Abstract
Objective
We identified significant expression of the matricellular protein, DEL1, in hypertrophic and mature cartilage during development. We hypothesized that this tissue-specific expression indicated a biological role for DEL1 in cartilage biology.
Methods
Del1 KO and WT mice had cartilage thickness evaluated by histomorphometry. Additional mice underwent medial meniscectomy to induce osteoarthritis, and were assayed at 1 week for apoptosis by TUNEL staining and at 8 weeks for histology and OA scoring. In vitro proliferation and apoptosis assays were performed on primary chondrocytes.
Results
Deletion of the Del1 gene led to decreased amounts of cartilage in the ears and knee joints in mice with otherwise normal skeletal morphology. Destabilization of the knee led to more severe OA compared to controls. In vitro, DEL1 blocked apoptosis in chondrocytes.
Conclusion
Osteoarthritis is among the most prevalent diseases worldwide and increasing in incidence as our population ages. Initiation begins with an injury resulting in the release of inflammatory mediators. Excessive production of inflammatory mediators results in apoptosis of chondrocytes. Because of the limited ability of chondrocytes to regenerate, articular cartilage deteriorates leading to the clinical symptoms including severe pain and decreased mobility. No treatments effectively block the progression of OA. We propose that direct modulation of chondrocyte apoptosis is a key variable in the etiology of OA, and therapies aimed at preventing this important step represent a new class of regenerative medicine targets.
Introduction
Osteoarthritis (OA) represents one of the most prevalent diseases in the United States. It is estimated that 85% of all people reaching the age of 75 will have some clinical evidence of OA.[1] The manifestations of the disease are significant with the symptoms ranging from pain to decreased mobility and disability. Beyond the impact of the disease on the musculoskeletal system, the lack of mobility contributes to exacerbation of heart and metabolic diseases due to decreased ability to engage in physical activity. Current management consists primarily of symptomatic relief ranging from exercise to maintain flexibility and mobility to non-steroidal anti-inflammatory drugs (NSAIDs) for pain control to joint replacement when no options remain. Despite the large number of people affected and the tremendous costs in morbidity, there are surprisingly few alternatives to these therapies.
Hyaline cartilage is unique for its avascular nature and for its limited ability to regenerate. It consists of mature chondrocytes sitting in a highly specialized matrix comprised of glycosaminoglycans that provide the surface required for friction-less motion in the joints. The process leading to clinical OA is believed to be triggered by some form of trauma resulting in inflammation with release of inflammatory mediators and matrix degrading enzymes into the articular space.[2] Among the key inflammatory mediators released is TNFα, a cytokine that promotes apoptosis in chondrocytes.[3] The combination of matrix degradation, chondrocytes apoptosis, and limited regeneration lead to fissures and erosions in the previously smooth articular surface. The primary clinical symptom of this is pain whose severity can lead to disability.
Among humans, there is a clear diversity of susceptibility to the disease. There are 45 year olds with severe enough disease to warrant joint replacement and 75 year olds running marathons. It is apparent that each individual has a different risk for development of the disease. Large-scale population studies looking to identify genetic markers have identified multiple genomic regions indicating that multiple genetic variables contribute to susceptibility.[4–6] Additionally, development of OA is complex and multifactorial with significant influence from environmental factors.
Animal studies have identified a number of genes that might contribute to development of OA and they fall into three broad categories: mutations in extracellular matrix (ECM) and matrix-modifying proteins (COL2A1, ADAMTS5, MMPs)[7–9] that compromise structural integrity, mutations that dysregulate the stress and inflammatory response (HIF-2α, NFκB, IL-1, TNF-α),[3,10,11] and mutations in developmentally regulated proteins (HH, CEBPβ, DKK)[12–14] which adversely affect cartilage development. There have been several mouse mutations of key regulatory genes that exhibit increased apoptosis within the articular chondrocytes in addition to a variety of other effects (SIRT-1, CHOP).[15,16] Mutations in ECM proteins often lead to mice with musculoskeletal abnormalities in the form of chondrodysplasias.[17]
We demonstrate here that DEL1, an ECM-associated, integrin-binding protein, has a potent biological function in chondrocytes where it serves as an anti-apoptotic factor. Furthermore, we show deletion of Del1 leads to decreased amounts of cartilage as measured by histomorphometry. Knockout mice also have increased susceptibility to OA associated with increased chondrocyte apoptosis.
Materials and Methods
Late developmental expression of Del1 mRNA and anatomic analysis of Del1 knockout mice
We used a previously described Del1-LacZ knock-in mouse.[18] Identification of areas of expression was performed in heterozygotes at the indicated dates with wild-type (WT) littermates as controls. Specimens were fixed in 4% paraformaldehye and placed in X-gal solution [400 μg/mL X-gal reagent (Invitrogen, Carlsbad, CA), 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, and 2 mM MgCl2 in 1x phosphate-buffered saline]. Specimens were incubated at 37°C incubator for 1 to 8 hrs until staining was apparent in test specimens but not control specimens, and post-fixed in 4% paraformaldehyde followed by embedding in paraffin, sectioning, and counterstaining with eosin. Characterization of the knockout (KO) phenotype was performed in male, age-matched controls. Knee joints and ears were harvested at 10 weeks of age, respectively, for basic histomorphometry. All animal protocols were approved by the Stanford University Institutional Animal Care and Use Committee in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Induction of osteoarthritis, TUNEL staining and immunohistochemistry
8-week old male KO and WT mice underwent surgery to remove the medial meniscus of the right knee. Briefly, mice underwent anesthesia with inhaled isoflurane prior to shaving and prep of the surgical site. An incision was made over the knee followed by resection of the medial meniscus and closure of the incision with 6–0 Vicryl. Mice were recovered under a warming lamp and observed until moving and feeding freely. Post-operative pain control was provided by subcutaneous injection of buprophenone q6 hrs for 48 hrs and as needed afterwards. Euthanasia was performed with CO2 inhalation followed by cervical dislocation. All animals survived until the endpoint with no early mortality.
Joints were harvested at 8 weeks after surgery and processed for histology with Safranin O-alcian blue staining. We obtained serial sections of 10 μm across the joint surface and used every third section for analysis resulting in 7–12 sections graded per joint. Grading was performed by a trained pathologist in a blinded manner using the OARSI method of scoring.[19]
TUNEL staining was performed using the in situ Cell Death Detection Kit (Roche, Indianapolis, IN). For these studies, mice were harvested at 1 week after surgery. Control was sham operation where the joint capsule was opened without resection of the medial meniscus. We chose site-matched areas to count number of apoptotic cells per high power field.
Immunohistochemistry was performed using antibodies directed to endothelial cells (anti-CD31, BD Biosciences, Franklin Lakes, NJ), lymphocytes (anti-CD45R, e-Bioscience, San Diego, CA), macrophages (anti-F4/80, e-Bioscience, San Diego, CA), and neutrophils (anti-Ly-6B.2, AbD Serotec, Raleigh, NC). Sections from mice 8 weeks after medial meniscectomy were used for angiogenesis and from 1 week after medial meniscectomy for inflammatory cells. For angiogenesis, we counted positive tubular structures per high power field. For immune cells, we counted positively stained cells per high power field. Controls for all immunohistochemistry consisted of incubation without primary antibody and with secondary antibody.
In vitro studies of DEL1 function
Normal human articular chondrocytes (NHACs) (Lonza, Walkersville, MD) in low passage numbers (3–4) were cultured in CGM (Lonza, Walkersville, MD) with 5% FBS and seeded at a density of 5x103 cells/100 μl in 96 well plates coated with 8 ng/mm2 Del1 or bovine serum albumin (BSA) coated. Proliferation was assessed by performing WST-8 assays at the indicated times (Sigma-Aldrich, St Louis, MO) and absorbance measured at OD450nm. Attachment was performed by first coating the plates with 8 ng/mm2 of BSA or DEL1. NHACs first suspended in CGM with 1% FBS with either 500 μM RGD or RGE peptide, 1:200 dilution of anti-integrin αvβ3 (ab 190147, LM609, Abcam, Cambridge, MA) or IgG1 isotype control, or 1:200 dilution of anti-integrin α1 (sc-271034, Santa Cruz Biotechnology, Dallas, TX) or IgG2b isotype control, and incubated at 37°C for 15 min prior to plating. After 6 hrs, unattached cells were washed off and the number of cells attached assayed by WST-8.
Apoptosis was induced with the addition of 10 μM doxorubicin (Sigma-Aldrich, St Louis, MO) or 10 ng/ml each of TNFα/actinomycin D (Sigma, St Louis, MO) in the presence of 500 μM RGD or RGE peptides (Bachem, Torrance, CA). Apoptosis was assayed by caspase 3/7 activity (Promega, Madison, WI). Cell viability was determined by trypan blue exclusion. Anoikis was induced using poly-HEMA coated plates to prevent attachment. NHACs were cultured at a density of 1x103 cells/100 μl in CGM (Lonza, Walkersville, MD) with 0.5% methyl cellulose (Sigma-Aldrich, St Louis, MO) added to avoid survival effects caused by clumping of cells.[20] 250 ng DEL1 or BSA was mixed with suspended chondrocytes for 10–16 hrs and cell survival assayed with trypan blue exclusion.
To examine factors inducing del1 expression, NHACs were cultured in the presence of recombinant human TNFα (10 ng/ml), IFNγ (10 ng/ml), IL-1α (10 ng/ml), IL-6 (50 ng/ml), TGF-β1 (10 ng/ml), VEGF (100 ng/ml), FGF2 (100 ng/ml) (all from Peprotech Inc., Rocky Hill, NJ) for 24 hr and RNA collected. We performed qPCR on an ABI PRISM 7900H (Applied Biosystems, Foster City, CA) with Cybergreen PCR reagents (Applied Biosystems, Foster City, CA) using primers specific for Del1 mRNA (forward primer: 5’- CTTTTATCGCCCTTCCCAAGA; reverse primer: 5’- CTTTTATCGCCCTTCCCAAGA).
To obtain primary mouse chondrocytes, 2-week old mice were sacrificed and the femoral head cartilage isolated. Fragments of cartilage were incubated in collagenase solution to obtain single cells. The resulting cellular suspension was centrifuged to pellet the chondrocytes before plating in DMEM with Glutamax (Thermo Scientific, Waltham, MA) and 10% FBS in an incubator at 37°C and 5% CO2.
Biomechanical testing
10 Del1 KO mice and 10 WT male mice, aged 10 weeks old, were euthanized and the femur was dissected free leaving the femoral head untouched. Tissues were analyzed while fresh, and kept hydrated and moist during the entire testing process. The femur was attached to a support using epoxy glue that was allowed to set for 2 hrs to ensure solid attachment. The stiffness, elasticity and resistance to penetration were measured by a microprobe system in an area on the femoral head toward the greater trochanter. A Keyence VHX-600 microscope was used with the microprobe system to image the sample as well as ensure the consistency of probe placement.
A high compliance microprobe metrology system was used to study the mechanical properties of the articular surface at the micron length scale. The system consists of a steel probe with a flat end mounted on a load cell with mN accuracy to measure the applied force that was used to push against the surface of the test sample. A test sample holder was mounted on a piezoelectric actuator, which allowed displacement control with sub-50 nm resolution. A micrometer-controlled x-y stage allowed the probe to be positioned with < 5 μm accuracy in the plane of the test die and another stage allowed positioning in the z direction in the direction perpendicular to the test die. The piezoelectric actuator was controlled by a LabView program, which allowed both displacement and displacement rate to be controlled. The entire system was mounted on a rigid steel frame to ensure maximum stiffness. 10 measurements for each femoral head were collected for data analysis. Data was further analyzed with Octave software.
Statistical analysis
For all comparisons of WT and KO animals, the minimum number of animals required for statistical significance was calculated using a significance level (alpha) of 0.05, and a power of 95%. For OARSI scores, statistical significance was calculated using Mann-Whitney U test. For in vitro and biomechanical studies, statistical significance was calculated using Student’s t test with p<0.05 considered statistically significant.
Results
Late developmental expression in cartilage
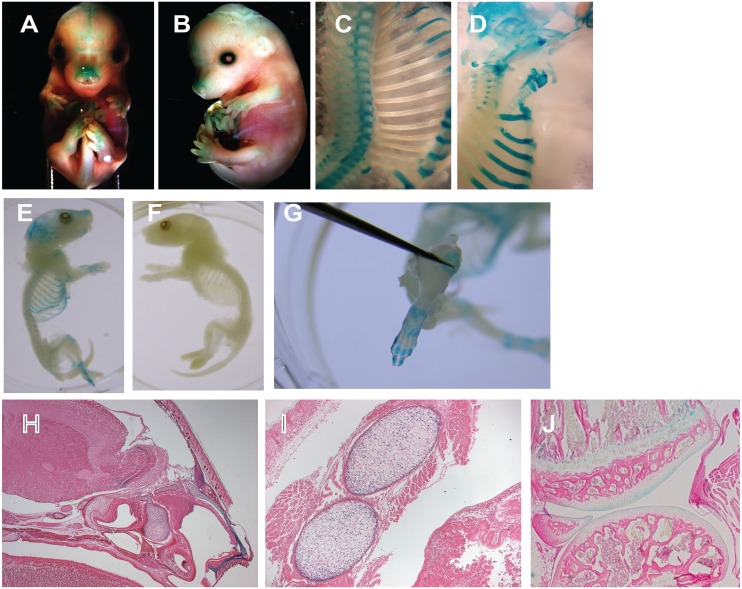
It has previously been shown that Del1 mRNA was expressed in a variety of tissues during early development including in the hypertrophic cartilage of developing long bone.[18] We looked at expression later during development and in the neonatal period to see if this persisted in mature cartilage. These mice have a LacZ gene inserted in the Del1 gene leading to a knockout of the native gene, and expression of LacZ under the control of the native Del1 promoter. Adding X-gal led to the presence of blue staining wherever LACZ was expressed. LacZ expression was found in many diverse areas of mature cartilage, including nose, rib, cranial suture, and trachea comprising both hyaline (joint) and elastic (ear) cartilage (Fig 1). This expression was present in newborn pups as well. Confirmation that the staining was within the cartilage was done with histology (Fig 1H–1J).
Fig 1. Del1 developmental expression.
Mice at the indicated developmental stage were sacrificed and underwent LACZ staining and whole mount preparation: (A) E14.5, frontal view; (B) E14.5, side view; (C) E17.5, ribs and vertebrae; (D) E17.5, skull base, larynx and trachea, and ribs; (E) D0 transgenic newborn; (F) D0 wildtype newborn; (G) D0 transgenic paw. E17.5 embryos were sectioned, stained for LACZ as indicated by blue staining, and counterstained with hematoxylin. Images are shown of staining in the nasal cartilage (H, 25x magnification), costal cartilage (I, 100x magnification), and knee (J, 25x magnification).
Knockout phenotype
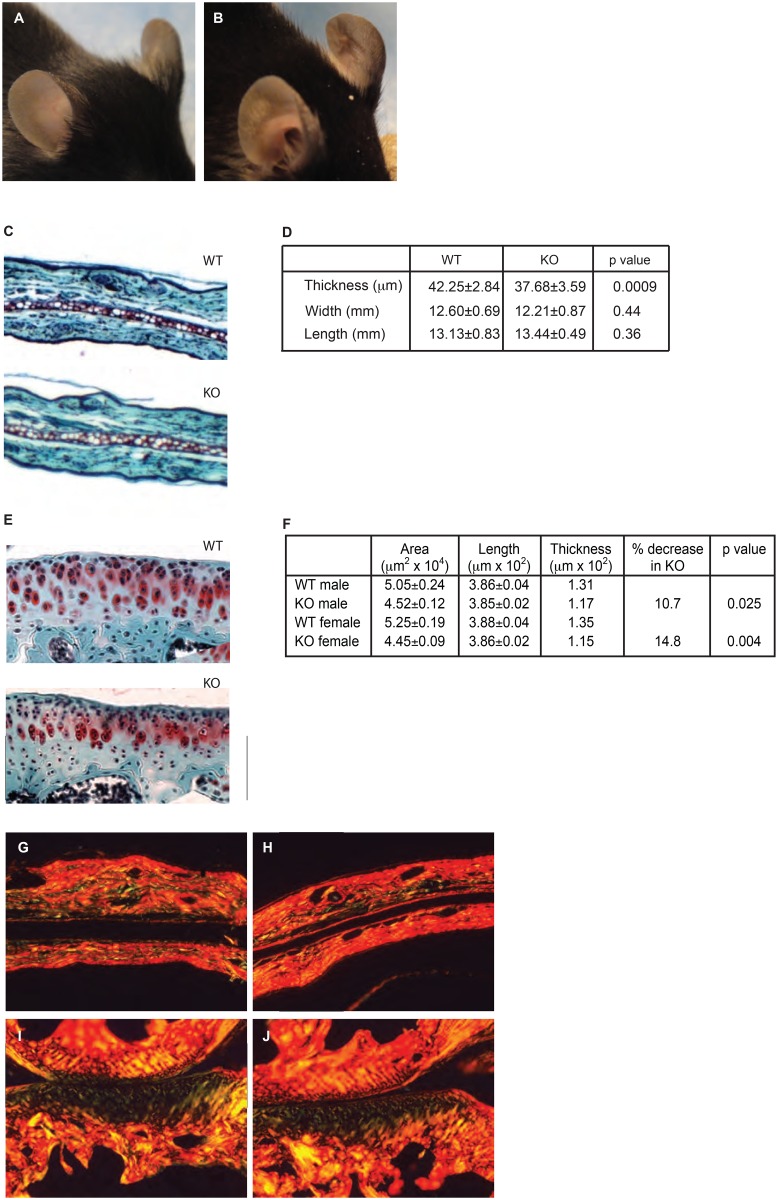
Del1 KO mice were born in normal Mendelian ratios, had normal fertility, and activity. Using both plain radiographs and microCT, we found no differences in the bony skeleton either in bone density or morphology. There was no difference in size between KO and WT mice based upon tibial length (1.82±0.01 cm KO vs 1.81±0.02 cm WT, p = 0.24, n = 26 KO, 12 WT). It was noticed that Del1 KO mice had floppy ears that were most noticeable in the first week of life (Fig 2A and 2B). Cartilage provides the structural framework for the ear and it was hypothesized that there was a difference in the auricular cartilage leading to the phenotype. Compared to age- and sex-matched controls, 10-week old KO mice demonstrated a 10% decrease in the thickness of the auricular cartilage (Fig 2C and 2D). Ear size did not vary as measurements of the length and width of the pinna did not show a difference (Fig 2D) indicating that the ears were not floppier simply because they were larger. Picrosirius red staining for collagens did not demonstrate any substantive difference in the matrix (Fig 2G and 2H). We concluded that the floppier ears in the KO mice were due to decreased amount of total cartilage.
Fig 2. Del1 KO phenoptype.
Appearance of the ear in WT (A) and KO (B) mice. Ears were harvested from 10-week old, male mice and stained (C) for measurement of auricular cartilage thickness (D, n = 12 WT and 18 KO). Width and length are measurements of the pinna. Photomicrographs shown are 40x magnification. Knees were harvested from 10-week old, male mice and stained (E) for measurement of tibial articular cartilage thickness (F, n = 4 for all groups). All values were normalized to tibial length. There was no difference in weight or tibial length between WT and KO mice. A bounding box at 200x magnification as shown was created and the area of cartilage within determined. Due to the variable thickness present within the ear and the undulating boundary between cartilage and bone in the knee, thickness was calculated by measuring the length and dividing into area. p value refers to difference between WT and KO mice. Picrosirius red staining of KO (G) and WT (H) ears and the medial surface of KO (I) and WT (J) knees. Representative sections are shown at 25x magnification.
We further investigated whether other sites in the cartilaginous skeleton were affected by Del1 deletion. Histomorphometric measurements of knee articular cartilage demonstrated that the thickness of the articular cartilage was reduced by 10–15% in the KO compared to the WT mice (Fig 2E and 2F). Given that we found decreased thickness of the articular and auricular cartilage, we reasoned that it could be due to decreased matrix production, decreased chondrocytes, or both. We hypothesized that if chondrocytes produced less matrix, cell density would increase since there is less matrix between them. We examined the cellular density of the cartilage by counting the number of chondrocytes present in a high power field. It was found to be practically identical (2.91±0.08 WT vs 2.89±0.08 KO, p = 0.93). We interpret these results to indicate there is no decreased matrix production, and suggest that the decreased thickness was due to decreased numbers of chondrocytes (S1 Fig). Picrosirius red staining was performed to look for gross differences in collagen content and structure within the cartilage and this demonstrated no difference (Fig 2I and 2J). Although we only surveyed two anatomic sites, the fact that these represented two different types of cartilage, elastic and hyaline, led us to conclude that there was a general decrease in cartilage throughout the skeleton despite no differences detected in the bony skeleton.
In vitro effect on chondrocytes
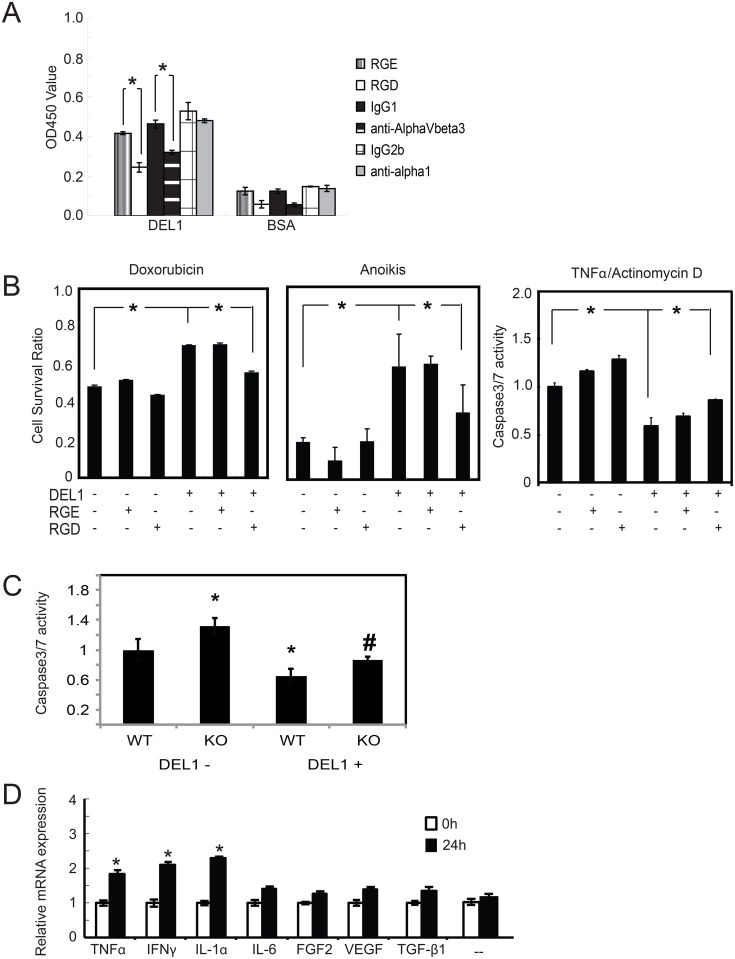
DEL1 is a matricellular protein as defined by its modular protein structure and its ability to interact with the ECM and cell surface receptors in the form of integrins.[21] Integrin binding can promote a variety of cellular functions including proliferation, attachment and apoptosis. We analyzed the impact of DEL1 on normal human articular chondrocytes (NHACs) to better understand what role it may be serving there. Although we chose to study NHACs, we recognize that mouse chondrocytes or chondrocytes from joints with OA could have different biology. DEL1 promoted chondrocyte attachment via its RGD motif as indicated by effect inhibition of attachment by RGD peptide, but not RGE, and attachment was mediated, at least in part, by integrin αvβ3 (Fig 3A).
Fig 3. DEL1 effect on apoptosis and induction.
(A) NHACs were pre-treated with the peptides or antibodies indicated and placed in plates coated with either BSA or DEL1. Cells attached after 6 hrs were determined by WST-8 assay. *p<0.05 between indicated values. (B) NHACs cultured with DEL1 have increased survival after pro-apoptotic stimuli that were inhibited by RGD, not RGE, peptides. For caspase 3/7 assays, untreated chondrocytes were arbitrarily assigned the value of 1. *p<0.05 between indicated values. (C) Primary chondrocytes from WT and KO mice had apoptosis induced with TNFα/actinomycin D in the presence or absence of purified DEL1 and assayed for caspase 3/7. *p<0.05 relative to WT without DEL1, #p<0.05 relative to KO without DEL1. (D) NHACs were treated with indicated factors (—indicates no treatment). RNA was assayed for Del1 mRNA expression by qPCR with amount at time 0 without treatment arbitrarily set at 1. Values are average of 3 separate experiments. *p<0.05 relative to untreated cells at 24 hrs.
We tested for the effect of DEL1 on NHACs after apoptosis was induced through either the extrinsic pathway using TNFα/actinomycin D or via the intrinsic pathway using doxorubicin (Fig 3B) and found it prevented apoptosis of NHACs. The anti-apoptotic effect of Del1 was blocked by RGD peptides indicating that integrin binding was the primary mediator of this effect. DEL1 had no effect on NHAC proliferation (S2 Fig).
Primary mammalian cells commonly need attachment to ECM for survival and the induction of apoptosis due to lack of ECM attachment is termed anoikis. Chondrocytes grown in suspension can avoid anoikis by aggregation due to interactions of cells with the ECM produced by other cells, and this process is integrin-dependent.[22] The addition of methyl cellulose prevents these cellular interactions in suspension and will induce anoikis in chondrocytes. In NHACs grown on polyHEMA-coated plates to force suspension culture and in the presence of methyl cellulose to prevent aggregation, DEL1 was highly protective against anoikis (Fig 3B).
Del1 KO mice had increased susceptibility to osteoarthritis
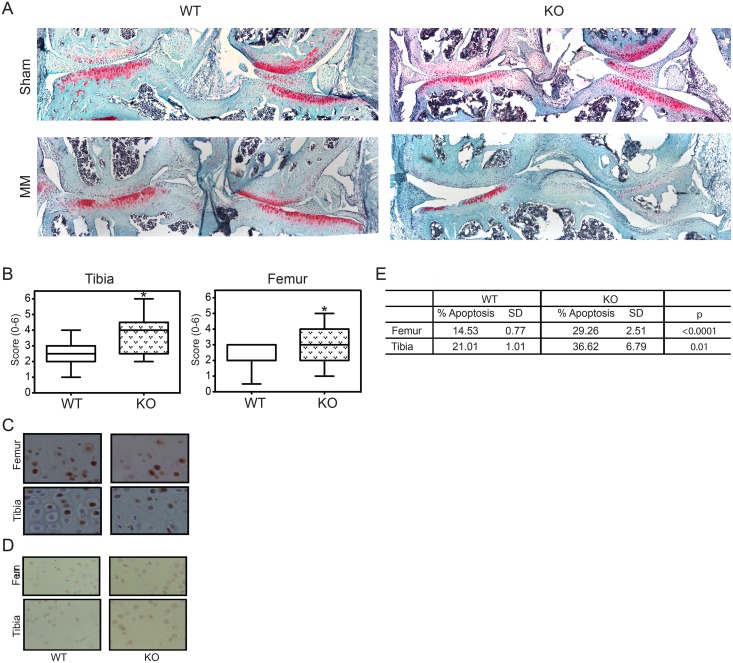
As noted above, apoptosis is an important step to developing OA. Because of the significant impact of DEL1 on chondrocyte apoptosis, we predicted that the KO mice would develop more severe OA in response to injury than WT mice. Normal laboratory mice rarely develop OA when allowed to live to relative old age without intervention.[7] We chose to use a model of post-traumatic OA because or relatively rapid and consistent progression of disease to assess whether KO mice had increased severity of disease. We performed a medial meniscectomy to destabilize the knee in 8-week-old male KO and WT mice.[7] Mice were harvested at 8 weeks after surgery and the degree of OA scored by a trained pathologist (KYJ) blinded to the mouse genotype using an established and validated system.[19] Representative photomicrographs of WT and KO mice after medial meniscectomy or sham surgery are shown (Fig 4A). KO mice had significantly worse destruction of the medial articular surface of the tibia and femur as determined by average score for OA severity (Fig 4B). The sham-operated knees had no evidence of OA.
Fig 4. Osteoarthritis susceptibility.
(A) 25x magnification view of knee joints from WT and KO mice after sham operation or medial meniscectomy (MM). (B) Box and whiskers plot of histologic scoring of medial tibial and femoral surfaces for OA. *p = 0.0206 for tibia, p = 0.0003 for femur, n = 18 WT and 17 KO. Representative photomicrographs of TUNEL staining of articular surfaces at 1 week after knee destabilization in the injured (C), and sham operated (D) knees. Apoptotic cells seen in the same area of the articular cartilage were counted at 200x magnification as shown and quantified (E). *p<0.001 for femur and p<0.00001 for tibia, n = 5 WT and 6 KO.
Exacerbation of osteoarthritis was associated with increased chondrocyte apoptosis
Apoptosis is an early event in the development of OA and precedes histologic evidence of articular surface damage. We hypothesized that we would see evidence of increased apoptosis in Del1 KO mice early after knee surgery so we harvested a separate group of animals after 1 week to evaluate for the degree of apoptosis within the articular chondrocytes. Using TUNEL staining we found significantly increased numbers of apoptotic cells on the medial tibial and femoral articular surfaces of KO knees consistent with the sites exhibiting the most severe histologic OA (Fig 4B–4E). There was essentially no apoptosis seen in sham-operated knees (Fig 4D). Collectively, these data suggest that DEL1 protein was protective against OA by preventing chondrocyte apoptosis.
We next asked whether chondrocytes from KO mice were more susceptible to apoptosis when compared to WT. We collected primary chondrocytes from the joints of WT and KO mice and induced apoptosis with TNFα/actinomycin D. Chondrocytes were grown in the absence or presence of purified DEL1 protein. WT chondrocytes showed increased resistance to apoptosis with added Del1. KO chondrocytes were more susceptible to apoptosis than WT in the absence of DEL1, and approached WT in the presence of DEL1 (Fig 3C).
No difference in angiogenesis and inflammation
The development of OA results from the complex interaction of many different cell types. While we could not exclude every other possible cellular mechanism by which DEL1 protects against OA, we did address some of the more relevant possibilities. TGF-β1 was shown to induce high levels of angiogenesis along with increased OA,[23] increased angiogenesis has been reported in the tissues around OA-affected joints, particularly the synovium[24,25] and DEL1 was reported to have angiogenic activity[26] creating the possibility that there were aberrations in angiogenesis around the knee that might have contributed to development of OA. Using immunohistochemistry with anti-CD31 antibodies to assess vascularity, we found no differences between WT and Del1 KO mice (S3 Fig).
When we examined other protein factors and cytokines that stimulated Del1 mRNA expression in chondrocytes, we found IL-1α, TNFα and IFNγ, all important inflammatory mediators implicated in OA,[3] significantly up-regulated expression (Fig 3D). Despite its initial identification as an angiogenic factor, Del1 mRNA was not up regulated by PDGF, VEGF or FGF2 in endothelial cells, or by VEGF or FGF2 in chondrocytes ([27]and Fig 3D).
In addition to angiogenesis, DEL1 facilitates leukocyte recruitment to areas of injury.[28] It was shown that Del1 KO mice had a greater accumulation of neutrophils in a lung injury model. MFGE8, the only known protein family member of DEL1, aids phagocytosis of apoptotic cells by binding exposed phosphotidyl serines on apoptotic cells through their discoidin-like domain and integrins on macrophages through the RGD motif to facilitate clearance.[29] A similar function has also been ascribed to DEL1.[30] We examined whether there were any differences in the inflammatory response using immunohistochemistry with antibodies directed against lymphocytes (anti-CD45R), macrophages (anti-F4/80) and neutrophils (anti-Ly-6B.2). Counting of positive cells per high power field demonstrated no differences in the presence of the various lineages of inflammatory cells in the injured joint (S3 Fig). There can be changes in immune function that we do not detect with this gross assay, but the papers describing the impact of DEL1 and MFGE8 on immune cell function noted there were differences in immune cell localization due to the effects on diapedesis and phagocytosis.[28,29]
Cartilage from Del1 KO mice was biomechanically similar to WT
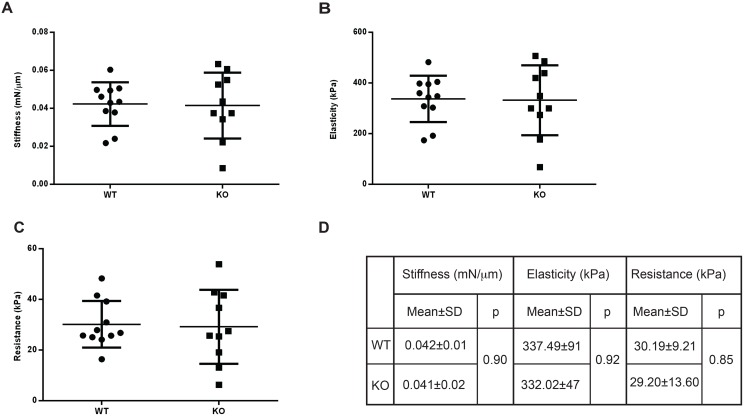
An alternative explanation for the Del1 KO mouse phenotype was simply that the cartilage was structurally weaker. Biomechanical testing was performed on the cartilage of the femoral head. The femoral head was chosen for analysis instead of the knee because the surface of the mouse knee joint was too small for adequate, reproducible measurements. We used 10 WT and KO male mice at 10 weeks of age for these studies. Specimens were analyzed using a microprobe system for stiffness, elasticity and resistance to penetration. No significant differences were seen in any of these parameters (Fig 5).
Fig 5. Biomechanical testing of cartilage.
Articular surfaces were tested to measure (A) stiffness, (B) elasticity, and (C) resistance to penetration. Numerical values are shown (D) and statistical significance calculated with Student’s t test with p<0.05 seen to be significant, n = 10 WT and 10 KO.
Discussion
Despite the expression of Del1 mRNA within cartilaginous structures during development and in the antenatal period, Del1 KO mice were not different in the bony skeleton. We did note the KO mice had floppy ears noticeable primarily in the first weeks of life due to decreased thickness of the auricular cartilage. Additional analysis of the knee joints showed there was also diminished cartilage there. The finding that both elastic and hyaline cartilage, the two major types within the body, were decreased led us to conclude that there was a general decrease in the amount of cartilage due to this mutation.
Using primary chondrocytes, we examined how DEL1 might affect their biology to result in this phenotype. We found that DEL1 promoted chondrocyte attachment and was strongly anti-apoptotic. It had no impact on chondrocyte proliferation. Given the importance of apoptosis in the development of OA and the significant expression of Del1 mRNA within cartilage, we proposed the Del1 KO mice develop more severe OA when compared to WT. We chose medial meniscectomy as a rapid and consistent trigger of post-traumatic OA as a model. Our data show Del1 KO mice had more severe OA in response to injury and this was correlated with increased apoptosis within chondrocytes in those areas. Among the proteins that induced Del1 mRNA expression, we found inflammatory mediators were the most prominent. These data led us to conclude that the phenotype was due to a positive survival signal provided by Del1 to chondrocytes, and may be a protective mechanism during periods of inflammation.
While we found increased chondrocyte apoptosis, there are a myriad other ways in which loss of DEL1 might lead to more severe OA. We examined a number of variables including angiogenesis, inflammatory cell infiltrate and biomechanical properties and found that we could not detect any significant differences. One limitation of these data is the unclear impact of the thinner cartilage found in Del1 KO mice, but we did find no difference in the biomechanical properties suggesting the primary function of joint cartilage in permitting smooth locomotion was not affected. We clearly note our work may not be able to detect more subtle effects, but our studies do point to the fact that preventing apoptosis was a major contributor to the phenotype.
Del1 KO mice are unique compared to most genetic mutants that have increased susceptibility to OA because they are grossly normal with the exception of a “floppy ear” phenotype early in life. Among the genetic mouse models of OA described,[7] mutations in major developmental regulatory genes typically required conditional knockouts due to embryonic lethality (i.e. HH).[13] Mutations in ECM proteins like COL2A1 display a variety of congenital malformations of the skeleton mirroring human pedigrees of patients with chondrodysplasias.[7] There are lines of mice that develop osteoarthritis spontaneously (SRT/Ort), but it is noted that this is not typical of human disease.[31] Del1 KO mice develop more severe OA than WT after an inciting trauma. This is similar to the clinical experience in humans where individuals suffering the same injury have very different outcomes with regards to development of OA, and we suggest that the Del1 KO mice represent a genetic model of susceptibility to OA that more closely mirrors the most common form of the human disease.
Previous genetic studies of non-syndromic OA susceptibility have indicated multiple genes contribute.[4–6] Our data suggest a recessive, single gene trait that is not readily recognized due to the subtle nature of the phenotype can cause more severe OA in response to trauma. Interestingly, a recent review of translational studies in OA specifically noted the post-traumatic model of OA used in our study most closely mimics actual human disease rather than those genetic mouse models that develop OA spontaneously, and this can have in impact in translational studies of new therapeutics.[32]
The role of apoptosis in the development of OA is well established. There are other mouse mutations described that lead to increased apoptosis of the articular chondrocytes. Mice with an inactivating point mutation in SIRT-1 had increased apoptosis of the chondrocytes, but were also noted to have reduced ECM components unlike the Del1 KO mice.[15] CHOP is a down stream target of the unfolded protein response (UPR) activated during periods of cellular stress that functions to regulate multiple components of the apoptotic pathway. In Chop-/- mice, there was decreased severity of OA following knee destabilization associated with decreased apoptosis.[16] The Chop-/- mice provide an interesting parallel to the Del1 KO mice in that both have a normal appearance with a phenotype expressed only after knee destabilization.
These data also show interesting parallels with the CCN family of matricellular proteins. Like DEL1, CCN proteins are ECM-associated and function in part through integrin binding. CCN1 promotes chondrocyte proliferation and aggregation, but has no known impact on skeletal development.[33,34] Ccn2 deletion leads to embryonic lethality due to severe skeletal dysmorphisms by impairing chondrocyte proliferation and ECM production.[35] Mutations in Ccn6 cause progressive pseudorhematoid dysplasia in humans, but have no impact on mouse skeletal development.[36,37] While DEL1 and CCN family members bind the same integrins, it is unclear what their relationship is to each other. Furthermore, the presence of multiple functional domains in these proteins increases the complexity of potential interactions.
Most OA therapies, including non-steroidal anti-inflammatory drugs (NSAIDs), behavior modification (weight loss, exercise) and surgical joint replacement, are to control OA symptoms rather than target the causal factors of the disease.[1,38] NSAIDs are capable of preventing inflammation, a significant component in the development of OA, but there are no data that NSAIDs can slow progression. A number of nutriceuticals (glucosamine/chondroitin) that looked promising in small studies have failed in larger, randomized trails to demonstrate efficacy at slowing OA disease progression.[39] Among the proteins identified which can contribute to OA, there are many that are less desirable targets for therapy because they are master regulatory proteins, and manipulation of their activity could have a host of unwanted side effects. Targeting enzymes involved in cartilage ECM degradation like MMPs is an active field of investigation. We have demonstrated that DEL1 protein was protective against OA, which suggests a new approach to OA treatment by using compounds that can directly block chondrocyte apoptosis.
Supporting Information
Calculated density of cells in cartilage of WT and KO mice performed by counting numbers of cells per high power field. N = 4 WT and KO mice.
(PDF)
Normal human chondrocytes were cultured on plates coated with 8 ng/mm2 of BSA (DEL1-) or DEL1 (DEL1+) and proliferation assayed using WST-8 assay with absorbance read at OD450nm.
(PDF)
Knee joints harvested at 8 weeks following medial meniscectomy were stained for CD31 as a marker of angiogenesis and at 1 week following medial meniscectomy for markers of lymphocytes (CD45R), macrophages (F4/80) and neutrophils (Ly-6B.2). Images shown are representative micrographs taken at 100x magnification. Positive cells per high power field were counted in the area of the medial compartment synovium and reported in the table below.
(PDF)
Acknowledgments
We would like to thank DM Kingsley and N Quarto for helpful discussions.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Funding was provided by the US Department of Veterans Affairs, American Heart Association, Oak Foundation, Hagey Laboratory for Pediatric Regenerative Medicine, and the Eilenberg Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Felson DT (2006) Clinical practice. Osteoarthritis of the knee. N Engl J Med 354: 841–848. [DOI] [PubMed] [Google Scholar]
- 2.Anderson DD, Chubinskaya S, Guilak F, Martin JA, Oegema TR, Olson SA, et al. (2011) Post-traumatic osteoarthritis: improved understanding and opportunities for early intervention. J Orthop Res 29: 802–809. 10.1002/jor.21359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernandes JC, Martel-Pelletier J, Pelletier JP (2002) The role of cytokines in osteoarthritis pathophysiology. Biorheology 39: 237–246. [PubMed] [Google Scholar]
- 4.Meulenbelt I, Bijkerk C, Breedveld FC, Slagboom PE (1997) Genetic linkage analysis of 14 candidate gene loci in a family with autosomal dominant osteoarthritis without dysplasia. J Med Genet 34: 1024–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyamoto Y, Mabuchi A, Shi D, Kubo T, Takatori Y, Saito S, et al. (2007) A functional polymorphism in the 5' UTR of GDF5 is associated with susceptibility to osteoarthritis. Nat Genet 39: 529–533. [DOI] [PubMed] [Google Scholar]
- 6.Valdes AM, Loughlin J, Timms KM, van Meurs JJ, Southam L, Wilson SG, et al. (2008) Genome-wide association scan identifies a prostaglandin-endoperoxide synthase 2 variant involved in risk of knee osteoarthritis. Am J Hum Genet 82: 1231–1240. 10.1016/j.ajhg.2008.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ameye LG, Young MF (2006) Animal models of osteoarthritis: lessons learned while seeking the "Holy Grail". Curr Opin Rheumatol 18: 537–547. [DOI] [PubMed] [Google Scholar]
- 8.Chambers MG, Cox L, Chong L, Suri N, Cover P, Bayliss MT, et al. (2001) Matrix metalloproteinases and aggrecanases cleave aggrecan in different zones of normal cartilage but colocalize in the development of osteoarthritic lesions in STR/ort mice. Arthritis Rheum 44: 1455–1465. [DOI] [PubMed] [Google Scholar]
- 9.Glasson SS, Askew R, Sheppard B, Carito B, Blanchet T, Ma HL, et al. (2005) Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature 434: 644–648. [DOI] [PubMed] [Google Scholar]
- 10.Saito T, Fukai A, Mabuchi A, Ikeda T, Yano F, Ohba S, et al. Transcriptional regulation of endochondral ossification by HIF-2alpha during skeletal growth and osteoarthritis development. Nat Med 16: 678–686. 10.1038/nm.2146 [DOI] [PubMed] [Google Scholar]
- 11.Yang S, Kim J, Ryu JH, Oh H, Chun CH, Kim BJ, et al. Hypoxia-inducible factor-2alpha is a catabolic regulator of osteoarthritic cartilage destruction. Nat Med 16: 687–693. 10.1038/nm.2153 [DOI] [PubMed] [Google Scholar]
- 12.Hirata M, Kugimiya F, Fukai A, Ohba S, Kawamura N, Ogasawara T, et al. (2009) C/EBPbeta Promotes transition from proliferation to hypertrophic differentiation of chondrocytes through transactivation of p57. PLoS One 4: e4543 10.1371/journal.pone.0004543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin AC, Seeto BL, Bartoszko JM, Khoury MA, Whetstone H, Ho L, et al. (2009) Modulating hedgehog signaling can attenuate the severity of osteoarthritis. Nat Med 15: 1421–1425. 10.1038/nm.2055 [DOI] [PubMed] [Google Scholar]
- 14.Weng LH, Wang CJ, Ko JY, Sun YC, Wang FS Control of Dkk-1 ameliorates chondrocyte apoptosis, cartilage destruction, and subchondral bone deterioration in osteoarthritic knees. Arthritis Rheum 62: 1393–1402. 10.1002/art.27357 [DOI] [PubMed] [Google Scholar]
- 15.Gabay O, Sanchez C, Dvir-Ginzberg M, Gagarina V, Zaal KJ, Song Y, et al. (2013) Sirtuin 1 enzymatic activity is required for cartilage homeostasis in vivo in a mouse model. Arthritis Rheum 65: 159–166. 10.1002/art.37750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uehara Y, Hirose J, Yamabe S, Okamoto N, Okada T, Oyadomari S, et al. (2014) Endoplasmic reticulum stress-induced apoptosis contributes to articular cartilage degeneration via C/EBP homologous protein. Osteoarthritis Cartilage 22: 1007–1017. 10.1016/j.joca.2014.04.025 [DOI] [PubMed] [Google Scholar]
- 17.Williams CJ, Jimenez SA (1995) Heritable diseases of cartilage caused by mutations in collagen genes. J Rheumatol Suppl 43: 28–33. [PubMed] [Google Scholar]
- 18.Hidai C, Zupancic T, Penta K, Mikhail A, Kawana M, Quertermous EE, et al. (1998) Cloning and characterization of developmental endothelial locus-1: an embryonic endothelial cell protein that binds the alphavbeta3 integrin receptor. Genes Dev 12: 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glasson SS, Chambers MG, Van Den Berg WB, Little CB The OARSI histopathology initiative—recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage 18 Suppl 3: S17–23. 10.1016/j.joca.2010.05.025 [DOI] [PubMed] [Google Scholar]
- 20.Kumar S, Park SH, Cieply B, Schupp J, Killiam E, Zhang F, et al. (2011) A pathway for the control of anoikis sensitivity by E-cadherin and epithelial-to-mesenchymal transition. Mol Cell Biol 31: 4036–4051. 10.1128/MCB.01342-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mosher DF, Adams JC (2012) Adhesion-modulating/matricellular ECM protein families: a structural, functional and evolutionary appraisal. Matrix Biol 31: 155–161. 10.1016/j.matbio.2012.01.003 [DOI] [PubMed] [Google Scholar]
- 22.Gigout A, Jolicoeur M, Nelea M, Raynal N, Farndale R, Buschmann MD (2008) Chondrocyte aggregation in suspension culture is GFOGER-GPP- and beta1 integrin-dependent. J Biol Chem 283: 31522–31530. 10.1074/jbc.M804234200 [DOI] [PubMed] [Google Scholar]
- 23.Zhen G, Wen C, Jia X, Li Y, Crane JL, Mears SC, et al. (2013) Inhibition of TGF-beta signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat Med 19: 704–712. 10.1038/nm.3143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Isozaki T, Rabquer BJ, Ruth JH, Haines GK 3rd, Koch AE (2013) ADAM-10 is overexpressed in rheumatoid arthritis synovial tissue and mediates angiogenesis. Arthritis Rheum 65: 98–108. 10.1002/art.37755 [DOI] [PubMed] [Google Scholar]
- 25.Weng LH, Ko JY, Wang CJ, Sun YC, Wang FS (2012) Dkk-1 promotes angiogenic responses and cartilage matrix proteinase secretion in synovial fibroblasts from osteoarthritic joints. Arthritis Rheum 64: 3267–3277. 10.1002/art.34602 [DOI] [PubMed] [Google Scholar]
- 26.Penta K, Varner JA, Liaw L, Hidai C, Schatzman R, Quertermous T (1999) Del1 induces integrin signaling and angiogenesis by ligation of alphaVbeta3. J Biol Chem 274: 11101–11109. [DOI] [PubMed] [Google Scholar]
- 27.Wang Z, Kundu RK, Longaker MT, Quertermous T, Yang GP (2012) The angiogenic factor Del1 prevents apoptosis of endothelial cells through integrin binding. Surgery 151: 296–305. 10.1016/j.surg.2011.07.013 [DOI] [PubMed] [Google Scholar]
- 28.Choi EY, Chavakis E, Czabanka MA, Langer HF, Fraemohs L, Economopoulou M, et al. (2008) Del-1, an endogenous leukocyte-endothelial adhesion inhibitor, limits inflammatory cell recruitment. Science 322: 1101–1104. 10.1126/science.1165218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S (2002) Identification of a factor that links apoptotic cells to phagocytes. Nature 417: 182–187. [DOI] [PubMed] [Google Scholar]
- 30.Hanayama R, Tanaka M, Miwa K, Nagata S (2004) Expression of developmental endothelial locus-1 in a subset of macrophages for engulfment of apoptotic cells. J Immunol 172: 3876–3882. [DOI] [PubMed] [Google Scholar]
- 31.Mason RM, Chambers MG, Flannelly J, Gaffen JD, Dudhia J, Bayliss MT (2001) The STR/ort mouse and its use as a model of osteoarthritis. Osteoarthritis Cartilage 9: 85–91. [DOI] [PubMed] [Google Scholar]
- 32.Little CB, Hunter DJ (2013) Post-traumatic osteoarthritis: from mouse models to clinical trials. Nat Rev Rheumatol 9: 485–497. 10.1038/nrrheum.2013.72 [DOI] [PubMed] [Google Scholar]
- 33.Mo FE, Muntean AG, Chen CC, Stolz DB, Watkins SC, Lau LF (2002) CYR61 (CCN1) Is Essential for Placental Development and Vascular Integrity. Molecular and Cellular Biology 22: 8709–8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong M, Kireeva ML, Kolesnikova TV, Lau LF (1997) Cyr61, product of a growth factor-inducible immediate-early gene, regulates chondrogenesis in mouse limb bud mesenchymal cells. Dev Biol 192: 492–508. [DOI] [PubMed] [Google Scholar]
- 35.Ivkovic S, Yoon BS, Popoff SN, Safadi FF, Libuda DE, Stephenson RC, et al. (2003) Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development 130: 2779–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hurvitz JR, Suwairi WM, Van Hul W, El-Shanti H, Superti-Furga A, Roudier J, et al. (1999) Mutations in the CCN gene family member WISP3 cause progressive pseudorheumatoid dysplasia. Nat Genet 23: 94–98. [DOI] [PubMed] [Google Scholar]
- 37.Kutz WE, Gong Y, Warman ML (2005) WISP3, the gene responsible for the human skeletal disease progressive pseudorheumatoid dysplasia, is not essential for skeletal function in mice. Mol Cell Biol 25: 414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarzi-Puttini P, Cimmino MA, Scarpa R, Caporali R, Parazzini F, Zaninelli A, et al. (2005) Osteoarthritis: an overview of the disease and its treatment strategies. Semin Arthritis Rheum 35: 1–10. [DOI] [PubMed] [Google Scholar]
- 39.Clegg DO, Reda DJ, Harris CL, Klein MA, O'Dell JR, Hooper MM, et al. (2006) Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med 354: 795–808. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Calculated density of cells in cartilage of WT and KO mice performed by counting numbers of cells per high power field. N = 4 WT and KO mice.
(PDF)
Normal human chondrocytes were cultured on plates coated with 8 ng/mm2 of BSA (DEL1-) or DEL1 (DEL1+) and proliferation assayed using WST-8 assay with absorbance read at OD450nm.
(PDF)
Knee joints harvested at 8 weeks following medial meniscectomy were stained for CD31 as a marker of angiogenesis and at 1 week following medial meniscectomy for markers of lymphocytes (CD45R), macrophages (F4/80) and neutrophils (Ly-6B.2). Images shown are representative micrographs taken at 100x magnification. Positive cells per high power field were counted in the area of the medial compartment synovium and reported in the table below.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.