Abstract
Altered functional connectivity has been associated with acute and chronic nicotine use. Connectivity alterations, specifically in the right and left executive control networks (RECN/LECN) and the default mode network (DMN), may contribute to the addiction cycle. The objective of this study was to determine if executive control network (ECN) and DMN connectivity is different between non‐smokers and smokers and whether reductions in connectivity are related to chronic cigarette use. The RECN, LECN, and DMN were identified in resting state functional magnetic resonance imaging data in 650 subjects. Analyses tested for group differences in network connectivity strength, controlling for age and alcohol use. There was a significant group effect on LECN and DMN connectivity strength with smokers (n = 452) having lower network strengths than non‐smokers (n = 198). Smokers had lower connectivity than non‐smokers associated with key network hubs: the dorsolateral prefrontal cortex, and parietal nodes within ECNs. Further, ECN connectivity strength was negatively associated with pack years of cigarette use. Our data suggest that chronic nicotine use negatively impacts functional connectivity within control networks that may contribute to the difficulty smokers have in quitting. Hum Brain Mapp 36:872–882, 2015. © 2014 Wiley Periodicals, Inc.
Keywords: cigarette, executive control network, default mode network, functional connectivity, nicotine, resting state, smoking
INTRODUCTION
Despite accepted scientific evidence that tobacco use is the leading cause of preventable illness and death in the United States [National Cancer Institute, 2013], and the fact that most smokers understand the negative health consequences associated with continued use, relapse rates are higher than 85 percent in those who attempt to quit on their own [National Institute on Drug Abuse, July 2012]. The complex interaction of nicotine's addictive liability and long‐term deleterious effects on cognitive processing in key brain networks likely contribute to this difficulty in breaking the addiction cycle [Jasinska et al., 2014].
Multiple large‐scale connected networks have been shown to have distinct functional and behavioral domains [Fair et al., 2007; Laird et al., 2011] that can be examined with functional connectivity analyses, quantifying the connections between brain regions based on temporal correlation [Wig et al., 2011]. These networks, referred to as intrinsic connectivity networks (ICNs), have been mapped to canonical functions: auditory; primary and higher visual; language; sensorimotor; anterior and posterior salience; basal ganglia; dorsal, ventral, and precuneus default mode; and bilateral executive control systems [Beckmann et al., 2005; Chakravarthy et al., 2010; Damoiseaux et al., [Link]; Greicius et al., 2003; Hampson et al., 2006; Kiviniemi et al., 2009; Seeley et al., 2007; Smith et al., 2009]. Recent research in substance users has primarily focused on networks putatively involved in addictions. In particular, the frontal‐parietal executive control networks [ECN; Goldstein and Volkow, 2011; Weiland et al., 2014]), as well as the internally focused default mode network (DMN), are neural circuits implicated in substance abuse [Sutherland et al., 2012].
To date only a limited number of studies have looked at these networks with specific focus on tobacco use [Andersen and Teicher, 2008]. Acute nicotine administration has been shown to depress activity in the DMN in non‐smokers [Tanabe et al., 2011], and, in smokers, to enhance cingulate‐cortical connectivity [Hong et al., 2009]. Nicotine replacement, in abstinent smokers, resulted in negative correlations between therapeutic effects and within‐network functional connectivity in both the ECN and DMN [Cole et al., 2010]. The latter study also found that between‐network functional connectivity changes, between the ECN and DMN, were positively correlated with the beneficial effects of nicotine therapy leading the authors to suggest that nicotine contributes to subjective improvements in cognition as well as reducing nicotine craving [Cole et al., 2010]. More recently, in evaluation of cessation aids in 24 smokers and 20 non‐smokers, Sutherland et al. [2013] found a decrease in connectivity between the insula and DMN regions following varenicline and nicotine administration in abstinent smokers, but not in non‐smokers, suggesting that cigarette use impacts connectivity which may contribute to withdrawal symptoms. In a follow‐up study, Lerman et al. found that in smokers deprived of cigarettes for 24 h, connectivity between the left ECN (LECN) and the salience network (SN) was reduced while that between DMN and SN increased [Lerman et al., 2014] demonstrating acute affects of nicotine withdrawal on functional brain systems.
Despite this work evaluating acute effects of nicotine and/or nicotine replacements, little work has been done to understand how chronic nicotine use may impact critical brain networks. It seems, in fact, crucial to first understand how repeated use of nicotine affects brain systems, which may then help clarify the mechanisms by which treatments may counteract the negative impacts of long‐term use and/or withdrawal. In that vein, Janes and coworkers evaluated differences between nicotine dependent female smokers and age‐matched controls finding enhanced left frontoparietal executive control network (LECN) connectivity with medial prefrontal cortical regions only in the smokers. This connectivity was correlated with striatal cue reactivity supporting a role for these networks in drug‐cue responding [Janes et al., 2012], which might contribute to the addictive cycle of nicotine. However, this study was limited in generalizability as the subjects were all female and, despite significant findings, was relatively low powered including only 13 smokers and 16 controls.
Given the limited work evaluating functional connectivity effects of long term nicotine use, and the small samples in the nicotine connectivity studies published to date, this study evaluated the functional connectivity strength across and between 14 ICNs that encompass the majority of the cortical and subcortical gray matter [Shirer et al., 2012] to look for differences between smokers and nonsmokers. Our primary interest, in light of previous research, were the dorsal DMN and the right and left executive control networks (RECN and LECN), also referred to as the frontoparietal networks [Shirer et al., 2012], which are involved in neurocognitive and rewarding responses to nicotine. Understanding underlying functional connectivity differences between smokers and non‐smokers may have important implications for the understanding and treatment of nicotine dependence. For example, the integrity of these networks, which we hypothesize will be negatively impacted by the quantity and chronicity of tobacco use, may underlie cognitive impairment more generally or contribute to cognitive impairments associated with nicotine withdrawal [Parrott et al., 1996; Xu, et al., 2005]. Therefore, using a large sample of 650 subjects, we investigated these networks in smokers compared to non‐smokers. Due to the high comorbidity of smoking with alcohol use [Kalman et al., 2005], and the fact that its use [Weiland et al., 2014], as well as subject motion during scanning [Van Dijk et al., 2012] have been shown to have an impact on functional connectivity, we included these as covariates in our analyses.
MATERIALS AND METHODS
Participants
Six hundred and sixty‐six individuals were recruited from the greater Albuquerque metropolitan region through advertisements in local print, online media, and radio advertisements. Exclusionary criteria were previous brain injury or loss of consciousness for more than 5 min, a history of severe alcohol withdrawal, or a positive pregnancy test [Claus et al., 2011]. Subjects were instructed not to smoke for 2 h or drink for 24 h prior to the functional magnetic resonance imaging (fMRI) scan and had to pass a breathalyzer to participate. Individuals with excessive motion (>3 mm translational or 0.053 radians rotational movement, n = 15) or technical problems (wrong acquisition plane, n = 1) were excluded. Subjects were paid $120 for participation in both the questionnaire and neuroimaging sessions. Written informed consent, approved by the University of New Mexico Human Research Committee, was obtained from all participants.
Substance Use
The 60‐day time line follow back [TLFB; Sobell et al., 1979], an interviewer‐administered assessment, was used to obtain estimates of daily cigarette, alcohol, and drug use. All participants were assessed with the alcohol use disorders identification test [AUDIT; Saunders et al., 1993] for use as a covariate. A subset of the smokers (n = 316) completed an additional smoking history questionnaire to collect information on frequency and duration of nicotine use. These data were used to calculate pack years (PY):
Image Acquisition
Whole brain resting state fMRI was performed on a 3‐T Siemens Trio scanner with a 12‐channel radio frequency coil. In the scanner, tape was placed across the participants' forehead to serve as feedback for movement reduction. T 2*‐weighted functional images were acquired using a gradient‐echo echo‐planer imaging (EPI) sequence: TE = 29 ms, TR = 2 s, flip angle = 75°, slice thickness = 3.5 mm, slice gap = 1.05 mm, field of view = 240 mm, 64 × 64 matrix, voxel size = 3.75 × 3.75 × 3.5 mm3. Resting‐state scans were 5 min in duration. Subjects were instructed to keep their eyes open and fixate on a cross. High‐resolution T1‐weighted structural image were acquired with a five‐echo multiecho MPRAGE sequence: TE = 1.64, 3.5, 5.36, 7.22, and 9.08 ms, TR = 2.53 s, TI = 1.2 s, flip angle = 7°, excitations = 1, slice thickness = 1 mm, field of view = 256 mm, resolution = 256 × 256 × 176, voxel size 1 × 1 × 1 mm, and pixel bandwidth = 650 Hz.
Image Preprocessing
Functional images were preprocessed using an automated pipeline based around SPM 5 (http://www.fil.ion.ucl.ac.uk/spm/software/spm5) including realignment, slice‐timing correction, spatial normalization to Montreal Neurological Institute space, reslicing, and smoothing with an 8 mm full‐width half‐max Gaussian kernel [Scott et al., 2011]. Time‐series of cerebrospinal fluid and white‐matter fluctuations, motion parameters and first derivative of motion parameters were progressively regressed from the time‐series data followed by band‐pass filtering (0.01–0.1Hz) using in house scripts [Welsh et al., 2010]. The mean square derivative (MSD), which measures the existence and severity of motion, was calculated for the processed image data for each subject for use as a covariant metric in subsequent analyses [Christodoulou et al., 2013].
Network Functional Connectivity Analysis
For the primary analysis, the LECN, RECN, and dorsal DMN networks were identified using a publicly available atlas of functionally defined regions of interest [ROIs; http://findlab.stanford.edu/functional_ROIs.html; Shirer et al., 2012]; see Figure 1. That study identified nodes for these networks by applying independent component analysis to resting state data from a sample of healthy control subjects. The nodes for the LECN include: dorsolateral prefrontal cortex (DLPFC), left middle/superior frontal gyrus (MFG), left superior parietal gyrus/angular gyrus (PAR), left inferior/middle temporal gyri (TL), right crus I/crus II/Lobule VI (CE), and left thalamus (TH). The RECN nodes include: right DLPFC, right MFG, right PAR, right medial superior frontal gyrus (mSFG), left CE, and right caudate (CU). The DMN nodes include: posterior cingulate cortex (pCC), medial prefrontal cortex/anterior cingulate cortex (mPFC), midcingulate cortex (mCC) left and right angular gyri (AG), thalamus (TH), left and right hippocampus (Hpc), and right superior frontal gyrus (SFG). The time‐series for each node was extracted from each subject's resting state data. Functional connections between nodes, or each pairwise correlation, was defined as an edge, and used to create a correlation matrix between the time‐series of all nodes within each network for each subject. A Fisher r‐to‐z transformation was applied to r‐values to yield z‐scores. Connectivity strength, as a global measure of connectivity for each network, was calculated as the mean of all pairwise correlations between nodes within each network [Lynall et al., 2010].
Figure 1.
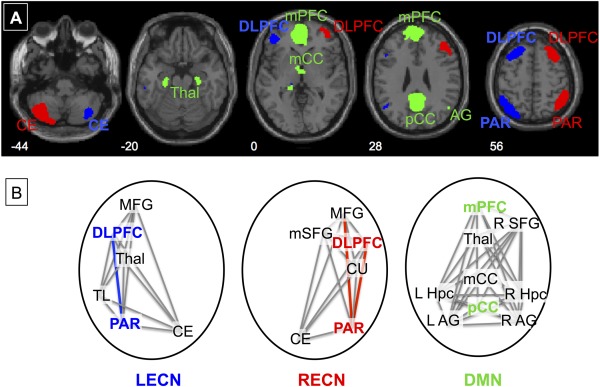
A) Networks of Interest. Networks investigated include the left and right executive (LECN—blue and RECN—red) and default mode (DMN—green) networks. ROIs defining these networks were downloaded from Stanford's FIND Lab [Shirer et al., 2012]. B) Key Nodes and Edges. Nodes and key hubs (bold) within the networks are shown with edges that were significantly less connected in smokers than non‐smokers colorized. Nodes include the dorsolateral prefrontal cortex (DLPFC), parietal lobe (PAR), middle frontal gyrus (MFG), temporal lobe (TL), thalamus (Thal), and cerebellum (CE) in the executive networks and the medial prefrontal cortex/anterior cingulate (mPFC), posterior cingulate cortex (pCC), midcingulate cortex (mCC), superior frontal gyrus (SFG), hippocampus (Hpc), angular gyri (AG), and thalamus (Thal) in the default network.
For exploratory analysis, we evaluated the connectivity strength within the other 11 networks as defined by Shirer et al. [2012]: auditory, basal ganglia, ventral default mode, language, precuneus, anterior and posterior salience, sensorimotor, primary and secondary visual, and visuospatial. Further, we calculated between network connectivity for all 14 ICNs to evaluate impact on nicotine on internetwork correlations. Connectivity strength between networks was calculated for each pair of networks as the average of all between‐network, node‐level pairwise correlations.
Data Analyses
SPSS Statistical Software version 21 (Chicago, IL) was used to perform univariate linear regressions to evaluate the effect of smoking group on network connectivity strength for each of the a priori networks (R/L ECN and DMN) as the dependent variable. AUDIT scores, MSD, age, and gender were included as covariates. Significance thresholds were false discovery rate (FDR) adjusted [Benjamini and Hochberg, 1995].
The exploratory analyses evaluated the connectivity strength within the other eleven ICNs (significance threshold: P = 0.05/11 = 0.0045), as well as between all networks (significance threshold: P = 0.05/(14 13/2) = 0.00055), was performed with the same regression covariates above.
As PYs was non‐normal (Kolmogorov‐Smirnov Test = 0.197, P = <0.001) and could not be normalized by transformation, Spearman's rho was used to test correlations with connectivity measures.
ROI/Hub Analyses
We hypothesized that within the networks of interest, specific nodes would be key hubs based on theories of functional systems [Bullmore and Sporns, 2009]. In particular, within the ECNs, the DLPF, and PAR nodes have been shown to be key hubs involved in cognitive control and adaptation and cognitive control [Cole et al., 2013], while within the DMN, the mPFC and pCC are considered key hubs [Buckner et al., 2009]. We first verified these nodes as key hubs within our sample by examining the edges, or pairwise correlations between all nodes within each network. We first calculated the average connectivity of each node, with all other nodes within each network: the key hubs were determined as those with average connectivity significantly higher than other hubs within the same network as tested with independent sample t tests. For these key hubs, we then tested for negative effects of smoking on the edges connected to these hubs using similar regressions as described above for network strengths, controlling for AUDIT, MSD, age, and gender. The significance thresholds for these analyses were based on the number of edges connected to these hubs (R/L ECN: P = 0.05/9 = 0.0056; DMN: P = 0.05/15 = 0.0033).
RESULTS
Demographic and Behavioral Measures
Table 1 shows sample characteristics for the two smoking groups which were determined from the TLFB data on cigarette use. The groups were well matched on age, gender, race, and drinks per drinking days. The non‐smokers, however, scored higher on the AUDIT.
Table 1.
Subject characteristics for subjects by smoking group
| Non‐smokers | Smokers | χ 2 or t | P | |
|---|---|---|---|---|
| N | 198 | 452 | ||
| Males:Females | 117:81 | 276:176 | 0.224 | 0.636 |
| Age | 30.1 (9.3) | 31.4 (9.4) | −1.649 | 0.100 |
| TLFB Data | ||||
| Cigarettes/Day | 0 | 12.2 (7.8) | −22.019 | 0.000 |
| Drinks/Drinking Day | 5.3 (2.9) | 5.4 (4.2) | −0.191 | 0.849 |
| AUDIT | 14.3 (7.3) | 11.8 (8.8) | 3.600 | 0.000 |
| Race/Ethnicity | 2.075 | 0.354 | ||
| Caucasian | 88 | 226 | ||
| Non‐Caucasian | 95 | 191 | ||
| Unknown | 15 | 35 | ||
| Image Motion Parameter | ||||
| MSD | 134854 (81578) | 140481 (97818) | −0.078 | 0.479 |
| N—Smoking History Data | 199 | 305 | ||
| PYs | 0 | 10.8 (7.0) | −21.528 | 0.000 |
TLFB, timeline follow back; AUDIT, alcohol use disorder identification test; MSD, mean square derivative.
Data presented as Mean (Standard Deviation) where applicable.
Group Network Connectivity Analyses
In the primary analyses, we found a significant effect of group on LECN and DMN connectivity, with non‐smokers having significantly higher network connectivity strength than smokers, (Table 2, Figs. 2 and 3). There was trend for an effect of group on the RECN connectivity (P = 0.0610). There were no significant effects of gender on the three primary networks of interest, but, in agreement with previous work [Weiland et al., 2014], an effect of AUDIT on the LECN. Motion significantly contributed to connectivity of all three networks while age had a significant effect on the ECNs.
Table 2.
Statistics for GLMs evaluating effect of smoking group on between network connectivity controlling for AUDIT score, motion, age, and gender
| NON‐SMOKERS | SMOKERS | Variance accounted for by Group | Effect Size | Variance accounted for by AUDIT | Effect Size | Variance accounted for by MSD | Effect Size | Variance Accounted for by Age | Effect Size | Variance Accounted for by Gender | Effect Size | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Network | Est'd Marginal Mean | Std Error | Est'd Marginal Mean | Std Error | F | P | Partial | F | P | Partial η 2 | F | P | Partial η 2 | F | P | Partial η 2 | F | P | Partial η 2 | |
| Primary Analyses | LECN | 0.3960 | 0.0093 | 0.3620 | 0.0061 | 8.9862 | 0.0028 | 0.0138 | 5.2980 | 0.0217 | 0.0082 | 70.3515 | <0.0001 | 0.0988 | 23.9559 | <0.0001 | 0.0360 | 2.5364 | 0.1117 | 0.0039 |
| RECN | 0.4270 | 0.0090 | 0.4060 | 0.0060 | 3.5230 | 0.0610 | 0.0055 | 2.1716 | 0.1411 | 0.0034 | 44.9622 | <0.0001 | 0.0655 | 30.9747 | <0.0001 | 0.0460 | 2.1217 | 0.1457 | 0.0033 | |
| DMN | 0.3930 | 0.0085 | 0.3690 | 0.0056 | 5.3941 | 0.0205 | 0.0083 | 0.6354 | 0.4257 | 0.0010 | 43.1017 | <0.0001 | 0.0629 | 2.2844 | 0.1312 | 0.0035 | 2.7558 | 0.0974 | 0.0043 | |
| Exploratory Analyses | AUD | |||||||||||||||||||
| HV | 0.4540 | 0.0112 | 0.4520 | 0.0074 | 0.0269 | 0.8698 | 0.0000 | 0.0002 | 0.9887 | 0.0000 | 1.1239 | 0.2895 | 0.0017 | 0.8124 | 0.3677 | 0.0013 | 0.1699 | 0.6804 | 0.0003 | |
| LNG | 1.2660 | 0.0186 | 1.2590 | 0.0123 | 0.0999 | 0.7520 | 0.0002 | 2.6936 | 0.1012 | 0.0042 | 1.3348 | 0.2484 | 0.0021 | 4.5564 | 0.0332 | 0.0070 | 21.0038 | <0.0001 | 0.0317 | |
| SM | 0.3430 | 0.0077 | 0.3310 | 0.0051 | 1.7615 | 0.1849 | 0.0027 | 4.4791 | 0.0347 | 0.0069 | 5.1992 | 0.0229 | 0.0080 | 14.0442 | 0.0002 | 0.0214 | 4.4834 | 0.0346 | 0.0069 | |
| PS | 0.2870 | 0.0084 | 0.3050 | 0.0055 | 3.0792 | 0.0798 | 0.0048 | 3.4102 | 0.0653 | 0.0053 | 3.2059 | 0.0738 | 0.0050 | 1.7064 | 0.1919 | 0.0027 | 0.3600 | 0.5487 | 0.0006 | |
| P | 0.2130 | 0.0051 | 0.1990 | 0.0034 | 4.8365 | 0.0282 | 0.0075 | 0.0173 | 0.8953 | 0.0000 | 1.7103 | 0.1914 | 0.0027 | 4.2144 | 0.0405 | 0.0065 | 0.6433 | 0.4228 | 0.0010 | |
| AS | 0.4910 | 0.0103 | 0.4910 | 0.0068 | 0.0004 | 0.9838 | 0.0000 | 1.7730 | 0.1835 | 0.0028 | 0.9614 | 0.3272 | 0.0015 | 5.7021 | 0.0172 | 0.0088 | 0.6388 | 0.4244 | 0.0010 | |
| VS | 0.4000 | 0.0085 | 0.3910 | 0.0056 | 0.7808 | 0.3772 | 0.0012 | 0.1337 | 0.7148 | 0.0002 | 11.8521 | 0.0006 | 0.0181 | 18.4579 | <0.0001 | 0.0279 | 1.1523 | 0.2835 | 0.0018 | |
| VDMN | 0.3900 | 0.0080 | 0.3980 | 0.0053 | 0.7649 | 0.3821 | 0.0012 | 1.8386 | 0.1756 | 0.0029 | 11.5736 | 0.0007 | 0.0177 | 23.7975 | <0.0001 | 0.0357 | 1.2212 | 0.2695 | 0.0019 | |
| BG | 0.3860 | 0.0078 | 0.3860 | 0.0051 | 0.0009 | 0.9758 | 0.0000 | 0.2410 | 0.6237 | 0.0004 | 11.2140 | 0.0009 | 0.0172 | 15.3078 | 0.0001 | 0.0233 | 4.0973 | 0.0434 | 0.0063 | |
| PV | 0.1760 | 0.0064 | 0.1740 | 0.0042 | 0.0460 | 0.8302 | 0.0001 | 1.1218 | 0.2899 | 0.0017 | 0.6124 | 0.4342 | 0.0010 | 0.3813 | 0.5371 | 0.0006 | 0.7111 | 0.3994 | 0.0011 | |
Networks in bold had significant effect of smoking group. Significance values in bold met thresholds for effects of other covariates, see text for details.
LECN, left executive control; RECN, right executive control; AS, anterior salience; PS, posterior salience; DMN, dorsal default mode; vDM, ventral default mode; P, precuneus; BG, basal ganglia; SM, sensorimotor; AUD, auditory; LGN, language; V1, primary visual; V2, high visual; VS, visuospatial; AUDIT, Alcohol Use Disorder Identification Test; MSD, mean square derivative.
Figure 2.
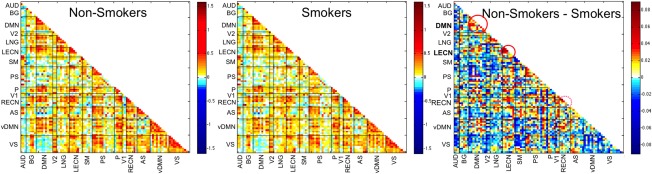
Average correlation matrices of functional connectivity across all 14 ICNs by group: non‐smokers (n = 198); smokers (n = 452). The difference matrix, non‐smokers‐smokers, identifies the networks that differed between groups with smokers having lower network functional connectivity strength: LECN and DMN. The RECN showed a trend for lower connectivity in smokers. No differences were found in internetwork connectivity between groups. LECN, left executive control; RECN, right executive control; AS, anterior salience; PS, posterior salience; DMN, dorsal default mode; vDM, ventral default mode; P, precuneus; BG, basal ganglia; SM, sensorimotor; AUD, auditory; LGN, language; V1, primary visual; V2, secondary visual; VS, visuospatial.
Figure 3.
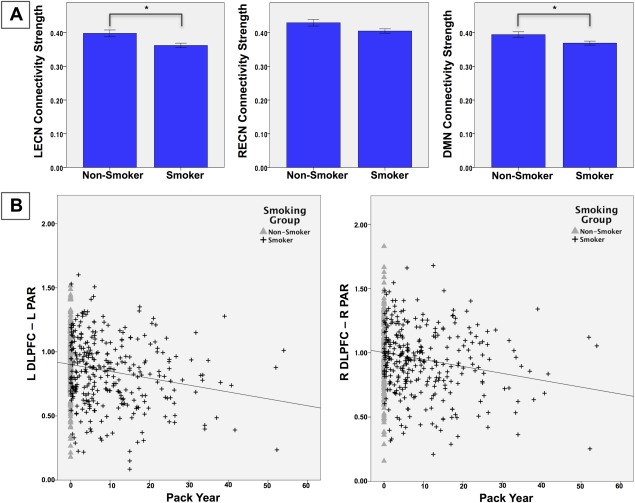
A) Network Connectivity Strength by Smoking Group. Network connectivity strength for non‐smokers (n = 198) and smokers (n = 452). *significant group difference, FDR corrected. B) Edges Associated with Chronic Cigarette Use. Scatter plots for the L/R ECN edges between the DLPFC and PAR nodes, which correlated with PYs of cigarette smoking. LECN, left executive control network; RECN, right executive control network; DMN, default mode network; DLPFC, dorsolateral prefrontal cortex; PAR, parietal lobe.
For the 514 subjects with smoking history data available, PYs was significantly negatively correlated with all three a priori networks, strength (LECN: rho = −0.153, P = 0.001; RECN: rho = −0.105, P = 0.029; DMN: rho = −0.089, P = 0.047). This effect remained significant for the LECN (rho = 0.128, P = −0.004) controlling for age and AUDIT, but not for the other two networks (RECN: rho = −0.067, P = 0.133; DMN: rho = −0.068, P = 0.130). These correlations were not significant when excluding non‐smokers (P's > 0.255).
For the exploratory analyses of the other 11 ICNs, no group effect was found. Gender had a significant effect on the secondary visual network. Age and motion contributed to the variance in the anterior salience, visuospatial, and ventral default networks with age also contributing to the variance in the language network (Table 2).
The between network exploratory analysis found no effect of smoking group on any interconnectivity strengths (Supporting Information Table 1 and Fig. 2). Significant effects of AUDIT, motion, and age were found between some networks but did not appear to follow a consistent pattern. An effect of gender was seen between the anterior SN and three networks: RECN, precuneus, and DMN, as well as between the DMN and the language network. Posthoc tests found that the internetwork connectivity was lower in all four cases in the females compared to male subject independent of smoking status.
Hub Analyses
Within the LECN, the PAR hub had the highest connectivity with other nodes, (0.52 ± 0.17) which was significantly higher than the next hub, the DLPFC (0.49 ± 0.18; t = 9.903, P < 0.001). Both of these hubs had significantly higher connectivity compared with all other LECN nodes (P's < 0.001). Within the LECN, there was a significant effect of group on the connections between the key hubs, PAR—DLPFC (P = 0.0054), with smokers having lower connectivity; no other edges met the significance threshold corrected for multiple comparisons but all edges had lower marginal means for smokers compared to non‐smokers. PAR—DLFPC edge strength negatively correlated with PYs (rho = −0.122, P = 0.006). This correlation remained at trend‐level (rho = −0.080, P = 0.063) when controlling for age and AUDIT, but was not significant when excluding non‐smokers (rho = 0.012, P = 0.838).
Within the RECN, the DLPFC hub had the highest connectivity with other nodes, (0.60 ± 0.16) followed by, and significantly higher than, the PAR hub (0.54 ± 0.16; t = 20.721, P < 0.001). Both of these hubs had significantly higher connectivity compared with all other RECN nodes (P's < 0.001). There was a significant group effect on the PAR—DPLFC and PAR—MFG (P's < 0.001), with lower connectivity in smokers compared to non‐smokers. These edges both had negative correlations with PYs (PAR—DLFPC: rho = −0.155, P = 0.001; PAR—MFG: rho = −0.096, P = 0.033). These correlations remained significant for PAR—DLPFC when controlling for age and AUDIT (rho = −0.112, P = 0.012) but not for PAR—MFG (rho = −0.051, P = 0.256). Neither edge correlation was significant when excluding non‐smokers (P's > 0.479).
For the DMN, the pCC hub had the highest connectivity with other nodes, (0.51 ± 0.15) followed by, and significantly higher than, the mPFC hub (0.49 ± 0.15; t = 6.595, P < 0.001); both having significantly higher connectivity than all other DMN nodes (P's < 0.001). There were no edges that met the significance threshold corrected for multiple comparisons. Results of GLMs for edges are presented in Supporting Information Table 2; Figure1 displays the edges different by smoking group and Figure 3 shows relationships between these edges and PYs.
DISCUSSION
Using a large sample of non‐smokers and regular smokers, this study investigated the relationship between nicotine use and resting state functional connectivity of brain networks critically involved in regulation and control: the LECN, RECN, and the DMN. We found a significant effect of group on network connectivity strength in both the LECN and the DMN with reduced connectivity strength within these networks in the smokers. Further, as recent neuroimaging research supports evidence of key hubs within networks [Buckner, et al., 2009; Bullmore and Sporns, 2009; Cole, et al., 2013], we identified the key hubs and evaluated edges connecting to these hubs within each of the networks of interest. Smokers, compared to non‐smokers, had significantly lower connectivity between the two key hubs, PAR and DLPFC within both ECNs. These results suggest that smoking may contribute to the degradation of key pathways within the executive systems and this may be a critical neurobiological mechanism contributing to the etiology of nicotine addiction. In addition, we report that increasing PYs of cigarette use are associated with decreased connectivity strength of the ECNs, suggesting a negative impact of chronic smoking on functional connectivity.
While acute nicotine administration may have enhancing effects on cognition [reviewed in Swan and Lessov‐Schlaggar, 2007], nicotine withdrawal is associated with negative symptoms ranging from craving, anxiety, and depression as well as cognitive deficits [Hughes, 2007]. Long term use of nicotine has also been associated with cognitive decline in several domains [Swan and Lessov‐Schlaggar, 2007] but may be age dependent [Reitz et al., 2005; Starr, et al., 2007] and mediated by genetic variation [Jacobsen et al., 2006; Reitz et al., 2005]. Understanding how continued nicotine use may impact brain networks involved in cognition and control is important as failure of executive control is thought to contribute to the addiction cycle [Goldstein and Volkow, 2002; Volkow et al., 2011]. Within both ECNs, the edge strength between the key hubs, PAR and DLPFC, was reduced in smokers compared to non‐smokers. This reduction in connectivity between these important nodes may represent degradation within the control systems that could contribute to the cognitive declines associated both with chronic smoking and withdrawal.
Of interest, other studies have found differences within the ECNs between smokers and non‐smokers. Smokers have been shown to have greater response to smoking cues in the right DLPFC [Zhang et al., 2011] and greater bilateral PAR activation during an inhibitory control task [Luijten et al., 2013] than non‐smokers. These results suggest that control networks in nicotine users may be compromised. Similarly, we suggest that the reduced connectivity of the DLPFC—PAR edge, may represent inefficient within network communication that could contribute to the difficulty smokers have in quitting, particularly when dealing with cue‐elicited craving [Hyman et al., 2006]. Our finding, however, is in disagreement with that of Janes et al. who reported increased within‐network connectivity in the LECN in their study of female smokers [Janes et al., 2012]. These differences may be attributable to differences in methodology, or network definitions, or to the fact their study was limited to women and had a significantly smaller sample size.
The other primary network of interest, the DMN, is hypothesized to contribute to internal mentation [Buckner et al., 2008] associated with self‐referential mental activity and emotional processing tasks [Gusnard et al., 2001]. Our analysis verified the mPFC and pCC as key hubs of this network in our subjects. Of particular relevance, compromised prefrontal cortical function has been linked with substance abuse disorders in both human [Bonson et al., 2002] and animal (McFarland and Kalivas, 2001] studies involving craving [Koob, 2006]. In contrast to our findings within the ECNs, however, we did not find particular edges driving the reduced connectivity within the DMN, but rather a global reduction in within network strength. We also failed to find significant group effects in any of the other eleven functional networks we explored though there was a trend for lower connectivity in the posterior salience network (SN) in smokers than non‐smokers. Neither were there significant differences in between‐network connectivity, which may not be unexpected given the exploratory nature of these analyses and the conservative significance threshold targets. As females showed less connectedness between the anterior SN and several other networks, including the DMN, gender effects need to be considered in future studies concerning these networks, particularly in studies that may utilize nodes within one of these networks as a seed.
Nicotine is known to bind to nicotinic acetylcholine receptors, found in many of the regions included in the ECNs and DMN particularly the frontal and cingulate cortices [Mamede et al., 2004]. Of interest, neuroanatomical studies have found structural abnormalities associated with chronic cigarette use in these same regions. Compared to non‐smokers, smokers had smaller grey matter volume and grey matter densities in bilateral PFC [Brody et al., 2004; Gallinat et al., 2006; Zhang et al., 2011] as well as the mPFC/ACC [Gallinat et al., 2006] with these volumes negatively correlated with lifetime PYs. Further, in smokers, nicotine has been shown to have detrimental effects on working memory [Park et al., 2000], a task associated with the DLPFC [Goldman‐Rakic, 1987]. Mechanistically, these negative effects of nicotine, on both structure and function, are posited to be mediated by the high density of nicotine binding sites in these areas [Nordberg et al., 1989]. This is supported by a study showing that smokers, treated with varenicline, a nicotinic receptor partial agonist, had increased DLPFC activation during a working memory task [Loughead et al., 2010]. While this study reports a small effect size of smoking on network connectivity strength, the edge analyses found stronger effects associated with prefrontal node connectivity within all three networks of interest, further supporting a potential negative impact on these important regions.
Several limitations of this study should be noted including the connectivity analysis technique used. The method used here does not evaluate causal or directional relationships, but only evaluates interregional temporal correlations. Further the current analyses made use of a publicly available set of ROIs to define networks implicated in addiction [Shirer et al., 2012]. The definition of brain networks is an ongoing research question and our future work may utilize other network definitions or data driven techniques. As smokers were asked to not smoke for 2 h prior to the scan, it is possible that acute withdrawal effects may have impacted the resting state data; future studies should more carefully control acute nicotine use. Further, as the associations between PYs and connectivity did not remain significant when non‐smokers were excluded, our data does not unequivocally support a cumulative cigarette exposure effect. One interpretation may be that there is some threshold (rather than a linear dose‐effect relationship) that determines the effect that all smokers in this study have reached with an end result that the correlation with the non‐smokers included may indeed simply mirror the group effects. Conversely, the relatively young age of our smokers (30.1 ± 9.3 years; 10.8 ± 7.0 PYs;) may be a factor in not finding a dose effect. It may be possible that with an older sample, with corresponding greater cumulative exposure, such an association might be detectable. Finally, as this study was cross sectional, it is also unclear whether differences in functional connectivity were preexisting in the smokers; however, the associations found with smoking history, when including non‐smokers, support negative impacts of chronic nicotine use on functional connectivity.
CONCLUSION
This study of 650 subjects indicates that smokers have overall reduced connectivity strength in the LECN network compared to non‐smokers. In addition, in the ECNs, smokers have lower connectivity between important nodes suggesting reduced within‐network communication and function associated with nicotine use. Reduced correlation of edges connected to the DLPFC node was associated with increased lifetime cigarette consumption suggesting an adverse impact on control networks with chronic smoking. Our data suggest that chronic cigarette use is negatively related to functional connectivity within control networks that may play a role in the addiction cycle and merits further study.
Supporting information
Supplementary Information Table 1.
Supplementary Information Table 2.
Conflict of interest: Nothing to report
REFERENCES
- Andersen SL, Teicher MH (2008): Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci 31:183–191. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM (2005): Investigations into resting‐state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci 360:1001–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y (1995): Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Ser B (Methodol) 57:289–300. [Google Scholar]
- Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, Kurian V, Ernst M, London ED (2002): Neural systems and cue‐induced cocaine craving. Neuropsychopharmacology 26:376–386. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Jarvik ME, Lee GS, Smith EC, Huang JC, Bota RG, Bartzokis G, London ED (2004): Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biol Psychiatry 55:77–84. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews‐Hanna JR, Schacter DL (2008): The brain's default network. Ann N Y Acad Sci 1124:1–38. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Andrews‐Hanna JR, Sperling RA, Johnson KA (2009): Cortical hubs revealed by intrinsic functional connectivity: Mapping, assessment of stability, and relation to Alzheimer's disease. The J Neurosci 29:1860–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E, Sporns O (2009): Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10:186–198. [DOI] [PubMed] [Google Scholar]
- Chakravarthy VS, Joseph D, Bapi R (2010): What do the basal ganglia do? A modeling perspective. Biol Cybern 103:237–253. [DOI] [PubMed] [Google Scholar]
- Christodoulou AG, Bauer TE, Kiehl KA, Feldstein Ewing SW, Bryan AD, Calhoun VD (2013): A quality control method for detecting and suppressing uncorrected residual motion in fMRI studies. Magn Reson Imaging 31:707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus ED, Ewing SWF, Filbey FM, Sabbineni A, Hutchison KE (2011): Identifying neurobiological phenotypes associated with alcohol use disorder severity. Neuropsychopharmacology 36:2086–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DM, Beckmann CF, Long CJ, Matthews PM, Durcan MJ, Beaver JD (2010): Nicotine replacement in abstinent smokers improves cognitive withdrawal symptoms with modulation of resting brain network dynamics. NeuroImage 52:590–599. [DOI] [PubMed] [Google Scholar]
- Cole MW, Reynolds JR, Power JD, Repovs G, Anticevic A, Braver TS (2013): Multi‐task connectivity reveals flexible hubs for adaptive task control. Nat Neurosci 16:1348–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF (2006): Consistent resting‐state networks across healthy subjects. Proc Natl Acad Sci USA 103:13848–13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NUF, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL (2007): Development of distinct control networks through segregation and integration. Proc Natl Acad Sci 104:13507–13512. 17679691 [Google Scholar]
- Gallinat J, Meisenzahl E, Jacobsen LK, Kalus P, Bierbrauer J, Kienast T, Witthaus H, Leopold K, Seifert F, Schubert F, Staedtgen M (2006): Smoking and structural brain deficits: A volumetric MR investigation. Eur J Neurosci 24:1744–1750. [DOI] [PubMed] [Google Scholar]
- Goldman‐Rakic PS (1987): Circuitry of primate prefrontal cortex and regulation of behavior by representational knowledge In: Plum F, Mountcasle V, editors. Handbook of Physiology—The Nervous System. Bethesda, MD: American Physiological Society; pp 373–417. [Google Scholar]
- Goldstein RZ, Volkow ND (2002): Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry 159:1642–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND (2011): Dysfunction of the prefrontal cortex in addiction: Neuroimaging findings and clinical implications. Nat Rev Neurosci 12:652–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V (2003): Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci USA 100:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME (2001): Medial prefrontal cortex and self‐referential mental activity: Relation to a default mode of brain function. Proc Natl Acad Sci 98:4259–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT (2006): Brain connectivity related to working memory performance. J Neurosci 26:13338–13343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong LE, Gu H, Yang Y, Ross TJ, Salmeron BJ, Buchholz B, Thaker GK, Stein EA (2009): Association of nicotine addiction and nicotine's actions with separate cingulate cortex functional circuits. Arch Gen Psychiatry 66:431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR (2007): Effects of abstinence from tobacco: Valid symptoms and time course. Nicotine Tob Res 9:315–327. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ (2006): Neural mechanisms of addiction: The role of reward‐related learning and memory. Annu Rev Neurosci 29:565–598. [DOI] [PubMed] [Google Scholar]
- Jacobsen L, Pugh K, Mencl WE, Gelernter J (2006): C957T polymorphism of the dopamine D2 receptor gene modulates the effect of nicotine on working memory performance and cortical processing efficiency. Psychopharmacology 188:530–540. [DOI] [PubMed] [Google Scholar]
- Janes AC, Nickerson LD, Frederick BD, Kaufman MJ (2012) Prefrontal and limbic resting state brain network functional connectivity differs between nicotine‐dependent smokers and non‐smoking controls. Drug Alcohol Depend 125:252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinska AJ, Zorick T, Brody AL, Stein EA (2014): Dual role of nicotine in addiction and cognition: A review of neuroimaging studies in humans. Neuropharmacology 83:111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalman D, Morissette SB, George TP (2005): Co‐morbidity of smoking in patients with psychiatric and substance use disorders. Am J Addict 14:106–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiviniemi V, Starck T, Remes J, Long X, Nikkinen J, Haapea M, Veijola J, Moilanen I, Isohanni M, Zang Y‐F, Tervonen O (2009): Functional segmentation of the brain cortex using high model order group PICA. Human Brain Mapp 30:3865–3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF (2006): The neurobiology of addiction: A neuroadaptational view relevant for diagnosis. Addiction 101(Suppl 1):23–30. [DOI] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Eickhoff SB, Turner JA, Ray KL, McKay DR, Glahn DC, Beckmann CF, Smith SM, Fox PT (2011): Behavioral interpretations of intrinsic connectivity networks. J Cogn Neurosci 23:4022–4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman C, Gu H, Loughead J, Ruparel K, Yang Y, Stein EA (2014): Large‐scale brain network coupling predicts acute nicotine abstinence effects on craving and cognitive function. JAMA Psychiatry 71:523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughead J, Ray R, Wileyto EP, Ruparel K, Sanborn P, Siegel S, Gur RC, Lerman C (2010): Effects of the alpha4beta2 partial agonist varenicline on brain activity and working memory in abstinent smokers. Biol Psychiatry 67:715–721. [DOI] [PubMed] [Google Scholar]
- Luijten M, O'Connor DA, Rossiter S, Franken IHA, Hester R (2013): Effects of reward and punishment on brain activations associated with inhibitory control in cigarette smokers. Addiction 108:1969–1978. [DOI] [PubMed] [Google Scholar]
- Lynall M‐E, Bassett DS, Kerwin R, McKenna PJ, Kitzbichler M, Muller U, Bullmore E (2010): Functional connectivity and brain networks in schizophrenia. J Neurosci 30:9477–9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamede M, Ishizu K, Ueda M, Mukai T, Iida Y, Fukuyama H, Saga T, Saji H (2004) Quantification of human nicotinic acetylcholine receptors with 123I‐5IA SPECT. J Nucl Med 45:1458–1470. [PubMed] [Google Scholar]
- McFarland K, Kalivas PW (2001) The circuitry mediating cocaine‐induced reinstatement of drug‐seeking behavior. J Neurosci 21:8655–8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Institute (2013): PDQ® Cigarette Smoking. Bethesda, MD. [Google Scholar]
- National Institute on Drug Abuse (2012): NIDA Research Report Series: Tobacco Addiction. Bethesda, Maryland. NIH Publication Number 12–4342. National Institute on Drug Abuse.
- Nordberg A, Hartvig P, Lundqvist H, Antoni G, Ulin J, Langstrom B (1989): Uptake and regional distribution of (+)‐(R)‐ and (‐)‐(S)‐N‐[methyl‐11C]‐nicotine in the brains of rhesus monkey. An attempt to study nicotinic receptors in vivo. J Neural Transm Park Dis Dement Sect 1:195–205. [DOI] [PubMed] [Google Scholar]
- Park S, Knopick C, McGurk S, Meltzer HY (2000): Nicotine impairs spatial working memory while leaving spatial attention intact. Neuropsychopharmacology 22:200–209. [DOI] [PubMed] [Google Scholar]
- Parrott A, Granham N, Wesnes K, Pinnock C (1996): Cigarette smoking and abstinence: Comparative effects upon cognitive task performance and mood state over 24 hours. Hum Psychopharmacol 11:391–400. [Google Scholar]
- Reitz C, Luchsinger J, Tang M‐X, Mayeux R (2005): Effect of smoking and time on cognitive function in the elderly without dementia. Neurology 65:870–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, De La Fuente JR, Grant, M (1993): Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption‐II. Addiction 88:791–804. [DOI] [PubMed] [Google Scholar]
- Scott A, Courtney W, Wood D, de la Garza R, Lane S, King M, Wang R, Roberts J, Turner JA, Calhoun VD (2011): COINS: An innovative informatics and neuroimaging tool suite built for large heterogeneous datasets. Front Neuroinform 5:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD (2007): Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD (2012): Decoding subject‐driven cognitive states with whole‐brain connectivity patterns. Cereb Cortex 22:158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF (2009): Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci 106:13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Maisto SA, Sobell MB, Cooper AM (1979): Reliability of alcohol abusers' self‐reports of drinking behavior. Behav Res Ther 17:157–160. [DOI] [PubMed] [Google Scholar]
- Starr JM, Deary IJ, Fox HC, Whalley LJ (2007): Smoking and cognitive change from age 11 to 66 years: A confirmatory investigation. Addict Behav 32:63–68. [DOI] [PubMed] [Google Scholar]
- Sutherland MT, McHugh MJ, Pariyadath V, Stein EA (2012): Resting state functional connectivity in addiction: Lessons learned and a road ahead. NeuroImage 62:2281–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MT, Carroll AJ, Salmeron BJ, Ross TJ, Hong LE, Stein EA (2013): Down‐regulation of amygdala and insula functional circuits by varenicline and nicotine in abstinent cigarette smokers. Biol Psychiatry 74:538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan GE, Lessov‐Schlaggar CN (2007): The effects of tobacco smoke and nicotine on cognition and the brain. Neuropsychol Rev 17:259–273. [DOI] [PubMed] [Google Scholar]
- Tanabe J, Nyberg E, Martin L, Martin J, Cordes D, Kronberg E, Tregellas J (2011): Nicotine effects on default mode network during resting state. Psychopharmacology 216:287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KRA, Sabuncu MR, Buckner RL (2012): The influence of head motion on intrinsic functional connectivity MRI. NeuroImage 59:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G‐J, Fowler JS, Tomasi D, Telang F (2011): Addiction: Beyond dopamine reward circuitry. Proc Natl Acad Sci USA 108:15037–15042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland BJ, Sabbineni A, Calhoun VD, Welsh RC, Bryan AD, Jung RE, Mayer AR, Hutchison KE (2014): Reduced left executive control network functional connectivity is associated with alcohol use disorders. Alcohol Clin Exp Res 38:2445–2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh RC, Chen AC, Taylor SF (2010): Low‐frequency BOLD fluctuations demonstrate altered thalamocortical connectivity in schizophrenia. Schizophr Bull 36:713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wig GS, Schlaggar BL, Petersen SE (2011): Concepts and principles in the analysis of brain networks. Ann N Y Acad Sci 1224:126–146. [DOI] [PubMed] [Google Scholar]
- Xu J, Mendrek A, Cohen MS, Monterosso J, Rodriguez P, Simon SL, Brody A, Jarvik M, Domier CP, Olmstead R, Ernst M, London ED (2005): Brain activity in cigarette smokers performing a working memory task: Effect of smoking abstinence. Biol Psychiatry 58:143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Salmeron BJ, Ross TJ, Gu H, Geng X, Yang Y, Stein EA (2011): Anatomical differences and network characteristics underlying smoking cue reactivity. NeuroImage 54:131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information Table 1.
Supplementary Information Table 2.


