Abstract
The insulin-like growth factors (IGFs), IGF-1 and IGF-2, have been implicated in the growth, survival, and metastasis of a broad range of malignancies including pediatric tumors. They bind to the IGF receptor type 1 (IGF-1R) and the insulin receptor (IR) which are overexpressed in many types of solid malignancies. Activation of the IR by IGF-2 results in increased survival of tumor cells. We have previously identified a novel human monoclonal antibody, m708.5, which binds with high (pM) affinity to both human IGF-1 and IGF-2, and potently inhibits phosphorylation of the IGF-1R and the IR in tumor cells. m708.5 exhibited strong anti-tumor activity as a single agent against most cell lines derived from neuroblastoma, Ewing family of tumor, rhabdomyosarcoma, and osteosarcoma. When tested in neuroblastoma cell lines, it showed strong synergy with temsirolimus, and synergy with chemotherapeutic agents in vitro. In xenograft models, the combination of m708.5 and temsirolimus significantly inhibited neuroblastoma growth and prolonged mouse survival. Taken together, these results support the clinical development of m708.5 for pediatric solid tumors with potential for synergy with chemotherapy and mTOR inhibitors.
Keywords: IGF-1, IGF-2, temsirolimus, chemotherapy, neuroblastoma
Introduction
The insulin-like growth factors (IGF) system consists of two ligands, IGF-1 and IGF-2, three cell-membrane receptors, IGF receptor type 1 (IGF-1R), insulin receptor (IR), IGF receptor type 2 (IGF-2R), and six high-affinity IGF binding proteins(1). The IGF-1 and IGF-2 have been shown to play important roles in neoplasm (2). Upon activation by the mitogenic ligands IGF-1 and IGF-2, the IGF-1R is phosphorylated, stimulating downstream intracellular pathways (the PI3K/AKT and Ras/MEK/ERK pathways) that lead totumor proliferation, survival, and metastasis (3). Although both IGF-1 and IGF-2 activate IGF-1R, the binding sites for IGF-1 and IGF-2 on the receptor are distinct. The IGF-1R shares a similar tetrameric α2β2 structure with IR. The IR isoform A (IR-A) binds to IGF-2 with the same affinity as it binds to insulin; the affinity of IR-B is about 10-fold lower although its interaction with IGF-2 leads to the activation of the same signaling pathways as that of IR-A. In addition, IR and IGF-1R subunits can form hybrid heterodimeric receptors. IGF-1R signaling has been shown to promote neuroblastoma (NB) tumorigenesis and inhibit apoptosis. IGF-1R is expressed in 86% of primary neuroblastoma tumors (4). It forms an autocrine loop where IGF-2 provides the activating ligand (5). In neuroblastoma, IGF-1 is supplied by the tumor stroma, whereby proliferation and motility (6) as well as survival (7) sare maintained. The coupling of IGF-1/2 to IGF-1R has also been shown to regulate neuroblastoma metastasis to bone (8).
There is substantially experimental and clinical evidence that targeting IGF components is a promising therapeutic strategy against cancers (9). Different strategies aimed at targeting the IGF system have been investigated, including small molecule tyrosine kinase inhibitors, and antibodies targeting the IGF-1R(10). In the Pediatric Preclinical Testing Program (PPTP) these therapeutics all show activity (≥intermediate) against neuroblastoma (11, 12). Much progress has also been made targeting IGF-1R in preclinical and clinical models (2). Although these IGF-1R antibodies and inhibitors have broad therapeutic implications for neuroblastoma, Ewings family of tumors, and osteosarcoma, their clinical activity as single agents is modest.
It is well known that IR is increased in primary malignant tumors and correlated with tumor survival (13, 14). The stimulation of IR-A as well IR-A/IGF-1R hybrid receptors by IGF-1, and/or IGF-2 leads to cell proliferation, motility, and metastasis, suggesting that IR can supplant IGF-1R when the latter is inhibited (15). IGF-2 signals through IR-A, promotes cancer survival (16), resulting in tumor resistance during treatment with IGF-1R inhibitors (17). However, most IGF-1R inhibitors partially inhibit IR-A activity by disrupting the IR-A/IGF-1R hybrid receptors, but fail to inhibit IR-A homodimers. Small molecules have been developed with targeting both IGF-1R and IR (15, 18). Since hyperglycemia is already observed when IGF-1R is inhibited (15), when both IGF-1R and IR are inhibited, glucose homeostasis could be severely dysregulated. More recently, several human antibodies against IGF-2 have been reported (19) and a IGF-1/2 cross-reactive antibody (MEDI-573) has also been described (20), which could deactivate the IGF-1R/IR and affect tumor growth.
We previously engineered a human monoclonal antibody (m708.5) with dual specificity for both human IGF-1 and IGF-2, that was affinity-matured by light chain shuffling, mutagenesis, yeast display, and reshaped as IgG1 with extremely high affinity to both IGF-1 and to IGF-2. The antibody m708.5 was shown to inhibit the activity of both IGF-1R and IR by neutralizing IGF-1 and IGF-2 in in vitro kinase assays. This antibody avoided the tumor escape mechanism that utilized IGF-2 to engage IGF-1R and IR signaling. In this study, we evaluated the anti-tumor activity of m708.5 IgG1 antibody alone and in combination with cytotoxic drugs or temsirolimus against a panel of pediatric solid tumor cell lines, with special emphasis on neuroblastoma and human tumor xenografts.
Materials and methods
Antibody production
The heavy and light variable regions of m708.5 were cloned into CHO GS expression vector, including human IgG1 constant regions. The vector was transfected into CHO-s cells and selected with G418 (Invitrogen) as previously described (21). The stable cell lines were cultured in Opticho serum free medium (Invitrogen) and the mature supernatant was harvested as previously described. The soluble IgG1 protein was purified using the MabSelect affinity chromatograph medium (GE Healthcare). Bound protein was eluted with 0.1 M citric acid/sodium citrate buffer, pH 3.9 and alkalinized (1:10 v/v ratio) in 25 mM sodium citrate, pH 8.5. The eluted IgG1 was subsequently concentrated using a 50,000 MWCO Vivaspin centrifuge tube (Sartorius Stedim). By HPLC and SDS-Gel, m708.5 IgG1 was >95% pure with <10% aggregates.
Drugs
Temsirolimus and four standard cytotoxic drugs for pediatric tumors (SN38, doxorubicin, vincristine, cisplatin) were obtained from Memorial Sloan-Kettering Cancer Center (MSKCC, New York, NY) clinical pharmacy, dissolved in dimethylsulphoxide (DMSO) and diluted in RPMI1640 medium for use in vitro or in vivo.
Neuroblastoma cell lines
NB cell lines BE(1)N, BE(2)N, BE(2)S, SK-N-JB, SK-N-JF, SK-N-FI and SK-N-MM were at MSKCC. LAN-1 was kindly provided by Dr. Robert Seeger, Children’s Hospital at Los Angeles, CA. These cells were maintained in the RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum (Life Technologies).
ELISA binding assay
The 25 ng of human IGF-1 (hIGF-1), IGF-2 (hIGF-2) or mouse IGF-1 (mIGF-1), IGF-2 (mIGF-2) (R&D System) per well were coated on 96-well ELISA plates overnight at 4°C. IgG1 m708.5 with different dilutions was incubated with antigens for 1 h at RT. Bound IgG1s were detected with anti-human Fc-HRP antibody (1:10,000, BD Biosciences). The 2,2′-azino-bis-(3-ethylbenzthiazoline-6-sulfonic acid) substrate (Sigma-Aldrich) was added and the reaction was read at 490 nm.
Affinity Determination by Surface Plasmon Resonance
Kinetics and affinities of various antibodies and IGF-1, IGF-2 were analyzed by surface plasmon resonance technology using a Biacore T100 instrument (GE healthcare). Biotinylated antigen was captured by streptavidin onto a sensor chip (CM5). A control reference surface was prepared for nonspecific binding and refractive index changes. For analysis of the kinetics of interactions, varying concentrations of antibodies were injected at flow rate of 30 μl/min using running buffer containing 10 mM HEPES, 150 mM NaCl, 3 mM EDTA, and 0.05% Surfactant P-20 (pH 7.4). The association and dissociation phase data were fitted simultaneously to a 1:1 model by using BIA evaluation 3.2. All the experiments were done at 25°C.
Flow cytometric analysis
NB cells were harvested in RMPI1640 medium. Mouse anti-IGF-1R or anti-IR-A antibody (BD Biosciences) was incubated with 106 NB cells for 30 min on ice. After washing 3 times, cells were incubated with secondary fluorescent anti-mouse IgG antibody. Analysis was performed using a BD Bioscience FACS calibur.
Cell proliferation assay
Tumor cells were seeded into tissue culture 96-well plates in RPMI1640 medium containing 2.5% FBS. The next day, cells were treated with m708.5 alone, the mixtures of m708.5 and drugs, or an isotype control IgG1 antibody (hu3F8 anti-GD2) (21), each serially diluted to required concentrations. Cell viability was determined after 48 h exposure using 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)- 2-(4-sulfophenyl)-2H-tetrazolium (MTS) and lysis solution (20% sodium dodecyl sulphate–dimethylformamide) by colorimetric measurement at 540 nm in microplate reader. Assays were performed in triplicate and experiments were repeated three times. The results were calculated as percent cell growth relative to growth of control cells. Dose response curves were constructed for each drug alone, m708.5 alone, and the combinations at fixed molar ratios defined as the ratio of the two agents at their maximally effective dose. The interaction between antibody and various chemotherapeutic agents was calculated by using the multiple drug effect analysis method (22), which quantitatively describes the interaction between two or more drugs (23).
In vivo tumor growth studies
Tumor xenografts were established by subcutaneous (s.c.) implantation of neuroblastoma cells into 5- to 6-week-old SCID mice. Mice were randomized into groups of 5 when tumors were 75 to 100 mm3. Tumor-bearing mice were treated with either 0.1 mg control IgG1 antibody (i.v. twice weekly for 3–4 weeks), 0.1 mg m708.5 (i.v., twice weekly for 3–4 weeks), 0.025 or 0.125 mg temsirolimus (i.p., 5 times weekly for 3 or 4 weeks), or both m708.5 and temsirolimus. Tumor volume (mm3) was measured 1 time per week and was calculated by: [length (mm) × width (mm)2]/2. Body weights were measured twice per week. Tumor growth inhibition (TGI) was calculated as (1 − T/C) × 100, where T = final tumor volumes from a treated group, and C = final tumor volumes from the control group. Statistical significance and survival of mice was determined using by log-rank Mantel-Cox or Mann–Whitney test and Prism software.
Results
Characterization of m708.5, a fully human antibody to IGF-1 and IGF-2
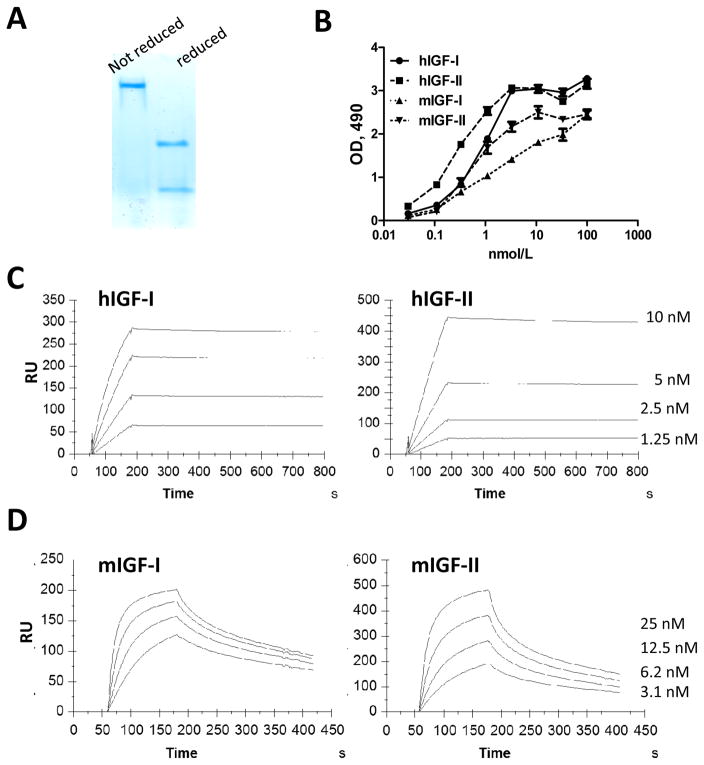
The m708.5 scFv bound with high affinity to hIGF-1 (KD = 200 pmol/L) and hIGF-2 (KD = 60 pmol/L), and it strongly inhibited both IGF-1- and IGF-2-induced phosphorylation of IGF-1R, as well as IGF-2-induced phosphorylation of the IR, and the growth of the breast cancer cell line MCF-7 (24, 25). No binding was detected against human insulin.
The scFv m708.5 was successfully reengineered into a human IgG1 format that was expressed in CHO cells. The purity of purified IgG1 protein was analyzed by HPLC (<10% aggregates) and SDS-PAGE (Figure 1A). Its affinity was measured by ELISA and surface plasmon resonance. In the ELISA result, m708.5 showed the cross-reactive binding to both human and mouse IGF-1, IGF-2 (Figure 1B). As shown in Table 1, the avidity of m708.5 as IgG1 was greatly increased, on the average ~10-fold (15pmol/L for hIGF-1 and 9 pmol/L for hIGF-2), compared to its scFv (200 pmol/L for hIGF-1 and 60 pmol/L for hIGF-2). It also showed high affinity for mouse IGF-1 (mIGF-1) (KD = 9.5nmol/L) and mIGF-2 (KD = 1.9nmol/L).
Figure 1. Characterization of IgG1 m708.5.
A, The purity of purified IgG1 m708.5 was analyzed by SDS-PAGE under native and denaturing conditions. B, Binding of IgG1 m708.5 to hIGF-1, huIGF-2 and mIGF-1, mIGF-2 was analyzed by ELISA. IgG1 m708.5 were serially diluted and added to wells coated with IGF-1 or IGF-2. Bound IgG1 was detected with an HRP conjugated anti-human Fc antibody and optical densities (O.D.) measured at 490 nm. C and D, sensorgrams of binding kinetics of IgG1 m708.5 as measured with Biacore. The hIGF-1, hIGF-2 (C) or mIGF-1, mIGF-2 (D) were immobilized and different concentrations of the antibodies were used as indicated in the figures. Details of the experiments were described in the Materials and Methods.
Table 1.
Binding affinities of m708.5 IgG1 and scFv (pM)
| Antibody Form | ||
|---|---|---|
| Target | IgG1 | scFv |
| hIGF-I | 15 | 200 |
| hIGF-II | 9 | 60 |
| mIGF-I | 9500 | NA |
| mIGF-II | 1900 | NA |
Anti-proliferative activity of m708.5 alone against tumor cell lines in vitro
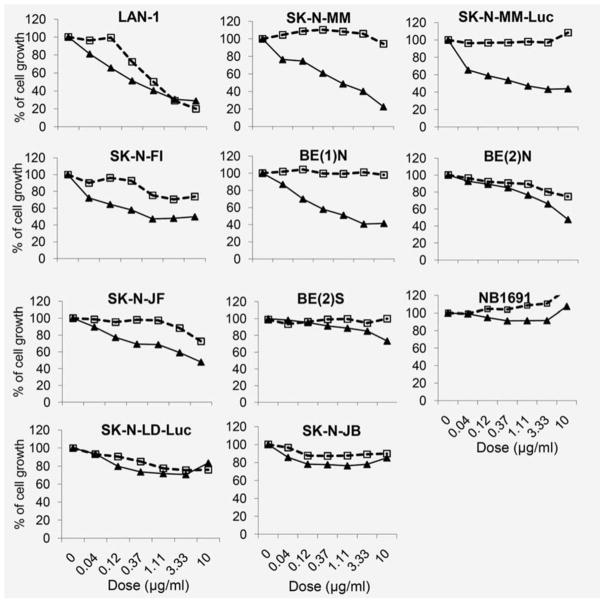
In order to investigate whether the neutralization of IGF by m708.5 is potent enough to inhibit the proliferation of tumor cells, we tested the bioactivity of m708.5 in the cell proliferation assay against cell lines from 10 (7+2+3) neuroblastomas, 7 (4+2+1) Ewing family of tumors, 3 (1+0+2) rhabdomyosarcomas, 2 (0+0+2) osteosarcomas, 2 (0+0+2) melanomas, and 1 (0+0+1) head and neck (H&M) cancers. We chose to use natural tumor cell lines instead of IGF-1 or IGF-2 transfected cell lines and did not add exogenous IGF-1 or IGF-2 in the culture or assay systems. After 2 days of treatment, m708.5 reduced cell proliferation of 8 neuroblastoma cell lines (Figure 2). As shown in Table S1, three cell lines, SK-N-MM, SK-MM-luc and LAN-1, were most sensitive with EC50 of 0.6, 0.6 and 0.46 μg/mL, respectively. Their maximum inhibition was >75% at 10 μg/mL. The other cell lines, SK-N-FI and BE(1)N, exhibited the same EC50 of 1.1 μg/mL. BE(2)N, SK-N-JF and Be(2)S cell lines showed modest values of EC50 from 9 to 15 μg/mL. But m708.5 failed to inhibit the cell growth of SK-N-JB. In comparison, the anti-GD2 3F8 antibody for neuroblastoma inhibited proliferation among 5 of 10 neuroblastoma cell lines under the same conditions.
Figure 2. Anti-proliferative activity of m708.5 alone in neuroblastoma cell lines.
Cell proliferation of neuroblastoma cell lines cultured in 2.5% FCS-conditioned medium after 48 h exposure to m708.5 IgG1 (solid line) or anti-GD2 hu3F8 IgG1(broken line) treatment at antibody concentration indicated in figures. Numbers of viable cells were determined using MTS method and expressed as the percentage of positive control (no antibody) on the Y axis.
As shown in Table S2, among the other solid tumor cells lines tested, the following were sensitive (EC50<10 μg/ml): Ewing family of tumors: SK-E-AW, TC71, SK-E-S1, and CHP100; Rhabdomyosarcoma: RH30. Moderately sensitive cell lines (10<EC50<30 μg/ml) included Ewing family of tumors: SK-E-RT, and A4573. The following cell lines were resistant (EC50>30 μg/ml) (1) Ewing family: SK-E-PR, (2) Rhabdomyosarcoma: Rh41 and Rh48, (3) Osteosarcoma: U2OS and CRL1427, (4) Melanoma: HTB63 and HTB67 (5) H&N cancer: SCC147T.
Synergistic effect of m708.5 in combination with temsirolimus and cytotoxic drugs against neuroblastoma cells in vitro
Temsirolimus (mTOR inhibitor) was tested in combination with m708.5 because of the importance of mTOR as an escape pathway during IGF-1R inhibition and its relative safety and clinical activity in neuroblastoma (26). To test whether m708.5 could enhance chemotherapy, it was tested in combination with four representative drugs used in the treatment of neuroblastoma, including SN38 (a topoisomerase II inhibitor), doxorubicin (anthracycline antibiotics, DOX), vincristine (a mitotic inhibitor), and cis-platinum (DNA alkylator, CDDP). For the MYCN-amplified p53-mutated neuroblastoma cell line LAN-1, which is widely used in drug studies, all combinations exhibited increased anti-proliferative activity over m708.5 by itself (Table 2). Synergism, with mean combination index values (CI) ranging from 0.37 to 0.6 (SN38=0.37, temsirolimus=0.49, doxorubicin=0.52, and vincristine=0.6) was seen. For the ATRX-mutated neuroblastoma cell line SK-N-MM, SN38, doxorubicin and vincristine showed synergistic effect with CI=0.48, 0.59 and 0.61, respectively, whereas cisplatinum showed moderate antagonism. Remarkably, the combination of m708.5 with the mTOR inhibitor temsirolimus yielded the strongest synergistic interactions across a wide concentration range. The mean combination index value for this combination was 0.18, suggesting that the anti-mTOR inhibitor combination was highly synergistic. These results suggest the greater efficacy of m708.5 when used in combination with mTOR inhibitor or chemotherapy in neuroblastoma.
Table 2.
Summary for m708.5 in combination with drugs against neuroblastoma.
| Drugs | LAN-1
|
SK-N-MM
|
||||||
|---|---|---|---|---|---|---|---|---|
| m708.5 | Drug | CIa ± SD | Effectb | m708.5 | Drug | CIa ± SD | Effectb | |
| SN38 | 0.3μg/ml | 0.4–100nmol/L | 0.37 ± 0.05 | synergism | 0.6μg/ml | 0.4–100nmol/L | 0.48 ± 0.05 | synergism |
| doxorubicin | 0.3μg/ml | 0.08–2μmol/L | 0.52 ± 0.03 | synergism | 0.6μg/ml | 0.08–2μmol/L | 0.61 ± 0.12 | synergism |
| vincristine | 0.3μg/ml | 0.2–60nmol/L | 0.6 ± 0.19 | synergism | 0.6μg/ml | 0.2–60 | 0.59 ± 0.02 | synergism |
| cisplatin | 0.3μg/ml | 0.4–100μmol/L | 0.80 ± 0.00 | moderate synergism | 0.6v | 0.4–100μmol/L | 1.28 ± 0.07 | moderate antagonism |
| temsirolimus | 0.3μg/ml | 0.2–5μmol/L | 0.49 ± 0.28 | synergism | 0.6μg/ml | 0.2–5μmol/L | 0.18 ± 0.04 | strong synergism |
CI, combination index
definition of CI values, <0.1(very strong synergism); 0.1–0.3 (strong synergism); 0.3–0.7 (synergism); 0.7–0.85 (modest synergism); 0.85–0.9 (slight synergism); 0.9–1.1 (nearly additive); 1.1–1.2 (slight antagonism); 1.2–1.45 (modest antagonism); 1.45–3.3 (antagonism);3.3–10 (strong antagonism); >10 (very strong antagonism).
IGF-1R/IR-A expression in neuroblastoma cells
Enhanced expression of IR-A was previously found to be a compensatory survival mechanism in cancer cells where IGF-1R signaling was inhibited (17). We tested if in vitro m708.5 sensitivity could be correlated with IGF-1R and IR-A expression in neuroblastoma cells. We use anti-IGF-1R or anti-IR-A antibodies to assay for receptor expression by flow cytometry, and the relative mean fluorescence index (MFI) summarized in Supplementary Table S2. Nine of eleven NB cells were observed to exhibit high expression of IGF-1R, consistent with the previous report of IGF-1R expression in 86% of primary neuroblastoma tumors. In contrast, IR-A was found to be expressed in 6 of 11 neuroblastoma cell lines. When these cell lines were grouped according to the IC50 values of m708.5 treatment (non-effective, modest and sensitive), sensitivity to m708.5 appeared to be dependent on the expression of both IGF-1R and IR-A in all cell lines. Thus, it appears that both IGF-1R and IR-A receptors were important for neuroblastoma growth and as potential targets for the antibody m708.5.
Inhibition of neuroblastoma growth by m708.5 alone in vivo
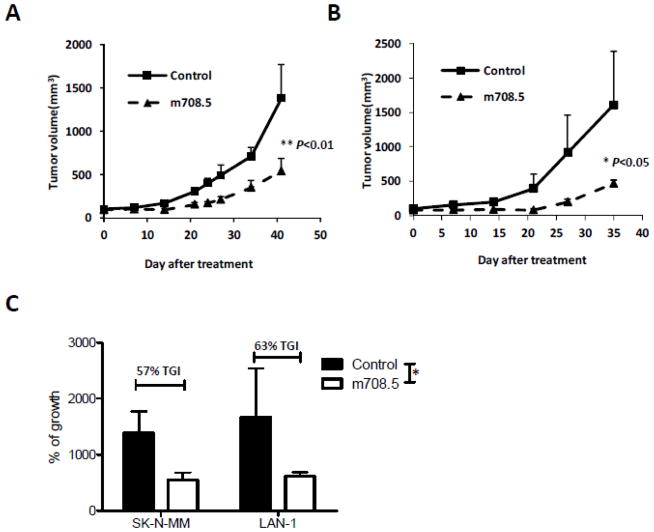
Antitumor activity of m708.5 was evaluated in vivo against neuroblastoma SK-N-MM and LAN-1 cell line when grown as xenografts in humanized SCID mice. Treatments were initiated when the average tumor size reached 75–100 mm3 after subcutaneous transplantation of SK-N-MM and LAN-1 tumors. Tumor-bearing mice were assigned to intravenous treatment with 0.1 mg/mouse of m708.5 (5 mg/kg/dose), twice weekly for 3 weeks. It was observed that m708.5 was highly active against SK-N-MM (P<0.01) and LAN-1 (P<0.05) xenograft models when administered as a single agent (Figures 3A and 3B), respectively. On day 42 after treatment initiation, the SK-N-MM xenograft tumor was inhibited by 57% when treated with m708.5 (Figure 3C). Tumors in the control grew with a median time to reach 700 mm3 of 34 days, whereas m708.5-treated tumors reached the same volume in 44 days, indicating a significant growth delay of 10 days (P<0.05). At day 35 after treatment initiation, the LAN-1 xenograft tumor was inhibited by 63% in the treatment with the same dose. Without treatment, time to reach 500 mm3 was 22 days, whereas with m708.5-treatment time was significantly delayed to 13 days (P<0.05). These data indicated that m708.5 could inhibit pediatric neuroblastoma cell proliferation as a single agent in vivo.
Figure 3. Therapy of neuroblastoma xenograft with m708.5.
A and B, SCID mice were inoculated s.c. with SK-N-MM (A) and LAN-1 (B) neuroblastoma cells. Tumor-bearing mice were assigned to intravenous treatment with either 0.1 mg control IgG1 antibody (4 mg/kg/dose) or 0.1 mg m708.5 (4 mg/kg/dose) twice weekly for 3 weeks. Mean tumor volume for each animal group are presented; bars represented standard errors of the means. C, Percent tumor growth inhibition was calculated for SK-N-MM (day 42) and LAN-1 (day 35) xenografts. The P values of the difference between treatment and control groups were significant (<0.05).
Inhibition of neuroblastoma growth by m708.5 in combination with temsirolimus in vivo
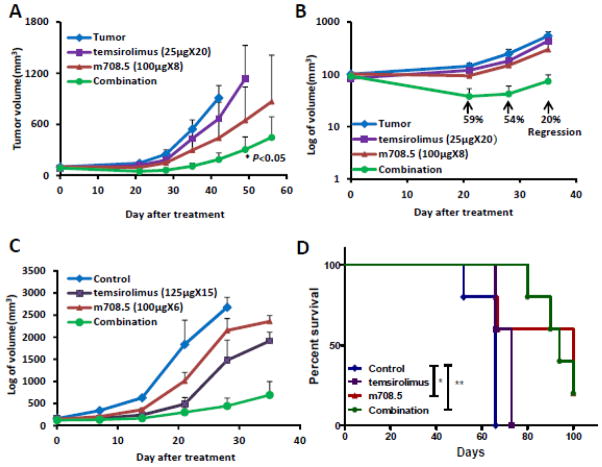
After s.c. SK-N-MM tumor implantation in SCID mice, treatment was initiated when the average tumor size reached 75–100 mm3. Mice were treated with either 0.1 mg (4 mg/kg/dose) m708.5 (i.v., 4 weeks), low dose 0.025mg temsirolimus (i.p., 4 weeks), or combination of m708.5 and temsirolimus. On day 42 after treatment, m708.5 alone inhibited tumor growth by 57% and temsirolimus alone by 27%. However, when m708.5 was combined with temsirolimus, inhibition increased to 80%, better than either single agent when used alone (Figure 4A). In control groups, time for the tumor to 500 mm3 size was 35 days. In contrast, m708.5 as a single agent extended that to 45 days, and in combination with temsirolimus to 63 days. By day 21 after treatment initiation, there was discernible tumor regression with the combination treatments; the combination of 0.025 mg temsirolimus with 0.1 mg m708.5 regressed tumors by 59%, 54% and 20%, respectively (P < 0.05), on day 21, 27 and 35 (Figure 4B). In contrast, treatment with m708.5 or temsirolimus alone as single agents did not result in tumor growth inhibition in this neuroblastoma xenograft model, although a dose titration was not carried out.
Figure 4. Therapy of neuroblastoma xenograft with m708.5 combined with temsirolimus.
A, SCID mice were inoculated s.c. with SK-N-MM neuroblastoma cells. Tumor-bearing mice were assigned to treatment with either 0.1 mg m708.5 (i.v., twice weekly for 4 weeks), 0.025 mg temsirolimus (i.p., 5 times weekly for 4 weeks), or both m708.5 and temsirolimus. B, Tumor growth regression was calculated on day 21, 28, 35 after treatment in (A). C, Tumor-bearing mice were treated with either 0.1 mg m708.5 (i.v., twice weekly for 3 weeks), 0.125 mg temsirolimus (i.p., 5 times weekly for 3 weeks), or both m708.5 and temsirolimus. D, Survival of mice in (A) was analyzed by log-rank Mantel-Cox test for groups of m708.5 alone (*P<0.05) and in combination with temsirolimus (**P<0.005) compared to untreated control animals.
In separate experiments, mice treated with decreased duration of m708.5 (3 weeks instead of 4 weeks), there was no regression observed in the combination group even though the dose of temsirolimus was increased to 0.125mg (Figure 4C). As shown in Figure 4D, m708.5 significantly prolonged survival of xenograft mice as single agent (P=0.014) and in combination with temsirolimus (P=0.003), respectively. No significant weight loss was observed in mice treated with m708.5 or temsirolimus alone or in combination throughout the course of study. These results, which are consistent with those observed in vitro, showed that the combination of temsirolimus plus m708.5 had substantially better antitumor efficacy in vivo than either agent alone.
Discussion
The IGF signaling system is important in tumorigenesis. Human IGF-1 and IGF-2 share 62% sequence identity and have overlapping functions: both IGF-1 and IGF-2 can activate the IGF-1R which can drive tumor cell proliferation. A remarkable feature of IGF-2 (but not IGF-1) is that it can also bind to the IR and enhance cell proliferation. IR is a known mitogenic driver for tumor cells, and IR activated by IGF-2, can compensate for IGF-1R disruption in tumor cells (17). IGF-2 could decrease the inhibitory effect of IGF-1R targeting antibodies (27), suggesting that IGF-1R antibodies as single agents are probably not sufficient for complete tumor inhibition, calling for combination therapies. IGF signaling pathway plays an important role in the development and maintenance of many pediatric tumors, including neuroblastoma (28). There is evidence to suggest that a significant subset of neuroblastoma, especially those with advanced stage disease, has an active IGF system that depends on signaling through the IGF-1R (4–5, 8). Available data also suggest that most neuroblastoma expresses IGF-II, which forms the autocrine loop with its IGF-1R receptor (5).
During the past decade, IGF-1R was deemed a promising target for cancer therapy with over 30 agents brought into early-phase clinical studies (29). Inhibitors of IGF-1R have broad therapeutic implications for neuroblastoma, Ewings family of tumors, and osteosarcoma. However, the failure of anti-IGF-1R antibodies in adult cancer patients has substantially dampened the initial enthusiasm (30–32). Although unclear as to why these clinical trials did not meet their expectations, one reasonable explanation is that the therapy against the IGF-1R induced upregulation of IR-A pathway as part of escape mechanism. In addition, one of the most common side effects from anti-IGF-1R agents was hyperglycemia through engagement of the hybrid heterodimeric receptors of IGF-1R/IR, even though insulin pathways were not interfered (33). In order to overcome these limitations, one strategy is to reduce the serum and tissue levels of ligands (IGF-1 and IGF-2) using neutralizing antibodies, thereby aborting the escape mechanism of IGF-2-mediated IR-A activation. In cancer therapy, the best example of ligand neutralizing antibody is bevacizumab specific for VEGF. IGF-1/2 neutralizing antibodies are promising in this class of agents. Several IGF-1 and IGF-2 neutralizing antibodies have been reported and show in vitro or in vivo antitumor activity (14, 34, 35). For neutralizing soluble ligands such as IGFs, high affinity of the antibodies is critical for the continuous sequestration of the ligand from its receptor. Since both the IGF-1 and IGF-2 activate these receptors, if both can be neutralized, the escape mechanism of IGF-2-mediated IR-A activation can be aborted besides shutting out IGF-1/IGF-2-activated IGF-1R pathway (34).
The dual specific m708.5 (24) exhibited extremely high avidity to hIGF-1 (KD = 15 pmol/L) and to hIGF-2 (KD = 9pmol/L). It strongly inhibited both hIGF-1- and hIGF-2-induced phosphorylation of IGF-1R, as well as hIGF-2-induced phosphorylation of the IR in breast cancer cell line. Recently, a similar antibody MEDI-573 against hIGF-1 and hIGF-2 was reported to inhibit in vivo growth of mouse embryonic fibroblast (MEF) tumors that were dependent on autocrine IGF stimulation (14). Mouse IGF-1 has nearly equivalent biological activity to human IGF-1 with respect to the activation of human IGF-1R. Human xenograft tumors exhibited only weak response to MEDI-573 partly due to paracrine mouse IGF. In comparison, m708.5 exhibited a better better KD to hIGF-1 (15pmol/L vs 294 pmol/L) with a comparable affinity for hIGF-2 (9 pmol/L vs 2 pmol/L). Importantly, although m708.5 also cross-reacted with mIGF-1 and mIGF-2, it was less avid compared to MEDI-573. Hence m708.5 was expected to have more difficulty to show activity in mouse models.
In this study, antitumor effects of m708.5 were evaluated in neuroblastoma as a single agent and in combination with cytotoxics in vitro and in vivo. This is the first study reporting significant anticancer activity of dual specific anti-IGF-1/2 antibody in human tumor derived cell lines, and specifically neuroblastoma. These studies contrast with previous reports where IGF transfected cell lines were used or where exogenous IGF was added (14). Our present results showed that m708.5 alone could inhibit the cell proliferation of most neuroblastoma cell lines. Notably, the sensitivity to m708.5 correlated with the high expression of IGF-1R and/or IR-A; however, this sensitivity to m708.5 was not uniform. Recent studies suggested that neuroblastoma cells were heterogeneous in their IGF-1R pathway-mediated cell proliferation (36). Constitutive activity of downstream signals (e.g. PI3K/Akt) independent of the IGF-1R or IRA pathways could explain part of the heterogeneity of response between neuroblastoma cell lines. In vivo, m708.5 alone (4 mg/kg/dose, twice weekly for 3–4 weeks) significantly delayed neuroblastoma growth. This contrasts with the relative lack of anti-tumor effect against Ewing’s sarcoma or rhabdomyosarcoma when MEDI-573 was used alone (30 mg/kg/dose, twice weekly for 6 weeks). One reason for the tumor resistance was the inability of MEDI-573 to completely neutralize IGF-1 (37). The addition of IGF-1R antibody did slow down tumor growth but only modestly (37). The difference in anti-tumor effect could be a result of the tumor type studied. Alternatively, the nearly 10 fold enhancement of affinity in m708.5 versus MEDI-573 towards IGF-1 could play a role.
The IGF-1R pathway is involved in a complex signaling cross-talk through multiple growth factors, receptors and down-stream effectors: two critical pathways, one through IRS, PI3K, AKT and mTOR for mitogenesis or survival, and one through Shc, RAS, RAF, MEK and ERK for inhibition of apoptosis. The known association of mTOR and IGF-1R signaling pathways, along with the correlation in IGFs and receptors expression patterns in tumors, provide a strong rationale for combination therapy (26). The combination of an anti-IGF-1R antibody with mTOR antagonists blocked the Akt-signaling pathway in pediatric cancers, Ewing family of tumor, breast, and prostate carcinomas, resulting in an additive increase in cell growth-inhibition (11, 38–40). It was noteworthy that the IR-A-mediated resistance was observed, when IGF-1R antibodies plus mTOR inhibitors were combined (41). There is also evidence that constitutive IGF-2 expression was implicated in retinoid-resistance in neuroblastoma cells (10, 42). However, no serious effort has been made in neuroblastoma. Our in vitro studies suggest not just a very strong synergistic effect of m708.5 with mTOR inhibitor temsirolimus, but also moderate synergism with SN38, doxorubicin, and vincristine. Importantly, the combination of m708.5 IgG1 and mTOR inhibitor could significantly result in a greater tumor inhibition than that observed for either single agent used alone. In conclusion, the dual specific anti-IGF-1/2 human monoclonal antibody m708.5 showed high preclinical activity against a broad spectrum of human cell lines and is a promising candidate therapeutic for further testing in pediatric tumors. Given the potent activity anti-IGF-1/2 antibody when combined with temsirolimus, both pathways commonly employed by these tumors, such a combination may be beneficial clinically in the therapy of tumors other than neuroblastoma.
Supplementary Material
Brief description.
IGF-1R and IR-A signaling driven by IGF-1/IGF-2 promotes neuroblastoma tumorigenesis. Anti-IGF-1/IGF-2 antibody m708.5 showed anti-tumor activity alone and strong synergy with mTOR inhibitor temsirolimus against neuroblastoma, Ewing family of tumor, rhabdomyosarcoma, and osteosarcoma. These results support the clinical development of m708.5 for pediatric solid tumors. The combination of anti-IGF-1/IGF-2 antibody and mTOR inhibitor may be beneficial clinically in the therapy of tumors.
Acknowledgments
We thank Ms. Hongfen Guo for her assistance in creating the IgG expression CHO-S cell line, and members of the Cheung laboratory for helpful discussions. This study was supported in part by the following: Mr. William H. Goodwin and Mrs. Alice Goodwin and the Commonwealth Foundation for Cancer Research and The Experimental Therapeutics Center of MSK (to NK Cheung), Cookies for Kids’ Cancer (to NK Cheung), the Robert Steel Foundation (to NK Cheung), and the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research (to DS Dimitrov).
Footnotes
Disclosure of Potential Conflicts of Interest: D. Dimitrov and Q. Zhao were named as inventors on a patent on m708.5 filed by the NIH.
References
- 1.Samani AA, Yakar S, LeRoith D, Brodt P. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev. 2007;28:20–47. doi: 10.1210/er.2006-0001. [DOI] [PubMed] [Google Scholar]
- 2.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915–28. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 3.Dupont J, LeRoith D. Insulin and insulin-like growth factor I receptors: similarities and differences in signal transduction. Horm Res. 2001;55(Suppl 2):22–6. doi: 10.1159/000063469. [DOI] [PubMed] [Google Scholar]
- 4.Tanno B, Mancini C, Vitali R, Mancuso M, McDowell HP, Dominici C, Raschella G. Down-regulation of insulin-like growth factor I receptor activity by NVP-AEW541 has an antitumor effect on neuroblastoma cells in vitro and in vivo. Clin Cancer Res. 2006;12:6772–80. doi: 10.1158/1078-0432.CCR-06-1479. [DOI] [PubMed] [Google Scholar]
- 5.Yee D, Favoni RE, Lebovic GS, Lombana F, Powell DR, Reynolds CP, Rosen N. Insulin-like growth factor I expression by tumors of neuroectodermal origin with the t(11;22) chromosomal translocation. A potential autocrine growth factor. J Clin Invest. 1990;86:1806–14. doi: 10.1172/JCI114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim B, van Golen CM, Feldman EL. Insulin-like growth factor-I signaling in human neuroblastoma cells. Oncogene. 2004;23:130–41. doi: 10.1038/sj.onc.1206924. [DOI] [PubMed] [Google Scholar]
- 7.Kim SY, Toretsky JA, Scher D, Helman LJ. The role of IGF-1R in pediatric malignancies. Oncologist. 2009;14:83–91. doi: 10.1634/theoncologist.2008-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Golen CM, Schwab TS, Kim B, Soules ME, Su Oh S, Fung K, van Golen KL, Feldman EL. Insulin-like growth factor-I receptor expression regulates neuroblastoma metastasis to bone. Cancer Res. 2006;66:6570–8. doi: 10.1158/0008-5472.CAN-05-1448. [DOI] [PubMed] [Google Scholar]
- 9.Gualberto A, Pollak M. Clinical development of inhibitors of the insulin-like growth factor receptor in oncology. Curr Drug Targets. 2009;10:923–36. doi: 10.2174/138945009789577945. [DOI] [PubMed] [Google Scholar]
- 10.Geoerger B, Brasme JF, Daudigeos-Dubus E, Opolon P, Venot C, Debussche L, Vrignaud P, Vassal G. Anti-insulin-like growth factor 1 receptor antibody EM164 (murine AVE1642) exhibits anti-tumour activity alone and in combination with temozolomide against neuroblastoma. Eur J Cancer. 2010;46:3251–62. doi: 10.1016/j.ejca.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Houghton PJ, Morton CL, Gorlick R, Kolb EA, Keir ST, Reynolds CP, Kang MH, Maris JM, Wu J, Smith MA. Initial testing of a monoclonal antibody (IMC-A12) against IGF-1R by the Pediatric Preclinical Testing Program. Pediatr Blood Cancer. 2010;54:921–6. doi: 10.1002/pbc.22367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolb EA, Gorlick R, Lock R, Carol H, Morton CL, Keir ST, Reynolds CP, Kang MH, Maris JM, Billups C, Smith MA, Houghton PJ. Initial testing (stage 1) of the IGF-1 receptor inhibitor BMS-754807 by the pediatric preclinical testing program. Pediatr Blood Cancer. 2011;56:595–603. doi: 10.1002/pbc.22741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Law JH, Habibi G, Hu K, Masoudi H, Wang MY, Stratford AL, Park E, Gee JM, Finlay P, Jones HE, Nicholson RI, Carboni J, et al. Phosphorylated insulin-like growth factor-i/insulin receptor is present in all breast cancer subtypes and is related to poor survival. Cancer Res. 2008;68:10238–46. doi: 10.1158/0008-5472.CAN-08-2755. [DOI] [PubMed] [Google Scholar]
- 14.Gao J, Chesebrough JW, Cartlidge SA, Ricketts SA, Incognito L, Veldman-Jones M, Blakey DC, Tabrizi M, Jallal B, Trail PA, Coats S, Bosslet K, et al. Dual IGF-I/II-neutralizing antibody MEDI-573 potently inhibits IGF signaling and tumor growth. Cancer Res. 2011;71:1029–40. doi: 10.1158/0008-5472.CAN-10-2274. [DOI] [PubMed] [Google Scholar]
- 15.Carboni JM, Wittman M, Yang Z, Lee F, Greer A, Hurlburt W, Hillerman S, Cao C, Cantor GH, Dell-John J, Chen C, Discenza L, et al. BMS-754807, a small molecule inhibitor of insulin-like growth factor-1R/IR. Mol Cancer Ther. 2009;8:3341–9. doi: 10.1158/1535-7163.MCT-09-0499. [DOI] [PubMed] [Google Scholar]
- 16.Belfiore A, Malaguarnera R. Insulin receptor and cancer. Endocr Relat Cancer. 2011;18:R125–47. doi: 10.1530/ERC-11-0074. [DOI] [PubMed] [Google Scholar]
- 17.Buck E, Gokhale PC, Koujak S, Brown E, Eyzaguirre A, Tao N, Rosenfeld-Franklin M, Lerner L, Chiu MI, Wild R, Epstein D, Pachter JA, et al. Compensatory insulin receptor (IR) activation on inhibition of insulin-like growth factor-1 receptor (IGF-1R): rationale for cotargeting IGF-1R and IR in cancer. Mol Cancer Ther. 2010;9:2652–64. doi: 10.1158/1535-7163.MCT-10-0318. [DOI] [PubMed] [Google Scholar]
- 18.Hou X, Huang F, Macedo LF, Harrington SC, Reeves KA, Greer A, Finckenstein FG, Brodie A, Gottardis MM, Carboni JM, Haluska P. Dual IGF-1R/InsR inhibitor BMS-754807 synergizes with hormonal agents in treatment of estrogen-dependent breast cancer. Cancer Res. 2011;71:7597–607. doi: 10.1158/0008-5472.CAN-11-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng Y, Dimitrov DS. Antibody-based therapeutics against components of the IGF system. Oncoimmunology. 2012;1:1390–1. doi: 10.4161/onci.20925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao J, Chang YS, Jallal B, Viner J. Targeting the insulin-like growth factor axis for the development of novel therapeutics in oncology. Cancer Res. 2012;72:3–12. doi: 10.1158/0008-5472.CAN-11-0550. [DOI] [PubMed] [Google Scholar]
- 21.Cheung NK, Guo H, Hu J, Tassev DV, Cheung IY. Humanizing murine IgG3 anti-GD2 antibody m3F8 substantially improves antibody-dependent cell-mediated cytotoxicity while retaining targeting in vivo. Oncoimmunology. 2012;1:477–86. doi: 10.4161/onci.19864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440–6. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 23.Pegram MD, Konecny GE, O’Callaghan C, Beryt M, Pietras R, Slamon DJ. Rational combinations of trastuzumab with chemotherapeutic drugs used in the treatment of breast cancer. J Natl Cancer Inst. 2004;96:739–49. doi: 10.1093/jnci/djh131. [DOI] [PubMed] [Google Scholar]
- 24.Zhao Q, Feng Y, Zhu Z, Dimitrov DS. Human monoclonal antibody fragments binding to insulin-like growth factors I and II with picomolar affinity. Mol Cancer Ther. 2011;10:1677–85. doi: 10.1158/1535-7163.MCT-11-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng Y, Zhao Q, Chen W, Wang Y, Crowder K, Dimitrov DS. A new bispecific antibody targeting non-overlapping epitopes on IGF2: Design, in vitro characterization and pharmacokinetics in macaques. Exp Mol Pathol. 2014;97:359–67. doi: 10.1016/j.yexmp.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cardillo TM, Trisal P, Arrojo R, Goldenberg DM, Chang CH. Targeting both IGF-1R and mTOR synergistically inhibits growth of renal cell carcinoma in vitro. BMC Cancer. 2013;13:170. doi: 10.1186/1471-2407-13-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bid HK, Zhan J, Phelps DA, Kurmasheva RT, Houghton PJ. Potent inhibition of angiogenesis by the IGF-1 receptor-targeting antibody SCH717454 is reversed by IGF-2. Mol Cancer Ther. 2012;11:649–59. doi: 10.1158/1535-7163.MCT-11-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cole KA, Maris JM. New strategies in refractory and recurrent neuroblastoma: translational opportunities to impact patient outcome. Clin Cancer Res. 2012;18:2423–8. doi: 10.1158/1078-0432.CCR-11-1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malempati S, Weigel B, Ingle AM, Ahern CH, Carroll JM, Roberts CT, Reid JM, Schmechel S, Voss SD, Cho SY, Chen HX, Krailo MD, et al. Phase I/II trial and pharmacokinetic study of cixutumumab in pediatric patients with refractory solid tumors and Ewing sarcoma: a report from the Children’s Oncology Group. J Clin Oncol. 2012;30:256–62. doi: 10.1200/JCO.2011.37.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fedele P, Calvani N, Marino A, Orlando L, Schiavone P, Quaranta A, Cinieri S. Targeted agents to reverse resistance to endocrine therapy in metastatic breast cancer: where are we now and where are we going? Crit Rev Oncol Hematol. 2012;84:243–51. doi: 10.1016/j.critrevonc.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Fidler MJ, Shersher DD, Borgia JA, Bonomi P. Targeting the insulin-like growth factor receptor pathway in lung cancer: problems and pitfalls. Ther Adv Med Oncol. 2012;4:51–60. doi: 10.1177/1758834011427576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strosberg JR, Chan JA, Ryan DP, Meyerhardt JA, Fuchs CS, Abrams T, Regan E, Brady R, Weber J, Campos T, Kvols LK, Kulke MH. A multi-institutional, phase II open-label study of ganitumab (AMG 479) in advanced carcinoid and pancreatic neuroendocrine tumors. Endocr Relat Cancer. 2013;20:383–90. doi: 10.1530/ERC-12-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weroha SJ, Haluska P. IGF-1 receptor inhibitors in clinical trials--early lessons. J Mammary Gland Biol Neoplasia. 2008;13:471–83. doi: 10.1007/s10911-008-9104-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feng Y, Zhu Z, Xiao X, Choudhry V, Barrett JC, Dimitrov DS. Novel human monoclonal antibodies to insulin-like growth factor (IGF)-II that potently inhibit the IGF receptor type I signal transduction function. Mol Cancer Ther. 2006;5:114–20. doi: 10.1158/1535-7163.MCT-05-0252. [DOI] [PubMed] [Google Scholar]
- 35.Dransfield DT, Cohen EH, Chang Q, Sparrow LG, Bentley JD, Dolezal O, Xiao X, Peat TS, Newman J, Pilling PA, Phan T, Priebe I, et al. A human monoclonal antibody against insulin-like growth factor-II blocks the growth of human hepatocellular carcinoma cell lines in vitro and in vivo. Mol Cancer Ther. 2010;9:1809–19. doi: 10.1158/1535-7163.MCT-09-1134. [DOI] [PubMed] [Google Scholar]
- 36.Qi L, Toyoda H, Shankar V, Sakurai N, Amano K, Kihira K, Iwasa T, Deguchi T, Hori H, Azuma E, Gabazza EC, Komada Y. Heterogeneity of neuroblastoma cell lines in insulin-like growth factor 1 receptor/Akt pathway-mediated cell proliferative responses. Cancer Sci. 2013;9:1162–71. doi: 10.1111/cas.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bid HK, London CA, Gao J, Zhong H, Hollingsworth RE, Fernandez S, Mo X, Houghton PJ. Dual targeting of the type 1 insulin-like growth factor receptor and its ligands as an effective antiangiogenic strategy. Clin Cancer Res. 2013;19:2984–94. doi: 10.1158/1078-0432.CCR-12-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spunt SL, Grupp SA, Vik TA, Santana VM, Greenblatt DJ, Clancy J, Berkenblit A, Krygowski M, Ananthakrishnan R, Boni JP, Gilbertson RJ. Phase I study of temsirolimus in pediatric patients with recurrent/refractory solid tumors. J Clin Oncol. 2011;29:2933–40. doi: 10.1200/JCO.2010.33.4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naing A, Lorusso P, Fu S, Hong D, Chen HX, Doyle LA, Phan AT, Habra MA, Kurzrock R. Insulin growth factor receptor (IGF-1R) antibody cixutumumab combined with the mTOR inhibitor temsirolimus in patients with metastatic adrenocortical carcinoma. Br J Cancer. 2013;108:826–30. doi: 10.1038/bjc.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naing A, LoRusso P, Fu S, Hong DS, Anderson P, Benjamin RS, et al. Insulin growth factor-receptor (IGF-1R) antibody cixutumumab combined with the mTOR inhibitor temsirolimus in patients with refractory Ewing’s sarcoma family tumors. Clin Cancer Res. 2012;18:2625–31. doi: 10.1158/1078-0432.CCR-12-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beltran PJ, Chung YA, Moody G, Mitchell P, Cajulis E, Vonderfecht S, Kendall R, Radinsky R, Calzone FJ. Efficacy of ganitumab (AMG 479), alone and in combination with rapamycin, in Ewing’s and osteogenic sarcoma models. J Pharmacol Exp Ther. 2011;337:644–54. doi: 10.1124/jpet.110.178400. [DOI] [PubMed] [Google Scholar]
- 42.Matsumoto K, Lucarelli E, Minniti C, Gaetano C, Thiele CJ. Signals transduced via insulin-like growth factor I receptor (IGF(R)) mediate resistance to retinoic acid-induced cell growth arrest in a human neuroblastoma cell line. Cell Death Differ. 1994;1:49–58. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.