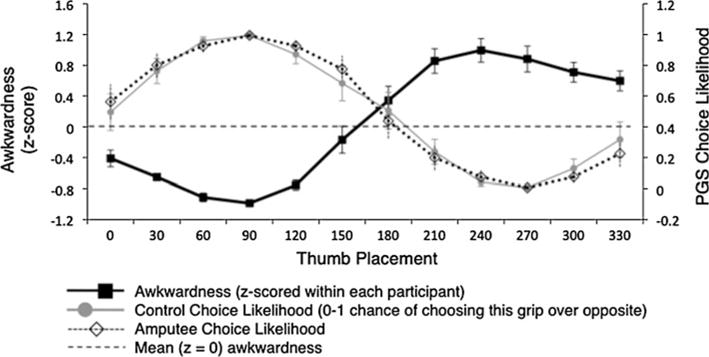
Relationship between awkwardness ratings and PGS choice likelihood, for affected hand in both amputees and controls. See Fig. 3 for format details
Posture effects
We expected to find hand-specific effects of shoulder posture on PGS, which would be reduced for amputees, compared to controls. We measured the effect of shoulder posture on onset time (OT), movement time (MT), response time (RT), and PGS accuracy. We quantified these effects via a 3 (posture) * 12 (orientation) * 2 (group) ANOVA for OGS; for PGS, because we obtained measurements for both hands, we added an additional two-level factor (hand: affected and intact). Rather than presenting these results in detail, we summarize them in Fig. 5. Crucially, arm posture never influenced PGS accuracy (F < 1.0), and we did not detect a significant group * posture interaction on RT [F(2,1739) = 3.25, P = 0.072]. The absence of either such effect suggests that shoulder posture, as manipulated here, does not substantially affect grip selection planning.
Fig. 5.
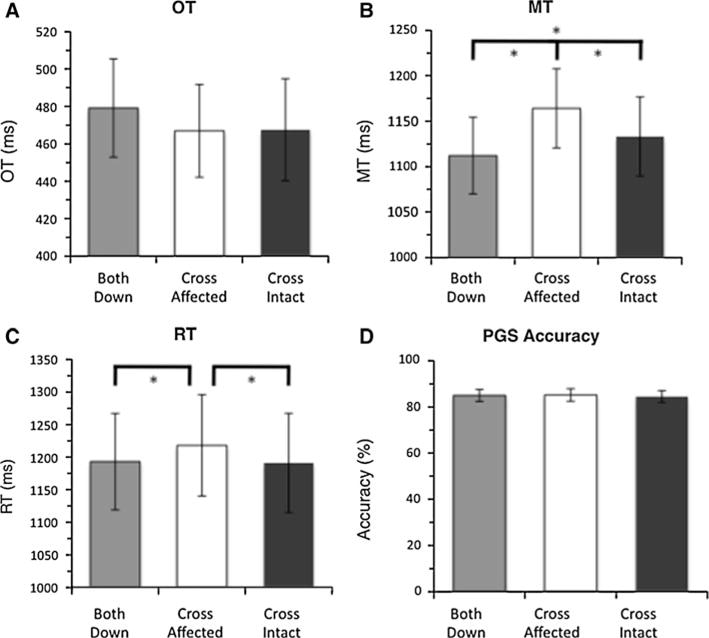
Effect of posture on task performance, group mean ± SEM. Results collapsed across hand and orientation and group, to show posture effect. * Significant difference (P < 0.05) by Tukey’s HSD. a OGS onset time (OT). b OGS movement time (MT). c PGS response time (RT). d PGS accuracy
Effects of amputation on grip selection
Having discounted any interaction effects of our posture manipulation on PGS, we collapsed across this factor and reanalyzed the data. This increased the power of our statistical tests by tripling the number of observations per condition. We expected to find decreased grip selection speed or accuracy for the affected hand. However, as detailed below, we found no evidence of performance deficits for amputees’ affected hand.
Amputation effects on PGS accuracy
We evaluated the accuracy of PGS by comparing responses with OGS grip preferences. If the accuracy of internal models involved in planning requires ongoing sensory feedback, then we expected to find decreased accuracy for amputees’ affected hand. However, we found no such evidence. Control participants selected movements with 85 ± 25% accuracy, while amputees reached 84 ± 27% accuracy (Fig. 6). Except for an effect of orientation [F(11, 453) = 13.79, P < 0.0001], a 2 (hand) * 12 (orientation) * 2 (group: amputee, control) between-groups ANOVA revealed neither main effects nor interactions (P > 0.1 in all cases).
Fig. 6.
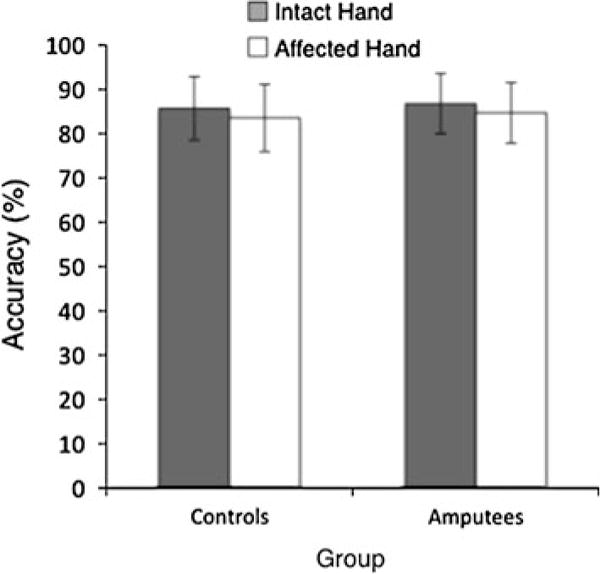
Effect of amputation on PGS selection accuracy, group mean ± SEM. No effect of group (P = 0.569), hand (P = 0.277), or interaction (P = 0.985)
Amputation effects on PGS timing
We also found no evidence for slowing of PGS based on the affected hand. A 2 (hand) * 12 (orientation) * 2 (group: amputee, control) between-groups ANOVA on RT revealed main effects of hand [F(1, 1793) = 7.96, P < 0.05] and orientation [F(11, 1793) = 7.12, P < 0.0001] on RT and an interaction between hand and orientation [F(11,1793) = 13.59, P < 0.0001). Critically, however, we failed to detect a main effect of group, F < 1.0 (Fig. 7a–b).
Fig. 7.
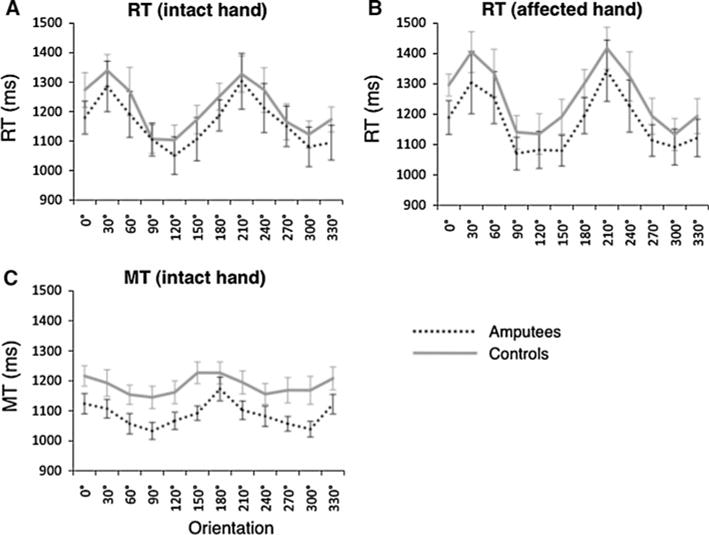
Orientation effects on measures of task speed, group mean ± SEM. a Effect of orientation on RT with intact hand. b Effect of orientation on RT with affected hand. c Effect of orientation on MT with intact hand. OT not shown, due to lack of orientation effect
Post hoc tests revealed that the effect of hand arose from slightly higher RT for the affected hand (1,213 ± 292 ms) than the intact hand (1,188 ± 277 ms) across groups.
Amputation effects on OGS timing
We expected to find no effect of amputation on OGS timing. A 2 (group: amputee, control) × 12 (orientation) repeated-measures ANOVA on OT detected a main effect of orientation [F(11, 813) = 2.15, P < 0.05], but no main effect of group, nor an interaction (F < 1.0). A similar analysis of MT showed main effects both for group [F(1, 813) = 7.06, P < 0.05] and for orientation [F(11, 813) = 9.51, P < 0.0001], but no interaction effect (F < 1.0). The effect of group arose from slightly lower MT for amputees, 1,088 ± 145 ms, compared to 1,184 ± 166 ms for controls. Figure 7c illustrates the effect of orientation on MT for each group.
Summary of amputation effects
In PGS, both amputees and controls selected grip movements just as accurately and quickly based on use of either hand. In other words, we found no evidence that that chronic amputation impacts the ability to plan precision grip movements. We found modestly slower OT and MT in OGS for amputees, but these effects did not depend on hand.
Effects of amputee pain, phantom mobility, age, and time since injury
We hypothesized that PGS accuracy might be better for participants with vivid and mobile phantom limbs, while PLP and/or time since amputation might adversely affect accuracy. However, we found no evidence of any such effects. Figure 8 illustrates the relationship between mean PGS accuracy and the VAS ratings or time since amputation. We looked for correlations between average behavioral measures (mean OT, mean MT, mean RT, and mean PGS accuracy) and participant characteristics such as VAS ratings (PLP or vividness of phantom mobility), years since amputation, age at amputation, and current age. We found a marginally non-significant trend toward a negative correlation between OT and phantom movement vividness (Kendall’s τ = −0.424, P = 0.051), and a significant positive correlation between OT and current age (τ = 0.597, P < 0.01), but we found no other statistically significant correlations between behavioral measures and any participant characteristics (absolute τ < 0.33 P > 0.14). These results suggest that the ability to accurately select movements based on the affected hand is not related to variations between participants’ pain levels and phantom mobility. Notably, we also found no indication that PGS accuracy deteriorates with time since amputation, contrary to expectations of experience dependence in PGS.
Fig. 8.
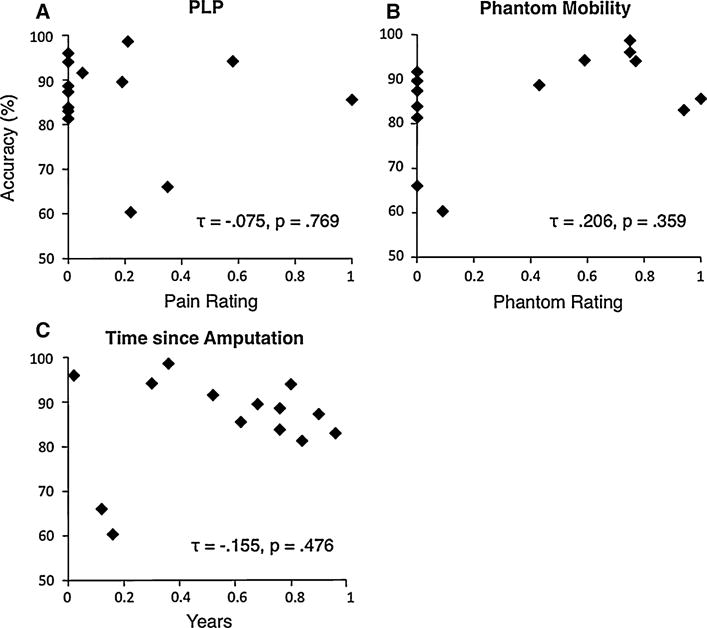
Correlation (Kendall τ) between PGS accuracy (participant mean) and amputee characteristics. a Phantom limb pain, 0–1. b Vividness of phantom mobility, 0–1. c Years since amputation
Effects of task order, hand laterality, and prosthesis usage
Throughout our analysis, we have averaged across two factors: laterality of hand used (left or right) and task order (PGS first or OGS first). Recall that all participants were right-hand dominant (Table 1). To confirm that these variables do not influence our findings, we performed a 2 (hand laterality) * 2 (task order) * 2 (group: amputee, control) between-groups ANOVA on the participant means of each behavioral measure (RT, OT, MT, and selection accuracy). We found a significant main effect of task order on OT [F(1,21) = 6.11, P < 0.05], produced by lower OT when participants performed OGS first. We also found a significant interaction between group and hand laterality on RT [F(1,21) = 5.01, P < 0.05]. We found no other significant main effects of hand laterality (P > 0.06 in all other cases) or task order (P > 0.1) on any variable, nor did we find any other interaction effects (P > 0.3). Table 2 summarizes the difference between left-hand and right-hand amputees. Crucially, the absence of any significant effects of hand or task order on selection accuracy (P > 0.2) demonstrates that preserved PGS accuracy depended neither on hand laterality nor on learning from the OGS task.
Table 2.
Amputee performance separated by affected hand (left vs. right), group means ± SEM
| Affected hand | Accuracy (%) | RT (ms) | MT (ms) | OT (ms) | |
|---|---|---|---|---|---|
| Left (n = 5) | Overall | 85.0 ± 2.9 | 1,258 ± 43 | 1,105 ± 27 | 1,258 ± 43 |
| Awk | 99.4 ± 0.4 | 1,234 ± 41 | 1,057 ± 22 | 1,234 ± 41 | |
| Comf | 99.9 ± 0.5 | 1,182 ± 37 | 1,046 ± 27 | 1,182 ± 37 | |
| Right (n = 8) | Overall | 86.6 ± 3.2 | 1,046 ± 70 | 1,065 ± 30 | 1,046 ± 70 |
| Awk | 99.3 ± 0.5 | 995 ± 60 | 1,056 ± 32 | 995 ± 60 | |
| Comf | 100 ± 0 | 962 ± 57 | 1,015 ± 32 | 962 ± 57 | |
| Difference (P) | Overall | 0.755 | 0.108 | 0.573 | 0.108 |
| Awk | 1.000 | 0.059 | 1.000 | 0.059 | |
| Comf | 0.615 | 0.059 | 0.755 | 0.059 |
Note that all amputees were right-hand dominant. PGS accuracy, PGS RT, OGS MT, and OGS OT measured across all thumb placements (Overall), most awkward thumb placement (Awk), and least awkward thumb placement (Comf). Difference between hands calculated by Mann– Whitney U-test for each placement and variable
We also analyzed the effect of prosthesis type, since prosthesis usage may affect cortical remapping and motor imagery (Lotze et al. 1999; Nico et al. 2004). Because we could not balance prosthesis type across participants, we used a Kruskal–Wallis test on participant means; this test provides non-parametric equivalent of the one-way ANOVA suitable for small sample sizes. Prosthesis type (none, mechanical, and cosmetic) had no significant effect on RT or PGS accuracy (P > 0.2) among amputees. However, prosthesis type did affect MT (H = 7.73, 2 df, P < 0.05) and OT (H = 6.19, 2 df, P = 0.045). Post hoc tests revealed that these effects arose from lower MT and OT for amputees with cosmetic prostheses, compared to amputees with no prosthesis (in the case of MT) or amputees with mechanical prostheses (in the case of OT). These statistically significant results must be interpreted with caution, since they both involve a group with an extremely small sample size (N = 2 for cosmetic prostheses).
Discussion
The current results demonstrate that chronic unilateral upper limb amputees retain the ability to accurately select precision grip movements that match the biomechanical constraints of their affected (absent) hand. This contradicts our hypothesis that amputees would show impaired ability to plan movements with the affected hand due to the chronic and complete absence of visual and somatosensory feedback. In addition, we found minimal effects of shoulder posture on prospective grip selection even for healthy controls, potentially indicating an ineffective posture manipulation. Regardless, the lack of an amputation effect on grip selection accuracy conflicts with long-standing beliefs about the role of experience in maintaining internal representations for upper limb movement and the functional effects of putative cortical map reorganizations. Below, we discuss potential mechanisms underlying these findings and potential implications for rehabilitation and prosthetic technologies.
Preserved internal models for the affected hand
While grip selection tasks generally minimize the use of non-motor strategies (Daprati et al. 2010), amputees could use alternative strategies to perform the task based on their affected hand. One such strategy might entail solving the task using visual or cognitive mechanisms. Our data do not support this possibility; amputees and healthy controls show nearly identical patterns of stimulus orientation dependence in PGS (Fig. 4) and that these patterns closely track awkwardness measured during OGS, all of which suggest that both groups use the same strategy. According to another alternative strategy, amputees could use the model of their opposite (intact) hand and transform its output to account for the opposite biomechanical constraints of their missing hand. However, this would entail an extra processing stage for the affected hand, in which the intact-hand model undergoes transformations to match the affected hand. Presumably, this transformation would increase response time for movement selections with the affected hand. Since we found no amputee-specific performance differences between hands, our data are inconsistent with amputees selecting movements for their affected hand by using and reversing the internal model of their intact hand. In sum, our evidence does not support the hypothesis that amputees use cognitive strategies in place of motor prediction and selection. Instead, it seems consistent with the hypothesis that amputees retain an accurate internal model of the affected hand that can be used to make accurate grip selection decisions.
Non-cognitive strategies such as speed-accuracy trade-offs might provide another alternative explanation for preserved grip selection accuracy. Under this explanation, amputees with grip selection deficits might maintain accuracy by reducing their speed. However, we found no evidence for reduced grip selection speed in amputees, demonstrating that amputees did not use a speed-accuracy tradeoff to maintain planning accuracy for movements with the affected hand.
While conceivably a more difficult task might reveal subtle limitations on the accuracy of these internal representations, we expected to see overt errors even for a simple task, due to the long post-amputation time (29 ± 16 years) in our amputees. Accurate prospective grip selection would appear to require the use of an internal model to predict the sensory consequences associated with the two grip options. One possibility is that internal forward models (e.g., Wolpert et al. 1998) also take part in such long-range forecasting (Frey 2010). Forward motor control appears to involve posterior parietal and cerebellar mechanisms (Desmurget et al. 1999; Buneo and Andersen 2006; Shadmehr and Krakauer 2008). One view is that the cerebellum supports forward internal models that predict sensory consequences of motor commands, while posterior parietal cortex represents the effects of these predictions on multisensory state representations of the effectors (see Shadmehr and Krakauer 2008, for review). Indeed, premotor, cerebellar and posterior parietal cortices consistently take part in prospective grip selection tasks similar to the one employed in the current experiment (Johnson et al. 2002b; Jacobs et al. 2010; Maragnon et al., in press), which supports the hypothesis that the current experiment may use the same mechanisms as forward models for online movement control. Our findings of intact planning post-amputation imply that internal representations constructed in these higher-level regions may not undergo functional reorganization in the same manner as primary sensory and motor cortices, as reviewed earlier. Put differently, these forward internal models may be relatively robust to the absence of sensory feedback. This is consistent with earlier work on prospective planning in stroke patients with dense hemiparesis (Johnson 2000a, b; Johnson et al. 2002a, b; Jenkinson et al. 2009). This intact performance in amputees seems to conflict directly with the aforementioned idea that accurate internal models require regular updates or maintenance. As such, more work is necessary to evaluate this hypothesis. Nevertheless, in the following section, we discuss how accurate internal models might persist in the absence of a limb.
Potential mechanisms for maintaining accurate internal models of the affected hand
We see three possible explanations that might account for a preserved internal model of the affected hand, separately or in concert: effector-independent representation, experience-independent representation, and cross-activation.
First, a single bilateral or hand-non-specific representation may underlie movements of either hand. Under this effector-independent representation hypothesis, the internal model for the affected hand would remain fully functional despite unilateral cortical map reorganization. Certainly, the human brain does encode movements in effector-independent ways, such as in eye-centered coordinate frames (e.g., Batista et al. 1999; Buneo and Andersen 2006). These effector-independent, potentially higher-level representations may be particularly important in the current task, since PGS and other action planning tasks engage premotor, cerebellar, and posterior parietal areas rather than primary sensorimotor areas, as described above. However, effector-dependent representations must also exist. Prior evidence strongly suggests that hand-specific movement representations exist, at least in the domain of motor skill learning and “cross-limb transfer.” In this phenomenon, unimanual motor training leads to partial performance increases with the opposite untrained hand. Importantly, this transfer occurs via a pattern known as “cross-activation” (e.g., Parlow and Kinsbourne 1989; Perez et al. 2007a; Lee et al. 2010), wherein learned adaptations for each hand are encoded in multiple cortical areas contralateral to the execution hand, not just the trained hand. While the brain may represent trained motor skills differently from how it represents biomechanical constraints, the current task shows sensitivity to the specific properties of each limb (i.e., the results of PGS, like OGS, are effector specific). This suggests that our task likely also relies, at least in part, on hand-specific unilateral cortical representations.
Second, the internal model for the affected hand may be relatively insensitive to experience in amputees. This experience-independent representation interpretation of a persistent hand representation is consistent with previous findings of preserved grip selection in densely hemiparetic stroke patients (Johnson 2000b; Johnson et al. 2002a; Jenkinson et al. 2009) and grossly intact arm-related activity in primary motor cortex for tetraplegic patients (Hochberg et al. 2006). An internal model could become functionally experience insensitive due to reduced error accumulation over time (if, for example, accumulated errors only arise concomitantly with feedback) or reduced sensitivity to accumulated errors (if the forward model ceases to be updated in the absence of sensory feedback and thus is preserved representation of the pre-amputation limb). An experience-insensitive internal model might entail an increased reliance on higher-level internal representations (e.g., in premotor, posterior parietal and cerebellar regions) that persist in the absence of sensory information, despite post-amputation cortical reorganization in primary sensorimotor areas. Regardless, this hypothesis may conflict with our prior understanding about the need for sensory or motor information to update certain aspects of biomechanical representations. Alternatively, it may point toward a divergence between internal forward models for online sensorimotor control (which seem to require updates to the state estimate to maintain accuracy) and internal forward models for movement planning and prediction (which, as shown here, may retain a certain level of accuracy despite the chronic absence of updates). Either way, we have no evidence here to distinguish whether the hypothesized experience-insensitivity is specific to amputees, an indicator that our task does not rely on the same internal models as used for online sensorimotor control, or a general property of internal models for movement planning and selection. While the hypothesis of a persistent internal model provides a potentially powerful explanation for our results, a conclusion of absolute experience independence (i.e., internal models maintained fully without updates) seems unwarranted, given prior knowledge about internal models.
Finally, the internal model for the affected hand may be maintained and updated by sensory and motor information related to the intact hand. Under this cross-activation hypothesis, feedback from the intact hand updates both the internal forward model for the intact hand and -to a limited degree- the internal forward model for the affected hand. Above, we described the “cross-activation” mechanism of cross-limb transfer as evidence for unilateral cortical representations; here, this mechanism serves as the foundation of this hypothesis (i.e., that unimanual training maintains bilateral cortical representations). The cross-activation mechanism rests on evidence for bilateral cortical activation during unilateral motor tasks (e.g., Kawashima et al. 1994; Koeneke et al. 2006), modulation of interhemispheric inhibition between primary motor cortex areas during unilateral motor practice (Perez et al. 2007b), and disruption of intermanual transfer from repetitive transcranial magnetic stimulation to the untrained hemisphere during execution (Lee et al. 2010). This cross-activation phenomenon could underlie maintenance of internal models for the affected hand in amputees, as sensory and motor signals related to the intact limb inform and maintain bilateral representations.
To summarize, our data suggest that amputees retain a relatively intact internal forward model for their absent limb, despite the complete absence of afferent proprioceptive and visual feedback. Possibly, the central nervous system maintains and updates this model based on a combination of non-updated higher-level representations in premotor, cerebellar, and posterior parietal areas and sensory/motor information arising from the intact hand (“cross-activation”). Future studies may test the cross-activation hypothesis of internal model maintenance by probing bilateral neurophysiological effects during motor learning in unilateral amputees or by testing movement selection in bilateral amputees.
Whatever the cause, our finding of preserved grip selection in chronic amputees sheds useful light on the high rates of rejection (29–38%) and low rates of satisfaction (5–25% per task) for arm prostheses (Wright et al. 1995; Datta et al. 2004; James et al. 2006). The preserved internal models for amputees’ affected limb suggest that this failure to use arm prostheses does not arise from a neurophysiological inability to select, imagine, plan, or predict movements with the affected hand. However, the possibility of experience independence suggests that existing models may have limited ability to adapt, which might interfere with prosthesis learning.
No effect of shoulder posture on grip selection
We found no consistent effect of shoulder and upper arm posture on grip selection performance. Posture did influence overt movement duration and prospective response time in amputees and controls, but not in a way that clearly addresses our hypothesis. We expected that posture would influence grip selection, as had been previously demonstrated with motor imagery tasks (e.g., Parsons 1994) and that amputees would show less influence of posture than healthy controls. Instead, we found posture effects that did not depend on which arm the participant used. Therefore, we cannot conclude that our shoulder posture manipulation affected grip selection planning. We chose our posture manipulation because it entailed a movement (upper arm adduction) that above-elbow amputees could perform; future studies might focus on below-elbow amputees to allow more hand-focused posture manipulations. For example, a rotation of the forearm, or an arm behind the back (e.g., Sirigu and Duhamel 2001), might produce a more substantial impact on grip selection performance.
Other effects on grip selection
We evaluated whether amputee performance depended on additional factors: hand laterality, prosthesis type, pain, phantom movement vividness, age, and time since amputation. Conceivably, phantom mobility could have a strong influence on amputees’ ability to perform the PGS task, as could time since amputation (i.e., time spent without proprioceptive feedback) or prosthesis usage. However, none of these factors showed any sign of a relationship with PGS speed or accuracy. As such, our main findings did not depend on the prosthesis usage, phantom experiences, pain, or time since limb loss of our individual amputees. A recent study of explicit imagery (Raffin et al. 2011) found broadly similar results for amputees, in that affected limbs did not differ significantly from intact limbs in the time to perform an explicit motor imagery task and pain predicted imagination times poorly. Ultimately, our results suggest that prospective grip selection capability for an affected hand neither decays over time (i.e., depends on time since amputation), nor does it depend on sensory experiences entailed by phantom movements or phantom pain.
Summary
We found preserved grip selection for the affected hand in chronic unilateral upper limb amputees, suggesting that amputees retain the ability to predict movement outcomes based on their affected hand’s biomechanical constraints. This preserved ability did not depend on phantom mobility, phantom pain, or time since amputation and cannot be accounted for by speed-accuracy tradeoff. These results suggest preservation of an internal model of the chronically absent hand. We propose two possible explanations for this finding: internal models for predictive motor control in amputees do not require sensorimotor updates to maintain accuracy, and/or amputees use feedback from their intact hand to maintain the forward internal model for their affected hand.
Overall, the preservation of motor planning abilities following chronic amputation has important potential implications for those seeking to assist patients with chronic immobility via brain–computer interfaces, neural prostheses, and conventional prostheses. Such prostheses should be able to capitalize on intact neural mechanisms for movement planning, even for long-term amputees.
Acknowledgments
This work was supported by a grant from the United States Army Medical Research Acquisition Activity (W81XWH-09-2-0114) to S.H.F. Panel B in Fig. 1 was adapted from Jacobs et al. (2010). Portions of these data were previously published in poster form as Philip and Frey (2010). This manuscript benefited from the constructive input of two anonymous reviewers.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Contributor Information
Benjamin A. Philip, Email: bphilip@uoregon.edu, Psychology Department, University of Oregon, Eugene, OR, USA.
Scott H. Frey, Psychology Department, University of Oregon, Eugene, OR, USA Department of Psychological Sciences, University of Missouri, Columbia, MO, USA.
References
- Batista AP, Buneo CA, Snyder LH, Andersen RA. Reach plans in eye-centered coordinates. Science. 1999;285(5425):257–260. doi: 10.1126/science.285.5425.257. [DOI] [PubMed] [Google Scholar]
- Buneo CA, Andersen RA. The posterior parietal cortex: sensorimotor interface for the planning and online control of visually guided movements. Neuropsychologia. 2006;44(13):2594–2606. doi: 10.1016/j.neuropsychologia.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Daprati E, Nico D, Duval S, Lacquaniti F. Different motor imagery modes following brain damage. Cortex. 2010;46(8):1016–1030. doi: 10.1016/j.cortex.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Datta D, Selvarajah K, Davey N. Functional outcome of patients with proximal upper limb deficiency—acquired and congenital. Clin rehabil. 2004;18(2):172–177. doi: 10.1191/0269215504cr716oa. [DOI] [PubMed] [Google Scholar]
- de Lange FP, Helmich RC, Toni I. Posture influences motor imagery: an fMRI study. NeuroImage. 2006;33(2):609–617. doi: 10.1016/j.neuroimage.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Epstein CM, Turner RS, Prablanc C, Alexander GE, Grafton ST. Role of the posterior parietal cortex in updating reaching movements to a visual target. Nat Neurosci. 1999;2(6):563–567. doi: 10.1038/9219. [DOI] [PubMed] [Google Scholar]
- Donoghue JP, Sanes JN. Organization of adult motor cortex representation patterns following neonatal forelimb nerve injury in rats. J Neurosci. 1988;8(9):3221–3232. doi: 10.1523/JNEUROSCI.08-09-03221.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey SH. Forecasting the long-range consequences of manual and tool use actions: neurophysiological, behavioral and computational considerations. In: Danion F, Latash ML, editors. Motor control: theories, experiments and applications. Oxford University Press; New York: 2010. pp. 295–313. [Google Scholar]
- Grush R. The emulation theory of representation: motor control, imagery, and perception. Behav Brain Sci. 2004;27(3):377–396. doi: 10.1017/s0140525x04000093. (discussion 396–442) [DOI] [PubMed] [Google Scholar]
- Haber WB. Reactions to loss of limb: physiological and psychological aspects. Ann NY Acad Sci. 1958;74(1):14–24. doi: 10.1111/j.1749-6632.1958.tb39524.x. [DOI] [PubMed] [Google Scholar]
- Hochberg LR, Serruya MD, Friehs GM, Mukand JA, Saleh M, Caplan AH, Branner A, Chen D, Penn RD, Donoghue JP. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006;442(7099):164–171. doi: 10.1038/nature04970. [DOI] [PubMed] [Google Scholar]
- Ionta S, Blanke O. Differential influence of hands posture on mental rotation of hands and feet in left and right handers. Exp Brain Res. 2009;195(2):207–217. doi: 10.1007/s00221-009-1770-0. [DOI] [PubMed] [Google Scholar]
- Jacobs S, Danielmeier C, Frey SH. Human anterior intraparietal and ventral premotor cortices support representations of grasping with the hand or a novel tool. J Cogn Neurosci. 2010;22(11):2594–2608. doi: 10.1162/jocn.2009.21372. [DOI] [PubMed] [Google Scholar]
- James MA, Bagley AM, Brasington K, Lutz C, McConnell S, Molitor F. Impact of prostheses on function and quality of life for children with unilateral congenital below-the-elbow deficiency. J Bone Jount Surg Am. 2006;88(11):2356–2365. doi: 10.2106/JBJS.E.01146. [DOI] [PubMed] [Google Scholar]
- Jeannerod M. The representing brain: neural correlates of motor intention and imagery. Behav Brain Sci. 1994;17(2):187–245. [Google Scholar]
- Jenkinson PM, Edelstyn NMJ, Ellis SJ. Imagining the impossible: motor representations in anosognosia for hemiplegia. Neuropsychologia. 2009;47(2):481–488. doi: 10.1016/j.neuropsychologia.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Johnson SH. Cerebral organization of motor imagery: contralateral control of grip selection in mentally represented prehension. Psychol Sci. 1998;9:219–222. [Google Scholar]
- Johnson SH. Thinking ahead: the case for motor imagery in prospective judgements of prehension. Cognition. 2000a;74(1):33–70. doi: 10.1016/s0010-0277(99)00063-3. [DOI] [PubMed] [Google Scholar]
- Johnson SH. Imagining the impossible: intact motor representations in hemiplegics. Neuro Report. 2000b;11(4):729–732. doi: 10.1097/00001756-200003200-00015. [DOI] [PubMed] [Google Scholar]
- Johnson SH, Sprehn G, Saykin AJ. Intact motor imagery in chronic upper limb hemiplegics: evidence for activity-independent action representations. J Cogn Neurosci. 2002a;14(6):841–852. doi: 10.1162/089892902760191072. [DOI] [PubMed] [Google Scholar]
- Johnson SH, Rotte M, Grafton ST, Hinrichs H, Gazzaniga MS, Henize J-H. Selective activation of a parietofrontal circuit during implicitly imagined prehension. NeuroImage. 2002b;17(4):1693–1704. doi: 10.1006/nimg.2002.1265. [DOI] [PubMed] [Google Scholar]
- Kaas JH. The reorganization of somatosensory and motor cortex after peripheral nerve or spinal cord injury in primates. Prog Brain Res. 2000;128:173–179. doi: 10.1016/S0079-6123(00)28015-1. [DOI] [PubMed] [Google Scholar]
- Kawashima R, Roland PE, O’Sullivan BT. Activity in the human primary motor cortex related to ipsilateral hand movements. Brain Res. 1994;663(2):251–256. doi: 10.1016/0006-8993(94)91270-x. [DOI] [PubMed] [Google Scholar]
- Koeneke S, Lutz K, Herwig U, Ziemann U, Jäncke L. Extensive training of elementary finger tapping movements changes the pattern of motor cortex excitability. Exp Brain Res. 2006;174(2):199–209. doi: 10.1007/s00221-006-0440-8. [DOI] [PubMed] [Google Scholar]
- Lee M, Hinder MR, Gandevia SC, Carroll TJ. The ipsilateral motor cortex contributes to cross-limb transfer of performance gains after ballistic motor practice. J Physiol. 2010;588(1):201–212. doi: 10.1113/jphysiol.2009.183855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotze M, Grodd W, Birbaumer N, Erb M, Huse E, Flor H. Does use of a myoelectric prosthesis prevent cortical reorganization and phantom limb pain? Nat Neurosci. 1999;2(6):501–502. doi: 10.1038/9145. [DOI] [PubMed] [Google Scholar]
- MacKenzie CL, Iberall T. The grasping hand. Elsevier; Amsterdam: 1994. [Google Scholar]
- Marangon M, Jacobs S, Frey SH. Context-sensitivity of grasp representations in human rostral inferior parietal lobule. J Neurophysiol. doi: 10.1152/jn.00796.2010. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier C, Reilly KT, Vargas CD, Aballea A, Sirigu A. Mapping phantom movement representations in the motor cortex of amputees. Brain. 2006;129(8):2202–2210. doi: 10.1093/brain/awl180. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Kaas JH, Wall J, Nelson RJ, Sur M, Felleman D. Topographic reorganization of somatosensory cortical areas 3b and 1 in adult monkeys following restricted deafferentation. Neuroscience. 1983;8(1):33–55. doi: 10.1016/0306-4522(83)90024-6. [DOI] [PubMed] [Google Scholar]
- Moore CJ, Price CJ. A functional neuroimaging study of the variables that generate category-specific object processing differences. Brain. 1999;122(5):943–962. doi: 10.1093/brain/122.5.943. [DOI] [PubMed] [Google Scholar]
- Moore CE, Schady W. Investigation of the functional correlates of reorganization within the human somatosensory cortex. Brain. 2000;123(9):1883–1895. doi: 10.1093/brain/123.9.1883. [DOI] [PubMed] [Google Scholar]
- Nico D, Daprati E, Rigal F, Parsons L, Sirigu A. Left and right hand recognition in upper limb amputees. Brain. 2004;127(1):120–132. doi: 10.1093/brain/awh006. [DOI] [PubMed] [Google Scholar]
- Parlow SE, Kinsbourne M. Asymmetrical transfer of training between hands: implications for interhemispheric communication in normal brain. Brain Cogn. 1989;11(1):98–113. doi: 10.1016/0278-2626(89)90008-0. [DOI] [PubMed] [Google Scholar]
- Parsons LM. Temporal and kinematic properties of motor behavior reflected in mentally simulated action. J Exp Psychol Hum Percept Perform. 1994;20(4):709–730. doi: 10.1037//0096-1523.20.4.709. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Peris M, Tormos JM, Pascual AP, Catalá MD. Reorganization of human cortical motor output maps following traumatic forearm amputation. NeuroReport. 1996;7(13):2068–2070. doi: 10.1097/00001756-199609020-00002. [DOI] [PubMed] [Google Scholar]
- Perez MA, Tanaka S, Wise SP, Sadato N, Tanabe HC, Willingham DT, Cohen LG. Neural substrates of intermanual transfer of a newly acquired motor skill. Curr Biol. 2007a;17(21):1896–1902. doi: 10.1016/j.cub.2007.09.058. [DOI] [PubMed] [Google Scholar]
- Perez M, Wise S, Willingham D, Cohen L. Neurophysiological mechanisms involved in transfer of procedural knowledge. J Neurosci. 2007b;27(5):1045–1053. doi: 10.1523/JNEUROSCI.4128-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip BA, Frey SH. Intact prediction of grip selection with an amputated hand. Society for Neuroscience; San Diego, CA: 2010. (Program No. 291.13. 2010 Neuroscience Meeting Planner). (Online) [Google Scholar]
- Raffin E, Giraux P, Reilly KT. The moving phantom: motor execution or motor imagery? Cortex. 2011 doi: 10.1016/j.cortex.2011.02.003. (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Sanes JN, Suner S, Lando JF, Donoghue JP. Rapid reorganization of adult rat motor cortex somatic representation patterns after motor nerve injury. Proc Natl Acad Sci USA. 1988;85(6):2003–2007. doi: 10.1073/pnas.85.6.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Krakauer J. A computational neuroanatomy for motor control. Exp Brain Res. 2008;185(3):359–381. doi: 10.1007/s00221-008-1280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenton JT, Schwoebel J, Branch HB. Mental motor imagery and the body schema: evidence for proprioceptive dominance. Neurosci Lett. 2004;370(1):19–24. doi: 10.1016/j.neulet.2004.07.053. [DOI] [PubMed] [Google Scholar]
- Sirigu A, Duhamel JR. Motor and visual imagery as two complementary but neurally dissociable mental processes. J Cogn Neurosci. 2001;13(7):910–919. doi: 10.1162/089892901753165827. [DOI] [PubMed] [Google Scholar]
- Tomasino B, Rumiati RI. Effects of strategies on mental rotation and hemispheric lateralization: neuropsychological evidence. J Cogn Neurosci. 2004;16(5):878–888. doi: 10.1162/089892904970753. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Flanagan JR. Motor prediction. Curr Biol. 2001;11(18):R729–R732. doi: 10.1016/s0960-9822(01)00432-8. [DOI] [PubMed] [Google Scholar]
- Wolpert D, Miall R. Forward models for physiological motor control. Neural Netw. 1996;9(8):1265–1279. doi: 10.1016/s0893-6080(96)00035-4. doi: papers://9ECEC99B-FEEC-4808-A2E1-EB1DC6D0033D/Paper/p503. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Goodbody SJ, Husain M. Maintaining internal representations: the role of the human superior parietal lobe. Nat Neurosci. 1998;1(6):529–533. doi: 10.1038/2245. [DOI] [PubMed] [Google Scholar]
- Wright TW, Hagen AD, Wood MB. Prosthetic usage in major upper extremity amputations. J Hand Surg Am. 1995;20(4):619–622. doi: 10.1016/S0363-5023(05)80278-3. [DOI] [PubMed] [Google Scholar]


