Abstract
Biological barriers to drug transport prevent successful accumulation of nanotherapeutics specifically at diseased sites, limiting efficacious responses in disease processes ranging from cancer to inflammation. Although substantial research efforts have aimed to incorporate multiple functionalities and moieties within the overall nanoparticle design, many of these strategies fail to adequately address these barriers. Obstacles, such as nonspecific distribution and inadequate accumulation of therapeutics, remain formidable challenges to drug developers. A reimagining of conventional nanoparticles is needed to successfully negotiate these impediments to drug delivery. Site-specific delivery of therapeutics will remain a distant reality unless nanocarrier design takes into account the majority, if not all, of the biological barriers that a particle encounters upon intravenous administration. By successively addressing each of these barriers, innovative design features can be rationally incorporated that will create a new generation of nanotherapeutics, realizing a paradigmatic shift in nanoparticle-based drug delivery.
Positive patient outcomes across a wide range of disease states rely heavily on the physician's ability to direct drugs to a specific site. Cancer represents the best example of a disease where the adequacy of delivery of chemotherapeutics with highly potent, yet toxic, mechanisms of action can mean the difference between efficacious responses and severe morbidity. Despite a century of perpetual discovery and development, present-day formulations leave drugs incapable of localizing en masse specifically at sites of interest. Drug molecules simply diffuse and distribute freely throughout the body, resulting in undesirable side effects and limiting achievement of proper doses required to bring about efficacious responses. This inability to reach target sites contributes to exceptionally high attrition rates of new chemical entities (NCEs) across all therapeutic areas, with only 1 in 9 drugs gaining approval by regulatory authorities1. Lack of efficacy and clinical safety remain principal causes of NCE failure in later-stage clinical trials.
Nanoparticle-based drug delivery platforms have emerged as suitable vehicles for overcoming pharmacokinetic limitations associated with conventional drug formulations. Nanoparticles, such as liposomes, have proven advantageous at solubilizing therapeutic cargos, substantially prolonging the circulation lifetimes of drugs2. Even so, it was Maeda and co-workers3, who, with their discovery of the enhanced permeability and retention (EPR) effect, demonstrated the potential for heightened accumulation of long-circulating macromolecules by extravasation through fenestrated blood vessels in tumors and opened several exciting avenues for site-specific localization of chemotherapeutics4. Consequently, over the past two decades, this characteristic of solid tumors has been a major impetus for extensive research efforts aimed at applying nanoparticles to chemotherapy. And with growing evidence of the EPR phenomenon in pathologies, ranging from infection5 to heart failure6, nanoparticle-based drug delivery is emerging as a powerful strategy in several distinct disease conditions, as demonstrated by clinical approval of nanoparticle formulations for fungal infections, hepatitis A, multiple sclerosis and end-stage renal disease7. Their long circulation lifetimes and ability to extravasate to disease sites largely improved the safety and tolerability of nanoparticle-formulated drugs, best shown by the reduced cardiotoxicity observed in patients after administration of liposomal doxorubicin compared with that in those undergoing treatment with the conventional formulation8. These improvements in patient morbidity led to the US Food and Drug Administration approval of liposomal doxorubicin (Doxil) for the treatment of Kaposi's sarcoma in 1995 (ref. 9), as well as approval of nanoparticle albumin-bound paclitaxel (Abraxane) 10 years later, which similarly reduced detrimental side effects associated with the conventional paclitaxel formulation by eliminating the excipient Cremophor EL10.
Although improvements in patient safety and morbidity led to clinical approval of nanoparticle platforms, such as doxorubicin and paclitaxel, efficacious patient responses remain modest; currently, these platforms offer only marginal improvements over conventional formulations11,12. Despite their potential for increased drug half-lives and improving a drug's propensity to accumulate at sites of injury, the platforms face a complex series of biological barriers that severely limit site-specific bioavailability, preventing achievement of proper therapeutic outcomes. These obstacles include opsonization and subsequent sequestration by the mononuclear phagocyte system (MPS), nonspecific distribution, hemorheological/blood vessel flow limitations, pressure gradients, cellular internalization, escape from endosomal and lysosomal compartments and drug efflux pumps13 (Fig. 1). In addition to the substantial challenges presented by each individual biological barrier, it is important to note that these vary in complexity depending on factors, such as administration route (oral versus intravenous), disease type (cancer versus infection) and state of disease progression (early- versus late-stage cancers).
Figure 1.
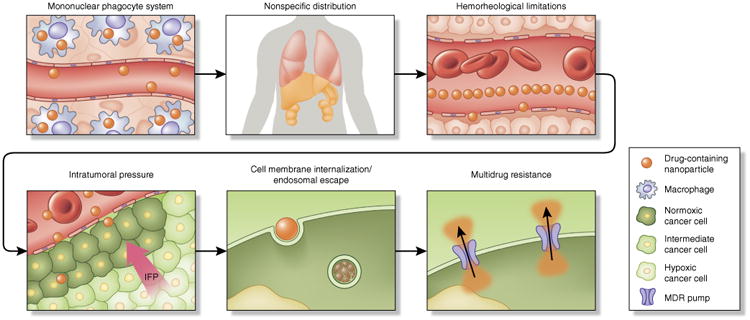
Framework of sequential biological barriers to nanoparticle drug delivery. Upon intravenous administration, drug-containing nanoparticles encounter a number of sequential obstacles hindering efficacious, site-specific delivery to tumors. Nanoparticles undergo opsonization and subsequent uptake by resident macrophages of the MPS. This results in high accumulation of nanoparticles in organs, such as the spleen and the liver, contributing to nonspecific distribution of nanotherapeutics to healthy organs. Under normal flow conditions in blood vessels, size and geometry have been shown to vastly influence margination dynamics to vascular walls. Spherical particles of small size migrate in a cell-free layer, at a considerable distance from endothelial surfaces, limiting both active targeting strategies and effective accumulation through passive targeting mechanisms (e.g., EPR). Another substantial barrier to nanoparticle accumulation in tumors is the high intratumoral pressure, resulting from interrupted vasculature, the aggressive nature of cellular growth, fibrosis, a dense extracellular matrix and impaired lymphatics. Cellular internalization and endosomal escape prove to be formidable barriers, with size and surface decoration affecting route of internalization (e.g., clathrin versus caveolin) and intracellular fate. Endosomal compartmentalization of internalized nanoparticles, subjected to a low pH environment and enzymes, proves detrimental to cargo, especially to genetic material. Last but not least, upon entry into the cell, drug efflux pumps that confer therapy resistance expel chemotherapeutics from the cell. IFP, interstitial fluid pressure.
The minimal therapeutic impact observed following nanoparticle delivery is a direct consequence of the nanoparticle's inability to overcome many of these barriers. A vast amount of research and resources are continually invested in the incorporation of innovative design features within traditional nanocarrier constructs for proper negotiation of biological barriers, resulting in the creation of multifunctional nanoparticles. Oftentimes, these features include incorporation of active targeting moieties for enhanced uptake in specific cells14 or constituent components for stimulus-responsive release (e.g., pH-sensitive, thermosensitive and ultrasound)15. Although these modifications highlight the impressive versatility and preclinical potential of nanomedicine, very few nanoparticles that simply address one or a few biological barriers progress to the clinical arena. This realization has led many experts to provocatively question, and challenge, the field of nanoparticle-based drug delivery in hopes of transitioning the discipline from platforms with mere potential to those capable of delivering positive clinical outcomes16,17.
Here, we present a conceptual framework of the biological barriers encountered by nanoparticles in a sequential fashion, from administration to arrival at sites of interest. For the major part of the following discussion, all nanoparticle drugs, irrespective of therapeutic cargo, share these barriers. However, certain types of therapeutic cargo, such as nucleic acid therapies, face additional limitations (Box 1). Our aim is to draw attention to the impact that these biological barriers have on the ultimate fate of administered nanoparticles, as well as strategies that may be helpful in overcoming these obstacles. We highlight the fact that if the majority of, if not all, biological barriers are not adequately addressed in a successive fashion at the time of nanoparticle design, the field of nanoparticle-based drug delivery will continue to fail to realize its clinical potential.
Box 1. Nanoparticle gene therapy and the nuclear membrane barrier.
The potential of genetic treatments for several life-threatening diseases, including B-cell leukemia, gliomas, Parkinson's disease and arthritis94, has undergone a resurgence thanks in part to the development and refinement of delivery strategies. Although nucleic acid therapies traditionally relied on the use of viral vectors as vehicles for transfection95, a parallel line of research has focused on the use of nanoparticle-based platforms for gene delivery, including liposomes96, magnetic nanoparticles97 and gold nanoparticles98.
In addition to the biological barriers limiting site-specific delivery, nanoparticle gene therapies face the additional challenges of the instability of genetic material, such as mRNAs, antisense oligonucleotides and siRNAs, and in the specific case of plasmid DNA, traversal of the nuclear envelope. Endosomal compartmentalization produces an enzyme-rich environment with low pH capable of degrading genetic material. Moreover, DNA that does survive endosomal escape is subjected to degradation by cytoplasmic nucleases99. Should DNA successfully overcome the degradative effects of the enzymes and nucleases comprising the intracellular environment, the next step is to gain entry into the nucleus. The nuclear envelope encases the cell genome and consists of an outer membrane and inner membrane that form a contiguous structure with the endoplasmic reticulum100. The inner and outer membranes are fused together at nuclear pore complex sites, which restrict traffic to macromolecules with molecular weights ∼40 kDa or smaller101.
Recently, Huang and co-workers102 designed a novel liposomal formulation capable of addressing the instability of genetic material and traversal of the nuclear envelope, with the end goal of using this vehicle to treat liver diseases, such as viral hepatitis and Wilson disease. The nanoparticle comprised DNA and octaarginine peptides encapsulated within a calcium phosphate core, and the surface was functionalized with PEG to ensure long circulation and a galactose-targeting ligand to guarantee hepatic cell uptake. It was hypothesized that the acid-sensitive calcium phosphate core would result in rapid endosomal escape before lysosomal fusion, whereas the reducible cyclic oligoarginine would facilitate nuclear importation of DNA. The ∼50-nm nanoparticles rapidly accumulated within hepatocytes, with approximately half of the injected dose recovered in the liver 6 h after intravenous administration. Encapsulated DNA translocated effectively to the nucleus, resulting in greatly enhanced gene expression.
Opsonization/sequestration by the mononuclear phagocyte system
The major limitation of nanotherapeutic delivery is its inability to reach therapeutic levels of drugs at disease sites owing to nonspecific uptake of nanoparticles in healthy organs. The MPS, which consists of a system of phagocytic cells, predominantly resident macrophages, in the spleen, lymph nodes and liver, sequesters nanoparticles immediately after injection18. The process of sequestration begins with opsonization of nanoparticles, involving the adsorption of plasma proteins, including serum albumin, apolipoproteins, complement components and immunoglobulins, onto the surface of circulating nanoparticles19. The formation of the protein corona around nanoparticles is dependent on several factors, including nanoparticle size, surface charge, hydrophobicity and surface chemistry20. Following protein adsorption, nanoparticles undergo attachment to specific receptors on the surface of phagocytes, after which nanoparticles are internalized, transported to phagosomes and fused with lysosomes21. In addition to increasing uptake by the MPS, opsonization often proves detrimental to active-targeting strategies for nanoparticles, as the adhered biological corona masks targeting ligands, resulting in a marked reduction in specificity. Dawson and co-workers22 effectively demonstrated this in a study wherein silica nanoparticles functionalized with the glycoprotein transferrin were unable to bind to corresponding receptors on A549 cells or soluble transferrin receptors following formation of a protein corona. Nanoparticles indeed represent dynamic entities immediately after systemic administration, warranting examination of the mechanism of protein corona formation and the effects these may have on nanoparticle stability, bioavailability, toxicity and fate23,24.
The strategy of functionalizing nanoparticles with poly(ethylene glycol) (PEG), or PEGylation, stemmed largely from the observation that nanoparticles had low circulation lifetimes following intravascular administration. PEGylation involves the grafting of PEG to the surface of nanoparticles, wherein ethylene glycol units form tight associations with water molecules, resulting in the formation of a hydrating layer25. This hydrating layer in turn hinders protein adsorption and subsequent clearance by the MPS (Fig. 2). The transformative potential of PEGylation to prolong the circulating lifetimes of nanoparticles was best exemplified by the PEGylation of liposomal doxorubicin, which increased the lifetime of the drug from minutes to hours26. Although functionalization of nanoparticles with materials that possess similar shielding effects has been attempted, such as with polaxamer, polyvinyl alcohol, poly(amino acid)s and polysac-charides27, PEG remains the most widely used material. Huang and co-workers28 demonstrated effective evasion of the MPS through the use of PEGylated liposome-polycation-DNA nanoparticles. The strategy consists of coating a negatively charged, nucleic acid–containing compact core with two cationic lipid bilayers, with the hypothesis that a supported and stabilized bilayer would tolerate a higher amount of distearoylphosphatidylethanolamine (DSPE)-PEG2000 and result in proper evasion of the MPS. Findings indeed demonstrated low liver sinusoidal uptake and a high amount of injected dose (∼33%) in NCI-H460 tumors.
Figure 2.
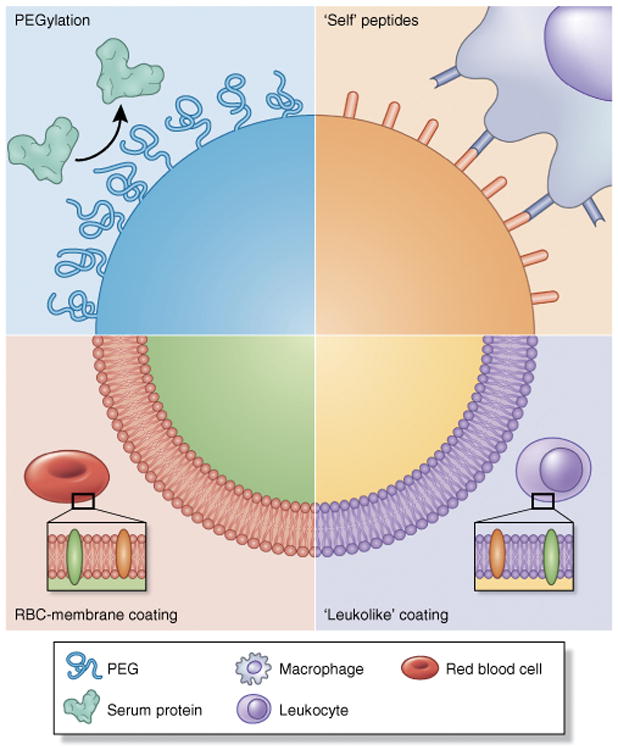
Strategies for nanoparticle biomimicry for MPS avoidance and prolonged circulation. Opsonization and sequestration by the MPS proves detrimental to long circulation times of nanoparticles. Several strategies have been used to ‘camouflage’ nanoparticles and prevent protein adsorption. PEGylation represents a classic strategy, wherein grafting of PEG to the surface provides a hydrating layer that hinders formation of a protein corona. In another strategy, CD47 peptides are attached to the surface of nanoparticles, after which macrophages identify the nanoparticle as ‘self’, whereby the nanoparticle avoids phagocytosis29. Lastly, coating of nanoparticles with cell membranes extracted from autologous leukocytes30 and red blood cells (RBC)31 provides a biomimetic surface shown to substantially prolong in vivo circulation.
An ‘active stealth’ strategy was explored by Discher and co-workers29, wherein ‘don't eat-me’ marker CD47 ‘self’ peptides were attached to the surface of nanoparticles in an attempt to avoid phagocytic clearance (Fig. 2). In this study, ‘self’ peptides were computationally designed, synthesized and attached to 160-nm nanobeads and, upon administration to NSG (non-obese diabetic (NOD) severe combined immunodeficient IL2rγnull) mice, the peptides substantially prolonged drug circulation by delaying phagocytic clearance by the liver and spleen. Moreover, nanoparticles functionalized with the self peptide showed greater accumulation in A549 tumors within 10 min of administration, with incorporation of paclitaxel within the nanobeads resulting in substantial tumor shrinkage compared with the conventional Cremophor EL formulation of the drug.
Tasciotti and co-workers30 recently developed a biomimetic particle coating consisting of cell membranes isolated from leukocytes with the objective of reducing opsonization and subsequent uptake by the MPS (Fig. 2). Upon surface functionalization with leukocytic membranes, particles showed about a tenfold decrease in protein (IgG and albumin) adsorption to the surface. Consequently, the biomimetic coating resulted in substantially less particle uptake in murine J774 macrophages (∼75% decrease) and human THP-1 phagocytic cells (∼50% decrease), especially when the coating was from the same donor species. When the platform was examined in murine models, functionalized particles accumulated to a lesser degree in the liver after systemic administration. Time-dependent accumulation of coated particles was shown to be independent of the phagocytic effects of Kupffer cells, with particles primarily associated with liver endothelium. This reduction in MPS uptake led to an enhanced accumulation of particles (about twofold) in a murine model of melanoma. A similar strategy of camouflaging nanoparticles was undertaken by Hu et al.31, who fashioned poly(lactic-co-glycolic acid) nanoparticles with membranes (both lipidic and protein components) isolated from red blood cells (Fig. 2), showing prolonged circulation in the blood at time points of up to 72 h.
Interestingly, research groups have attempted to use the ubiquitous protein corona to their advantage to target specific cells and/or disease sites. Kreuter et al.32, using poly(butyl cyanoacrylate) nanoparticles coated with polysorbate 80, demonstrated enhanced drug delivery beyond the blood-brain barrier. Through adsorption of apolipoproteins to the polysorbate 80 surface, nanoparticles effectively act as ‘Trojan horses’ that mimic lipoprotein particles, traversing the blood-brain barrier through low-density lipoprotein–mediated endocytosis. Macrophages are heavily involved in inflammation and consequent disease processes, including cancer33 and atherosclerosis34, making these cells attractive targets for therapeutic applications. Wentworth and co-workers35 functionalized the surface of CdSe/ZnS quantum dots with the inflammatory metabolite cholesterol 5,6-secosterol atheronal-B. The coating induced a conformational change (misfolding/aggregation) of proteins that constitute the protein corona, in particular apolipoprotein B, the protein portion of low-density lipoprotein, which in turn enhanced nanoparticle uptake in macrophages. This same strategy can potentially be employed to enhance nanoparticle uptake in other cells through engineering or programming of the protein corona.
Hemorheology and blood vessel fluid dynamics
Nanoparticle fluid dynamics in blood vessels is highly dependent on the size and geometry of the construct. Conventional nanoparticle platforms, such as liposomes and polymer nanoparticles, typically possess a spherical geometry, generally 10–100 nm in diameter, and are designed specifically for intravenous delivery. Recently, our laboratory36 has divided the intravascular trek of nanoparticles following administration into circulation, margination, adhesion to vascular walls and internalization. Circulation time in blood vessels is highly dependent on the aforementioned opsonization and sequestration by the MPS. Surface properties, such as charge, play a crucial role in protein adsorption, which in turn affects pharmacokinetics and biodistribution of nanoparticles. Highly cationic nanoparticles are rapidly cleared from circulation37, to a greater extent than highly anionic nanoparticles. In contrast, neutral nanoparticles, as well as those with a slight negative charge, show significantly prolonged circulating half-lives. This translates to improved accumulation in tumors, which in turn has led to recent research efforts aimed at functionalizing nanoparticles with zwitterionic surfaces38. Nanocarrier size also affects its in vivo fate, with larger particles (>200 nm) shown to accumulate in the liver and spleen. Margination dynamics, or the lateral drift of nanoparticles to endothelial walls, is a very important nanoparticle design consideration, as association with vessel walls favors particle-cell binding and receptor-ligand interactions in active targeting strategies and enables extravasation through the fenestrated vasculature of tumors. Of note is the fact that small spherical particles, such as liposomes, are found in a particular region of the vessel known as the cell-free layer, which is a direct result of the tendency of red blood cells to accumulate preferentially within the core of a vessel (Fig. 3). Our laboratory39, along with other several other research groups40,41, has shown that nanoparticles possessing traditional spherical geometries exhibit minimal lateral drift and were less likely to marginate to vessel walls and establish contact/binding points with endothelial cells. Needless to say, this hemorheological characteristic of nanoparticles in circulation does little to assist site-specific delivery, unless specific external forces, such as magnetic guidance42, are applied.
Figure 3.
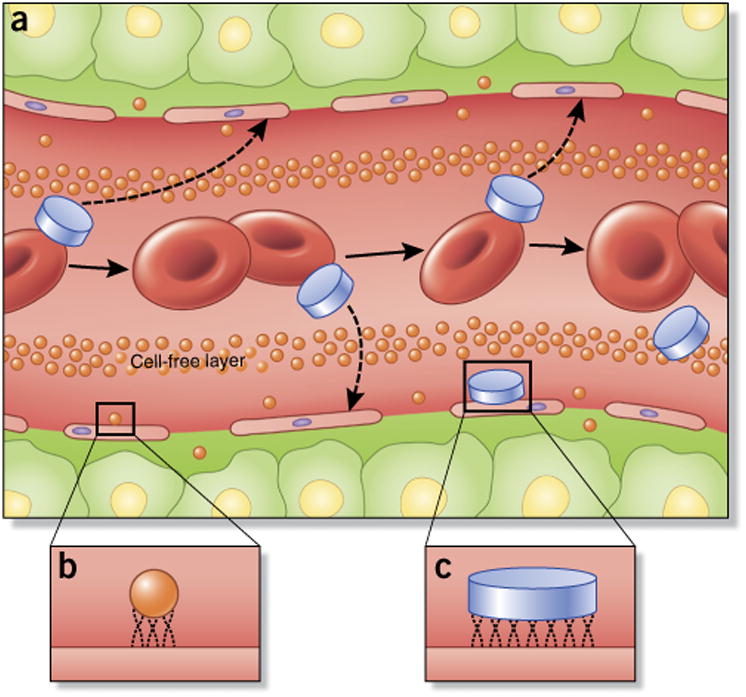
Nanoparticle flow, margination and adhesive properties in blood vessels are dependent on particle size and geometry. (a) Unlike spherical nanoparticles, nonspherical particles, such as those possessing discoidal geometries, are more prone to tumbling and oscillatory effects in vasculature, increasing greatly the propensity of nanoparticle–cell wall contact and potential extravasation through fenestrations in vasculature. (b,c) Once in contact with endothelial cells, the small size and surface area of conventional spherical nanoparticles (b) reduce the number of binding and contact points compared with larger, discoidal nanoparticles (c; as well as other nonspherical geometries), which can affect tumor accumulation and active targeting strategies.
Several strategies have been designed to increase margination of nanoparticles in attempts to enhance both interactions with vessel walls and extravasation to disease sites. Recent research has focused on a reimagining of nanoparticle geometry that represents a departure from classical spherical shapes. Nonspherical particles under flow exhibit tumbling and rolling dynamics, with lateral drifting velocity shown to be directly proportional to the aspect ratio of the nanoparticle36. Whereas a spherical nanoparticle flows a certain distance parallel to and away from the vessel wall, a particle with an ellipsoidal geometry is capable of tumbling and oscillating from one wall to the opposite wall in a vessel.
Our laboratory43 demonstrated by parallel-plate flow chamber experiments under controlled hydrodynamic conditions that discoidal particles marginate to vessel walls more often than quasi-hemispherical and spherical particles (Fig. 3). These findings have provided the rational basis for our own exploration of nonspherical design considerations for nanoparticle drug delivery. We have developed a novel multistage delivery vector (MSV), designed specifically to successfully circumnavigate and successively address several biobarriers encountered by nanoparticles after intravascular delivery13,44. The platform aims to enhance site-specific delivery and release of therapeutics in tumors by encapsulating drug-containing nanoparticles within a carrier construct composed of mesoporous silicon. Photolithographic methods were adopted to fabricate a variety of nanoporous particle shapes (e.g., hemispherical, discoidal) with dimensions ranging from the nanometer to the micron scale44,45. A rational design approach, wherein maximal margination and firm adhesion were taken into consideration, was used to arrive at ideal particle geometries. Mathematical modeling combined with in vitro and in vivo experimentation demonstrated that discoidal geometries possessed the most favorable margination dynamics36. Given its ability to negotiate several distinct biological barriers, including proper protection of cargo, margination to endothelial walls43, accumulation at disease sites46 and controlled release of cargo, the multistage delivery approach has been applied primarily toward chemotherapeutic applications and also shows potential in RNA interference (RNAi) strategies in mouse models of ovarian47 and breast cancer48,49.
Recent research has focused on innovative means with which to amass nanoparticles at specific sites. Bhatia and co-workers50 developed nanoparticles capable of ‘communicating’ with one another to enhance tumor accumulation. As part of this strategy, plasmonic gold nanorods or tumor-targeted truncated tissue factor proteins that initiate the coagulation cascade carry out the signaling. Another nanoparticle, in this case, inorganic nanoworms or liposomes, receives the signal in the form of coagulation transglutaminase factor XIII (FXIII) or through targeting of polymerized fibrin, leading to accumulation in tumors.
Intratumoral pressure and nanoparticle extravasation
A large part of the excitement generated by nanoparticles for drug delivery arises from their potential to preferentially accumulate at sites of injury, infection and inflammation. This passive targeting is due primarily to the presence of endothelial dysfunction and blood vessel fenestrations, the aforementioned EPR effect elegantly delineated by Maeda and co-workers3,4. This phenomenon is highly pronounced in cancer, where chaotic and disorganized vasculature stems from the aggressive angiogenic nature of tumors. This feature of tumors, by no means unique to cancer, has led to strategies aimed at targeting angiogenic vessels with the aim of enhancing site-specific accumulation of nanoparticles. Several groups have fashioned nanoparticle surfaces with moieties specific to overexpressed receptors such as αvβ3 on endothelial cells of tumor vasculature51. McDonald and co-workers52 showed that positively charged liposomes experience higher binding and internalization (15- to 33-fold) by endothelial cells associated with angiogenic tumor vessels than corresponding normal vasculature.
Although EPR is principally associated with fenestrations in vasculature, the unique tumor microenvironment plays an important role in nanoparticle accumulation. Yokoi et al.53 have recently demonstrated that enhanced permeability and retention of nanotherapeutics is dependent on tumor type and the organ in which the disease is located. By examining liposomal accumulation in 4T1 (breast cancer), 3LL (lung cancer) and CT26 (colon cancer) cells at different primary and metastatic sites, the relative ratio of matrix metalloproteinase 9 (MMP-9) and tissue inhibitor of metalloproteinase 1 (TIMP-1) was found to correlate with nanoparticle accumulation; increased levels of MMP-9 were indicative of increased vascular permeability (Fig. 4). These findings suggest that these metalloproteinases may be used as potential biomarkers for EPR and a means by which to identify patients who would benefit the most from nanoparticle-based drug delivery. The same group later employed computational modeling combined with in vivo tumor models to examine the effect of collagen structure on diffusion of therapeutics of varying size54. The study highlights a direct correlation between collagen content in tumor vasculature and nanoparticle permeability (Fig. 4) and suggests another potential marker for patient stratification.
Figure 4.
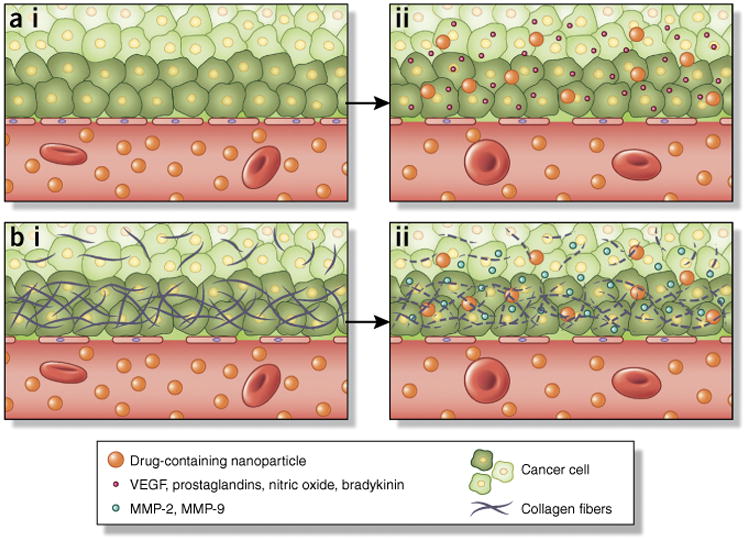
Determinants of enhanced permeability of nanoparticles into tumor tissues. The EPR effect3 is a transport phenomenon characterized primarily by the presence of fenestrations in tumor vasculature that enables passive accumulation of nanoparticles in tumors. (a,i) Normal vasculature typically possesses tight interendothelial junctions that prevent extravasation of particles into tissues. The imbalance of several factors influences the size of fenestrations in tumor vasculature. (a,ii) As an example, VEGF and nitric oxide have been shown to increase the size of gaps in the endothelia. (b,i) Tumors oftentimes possess a dense extracellular matrix that prevents adequate penetration of nanoparticles into the tumor. (b,ii) MMP-2 and MMP-9 degrade the dense collagen matrix comprising the basement membrane. These in turn can be exploited pharmacologically (e.g., by administration of antifibrotics) to enhance the EPR effect for increased nanoparticle accumulation in tumors.
Although the EPR effect has the potential to result in accumulation of nanoparticles, interstitial fluid pressures may still prove detrimental to the flow of nanoparticles into sites of interest. In tumors, normal hydrostatic and osmotic pressures necessary for transit of small molecules and solutes into and out of vessels along gradients are severely compromised owing to the local tumor microenvironment and disrupted vasculature55. Moreover, poor lymphatic drainage, brought about by the highly aggressive replicative nature of cancer cells, as well as extensive fibrosis and a dense extracellular matrix, result in elevated interstitial fluid pressures. These high intratumoral pressures prevent proper extravasation of macromolecules and nanoparticles to distal regions in the tumor, resulting in suboptimal delivery of chemotherapeutics.
Several strategies have been proposed to overcome high interstitial fluid pressures in tumors, including normalization of the tumor vasculature56. Jain and co-workers57,58 have investigated the administration of antiangiogenic, as well as angiogenic agents, in attempts to normalize tumor-associated vessels to facilitate the diffusion of drugs, polymer-drug conjugates and nanoparticles to tumors. One study demonstrated that administration of vascular endothelial growth factor (VEGF) enhanced transvascular delivery of nanoparticles in murine tumor models59. Another strategy aimed at overcoming high interstitial fluid pressure to increase nanoparticle accumulation in tumors involved dramatically reducing the collagen density in tumors. In another study, Jain and co-workers60 show that losartan, an angiotensin II receptor antagonist and antifibrotic agent, inhibits collagen I production by carcinoma-associated fibroblasts and improves the accumulation and efficacy of doxorubicin-containing liposomes.
Cellular membrane traversal and subsequent endosomal compartmentalization
Following site-specific extravasation, nanoparticles are expected to undergo cellular internalization, after which cargo (i.e., drugs, proteins, peptides or nucleic acids) can be released to exert therapeutic effects on cytoplasmic and nuclear targets. Although low molecular weight, hydrophobic molecules are capable of simple diffusion through the lipid bilayer membrane of cells, microscale and nanoscale supramolecular constructs require active uptake mechanisms. Surface charge of nanoparticles has proven to be a major determinant of cellular internalization, with charge-based uptake highly dependent on cell type. Several groups have reported heightened internalization of positively charged nanoparticles compared with their negatively charged counterparts in different cancer cell types, such as HeLa cells61,62, MCF-7 (ref. 63) cells and endothelial cells64. This observation has led to innovative ‘charge-conversion’ strategies aimed at site-specifically switching the charge of nanoparticles in response to environmental stimuli, such as pH65. As an example, Yuan et al.66 fabricated doxorubicin-containing zwitterionic nanoparticles that enabled prolonged circulation. Upon extravasation into tumor microenvironments with lower pH values of ∼6.8, the anionic component of the surface was shed, leaving a positive surface charge on nanoparticles that facilitated heightened tumor cell entry and improved in vivo responses.
In nonspecialized mammalian cells, clathrin-mediated endocytosis represents the classic mechanism governing uptake of nanoparticles67. Endocytosis of nanoparticles involves engulfment in membrane invaginations and formation of intracellular vesicles (endosomes) that eventually fuse with lysosomes68. The highly acidic environment of lysosomes, rich with enzymes, contributes to the degradation of organic nanoparticles, drugs and especially genetic material69. Although the phagosomes and endosomes associated with phagocytotic and clathrin-mediated endocytotic pathways are ultimately routed toward lysosomes, the caveolae-mediated mechanism of endocytosis results in the formation of caveolae that pinch off from the membrane and are fused with caveosomes of neutral pH, which in certain instances have been shown to bypass lysosomes21,70.
In light of the highly degradative environment of the endosomes/lysosomes after internalization, recent research has focused on strategies aimed at promoting endosomal escape or lysosomal avoidance altogether. Membrane-destabilizing peptides, such as INF7, H5WYG and GALA, represent a viable strategy for inducing endosomal escape71. The incorporation of cationic polymers, such as poly(ethylene imine) (PEI) and poly(l-lysine) (PLL), in nanoparticle design has also led to effective release of therapeutics from endosomal compartments. The cationic charge of the nanoparticle interacts with the outer negatively charged surface of the endosomal membrane, resulting in membrane flipping and consequent destabilization (i.e., the ‘flip-flop’ mechanism)72. Polymers containing protonatable secondary and/or tertiary amine groups (i.e., PEI, histidine) can absorb protons, resulting in swelling from an influx of water into the endosomal compartment leading to eventual rupture, also known as the ‘proton sponge effect’73.
Another viable strategy to prevent degradation of nanoparticles by lysosomes is to enable their cellular internalization by caveolae-mediated endocytosis. Nanoparticle surface functionalization with ligands, such as folic acid, albumin and cholesterol, has been shown to result in uptake of nanoparticles by caveolin-mediated endocytosis74. The best example of this is nanoparticle albumin-bound paclitaxel. Nanoparticle albumin-bound paclitaxel undergoes caveolae-mediated uptake by binding to glycoprotein 60 (gp60), the albumin receptor present in caveolae of endothelial cells75. This in turn facilitates transport across the vascular walls to the tumor interstitial space, which has been shown to result in higher safety profiles and efficacy in metastatic breast cancer patients76.
The study by Tasciotti and co-workers30 regarding nanoparticle surface functionalization with biomimetic leukocyte membranes highlighted the advantage of this coating for cellular entry and lysosomal avoidance. Unlike internalization of uncoated particles, internalization of coated particles resulted in cytoskeleton rearrangement around the particles, with actin filaments organized in channel formations. Transmission electron microscopy analysis of internalized coated particles indeed showed they were not sequestered in lysosomes, whereas uncoated particles were trapped within endolysosomal compartments after 24 h.
Multidrug resistance from drug efflux pumps
Resistance to drugs represents a major hurdle in the treatment of several disease processes, including infection, inflammation and cancer. Multidrug resistance (MDR), either intrinsic or acquired through prolonged exposure, involves the efflux of drugs from cells that results in a lowering of intracellular concentrations and consequent dampening of the therapeutic impact. In cancer, resistance to chemotherapeutics by malignant cells is independent of structure or mechanism of action, as is the case with anthracyclines, taxanes and vinca alkaloids77. The end result is increased local toxicity due to exposure of healthy cells to the expelled drug, as well as the need to increase treatment doses, often to levels that lead to extreme patient morbidity, contributing to nonresponsive recurrence and subsequent failure of select chemotherapeutic regimens.
Although drug resistance can indeed be multifactorial, arising from combined mechanisms, such as activation of detoxifying systems and defective apoptotic pathways, classic MDR is effected through the efflux action of ATP-dependent transporters that are members of a superfamily of proteins that possess an ATP-binding cassette (ABC)78. MDR in cancer frequently arises from the overexpressed ABC transporter, P-glycoprotein, an efflux pump capable of binding several distinct hydrophobic chemotherapeutics79. Although it is found overexpressed in cancer and arises from cellular adaptations to stress, such as hypoxia, P-glycoprotein is normally found in organs, such as the brain, testis, placenta, liver, gastrointestinal tract and kidneys, tasked with protecting these organs from toxins80. Further insights into MDR in cells not expressing P-glycoprotein led to the discovery of MDR-associated protein-1 and the breast cancer resistance protein (BCRP)81. Consequently, research efforts have been devoted to the investigation of efflux pump inhibitors, with verapamil (Covera) and cyclosporine A emerging as first-generation antagonists82.
Strategies aimed at overcoming MDR have long involved nanoparticle encapsulation of chemotherapeutics and an MDR modulator. As examples, Lee and co-workers83 have formulated targeted liposomes with doxorubicin and verapamil, a P-glycoprotein inhibitor, and Wu and co-workers84 have developed hybrid nanoparticles comprising lipids and polymers co-loaded with doxorubicin and GG918, a BCRP inhibitor. Compared with free-drug controls and chemotherapeutic nanoparticles without MDR inhibitors, the doxorubicin/verapamil and doxorubicin/GG918 formulations were more cytotoxic to leukemia and MDR breast cancer cell lines, respectively. Studies have also examined combinations of drugs, such as paclitaxel with P-glycoprotein inhibitors, like tariquidar85. Recently, RNAi strategies to inhibit efflux pumps, such as P-glycoprotein, have also been explored. As an example, Lavasanifar and co-workers86 developed a multifunctional polymer micelle system comprising poly(ethylene oxide)-block-poly(ε-caprolactone) (PEO-b-PCL) encapsulating doxorubicin and a short interfering RNA (siRNA) targeting MDR-1 for gene silencing of P-glycoprotein expression.
It would be impossible to discuss nanoparticle-based strategies aimed at overcoming MDR without mentioning the work of Kabanov and co-workers87 regarding poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) (PEO-PPO-PEO; Pluronic) block copolymers. Initially, carcinoma cells overexpressing P-glycoprotein were found to be hypersensitized to daunorubicin (Cerubidin) upon coadministration with Pluronic88. It was then discovered that Pluronic formulations, particularly Pluronic P85, abrogated MDR through a multitude of different mechanisms, including membrane incorporation, inhibition of efflux transporters, reduction in ATP levels in cells, enhanced pro-apoptotic signaling and reduced accumulation of drug molecules in cytoplasmic vesicles87. Consequently, several Pluronic polymeric micelle formulations encapsulating chemotherapeutics (e.g., doxorubicin) have been explored89,90.
Conclusions
First-generation nanotherapeutics arose from an urgent need to address the limitations and deleterious effects of formulation excipients (e.g., Cremophor EL) that resulted in rapid and indiscriminate tissue distribution as well as vehicle-associated toxicities. Simplified in their design, these nanoparticles were viewed as vectors for drug solubilization that improved pharmacokinetic parameters, such as blood-residence times, providing an effective means to an end. And although these improvements proved beneficial in terms of reducing drug-associated morbidity, they have yet to translate to substantially improved patient outcomes.
Currently, the field of nanoparticle-based drug delivery is extending beyond the confines of convention (e.g., traditional geometries, sizes or chemistries) so as to rationally design entities specifically tasked with overcoming sequential biological barriers (Box 2). There is the growing realization that although the distinct obstacles that hinder adequate delivery of therapeutics to tumors are indeed complex, they are by no means insurmountable. As highlighted here, innovative design implementations, such as the use of nontraditional geometries for improved vascular dynamics or functionalization with biomimetic membranes for avoidance of phagocytic uptake, have shown distinct advantages over preexisting conventional nanoparticle formulations. And although it is evident that the field is transitioning toward more rational approaches that take into consideration biological barriers, complications will arise that may hinder clinical translation. These revolve around the additional complexities associated with these systems, which will directly affect ease of scale-up, mass-production and associated costs. Moreover, depending on design implementations (e.g., addition of autologous cell-derived biomimetic surfaces), regulatory approvals concerning quality control, reproducibility and toxicity may represent additional hurdles.
An enhanced understanding of biological processes governing these barriers, and how they evolve in various disease states, coupled with innovations in materials science, will continue to enable the development of nanoparticles capable of sequential negotiation of these obstacles for efficacious, site-specific delivery. This may not only result in successful translation of novel therapeutics, but will also elevate nanoparticle-based drug delivery from a promising field to a viable and commonplace strategy for the treatment of several diseases.
Figure 5.
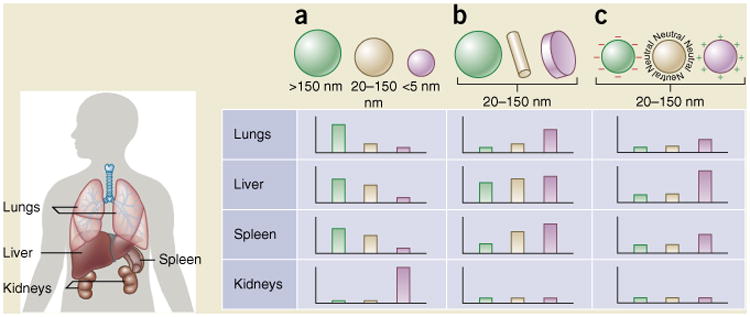
Nanoparticle size, shape and surface charge dictate biodistribution among the different organs including the lungs, liver, spleen and kidneys. (a) Spherical particles, including gold nanoparticles, liposomes and polymeric micelles/nanoparticles can vary in size and display disparate in vivo fates. Large rigid particles with diameters >2,000 nm accumulate readily within the spleen and liver, as well as in the capillaries of the lungs. Nanoparticles in the range of 100–200 nm have been shown to extravasate through vascular fenestrations of tumors (the EPR effect) and escape filtration by liver and spleen. As size increases beyond 150 nm, more and more nanoparticles are entrapped within the liver and spleen. Small-sized nanoparticles (<5 nm) are filtered out by the kidneys91. (b) Novel ‘top-down’ and ‘bottom up’ fabrication techniques have enabled the exploration of different geometries of nanoparticles, including cylindrical and discoidal shapes, which have been shown to exhibit pronounced effects on pharmacokinetics and biodistribution. Different nanoparticle shapes exhibit unique flow characteristics that substantially alter circulating lifetimes, cell membrane interactions and macrophage uptake, which in turn affect biodistribution among the different organs92. (c) Charge of nanoparticles stemming from distinct surface chemistries influences opsonization, circulation times and interaction with resident macrophages of organs comprising the MPS, with positively charged particles more prone to sequestration by macrophages in the lungs, liver and spleen. Neutral and slightly negatively charged nanoparticles have longer circulation lifetimes and less accumulation in the aforementioned organs of the MPS93. In both b and c, the size of the nanoparticles is assumed to range from 20–150 nm. Individual panels represent in vivo fates of nanoparticles, taking into account singular design parameters of size, shape and surface charge independent of one another, and for this reason, respective scales vary from one panel to the next. It is important to note that in vivo biodistribution will undoubtedly vary based on the interplay of several of the above parameters.
Box 2. Nanoparticle rational design implementation for overcoming delivery barriers.
Taking into consideration the framework of sequential barriers encountered by nanoparticles upon intravenous administration, one can easily appreciate the many obstacles hindering site-specific accumulation of therapeutics. A heightened understanding of these barriers, as well as notable advances in materials science at the micro- and nano-scale (e.g., photolithography, top-down fabrication and self-assembly techniques), has led to the potential to optimize several nanoparticle design parameters, such as size and shape, with the purpose of successfully overcoming these barriers.
Particle size
The size of a nanoparticle, which can now be engineered to precise dimensions and high monodispersity, is an important design parameter that can be tailored for purposes of directing particle distribution in vivo (Fig. 5). Size drives several biological phenomena with discrete cut-off size ranges that include circulation half-lives, extravasation through leaky vasculature and macrophage uptake. As an example, nanoparticles with diameters <∼5 nm rapidly undergo renal clearance upon intravenous administration103. Noncontinuous endothelia with vascular fenestrations measuring 50–100 nm (ref. 104) are present in the liver, leading to nonspecific accumulation of larger particles. Moreover, splenic filtration accounts for retention of particles >200 nm, due to the 200–500 nm (ref. 105) size range of interendothelial cell slits. Particles in the micrometer range (2–5 μm) have been shown to accumulate readily within capillaries of the lungs, providing possibly a distinct advantage when targeting one of the predominant sites of metastatic disease. Lastly, resident macrophages of the liver, spleen and lungs contribute to substantial particle uptake. Consideration of shape and size in nanoparticle design should highlight that although geometry drives initial internalization, size ultimately determines successful completion of uptake.
Taken together, nanoparticles averaging ∼100 nm generally prove long-lasting in the circulation. Long half-lives in blood increase the propensity of nanoparticles to extravasate through fenestrations in tumor vasculature, which range in size from 380–780 nm (ref. 106). Although the EPR effect in tumors has propelled the field of nanoparticle-based drug delivery, the phenomenon has been shown to vary dramatically with regards to the degree of tumor vascularity. As an example, Kataoka and co-workers107 have demonstrated that a variety of sub-100 nm polymer micelles of different sizes (30 nm, 50 nm, 70 nm and 100 nm) penetrate well within highly permeable tumors. However, in a poorly permeable human pancreatic adenocarcinoma, characterized by low vascularity and dense fibrosis, only small size nanoparticles <50 nm in diameter are able to accumulate in tumors. The extent of nanoparticle accumulation also varies depending on tumor type due to the interplay of various microenvironmental factors. Several solid tumors have increased levels of permeability factors, such as bradykinin, prostaglandins, VEGF and MMPs108. However, leukemia represents a nonsolid cancer of the blood and bone marrow devoid of the EPR effect, one where efficacious nanoparticle treatments typically rely on long-circulating nanoparticles with active targeting to specific cell populations responsible for disease progression.
Particle shape
The principle of form follows function has heavily influenced nanoparticle architecture, with distinct geometries affecting hemorheological dynamics, cellular uptake and in vivo fate (Fig. 5). As an example, discoidal particles exhibit unique tumbling and margination dynamics that favor vessel wall interaction substantially more than spherical particles, with implications for particle binding and adhesion to endothelium43. The circulation half-life of a particle is also heavily affected by shape. As an example, Discher and co-workers109 have demonstrated that filamentous polymer micelles (filomicelles) have long-circulating lifetimes (>1 week after administration) compared with spherical counterparts (2–3 days), owing largely to the tendency of these particles to align with blood flow. With regards to macrophage internalization, geometrical parameters, such as curvature and aspect ratio, affect uptake, with Mitragotri and co-workers110 demonstrating the importance of particle shape at the point of first contact with cells. These findings have highlighted the effect of curvature on kinetics of phagocytosis, with particles possessing a length of normalized curvature, denoted Ω, less than or equal to 45° (spherical particles) undergoing faster internalization than particles with a Ω ≥ 45°. Interestingly, the aforementioned design parameter of size largely dictated successful fruition of phagocytosis. These results provided the rationale for the exploration of ellipsoidal, cylindrical and discoidal particle shapes, all constructs that possess high aspect ratios and minimal regions of curvature, such as worm-like particles111 and the aforementioned filomicelles, for enhanced accumulation of therapeutics within tumors. Upon intravenous administration, filomicelles containing paclitaxel showed higher accumulation in tumors than spherical micelles112, a similar finding to that observed by Sailor and co-workers113 using nanoworm iron-oxide particle formulations.
Surface charge
Nanoparticle surface charge represents another design feature that can be tailored to prolong circulation lifetimes and selectively enhance accumulation at specific sites of interest (Fig. 5). Nanoparticles with neutral and negative surface charges have been shown to reduce the adsorption of serum proteins, resulting in longer circulation half-lives114. Kataoka and co-workers115 demonstrated the advantage of neutral (1.3 mV) and anionic (-10.6 mV) polymer micelle surfaces for long circulation and showed that negatively charged nanoparticles resulted in lower accumulation in livers and spleen. Positively charged nanoparticles, on the other hand, have a higher rate of nonspecific uptake in the majority of cells. Interestingly, McDonald and co-workers52 showed that cationic liposomes are preferentially bound and internalized by tumor-associated angiogenic endothelial cells compared with normal vasculature, a phenomenon also observed in sites of chronic inflammation. Importantly, positively charged particles facilitate endosomal release through mechanisms, such as the ‘proton sponge effect’20, hindering degradative effects of the endosomal compartment on drug cargo. Thus, for effective nanoparticle delivery to tumors, one would desire a neutral or slightly negative nanoparticle surface charge upon intravenous administration, but a switch to a positive charge upon arrival at the tumor site. This makes the aforementioned work by Wang and co-workers66 of high significance, given the rational design that was implemented to maximize tumor accumulation and cellular uptake by designing zwitterionic nanoparticles with switchable charge based on environmental stimulus.
Deformability and degradability
Other features, such as deformability and biodegradability, should also be considered when assessing the in vivo fate of therapeutics. As mentioned previously, organs, such as the liver and spleen, have discontinuous or fenestrated endothelia tasked with filtration of particulates from circulation. As an example, rigid particles with diameters that exceed the cut-off limit of splenic interendothelial slits are easily cleared by these organs116. Several research groups have examined the effect of varying nanoparticle stiffness on biodistribution and circulation by modulating degree of crosslinking. Jiang and co-workers117 designed nanogels of varying rigidity using zwitterionic monomers and cross-linkers, with results demonstrating that ‘softer’ nanoparticles more prone to deformability have prolonged circulation lifetimes and reduced accumulation in the spleen. DeSimone and co-workers118 have found similar findings with soft hydrogel particles of varying diameters (including those approximating the size of red blood cells), fabricated using a PRINT (particle replication in nonwetting templates) method. Upon intravenous administration, highly deformable particles demonstrate long circulation lifetimes exceeding 30 h. Interestingly, although particles did accumulate in the spleen at early time points, their ability to migrate through interendothelial slits was evident by a decrease in the amount of particles in the organ over time, which correlated with an increase in particle amount in the blood at later time points. Deformability may also play a role in assisting transport of particles through small capillaries, such as those found in the lung, as demonstrated recently by experiments in microfluidic blood capillary models119.
Particle stability has a major impact on the in vivo fate of therapeutic payloads. Although conventional nanoparticles for drug delivery generally include those of lipidic or polymeric origin, novel constituent materials are emerging that indeed adhere to the caveat that degradation components not cause adverse effects. Given that release of drugs is dependent on the degradation kinetics of the platform, biodegradation represents a critical nanoparticle design consideration. It is of the utmost importance that particles remain stable while in circulation so as to prevent nondiscriminate drug accumulation in healthy organs and to maximize bioavailability at the intended site. Thus, the critical micelle concentration (CMC), or the thermodynamic stability threshold above which micellization occurs, as well as kinetic stability, or how fast nanoparticles dissociate into component parts, all become important parameters that ultimately dictate the feasibility of the carrier platform. Yang and co-workers120 recently fabricated various mixed micelle formulations based on aliphatic polycarbonates, consisting of urea-containing block copolymers blended with acid-functionalized block copolymers, and demonstrated that particle formulations with higher kinetic stability accumulate in tumors to a greater extent and more rapidly than formulations with lower kinetic stability. Recent research efforts have also focused on covalent cross-linking of constructs121, demonstrating improvements with regards to pharmacokinetics and site-specific accumulation of therapeutics.
Several of these rational design strategies have been combined into single nanoparticle entities in hopes of overcoming as many biological barriers as possible. Our own laboratory has recently worked toward the development of a platform incorporating several rational design components to sequentially overcome biological barriers. The aforementioned multistage vector was optimized with regards to shape to enhance vascular dynamics including margination dynamics and endothelial binding interactions, as well as to minimize nonspecific uptake by resident macrophages of the MPS. Tailoring of size to the micrometer-scale has resulted in our ability to guide natural tropism of the platform to sites of lung and liver tumor metastasis. Surface functionalization with PEG and biomimetic surfaces, such as the aforementioned leuko-like approach, has resulted in prolonged circulation times and further avoidance of uptake by the MPS. The encapsulation of therapeutics within nanoparticles, which are in turn housed within nanopores of the multistage vector carrier, allows an additional level of rational design for overcoming localized, site-specific biological barriers. As an example, encapsulation of drugs within positively charged nanoparticles released in the tumor microenvironment results in enhanced uptake by cancer cells, while facilitating endosomal escape of therapeutic payloads.
Acknowledgments
M.F. is grateful for the Ernest Cockrell Jr. Distinguished Endowed Chair in the Department of Nanomedicine at the Houston Methodist Research Institute. The authors acknowledge financial support from Department of Defense grant W81XWH-12-1-0414, and US National Institutes of Health grants NIH U54CA143837 and NIH U54CA151668. E.B. is grateful for the support of the Susan G. Komen Breast Cancer Foundation (grant KG101394). The authors are grateful for the assistance of F.E. Cara, V. Segura-Ibarra and Suhong Wu in manuscript preparation. M.G. Landry is acknowledged for manuscript schematics.
Footnotes
Competing Financial Interests: The authors declare no competing financial interests.
References
- 1.Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov. 2004;3:711–716. doi: 10.1038/nrd1470. [DOI] [PubMed] [Google Scholar]
- 2.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 3.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–6392. A seminal work on the EPR effect in cancer that essentially laid the foundation for the use of nanotherapeutics, including liposomes and polymer-drug conjugates, as treatment modalities. [PubMed] [Google Scholar]
- 4.Maeda H, Nakamura H, Fang J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv Drug Deliv Rev. 2013;65:71–79. doi: 10.1016/j.addr.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Azzopardi EA, Ferguson EL, Thomas DW. The enhanced permeability retention effect: a new paradigm for drug targeting in infection. J Antimicrob Chemother. 2013;68:257–274. doi: 10.1093/jac/dks379. [DOI] [PubMed] [Google Scholar]
- 6.Marti CN, et al. Endothelial dysfunction, arterial stiffness, and heart failure. J Am Coll Cardiol. 2012;60:1455–1469. doi: 10.1016/j.jacc.2011.11.082. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L, et al. Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther. 2008;83:761–769. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- 8.Safra T, et al. Pegylated liposomal doxorubicin (doxil): reduced clinical cardiotoxicity in patients reaching or exceeding cumulative doses of 500 mg/m2. Ann Oncol. 2000;11:1029–1033. doi: 10.1023/a:1008365716693. [DOI] [PubMed] [Google Scholar]
- 9.Barenholz Y. Doxil(R)–the first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160:117–134. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 10.Hawkins MJ, Soon-Shiong P, Desai N. Protein nanoparticles as drug carriers in clinical medicine. Adv Drug Deliv Rev. 2008;60:876–885. doi: 10.1016/j.addr.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 11.Gradishar WJ, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23:7794–7803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 12.O'Brien ME, et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol. 2004;15:440–449. doi: 10.1093/annonc/mdh097. [DOI] [PubMed] [Google Scholar]
- 13.Ferrari M. Frontiers in cancer nanomedicine: directing mass transport through biological barriers. Trends Biotechnol. 2010;28:181–188. doi: 10.1016/j.tibtech.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv Drug Deliv Rev. 2014;66:2–25. doi: 10.1016/j.addr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12:991–1003. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- 16.Park K. Facing the truth about nanotechnology in drug delivery. ACS Nano. 2013;7:7442–7447. doi: 10.1021/nn404501g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Venditto VJ, Szoka FC. Cancer nanomedicines: so many papers and so few drugs! Adv Drug Deliv Rev. 2013;65:80–88. doi: 10.1016/j.addr.2012.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel HM, Moghimi SM. Serum-mediated recognition of liposomes by phagocytic cells of the reticuloendothelial system - The concept of tissue specificity. Adv Drug Deliv Rev. 1998;32:45–60. doi: 10.1016/s0169-409x(97)00131-2. [DOI] [PubMed] [Google Scholar]
- 19.Tenzer S, et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat Nanotechnol. 2013;8:772–781. doi: 10.1038/nnano.2013.181. [DOI] [PubMed] [Google Scholar]
- 20.Nel AE, et al. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater. 2009;8:543–557. doi: 10.1038/nmat2442. One of the most comprehensive reviews on the subject of nanoparticle protein coronas and implications on nanoparticle fate. [DOI] [PubMed] [Google Scholar]
- 21.Sahay G, Alakhova DY, Kabanov AV. Endocytosis of nanomedicines. J Control Release. 2010;145:182–195. doi: 10.1016/j.jconrel.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salvati A, et al. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat Nanotechnol. 2013;8:137–143. doi: 10.1038/nnano.2012.237. Excellent study highlighting the dynamic nature of nanoparticles following protein corona formation, and the detrimental effects this has on active targeting strategies. [DOI] [PubMed] [Google Scholar]
- 23.Docter D, et al. Quantitative profiling of the protein coronas that form around nanoparticles. Nat Protoc. 2014;9:2030–2044. doi: 10.1038/nprot.2014.139. [DOI] [PubMed] [Google Scholar]
- 24.Röcker C, Potzl M, Zhang F, Parak WJ, Nienhaus GU. A quantitative fluorescence study of protein monolayer formation on colloidal nanoparticles. Nat Nanotechnol. 2009;4:577–580. doi: 10.1038/nnano.2009.195. [DOI] [PubMed] [Google Scholar]
- 25.Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov. 2003;2:214–221. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 26.Hamilton A, et al. EORTC 10968: a phase I clinical and pharmacokinetic study of polyethylene glycol liposomal doxorubicin (Caelyx, Doxil) at a 6-week interval in patients with metastatic breast cancer. European Organization for Research and Treatment of Cancer. Ann Oncol. 2002;13:910–918. doi: 10.1093/annonc/mdf157. [DOI] [PubMed] [Google Scholar]
- 27.Guo ST, Huang L. Nanoparticles escaping RES and endosome: challenges for siRNA delivery for cancer therapy. J Nanomater. 2011;2011:742895. [Google Scholar]
- 28.Li SD, Huang L. Nanoparticles evading the reticuloendothelial system: role of the supported bilayer. Biochim Biophys Acta. 2009;1788:2259–2266. doi: 10.1016/j.bbamem.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez PL, et al. Minimal “Self” peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science. 2013;339:971–975. doi: 10.1126/science.1229568. An excellent study involving nanoparticle ‘camoufaging’ or ‘biomimicry’ for evasion of the MPS, highlighting consequent enhanced tumor accumulation and efficacy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parodi A, et al. Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nat Nanotechnol. 2013;8:61–68. doi: 10.1038/nnano.2012.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu CM, et al. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci USA. 2011;108:10980–10985. doi: 10.1073/pnas.1106634108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kreuter J, et al. Apolipoprotein-mediated transport of nanoparticle-bound drugs across the blood-brain barrier. J Drug Target. 2002;10:317–325. doi: 10.1080/10611860290031877. [DOI] [PubMed] [Google Scholar]
- 33.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007;117:1155–1166. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol. 2010;7:77–86. doi: 10.1038/nrcardio.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prapainop K, Witter DP, Wentworth P., Jr A chemical approach for cell-specific targeting of nanomaterials: small-molecule-initiated misfolding of nanoparticle corona proteins. J Am Chem Soc. 2012;134:4100–4103. doi: 10.1021/ja300537u. [DOI] [PubMed] [Google Scholar]
- 36.Decuzzi P, Pasqualini R, Arap W, Ferrari M. Intravascular delivery of particulate systems: does geometry really matter? Pharm Res. 2009;26:235–243. doi: 10.1007/s11095-008-9697-x. [DOI] [PubMed] [Google Scholar]
- 37.Arvizo RR, et al. Modulating pharmacokinetics, tumor uptake and biodistribution by engineered nanoparticles. PLoS ONE. 2011;6:e24374. doi: 10.1371/journal.pone.0024374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yao C, et al. Highly biocompatible zwitterionic phospholipids coated upconversion nanoparticles for efficient bioimaging. Anal Chem. 2014;86:9749–9757. doi: 10.1021/ac5023259. [DOI] [PubMed] [Google Scholar]
- 39.Decuzzi P, Lee S, Bhushan B, Ferrari M. A theoretical model for the margination of particles within blood vessels. Ann Biomed Eng. 2005;33:179–190. doi: 10.1007/s10439-005-8976-5. [DOI] [PubMed] [Google Scholar]
- 40.Tan J, Shah S, Thomas A, Ou-Yang HD, Liu Y. The influence of size, shape and vessel geometry on nanoparticle distribution. Microfuid Nanofluidics. 2013;14:77–87. doi: 10.1007/s10404-012-1024-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shah S, Liu Y, Hu W, Gao J. Modeling particle shape-dependent dynamics in nanomedicine. J Nanosci Nanotechnol. 2011;11:919–928. doi: 10.1166/jnn.2011.3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alexiou C, et al. Locoregional cancer treatment with magnetic drug targeting. Cancer Res. 2000;60:6641–6648. [PubMed] [Google Scholar]
- 43.Gentile F, et al. The effect of shape on the margination dynamics of non-neutrally buoyant particles in two-dimensional shear flows. J Biomech. 2008;41:2312–2318. doi: 10.1016/j.jbiomech.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 44.Tasciotti E, et al. Mesoporous silicon particles as a multistage delivery system for imaging and therapeutic applications. Nat Nanotechnol. 2008;3:151–157. doi: 10.1038/nnano.2008.34. [DOI] [PubMed] [Google Scholar]
- 45.Chiappini C, et al. Tailored porous silicon microparticles: fabrication and properties. ChemPhysChem. 2010;11:1029–1035. doi: 10.1002/cphc.200900914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van de Ven AL, et al. Rapid tumoritropic accumulation of systemically injected plateloid particles and their biodistribution. J Control Release. 2012;158:148–155. doi: 10.1016/j.jconrel.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanaka T, et al. Sustained small interfering RNA delivery by mesoporous silicon particles. Cancer Res. 2010;70:3687–3696. doi: 10.1158/0008-5472.CAN-09-3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen X, et al. XBP1 promotes triple-negative breast cancer by controlling the HIF1alpha pathway. Nature. 2014;508:103–107. doi: 10.1038/nature13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dave B, et al. Targeting RPL39 and MLF2 reduces tumor initiation and metastasis in breast cancer by inhibiting nitric oxide synthase signaling. Proc Natl Acad Sci USA. 2014;111:8838–8843. doi: 10.1073/pnas.1320769111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.von Maltzahn G, et al. Nanoparticles that communicate in vivo to amplify tumour targeting. Nat Mater. 2011;10:545–552. doi: 10.1038/nmat3049. An inventive approach for targeting of nanoparticles involving ‘biological communication’ between different injectable constructs for enhanced uptake into tumors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murphy EA, et al. Nanoparticle-mediated drug delivery to tumor vasculature suppresses metastasis. Proc Natl Acad Sci USA. 2008;105:9343–9348. doi: 10.1073/pnas.0803728105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thurston G, et al. Cationic liposomes target angiogenic endothelial cells in tumors and chronic inflammation in mice. J Clin Invest. 1998;101:1401–1413. doi: 10.1172/JCI965. An interesting study demonstrating the potential of using surface charge of nanoparticles, in this case cationic liposomes, as a means to target therapeutics to angiogenic tumor vasculature by means of enhanced endothelial cell uptake. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yokoi K, et al. Serum biomarkers for personalization of nanotherapeutics-based therapy in different tumor and organ microenvironments. Cancer Lett. 2014;345:48–55. doi: 10.1016/j.canlet.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yokoi K, et al. Capillary-wall collagen as a biophysical marker of nanotherapeutic permeability into the tumor microenvironment. Cancer Res. 2014;74:4239–4246. doi: 10.1158/0008-5472.CAN-13-3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heldin CH, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure - an obstacle in cancer therapy. Nat Rev Cancer. 2004;4:806–813. doi: 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- 56.Chauhan VP, et al. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat Nanotechnol. 2012;7:383–388. doi: 10.1038/nnano.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jain RK. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J Clin Oncol. 2013;31:2205–2218. doi: 10.1200/JCO.2012.46.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol. 2010;7:653–664. doi: 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Monsky WL, et al. Augmentation of transvascular transport of macromolecules and nanoparticles in tumors using vascular endothelial growth factor. Cancer Res. 1999;59:4129–4135. [PubMed] [Google Scholar]
- 60.Diop-Frimpong B, Chauhan VP, Krane S, Boucher Y, Jain RK. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc Natl Acad Sci USA. 2011;108:2909–2914. doi: 10.1073/pnas.1018892108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miller CR, Bondurant B, McLean SD, McGovern KA, O'Brien DF. Liposome-cell interactions in vitro: effect of liposome surface charge on the binding and endocytosis of conventional and sterically stabilized liposomes. Biochemistry. 1998;37:12875–12883. doi: 10.1021/bi980096y. [DOI] [PubMed] [Google Scholar]
- 62.Gratton SE, et al. The effect of particle design on cellular internalization pathways. Proc Natl Acad Sci USA. 2008;105:11613–11618. doi: 10.1073/pnas.0801763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Osaka T, Nakanishi T, Shanmugam S, Takahama S, Zhang H. Effect of surface charge of magnetite nanoparticles on their internalization into breast cancer and umbilical vein endothelial cells. Colloids Surf B Biointerfaces. 2009;71:325–330. doi: 10.1016/j.colsurfb.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 64.Serda RE, et al. The association of silicon microparticles with endothelial cells in drug delivery to the vasculature. Biomaterials. 2009;30:2440–2448. doi: 10.1016/j.biomaterials.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 65.Lee Y, et al. A protein nanocarrier from charge-conversion polymer in response to endosomal pH. J Am Chem Soc. 2007;129:5362–5363. doi: 10.1021/ja071090b. [DOI] [PubMed] [Google Scholar]
- 66.Yuan YY, et al. Surface charge switchable nanoparticles based on zwitterionic polymer for enhanced drug delivery to tumor. Adv Mater. 2012;24:5476–5480. doi: 10.1002/adma.201202296. A highly innovative approach towards site-specific, externally stimulated nanoparticle surface charge conversion that permits for prolonged circulation and heightened tumor cell internalization: a perfect example of design considerations that take into account different barriers to nanoparticle drug delivery. [DOI] [PubMed] [Google Scholar]
- 67.Le Roy C, Wrana JL. Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat Rev Mol Cell Biol. 2005;6:112–126. doi: 10.1038/nrm1571. [DOI] [PubMed] [Google Scholar]
- 68.Gould GW, Lippincott-Schwartz J. New roles for endosomes: from vesicular carriers to multi-purpose platforms. Nat Rev Mol Cell Biol. 2009;10:287–292. doi: 10.1038/nrm2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Luo D, Saltzman WM. Synthetic DNA delivery systems. Nat Biotechnol. 2000;18:33–37. doi: 10.1038/71889. [DOI] [PubMed] [Google Scholar]
- 70.Bareford LM, Swaan PW. Endocytic mechanisms for targeted drug delivery. Adv Drug Deliv Rev. 2007;59:748–758. doi: 10.1016/j.addr.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martens TF, Remaut K, Demeester J, De Smedt SC, Braeckmans K. Intracellular delivery of nanomaterials: how to catch endosomal escape in the act. Nano Today. 2014;9:344–364. [Google Scholar]
- 72.Wasungu L, Hoekstra D. Cationic lipids, lipoplexes and intracellular delivery of genes. J Control Release. 2006;116:255–264. doi: 10.1016/j.jconrel.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 73.Chou LY, Ming K, Chan WC. Strategies for the intracellular delivery of nanoparticles. Chem Soc Rev. 2011;40:233–245. doi: 10.1039/c0cs00003e. [DOI] [PubMed] [Google Scholar]
- 74.Dauty E, Remy JS, Zuber G, Behr JP. Intracellular delivery of nanometric DNA particles via the folate receptor. Bioconjug Chem. 2002;13:831–839. doi: 10.1021/bc0255182. [DOI] [PubMed] [Google Scholar]
- 75.Gradishar WJ. Albumin-bound paclitaxel: a next-generation taxane. Expert Opin Pharmacother. 2006;7:1041–1053. doi: 10.1517/14656566.7.8.1041. [DOI] [PubMed] [Google Scholar]
- 76.Ibrahim NK, et al. Multicenter phase II trial of ABI-007, an albumin-bound paclitaxel, in women with metastatic breast cancer. J Clin Oncol. 2005;23:6019–6026. doi: 10.1200/JCO.2005.11.013. Clinical trial of nanoparticle albumin-bound paclitaxel in metastatic breast cancer patients, highlighting not only the therapeutic potential of paclitaxel, but its benefits with regards to reduced side effects compared to the conventional formulation containing Cremophor EL. [DOI] [PubMed] [Google Scholar]
- 77.Szakács G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–234. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 78.Rees DC, Johnson E, Lewinson O. ABC transporters: the power to change. Nat Rev Mol Cell Biol. 2009;10:218–227. doi: 10.1038/nrm2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aller SG, et al. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science. 2009;323:1718–1722. doi: 10.1126/science.1168750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 81.Fletcher JI, Haber M, Henderson MJ, Norris MD. ABC transporters in cancer: more than just drug efflux pumps. Nat Rev Cancer. 2010;10:147–156. doi: 10.1038/nrc2789. [DOI] [PubMed] [Google Scholar]
- 82.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 83.Wu J, et al. Reversal of multidrug resistance by transferrin-conjugated liposomes co-encapsulating doxorubicin and verapamil. J Pharm Pharm Sci. 2007;10:350–357. [PubMed] [Google Scholar]
- 84.Wong HL, Bendayan R, Rauth AM, Wu XY. Simultaneous delivery of doxorubicin and GG918 (Elacridar) by new polymer-lipid hybrid nanoparticles (PLN) for enhanced treatment of multidrug-resistant breast cancer. J Control Release. 2006;116:275–284. doi: 10.1016/j.jconrel.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 85.Patel NR, Rathi A, Mongayt D, Torchilin VP. Reversal of multidrug resistance by co-delivery of tariquidar (XR9576) and paclitaxel using long-circulating liposomes. Int J Pharm. 2011;416:296–299. doi: 10.1016/j.ijpharm.2011.05.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xiong XB, Lavasanifar A. Traceable multifunctional micellar nanocarriers for cancer-targeted co-delivery of MDR-1 siRNA and doxorubicin. ACS Nano. 2011;5:5202–5213. doi: 10.1021/nn2013707. [DOI] [PubMed] [Google Scholar]
- 87.Batrakova EV, Kabanov AV. Pluronic block copolymers: evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J Control Release. 2008;130:98–106. doi: 10.1016/j.jconrel.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sosnik A. Reversal of multidrug resistance by the inhibition of ATP-binding cassette pumps employing “Generally Recognized As Safe” (GRAS) nanopharmaceuticals: A review. Adv Drug Deliv Rev. 2013;65:1828–1851. doi: 10.1016/j.addr.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 89.Lee Y, Park SY, Mok H, Park TG. Synthesis, characterization, antitumor activity of pluronic mimicking copolymer micelles conjugated with doxorubicin via acid-cleavable linkage. Bioconjug Chem. 2008;19:525–531. doi: 10.1021/bc700382z. [DOI] [PubMed] [Google Scholar]
- 90.Tian Y, Bromberg L, Lin SN, Hatton TA, Tam KC. Complexation and release of doxorubicin from its complexes with pluronic P85-b-poly(acrylic acid) block copolymers. J Control Release. 2007;121:137–145. doi: 10.1016/j.jconrel.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 91.Longmire M, Choyke PL, Kobayashi H. Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. Nanomedicine (Lond) 2008;3:703–717. doi: 10.2217/17435889.3.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Black KC, et al. Radioactive 198Au-doped nanostructures with different shapes for in vivo analyses of their biodistribution, tumor uptake, and intratumoral distribution. ACS Nano. 2014;8:4385–4394. doi: 10.1021/nn406258m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xiao K, et al. The effect of surface charge on in vivo biodistribution of PEG-oligocholic acid based micellar nanoparticles. Biomaterials. 2011;32:3435–3446. doi: 10.1016/j.biomaterials.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sheridan C. Gene therapy finds its niche. Nat Biotechnol. 2011;29:121–128. doi: 10.1038/nbt.1769. [DOI] [PubMed] [Google Scholar]
- 95.Kay MA, Glorioso JC, Naldini L. Viral vectors for gene therapy: the art of turning infectious agents into vehicles of therapeutics. Nat Med. 2001;7:33–40. doi: 10.1038/83324. [DOI] [PubMed] [Google Scholar]
- 96.Levine RM, Pearce TR, Adil M, Kokkoli E. Preparation and characterization of liposome-encapsulated plasmid DNA for gene delivery. Langmuir. 2013;29:9208–9215. doi: 10.1021/la400859e. [DOI] [PubMed] [Google Scholar]
- 97.Dobson J. Gene therapy progress and prospects: magnetic nanoparticle-based gene delivery. Gene Ther. 2006;13:283–287. doi: 10.1038/sj.gt.3302720. [DOI] [PubMed] [Google Scholar]
- 98.Pissuwan D, Niidome T, Cortie MB. The forthcoming applications of gold nanoparticles in drug and gene delivery systems. J Control Release. 2011;149:65–71. doi: 10.1016/j.jconrel.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 99.Lechardeur D, et al. Metabolic instability of plasmid DNA in the cytosol: a potential barrier to gene transfer. Gene Ther. 1999;6:482–497. doi: 10.1038/sj.gt.3300867. [DOI] [PubMed] [Google Scholar]
- 100.Anderson DJ, Hetzer MW. Nuclear envelope formation by chromatin-mediated reorganization of the endoplasmic reticulum. Nat Cell Biol. 2007;9:1160–1166. doi: 10.1038/ncb1636. [DOI] [PubMed] [Google Scholar]
- 101.Raices M, D'Angelo MA. Nuclear pore complex composition: a new regulator of tissue-specific and developmental functions. Nat Rev Mol Cell Biol. 2012;13:687–699. doi: 10.1038/nrm3461. [DOI] [PubMed] [Google Scholar]
- 102.Hu Y, Haynes MT, Wang Y, Liu F, Huang L. A highly efficient synthetic vector: nonhydrodynamic delivery of DNA to hepatocyte nuclei in vivo. ACS Nano. 2013;7:5376–5384. doi: 10.1021/nn4012384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Choi HS, et al. Renal clearance of quantum dots. Nat Biotechnol. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Braet F, et al. Contribution of high-resolution correlative imaging techniques in the study of the liver sieve in three-dimensions. Microsc Res Tech. 2007;70:230–242. doi: 10.1002/jemt.20408. [DOI] [PubMed] [Google Scholar]
- 105.Chen LT, Weiss L. The role of the sinus wall in the passage of erythrocytes through the spleen. Blood. 1973;41:529–537. [PubMed] [Google Scholar]
- 106.Hobbs SK, et al. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci USA. 1998;95:4607–4612. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cabral H, et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat Nanotechnol. 2011;6:815–823. doi: 10.1038/nnano.2011.166. Excellent study that highlights the effect of nanoparticle size on accumulation and penetration of nanoparticles in hypovascular tumors. [DOI] [PubMed] [Google Scholar]
- 108.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 109.Geng Y, et al. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nanotechnol. 2007;2:249–255. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci USA. 2006;103:4930–4934. doi: 10.1073/pnas.0600997103. Seminal work on the effect of particle geometry on macrophage uptake, demonstrating that the architectural design parameter of curvature drives phagocytosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Champion JA, Mitragotri S. Shape induced inhibition of phagocytosis of polymer particles. Pharm Res. 2009;26:244–249. doi: 10.1007/s11095-008-9626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Christian DA, et al. Flexible filaments for in vivo imaging and delivery: persistent circulation of filomicelles opens the dosage window for sustained tumor shrinkage. Mol Pharm. 2009;6:1343–1352. doi: 10.1021/mp900022m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Park JH, et al. Systematic surface engineering of magnetic nanoworms for in vivo tumor targeting. Small. 2009;5:694–700. doi: 10.1002/smll.200801789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm. 2008;5:505–515. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yamamoto Y, Nagasaki Y, Kato Y, Sugiyama Y, Kataoka K. Long-circulating poly(ethylene glycol)-poly(d,l-lactide) block copolymer micelles with modulated surface charge. J Control Release. 2001;77:27–38. doi: 10.1016/s0168-3659(01)00451-5. [DOI] [PubMed] [Google Scholar]
- 116.Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specifc nanoparticles: theory to practice. Pharmacol Rev. 2001;53:283–318. [PubMed] [Google Scholar]
- 117.Zhang L, et al. Softer zwitterionic nanogels for longer circulation and lower splenic accumulation. ACS Nano. 2012;6:6681–6686. doi: 10.1021/nn301159a. [DOI] [PubMed] [Google Scholar]
- 118.Merkel TJ, et al. The effect of particle size on the biodistribution of low-modulus hydrogel PRINT particles. J Control Release. 2012;162:37–44. doi: 10.1016/j.jconrel.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cui J, et al. Super-soft hydrogel particles with tunable elasticity in a microfluidic blood capillary model. Adv Mater. 2014;26:7295–7299. doi: 10.1002/adma.201402753. [DOI] [PubMed] [Google Scholar]
- 120.Attia ABE, et al. The effect of kinetic stability on biodistribution and anti-tumor efficacy of drug-loaded biodegradable polymeric micelles. Biomaterials. 2013;34:3132–3140. doi: 10.1016/j.biomaterials.2013.01.042. [DOI] [PubMed] [Google Scholar]
- 121.van Nostrum CF. Covalently cross-linked amphiphilic block copolymer micelles. Soft Matter. 2011;7:3246–3259. [Google Scholar]


