Abstract
Background
Alcohol-related peripheral neuropathy (ALN) is generally characterized as an axonal large-fiber polyneuropathy caused by thiamine deficiency. We hypothesized, based on clinical observations, that ALN is associated with a small-fiber polyneuropathy that can be diagnosed with skin biopsy in heavy alcohol drinking subjects with normal thiamine status.
Methods
Eighteen individuals (9 heavy alcohol drinking subjects and 9 healthy control subjects) were assessed for the potential utility of skin biopsies in detecting ALN-associated small nerve fiber degeneration. Heavy drinking was defined as greater than 4 drinks/d and 5 drinks/d in women and men, respectively, as determined by the Timeline Follow-Back and lifetime drinking history. All subjects underwent neurological examination, nerve conduction studies, and skin biopsies to quantify end nerve fiber densities (ENFD). Other causes of neuropathy were excluded and thiamine status was assessed.
Results
Average ENFD were significantly decreased at the calf in the alcohol group as compared with control group (p < 0.0001). Histological sections demonstrated striking attrition and architectural simplification of intraepidermal nerve fibers in the heavy alcohol drinking subjects. There were no significant intergroup differences with respect to clinical assessments of neuropathy or thiamine status.
Conclusions
ALN is associated with a small-fiber neuropathy that can be detected with skin biopsy in heavy alcohol drinking individuals with normal thiamine status. Skin biopsy is a useful, minimally invasive biomarker that could extend studies to understand the effect of alcohol on the peripheral nerves and to evaluate potential therapeutic agents in larger clinical trials.
Keywords: Peripheral Neuropathy, Alcohol, Nerve, Small Fiber
Alcohol-related peripheral neuropathy (ALN) is an under recognized and significant neurological complication that exacerbates the substantial morbidity caused by chronic alcoholism. The rate of ALN in alcoholic individuals has been estimated as high as 66% (Ammendola et al., 2001; Laker and Sullivan, 2009). Although excessive alcohol intake is clearly the critical variable in the development of ALN, the mechanisms of this disease process are not well understood; and therefore, effective treatments have not emerged. In the past, considerable attention was paid to the role of malnutrition, particularly thiamine deficiency (Abe and Itokawa, 1977; Fennelly et al., 1967; Frank et al., 1971; Hoyumpa, 1980; Hoyumpa et al., 1975; Leevy et al., 1965; Tomasulo et al., 1968). This concept had been challenged as several studies failed to show significant and specific associations between ALN and thiamine deficiency (Behse and Buchthal, 1977; Hilz et al., 1995; Koike et al., 2001, 2003; Zambelis et al., 2005) and clinical trials of vitamin supplementation alone did not reverse symptoms of ALN (Chopra and Tiwari, 2012). Additionally, experimental models indicated chronic high-dose ethanol administration, in the absence of nutritional deficiency, was sufficient to cause ALN (Mellion et al., 2013; Nguyen et al., 2012).
ALN is generally regarded as a predominantly length-dependent axonal large-fiber polyneuropathy caused by thiamine deficiency (Behse and Buchthal, 1977; Blackstock et al., 1972; Dyck et al., 1968; Koike et al., 2003; Mawdsley and Mayer, 1965; Walsh and McLeod, 1970; Zambelis et al., 2005). However, the typical clinical presentation of ALN as a painful, burning neuropathy followed by motor and autonomic instability is suggestive of small-fiber involvement at the onset of disease that progresses to involve larger nerve fibers. Several studies of sural nerve biopsies have shown that patients with recent onset of ALN had associated small-fiber axonal loss, whereas those with long-standing disease had abundant regenerating fibers and marked axonal sprouting consistent with large-fiber damage (Behse and Buchthal, 1977; Koike et al., 2003; Zambelis et al., 2005). Furthermore, recent studies, also involving sural nerve biopsies, showed that small myelinated and unmyelinated fiber densities were more severely reduced than large myelinated fiber densities in the early stages of ALN (Koike et al., 2001, 2003). The observation of increased thermal thresholds in patients with ALN also supports the concept that small myelinated and unmyelinated nerve fibers may be selectively vulnerable to degeneration in ALN (Zambelis et al., 2005). Together, these studies suggest that small, unmyelinated nerve fibers are significantly affected (Koike et al., 2001). Until recently, damage to small nerve fibers was inferred by decreased thermal thresholds, abnormal autonomic testing, or axonal loss observed in sural nerve biopsies (Hilz et al., 1995; Zambelis et al., 2005). Nerve biopsies are invasive and can lead to significant morbidity due to loss of sensory function in the previously innervated structures. Skin biopsy is currently the standard approach in diagnosing small-fiber neuropathies, though no studies have been conducted to validate the use of skin biopsy in ALN (Ebenezer et al., 2007; Gibbons et al., 2006; McArthur et al., 1998; McCarthy et al., 1995).
The primary objective of this pilot study is to demonstrate that ALN is associated with a small-fiber neuropathy and that this neuropathy can be diagnosed by skin biopsy in heavy alcohol drinking individuals with normal thiamine status. Skin biopsy is relatively benign, minimally invasive, repeatable over time, and could become an important tool in the study of the pathogenesis and assessment of the effectiveness of treatments in ALN.
MATERIALS AND METHODS
Patients
Eighteen individuals, 9 heavy alcohol drinking subjects and 9 control subjects, were recruited from the community and neurology clinics for a one-time visit with the research team. After a brief phone prescreening, potential participants came to our Neurology Unit at Rhode Island Hospital (RIH) for a comprehensive screening assessment. Inclusion criteria were: (i) age between 18–70 (inclusive); (ii) no detectable blood alcohol level at the time of the study as determined by breathalyzer (AlcoHawk Ultraslim; Q3 Innovations, Independence, IA); and (iii) for the alcohol group, current heavy alcohol drinking, determined as an average of ≥4 drinks/d for woman and ≥ 5 drinks/d for men during 30 days within the 90 days prior to screening. The definition of heavy drinking was determined by limits set by the National Institutes on Alcohol Abuse and Alcoholism (NIAAA, 2013). Exclusion criteria were evidence of hepatic disease (including hepatitis B, hepatitis C, alcoholic hepatitis, or abnormal liver functions), thyroid disease, risk of diabetes mellitus (as defined as a hemoglobin A1c [HbA1c] > 5.8), HIV positivity, neuropathy of any other cause, and women who were pregnant or lactating. After complete description of the study, written informed consent was obtained following a negative alcohol breath test to ensure the potential participants’ understanding and risks of participating in the study. The RIH Institutional Review Board reviewed and approved the protocol.
Demographics
Each participant provided demographic information including age, gender, ethnicity, employment, and annual income (Table 1).
Table 1.
Demographic Characteristics
| Alcohol group (AG) |
Control group (CG) |
p-Value | |
|---|---|---|---|
| Male : Female | 6:3 | 3:6 | |
| Ethnicity | |||
| Caucasian | 4 | 5 | |
| African-American | 2 | 3 | |
| Asian | 0 | 1 | |
| Latino/Hispanic | 3 | 0 | |
| BMI(mean [95% CI]) |
34.6 (26.8–42.3) | 27.8 (23.4–32.0) | |
| Age(mean [95% CI]) |
35.4 (30.4–40.4) | 43.3 (38.3–48.2) | 0.03 |
| Male | 39.5 (32.6–46.3) | 37.3 (28.7–45.9) | |
| Female | 31.3 (24.2–38.5) | 49.1 (44.2–54.1) | 0.0006 |
| TLFB(drinks/d) (mean [95% CI]) |
13.5 (8.7–20.1) | 1.3 (0.6–2.3) | <0.0001 |
| Male | 12.4 (8.6–18) | 2.6 (1.9–3.6) | <0.0001 |
| Female | 14.7 (6.6–32.4) | 0.7 (0.2–2.8) | 0.001 |
| Ethanol consumed daily (grams) |
189 (121.8–281.4) | N/A | |
| Average duration heavy drinking (years) |
20.7 (15.9–25.3) | N/A |
BMI, body mass index; TLFB, Timeline Follow-Back.
A total of 18 subjects were enrolled in this study. Main effect p-values and least-squares mean estimates with 95% confidence interval (CI) of AG vs.CG were calculated. There was a significant difference in age and with the CG as a whole and the female CG patients being older than the AG (p = 0.03 and 0.0006, respectively), while there was no significant difference in age between male drinkers and nondrinkers. As expected, there was also a significant difference in the amount of alcohol consumed daily (p < 0.0001 for the AG as a whole and males, and p = 0.001 for females).
Assessment of Alcohol Consumption
Semi-structured interview tools were administered to assess drinking habits and history. The alcohol Timeline Follow-Back (TLFB) is a validated structured interview that allows participants to retrospectively self-report their drinking histories. The TLFB has become state-of-the-science for the collection of daily alcohol use (Sobell and Sobell, 1992). A drink is defined as a standard drinking unit (SDU), where 1.0 SDU equals the U.S. equivalent of 14 g of pure alcohol. Data was gathered for 90 days prior to the date of enrollment. The lifetime drinking history (LDH) is also a validated structured interview tool where participants report patterns of alcohol consumption from the onset of regular drinking. In addition, participants are asked to correlate changes in drinking behaviors with other significant life changes (Skinner and Sheu, 1982). Both TLFB and LDH were administered by trained research staff.
Laboratory Studies
Serological studies were obtained to rule out other causes of neuropathy and to assess overall nutritional status. Specific blood tests included aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma glutamyl transpeptidase (GGT), thiamine pyrophosphate, thyroid stimulating hormone (TSH), erythrocyte sedimentation rate (ESR), glucose, HbA1c, Lyme ELISA, HIV, albumin, prothrombin time (PT), and international normalized ratio (INR). Thiamine pyrophosphate was checked as it has been shown to be a sensitive and accurate measure of thiamine status (Poupon et al., 1990). Nutritional status was assessed with serum albumin and body mass index (BMI).
Clinical Neuropathy Assessment
All participants had a full neurological examination performed by a trained neurologist. The Utah Early Neuropathy Scale (UENS) is a sensitive, objective, and reproducible validated scale used to assess for large- and small-fiber neuropathy (Singleton et al., 2008). Patient strength, pin sensation, allodynia and hyperesthesia, large-fiber sensation, and deep tendon reflexes were scored by a trained neurologist.
Nerve Conduction Studies
Nerve conduction studies (NCS) were recorded with a Medtronic Keypoint Electromyography System (Medtronic, Boston, MA) using standard filter settings and techniques for motor NCS (MNCS) and sensory NCS (SNCS). All nerve conductions were recorded in the right arm and leg. MNCS included median nerve to the abductor pollicis brevis, ulnar nerve to the adductor digiti minimi, fibular nerve to the extensor digitorum brevis, and tibial nerve to the abductor hallucis. Measurements were taken distally and proximally according to standardized protocol. Orthodromic sensory NCS included median, ulnar, and sural nerves. Distal latency measured in milliseconds (ms), velocity measured in meters/second (m/s), compound motor amplitudes measured in millivolts (mV), and sensory nerve amplitudes measured in microvolts (µV) were recorded and calculated according to standard protocol (Oh, 2003).
Skin Biopsy
Small-fiber neuropathy was assessed using immunohistochemical staining of skin punch biopsies. Four 3-mm skin punch biopsies were taken. Two from the calf 10 cm proximal to the lateral malleolus and 2 from the ipsilateral proximal thigh. The biopsy sites were prepped and cleaned using povidone-iodine solution and anesthetized using 1% lidocaine. Biopsies were taken using sterile, single-use tools provided by Therapath (Therapath Laboratories, New York, NY). One biopsy from the calf and 1 biopsy from the thigh were harvested into 2% paraformaldehyde-lysine-periodate buffer and immediately mailed to a commercial laboratory (Therapath) for further assessment. The sample must sit in the fixative solutions for 24 hours. Upon arrival of the specimen at the laboratory, the sample is grossed, inked, and assessed. The second step is to immerse the 3-mm punch biopsy sample into a cryo-protectant solution, 20% glycerol in Sorensen’s buffer with 1% Fast Green. The sample must sit in this cryo-protectant for at least 12 hours. The glycerol serves as a protectant for the cells of the sample and prevents it from rupturing and bursting during freezing. The remaining 2 punch biopsies were stored in RNAlater (Ambion, Grand Island, NY) for further biochemical studies. Following biopsy, pressure was applied to all sites until hemostasis was achieved. Each site was bandaged using a pressure dressing and subjects were given specific written and verbal directions for wound management.
Biopsy samples were cut into 50-micron sections, and stained using panaxonal marker PGP 9.5, hematoxylin-eosin to assess for histological abnormalities, and Congo Red to assess for evidence of amyloid. Samples were evaluated using light microscopy to determine end nerve fiber densities (ENFD) as well as sweat gland nerve fiber densities (SGNFD) according to standard protocol (Lauria et al., 2005).
Statistical Analysis
Generalized linear models for Gaussian, lognormal, Poisson, and binomial distributions were used to test for main effects of prolonged drinking on physiological indices of neuropathy including clinical assessment of neuropathy (i.e., UENS), serological studies, NCS, and analyses of ENFD and SGNFD. Differences between the alcohol group (AG) and the control group (CG) patients were assessed. Model parameters (mean, 95% confidence interval) were back translated for presentation. Classical sandwich estimation was used to adjust for any model misspecification in residual variance. Effects on nerve latencies were modeled using Kaplan–Meier estimates of survival functions to right censor observations at their upper limit without introducing bias.
RESULTS
Patients
Of 30 subjects screened for potential eligibility, 18 patients qualified for the study and were enrolled. In the AG (n = 9) there were 6 men and 3 women, and in the CG (n = 9) there were 3 men and 6 women. Recruitment was discontinued at this point because the end point of detecting a significant difference in ENFD between the 2 groups had been attained. There was a significant difference in age and with the female CG patients being older than the AG, while there was no significant difference in age between male drinkers and non-drinkers. As expected, there was also a significant difference in the amount of alcohol consumed daily. There was no difference in nutritional status as assessed by BMI and albumin (Table 1). Most patients who were ineligible for the study had evidence of other causes of neuropathy, mostly elevated HbA1c consistent with insulin resistance or diabetes.
Objective clinical assessment with the UENS and its subsections including motor examination, pin sensation, allodynia/hyperesthesia, large-fiber sensation (vibration/position sense), and deep tendon reflexes did not reveal any statistically significant differences between the 2 groups. There was no difference between the 2 groups in serologic evaluations including thiamine pyrophosphate, TSH, AST, ALT, GGT, ESR, Lyme titer, HIV, glucose, HbA1c, albumin, PT, and INR (Table 2).
Table 2.
Serological Laboratory Values (Mean [95% CI])
| Alcohol group (AG) |
Control group (CG) |
Normal values |
|
|---|---|---|---|
| Thiamine pyrophosphate (nmol/l) |
17.1 (13.0–22.6) | 17.1 (14.6–20.0) | 9–44 |
| TSH (uIU/ml) | 1.1 (0.8–1.4) | 1.2 (0.9–1.6) | 0.350–5.500 |
| AST (IU/l) | 20.2 (17.5–23.1) | 23 (19.3–27.4) | 10–42 |
| ALT (IU/l) | 19.5 (16–23.8) | 17.2 (15–20) | 6–45 |
| GGT (IU/l) | 21.5 (17–26.9) | 18 (13.2–24.6) | 10–70 |
| ESR (mm/h) | 14.0 (6.3–31.3) | 12.9 (9.2–17.9) | 0–15 |
| Lyme | Negative | Negative | |
| HIV | Negative | Negative | |
| Glucose (mg/dl) | 96.5 (90.9–102.4) | 97.2 (92.2–102.3) | 67–99 |
| HbA1c (%) | 5.2 (4.8–5.6) | 5.4 (5.2–5.6) | 4.3–5.8 |
| Albumin (g/dl) | 3.6 (3.5–3.8) | 3.7 (3.5–4) | 3.5–5.0 |
| PT (sec) | 12.4 (11.5–13.4) | 12.6 (12.2–13.1) | 11.0–13.2 |
| INR | 1.0 (0.9–1.1) | 1.0 (0.9–1.1) | 0.8–1.2 |
AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma glutamyl transpeptidase; TSH, thyroid stimulating hormone; ESR, erythrocyte sedimentation rate; HbA1c, hemoglobin A1c; PT, prothrombin time; INR, international normalized ratio.
Patients were subjectively evaluated for small-fiber neuropathic symptoms. Of the 9 subjects in the AG, 2 endorsed symptoms, while 3 subjects in the CG endorsed symptoms consistent with small-fiber neuropathy.
Nerve Conduction Studies
All recorded MNCS of the right tibial and fibular nerves were within normal limits in both groups. Despite all of the recorded latencies in both groups falling within normal limits, there was a mild prolongation of distal latency in the tibial nerve of the AG as compared to the CG (p = 0.047). There was also a significant difference in median amplitudes, with amplitudes being lower in the AG group as compared with the CG (p = 0.0013). Analysis of velocity did not reveal any difference between the 2 groups (Table 3).
Table 3.
Motor Nerve Conduction Studies
| Nerve | Alcohol group (AG) |
Control group (CG) |
p-Value | Normal values |
|---|---|---|---|---|
| Tibial (mean [95% CI]) | ||||
|
Distal Latency (ms) |
4.9 (4.1–5.6) | 4.2 (2.9–5.0) | 0.047 | <5.7 |
| Amplitude (mV) |
8.5 (4.8–12.1) | 13.1 (8.2–18) | >2.8 | |
| Velocity (m/s) | 48.1(38.8–57.6) | 51.7(50–53.4) | >41 | |
| Fibular (mean [95% CI]) | ||||
| Distal latency (ms) |
4.2 (3.0–4.8) | 3.7 (2.9–4.1) | <5.7 | |
| Amplitude (mV) |
5.9 (4.2–6.8) | 7.7 (6.1–9.4) | >2.2 | |
| Distal velocity (m/s) |
48.0 (35.6–50.4) | 49.9 (48.9–50.9) | >41 | |
| Median (mean [95% CI]) | ||||
| Distal latency (ms) |
4.3 (3.2–5.3) | 3.5 (2.5–4.3) | <4.4 | |
|
Amplitude (mV) |
9.4 (8.5–10.3) | 12.8 (11.2–14.4) | 0.0013 | >4.2 |
| Distal velocity (m/s) |
56.6 (53.2–59.9) | 57.3 (56.1–58.5) | >49 | |
| Ulnar (mean [95% CI]) | ||||
| Distal latency (ms) |
2.5 (2.1–3.3) | 2.4 (2.2–2.6) | <3.5 | |
| Amplitude (mV) | 9.4 (8.3–10.5) | 10.9 (8–11.9) | >5.6 | |
| Distal velocity (m/s) |
58.7 (54–63.3) | 62 (59.7–64.3) | >49 | |
Motor nerve conduction studies (MNCS) were performed on the tibial, fibular, median, and ulnar nerves. Main effect p-values and least-squares mean estimates with 95% confidence interval (CI) of AG vs. CG were calculated (mean (95% CI)). All recorded motor nerve conduction studies (MNCS) of the right tibial and fibular nerves were within normal limits in both groups. Despite all of the recorded latencies in both groups falling within normal limits, there was a mild prolongation of distal latency in the tibial nerve and decreased amplitude of the median nerve of the AG as compared to the CG (p = 0.047, 0.0013 respectively; bold).
Sural sensory nerve conduction studies revealed that AG subjects had significantly prolonged distal latencies (p = 0.006), decreased amplitudes (p = 0.005), and slowed velocities (p = 0.04). Within AG, 3 sural sensory potentials were nonrecordable, 1 showed decreased amplitude with normal latency and velocity, while the other 5 were normal. All of the controls had normal sural nerve sensory studies. Median and ulnar sensory nerve conduction studies did not reveal any differences (Table 4).
Table 4.
Sensory Nerve Conduction Studies
| Nerve | Alcohol group (AG) |
Control group (CG) |
p-Value | Normal values |
|---|---|---|---|---|
| Sural (mean [95% CI]) | ||||
| Distal | 4.1 (3.5–5.6) | 3.6 (3.2–3.8) | 0.006 | <4.2 |
| Latency (ms) | ||||
| Amplitude (µV) | 9.1 (5.3–12.9) | 16.5 (9.9–22.9) | 0.005 | >5.0 |
| Velocity (m/s) | 36.6 (34.0–39.1) | 40.6 (37.6–43.5) | 0.04 | >33 |
| Median (mean [95% CI]) | ||||
| Distal | 2.03 (1.7–3.3) | 2.1 (1.6–2.9) | NS | <2.2 |
| Latency (ms) | ||||
| Amplitude (µV) | 50.7 (44.1–57.3) | 66.9 (45.5–88.3) | >40 | |
| Velocity (m/s) | 39.4 (35.2–43.7) | 40.6 (35.1–46.1) | >36 | |
| Ulnar (mean [95% CI]) | ||||
| Distal | 1.9 (1.6–2.2) | 1.8 (1.6–1.9) | NS | <2.2 |
| Latency (ms) | ||||
| Amplitude (µV) | 21.6 (13.0–30.3) | 39.9 (18.7–61.0) | >20 | |
| Velocity (m/s) | 44.4 (41–48) | 44.8 (42.6–47) | >36 | |
Sensory nerve conduction studies (SNCS) were performed on the right sural, median, and ulnar nerves. Main effect p-values and least-squares mean estimates with 95% confidence interval (CI) of AG vs. CG were calculated (mean (95% CI)). SNCS revealed that there was a statically significant prolongation of the sural nerve latency, decrease in amplitude, and slowed velocity of the AG as compared with the CG. Five subjects in the AG had abnormal SNCS with nonrecordable signals in 3 patients, prolonged distal latency in 1 subject, and decreased amplitude in 1 subject. All SNCS of the sural nerve in the CG were normal. Sural sensory nerve conduction studies revealed that AG subjects had significantly prolonged distal latencies (p = 0.006; bold), decreased amplitudes (p = 0.005; bold), and slowed velocities (p = 0.04; bold).
Skin Biopsy
ENFD were significantly decreased at the calf in the AG group as compared with the CG (nerve fibers/mm; mean [95% CI] of 4.85 [4.2–5.7] and 8.83 [7.5–10.4], p < 0.0001, respectively). There was no statistically significant difference in proximal ENFD.
There was also a significant decrease in SGNFD at the calf in the AG group as compared with CG (nerve fibers/mm; mean [95% CI] of 30.7 [23.3–40.5] and 41.9 [37.0–47.7], p = 0.04, respectively). There was insufficient tissue for SGNFD in 2 AG and 2 CG subjects. There was insufficient tissue for analysis of proximal SGNFD (Table 5).
Table 5.
End Nerve Fiber Densites (ENFD) and Sweat Gland Nerve Fiber Densities (SGNFD)
| Alcohol group (AG) |
Control group (CG) |
p-Value | Normal values |
|
|---|---|---|---|---|
| ENFD (nerve fibers/mm) mean (95% CI) | ||||
| Thigh | 7.95 (6.6–9.5) | 9.74 (7.9–12) | NS | <6.8 |
| Calf | 4.85 (4.2–5.7) | 8.83 (7.5–10.4) | <0.0001 | <5.4 |
| SGNFD (nerve fibers/mm) mean (95% CI) | ||||
| Thigh* | N/A | N/A | N/A | <37.8 |
| Calf** | 30.7 (23.3–40.5) | 41.9 (37.0–47.7) | 0.04 | <36.5 |
Not all subjects had sufficient tissue for analysis.
Four subjects, 2 males from each group, had insufficient tissue for analysis.
All subjects underwent skin biopsy at the calf and at the thigh. End nerve fiber densities (ENFD) and sweat gland nerve fiber densities (SGNFD) were calculated according to established counting rules. Main effect p-values and least-squares mean estimates with 95% confidence interval (CI) of AG vs. CG were calculated. In aggregate, there was a significant decrease in both ENFD and SGNFD of the AG as compared to the CG. There was no difference in proximal nerve fiber density. Analysis of proximal SGNFD could not be performed because of inadequate tissue sampling. ENFD were significantly decreased at the calf in the AG group as compared with CG (nerve fibers/mm; mean [95% CI] of 4.85 [4.2–5.7] and 8.83 [7.5–10.4], p < 0.0001, respectively; bold). There was also a significant decrease in SGNFD at the calf in the AG group as compared with the CG (nerve fibers/mm; mean [95% CI] of 30.7 [23.3–40.5] and 41.9 [37.0–47.7], p = 0.04, respectively; bold).
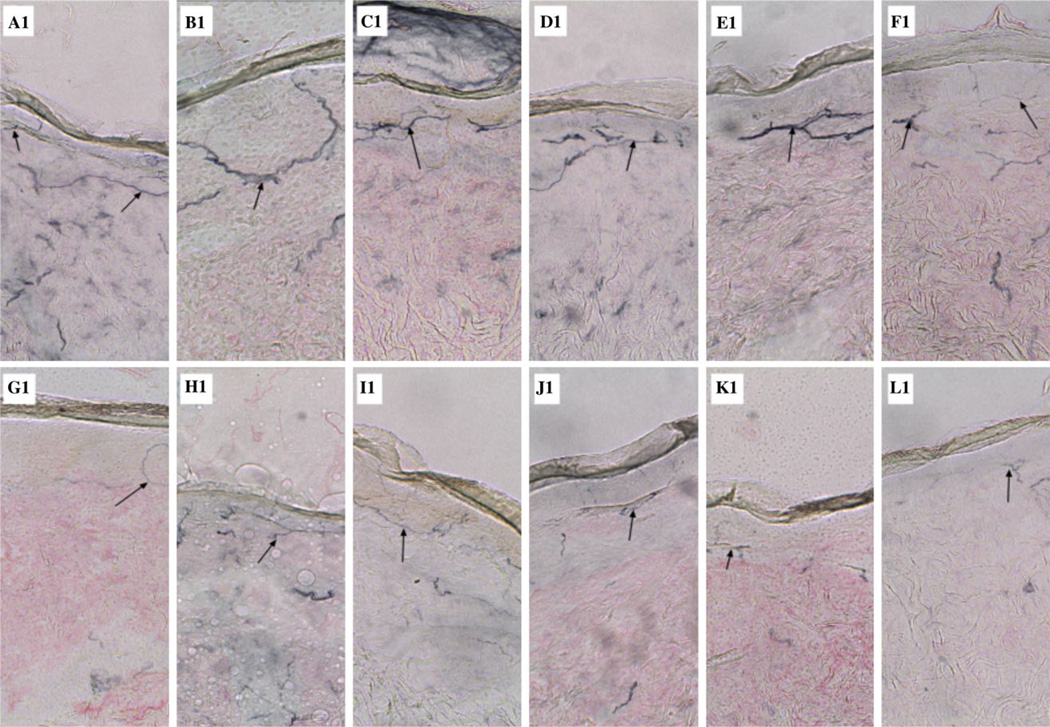
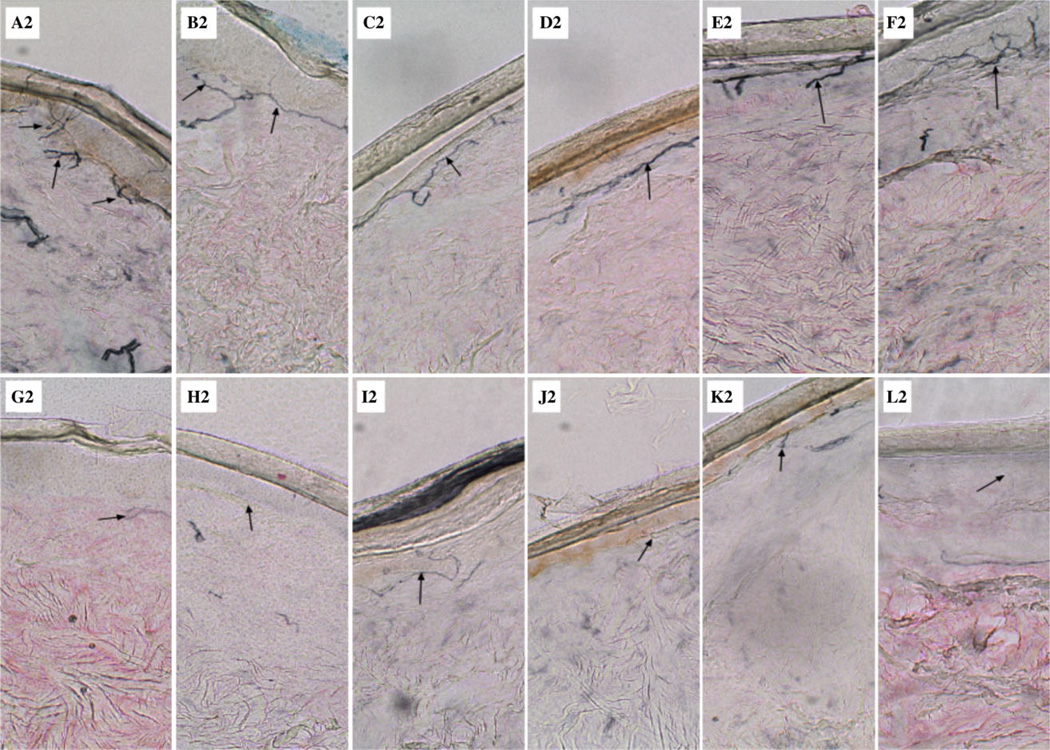
Light microscopic examination of the dermal biopsies in the CG showed a greater abundance, thickness, and complexity of intraepidermal nerve fibers as compared with the AG proximally and distally (black arrows) (Figs 1 and 2).
Fig. 1.
Proximal small-fiber neuropathy in alcoholics. Lateral thigh skin biopsies (3-mm punch) were obtained from 9 controls and 9 alcoholics. Specimens were harvested into 2% paraformaldehyde-lysine-periodate buffer. Biopsy samples were cut into 50-micron sections, and stained using panaxonal marker PGP 9.5 and hematoxylin-eosin to assess for histological abnormalities. Representative images from 6 of the controls (A1-F1) and 6 alcoholics (G1-L1) are shown. Note the greater abundance, thickness, and complexity of intraepidermal nerve fibers (arrows) in control (A1-F1) versus alcoholic (G1-L1) biopsy specimens.
Fig. 2.
Distal small-fiber neuropathy in alcoholics. Lateral lower calf skin biopsies (3-mm punch) were obtained from 9 controls and 9 alcoholics. Specimens were harvested into 2% paraformaldehyde-lysine-periodate buffer. Biopsy samples were cut into 50-micron sections and stained using panaxonal marker PGP 9.5 and hematoxylin-eosin to assess for histological abnormalities. Representative images from 6 of the controls (A2-F2) and 6 alcoholics (G2-L2) are shown, and they correspond with the same subjects shown in Fig. 1. Note the greater abundance, thickness, and complexity of intraepidermal nerve fibers (arrows) in control (A1-F1) versus alcoholic (G1-L1) biopsy specimens.
DISCUSSION
This study demonstrates that ALN is associated with a remarkable small-fiber neuropathy despite the absence of any objective clinical evidence of large- or small-fiber neuropathy as demonstrated by the UENS. Contrary to the traditional belief of ALN as a disease that exclusively affects distal large nerve fibers, we were able to show that alcohol affects small fibers not only distally, but also proximally. This small-fiber neuropathy can be diagnosed by skin biopsy in heavy alcohol drinking individuals with normal thiamine status. There was no evidence of malnutrition as all objective measures of nutritional status assessed in this study (i.e., BMI and albumin) were normal. All other causes of neuropathy were ruled out with serological studies. ENFD were significantly decreased in the AG. Light microscopy examination of dermal biopsies show substantial decrement in the ENFD distally as well as axonal abnormalities both distally and proximally. The observation of the difference in SGNFD supports the fact that alcohol also appreciably affects the autonomic nervous system. This significant damage in the AG suggests that small fibers are severely affected leading to the usual clinical complaints of burning and throbbing pain as well as autonomic dysfunction. With continued alcohol ingestion, it is likely that nerve damage insidiously progresses to damage larger, myelinated nerve fibers, manifesting clinically with the typical debilitating neuropathic symptoms associated with the later stages of ALN including weakness, sensory loss, repeated injury to the extremities, head trauma from falls, bowel, bladder, and sexual dysfunctions (Ammendola et al., 2001; Laker and Sullivan, 2009).
There was no objective clinical difference in our study population as evaluated by a trained neurologist and a validated neuropathy scale (UENS). Despite the lack of clinical evidence for neuropathy, NCS, as expected, did reveal significant differences in the SNCS of the sural nerve; however, this effect of alcohol on large-fiber peripheral nerves, unlike the effect of the on small fibers, could not be appreciated in all AG patients. Other than the sural nerve, all other nerve conductions results were within normal limits. This pattern is consistent with the well-documented distal axonal neuropathy associated with ALN. The results observed in the SNCS supports the fact that there is an insidious progressive, large-fiber toxic neuropathy that may take years to detect with our current conventional methods, mainly NCS. The motor nerve conductions were all essentially normal with a significant decrease in median amplitude that could be due to either technique or to the proclivity of patients with neuropathy to develop mononeuroapthies. The degree of large-fiber involvement is clearly not as impressive as that observed in smaller nerve fibers, which shows pathological involvement both distally and proximally, suggesting that skin biopsy may be a more sensitive measure for assessment and earlier detection of ALN.
Alcohol’s effect on the central nervous system (CNS) and liver provides possible clues as to how it may affect peripheral nervous system (PNS), but the primary targeted structure is still under debate. It is reasonable to hypothesize that ALN is in fact the result of a multifactorial process primarily mediated by the toxic effect of alcohol, but whose effect may be modulated by other factors such as thiamine deficiency, altered thiamine metabolism, malnutrition, or impurities such as lead (Chopra and Tiwari, 2012). While thiamine may have a role, especially in axonal large-fiber polyneuropathy, here, we only enrolled patients with normal thiamine status, and still we were able to detect a small-fiber polyneuropathy. This observation supports the hypothesis that alcohol has a direct neurotoxic effect on small nerve fibers. The direct toxic effect of alcohol on the structural integrity of nerves as well as on nerve function and metabolism has already been demonstrated in the CNS (Balduini and Costa, 1989; Balduini et al., 1991; Malatova and Cizkova, 2002). Notably, emerging data has pointed to the roles of impaired insulin and insulin-like growth factor signaling and oxidative stress in the pathogenesis of alcohol-related diseases of the CNS, liver, and most recently in our animal model of ALN (Cohen et al., 2007; Mellion et al., 2013; de la Monte et al., 2008a,b; Nguyen et al., 2012). Translational studies are warranted to shed light on the role alcohol plays in the generation of small-fiber polyneuropathy.
This study provides novel evidence that ALN is associated with both a distal and proximal small-fiber neuropathy. Furthermore, this study successfully piloted the use of skin biopsy to detect small-fiber neuropathy in ALN. Skin biopsy is a valuable tool with which the pathogenesis of this disease process can be evaluated, specifically alcohol’s toxic effect on the PNS. However, important limitations of the study should be taken into account. First of all, the small sample size does not allow for drawing definitive conclusions, although the differences between the CG and AG were highly significant. It serves as a proof-of-concept study for future larger studies. The CG was slightly older than the AG, which makes our results even more relevant given that neuropathy is more prevalent in older individuals. Additionally, although not statistically significant, the calculated BMI of the CG is overweight, while the BMI of the AG was obese by WHO (2013) definitions. Both groups may be predisposed to insulin resistance, one of the proposed mechanisms for ALN, but neither met criteria for insulin resistance as measured by HbA1c. Future studies will utilize the glucose tolerance test, a more accurate measure of insulin resistance. Also, consistent with known epidemiological studies, only one-third of the AG and the majority of the CG were female in our sample. It is known that there is a greater vulnerability of alcohol-related damage in multiple systems in females compared to males (Hommer et al., 1996; Kwo et al., 1998). This study was not designed to assess the differential effect of gender. Age- and gender- matched studies are needed (and already planned by our team) to further evaluate the role gender may play in ALN.
It is important to note that we enrolled a population of heavy alcohol drinking individuals with no important medical complications (e.g., alcoholic hepatitis, diabetes). It is conceivable that alcoholics at more advanced stages may be more prone to have both small- and large-fiber polyneuropathies, the latter probably due to not only alcohol per se but also abnormal thiamine status. By contrast, here, we enrolled a population of heavy drinking subjects with normal thiamine status who, by objective examination with the UENS were not different from controls, but still we detected a significant small-fiber polyneuropathy. While future studies are needed to fully investigate these aspects, this pilot study provides two preliminary but important clinical take-home messages: one being the presence of small-fiber polyneuropathy as a manifestation of alcohol-related neurological damage; and the other being the sensitivity of skin biopsy for diagnosis.
For nearly a century, ALN has been classified as a nutritional neuropathy based on the observations and studies utilizing insensitive clinical and electrophysiological tests. Skin biopsy will be a valuable biomarker not only for detection of small-fiber involvement in ALN, but also for understanding the causative mechanisms and development of more effective treatments for this potentially disabling disease. This technique, which is now the established gold standard to evaluate small-fiber neuropathy, is minimally invasive and repeatable over time. These characteristics make skin biopsy an ideal biomarker with which to study ALN and potential treatments. To fully understand the effect of alcohol on peripheral nerves, an adequately powered clinical trial with gender- and age-matched populations utilizing skin biopsy is necessary to fully comprehend the pathogenesis of ALN and to develop effective treatments.
Acknowledgments
This research was funded by the Alcoholic Beverage Medical Research Foundation (ABMRF) (PI: Michelle L. Mellion, MD) and an internal departmental research fund from Rhode Island Hospital, Providence RI (PI: Michelle L. Mellion, MD).
REFERENCES
- Abe T, Itokawa Y. Effect of ethanol administration on thiamine metabolism and transketolase activity in rats. International journal for vitamin and nutrition research. Internationale Zeitschrift fur Vitamin- und Ernahrungsforschung. J Int Vitaminol Nutr. 1977;47:307–314. [PubMed] [Google Scholar]
- Ammendola A, Tata MR, Aurilio C, Ciccone G, Gemini D, Ammendola E, Ugolini G, Argenzio F. Peripheral neuropathy in chronic alcoholism: a retrospective cross-sectional study in 76 subjects. Alcohol Alcohol. 2001;36:271–275. doi: 10.1093/alcalc/36.3.271. [DOI] [PubMed] [Google Scholar]
- Balduini W, Candura SM, Manzo L, Cattabeni F, Costa LG. Time-, concentration-, and age-dependent inhibition of muscarinic receptor-stimulated phosphoinositide metabolism by ethanol in the developing rat brain. Neurochem Res. 1991;16:1235–1240. doi: 10.1007/BF00966701. [DOI] [PubMed] [Google Scholar]
- Balduini W, Costa LG. Effects of ethanol on muscarinic receptor-stimulated phosphoinositide metabolism during brain development. J Pharmacol Exp Therapeut. 1989;250:541–547. [PubMed] [Google Scholar]
- Behse F, Buchthal F. Alcoholic neuropathy: clinical, electrophysiological and biopsy findings. Ann Neurol. 1977;2:95–110. [Google Scholar]
- Blackstock E, Rushworth G, Garth D. Electrophysiological studies in alcoholism. J Neurol Neurosurg Psychiatry. 1972;35:326–334. doi: 10.1136/jnnp.35.3.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra K, Tiwari V. Alcoholic neuropathy: possible mechanisms and future treatment possibilities. Br J Clin Pharmacol. 2012;73:348–362. doi: 10.1111/j.1365-2125.2011.04111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AC, Tong M, Wands JR, de la Monte SM. Insulin and insulin-like growth factor resistance with neurodegeneration in an adult chronic ethanol exposure model. Alcohol Clin Exp Res. 2007;31:1558–1573. doi: 10.1111/j.1530-0277.2007.00450.x. [DOI] [PubMed] [Google Scholar]
- Dyck PJ, Gutrecht JA, Bastron JA, Karnes WE, Dale AJ. Histologic and teased-fiber measurements of sural nerve in disorders of lower motor and primary sensory neurons. Mayo Clin Proc. 1968;43:81–123. [PubMed] [Google Scholar]
- Ebenezer GJ, Hauer P, Gibbons C, McArthur JC, Polydefkis M. Assessment of epidermal nerve fibers: a new diagnostic and predictive tool for peripheral neuropathies. J Neuropathol Exp Neurol. 2007;66:1059–1073. doi: 10.1097/nen.0b013e31815c8989. [DOI] [PubMed] [Google Scholar]
- Fennelly J, Frank O, Baker H, Leevy CM. Red blood cell-transketolase activity in malnourished alcoholics with cirrhosis. Am J Clin Nutr. 1967;20:946–949. doi: 10.1093/ajcn/20.9.946. [DOI] [PubMed] [Google Scholar]
- Frank O, Luisasa-Opper MF, Sorrell A, Thompson D, Baker H. Vitamin deficits in severe alcohol fatty liver of man calculated from multiple reference points. Exp Mol Pathol. 1971;15:191–197. doi: 10.1016/0014-4800(71)90098-0. [DOI] [PubMed] [Google Scholar]
- Gibbons CH, Griffin JW, Polydefkis M, Bonyhay I, Brown A, Hauer PE, McArthur JC. The utility of skin biopsy for prediction of progression in suspected small fiber neuropathy. Neurology. 2006;66:256–258. doi: 10.1212/01.wnl.0000194314.86486.a2. [DOI] [PubMed] [Google Scholar]
- Hilz MJ, Zimmermann P, Claus D, Neundorfer B. Thermal threshold determination in alcoholic polyneuropathy: an improvement of diagnosis. Acta Neurol Scand. 1995;91:389–393. doi: 10.1111/j.1600-0404.1995.tb07026.x. [DOI] [PubMed] [Google Scholar]
- Hommer D, Momenan R, Rawlings R, Ragan P, Williams W, Rio D, Eckardt M. Decreased corpus callosum size among alcoholic women. Arch Neurol. 1996;53:359–363. doi: 10.1001/archneur.1996.00550040099019. [DOI] [PubMed] [Google Scholar]
- Hoyumpa AM., Jr Mechanisms of thiamin deficiency in chronic alcoholism. Am J Clin Nutr. 1980;33:2750–2761. doi: 10.1093/ajcn/33.12.2750. [DOI] [PubMed] [Google Scholar]
- Hoyumpa AM, Jr, Breen KJ, Schenker S, Wilson FA. Thiamine transport across the rat intestine. II. Effect of ethanol. J Lab Clin Med. 1975;86:803–816. [PubMed] [Google Scholar]
- Koike H, Iijima M, Sugiura M, Mori K, Hattori N, Ito H, Hirayama M, Sobue G. Alcoholic neuropathy is clinicopathologically distinct from thiamine-deficiency neuropathy. Ann Neurol. 2003;54:19–29. doi: 10.1002/ana.10550. [DOI] [PubMed] [Google Scholar]
- Koike H, Mori K, Misu K, Hattori N, Ito H, Hirayama M, Sobue G. Painful alcoholic polyneuropathy with predominant small-fiber loss and normal thiamine status. Neurology. 2001;56:1727–1732. doi: 10.1212/wnl.56.12.1727. [DOI] [PubMed] [Google Scholar]
- Kwo PY, Ramchandani VA, O’Connor S, Amann D, Carr LG, Sandrasegaran K, Kopecky KK, Li TK. Gender differences in alcohol metabolism: relationship to liver volume and effect of adjusting for body mass. Gastroenterology. 1998;115:1552–1557. doi: 10.1016/s0016-5085(98)70035-6. [DOI] [PubMed] [Google Scholar]
- Laker SR, Sullivan WJ. Alcoholic neuropathy. [Accessed May 13, 2009];2009 Available at: http://emedicine.medscape.com/article/315159. [Google Scholar]
- Lauria G, Cornblath DR, Johansson O, McArthur JC, Mellgren SI, Nolano M, Rosenberg N, Sommer C European Federation of Neurological Societies. EFNS guidelines on the use of skin biopsy in the diagnosis of peripheral neuropathy. Eur J Neurol. 2005;12:747–758. doi: 10.1111/j.1468-1331.2005.01260.x. [DOI] [PubMed] [Google Scholar]
- Leevy CM, Baker H, Ten Hove W, Frank O, Cherrick GR. B-complex vitamins in liver disease of the alcoholic. Am J Clin Nutr. 1965;16:339–346. doi: 10.1093/ajcn/16.4.339. [DOI] [PubMed] [Google Scholar]
- Malatova Z, Cizkova D. Effect of ethanol on axonal transport of cholinergic enzymes in rat sciatic nerve. Alcohol. 2002;26:115–120. doi: 10.1016/s0741-8329(01)00207-5. [DOI] [PubMed] [Google Scholar]
- Mawdsley C, Mayer RF. Nerve conduction in alcoholic polyneuropathy. Brain. 1965;88:335–356. doi: 10.1093/brain/88.2.335. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Stocks EA, Hauer P, Cornblath DR, Griffin JW. Epidermal nerve fiber density: normative reference range and diagnostic efficiency. Arch Neurol. 1998;55:1513–1520. doi: 10.1001/archneur.55.12.1513. [DOI] [PubMed] [Google Scholar]
- McCarthy BG, Hsieh ST, Stocks A, Hauer P, Macko C, Cornblath DR, Griffin JW, McArthur JC. Cutaneous innervation in sensory neuropathies: evaluation by skin biopsy. Neurology. 1995;45:1848–1855. doi: 10.1212/wnl.45.10.1848. [DOI] [PubMed] [Google Scholar]
- Mellion ML, Nguyen V, Tong M, Gilchrist J, De La Monte S. Experimental model of alcohol-related peripheral neuropathy. Muscle Nerve. 2013;48:204–211. doi: 10.1002/mus.23744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM, Tong M, Cohen AC, Sheedy D, Harper C, Wands JR. Insulin and insulin-like growth factor resistance in alcoholic neurodegeneration. Alcohol Clin Exp Res. 2008a;32:1630–1644. doi: 10.1111/j.1530-0277.2008.00731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM, Yeon JE, Tong M, Longato L, Chaudhry R, Pang MY, Duan K, Wands JR. Insulin resistance in experimental alcohol-induced liver disease. J Gastroenterol Hepatol. 2008b;23:e477–e486. doi: 10.1111/j.1440-1746.2008.05339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen VA, Le T, Tong M, Mellion M, Gilchrist J, de la Monte SM. Experimental alcohol-related peripheral neuropathy: role of insulin/IGF resistance. Nutrients. 2012;4:1042–1057. doi: 10.3390/nu4081042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIAAA. [Accessed September 28, 2013];2013 Available at: http://www.niaaa.nih.gov/alcohol-health/over view-alcohol-consumption/moderate-binge-drinking.
- Oh SJ. Nerve Conduction Studies. 3rd. Philadelphia, PA: Lippincott, Williams and Wilkins; 2003. Clinical Electromyography. [Google Scholar]
- Poupon RE, Gervaise G, Riant P, Houin G, Tillement JP. Blood thiamine and thiamine phosphate concentrations in excessive drinkers with or without peripheral neuropathy. Alcohol Alcohol. 1990;25:605–611. doi: 10.1093/oxfordjournals.alcalc.a045056. [DOI] [PubMed] [Google Scholar]
- Singleton JR, Bixby B, Russell JW, Feldman EL, Peltier A, Goldstein J, Howard J, Smith AG. The Utah Early Neuropathy Scale: a sensitive clinical scale for early sensory predominant neuropathy. J Peripher Nerv Syst. 2008;13:218–227. doi: 10.1111/j.1529-8027.2008.00180.x. [DOI] [PubMed] [Google Scholar]
- Skinner HA, Sheu WJ. Reliability of alcohol use indices. The Lifetime Drinking History and the MAST. J Stud Alcohol. 1982;43:1157–1170. doi: 10.15288/jsa.1982.43.1157. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline Follow-Back: A Technique for Assessing Self Reported Alcohol Consumption. Totowa, NJ: Humana Press; 1992. [Google Scholar]
- Tomasulo PA, Kater RM, Iber FL. Impairment of thiamine absorption in alcoholism. Am J Clin Nutr. 1968;21:1341–1344. doi: 10.1093/ajcn/21.11.1341. [DOI] [PubMed] [Google Scholar]
- Walsh JC, McLeod JG. Alcoholic neuropathy. An electrophysiological and histological study. J Neurol Sci. 1970;10:457–469. doi: 10.1016/0022-510x(70)90025-0. [DOI] [PubMed] [Google Scholar]
- WHO. [Accessed May 25, 2013];2013 Available at: http://www.who.int/mediacentre/factsheets/fs311/en/
- Zambelis T, Karandreas N, Tzavellas E, Kokotis P, Liappas J. Large and small fiber neuropathy in chronic alcohol-dependent subjects. J Peripher Nerv Syst. 2005;10:375–381. doi: 10.1111/j.1085-9489.2005.00050.x. [DOI] [PubMed] [Google Scholar]