Abstract
Hyperalgesia arising from sensitization of pain relays in the spinal dorsal horn shares many mechanistic and phenotypic parallels with memory formation. We discover that mechanical hyperalgesia can be rendered labile and reversible in mice after reactivation of spinal pain pathways in a process analogous to memory reconsolidation. These findings reveal a novel regulatory mechanism underlying hyperalgesia and demonstrate the existence of reconsolidation-like processes in a sensory system.
Long-lasting changes in the processing of nociceptive information within the dorsal horn of the spinal cord may contribute to pathological pain sensation1, 2. These changes mechanistically and phenotypically resemble hippocampus-dependent memory formation3, 4. The parallels discovered between hyperalgesia and memory raise the possibility that hyperalgesia may also exhibit a phenomenon similar to memory reconsolidation: a protein synthesis-dependent process by which memories are rendered labile after reactivation and susceptible to erasure5, 6. However, the existence of a process analogous to memory reconsolidation has not yet been observed outside defined memory systems in mammals. To address the possibility that such a process exists in pain pathways, we directly tested the hypothesis that hyperalgesia becomes labile and reversible after re-sensitization.
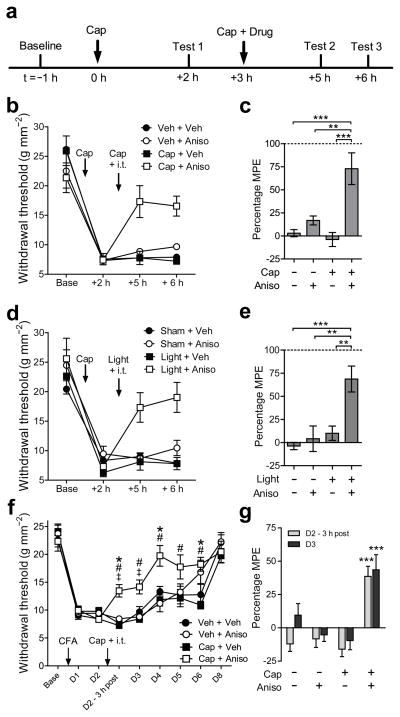
We first administered an intraplantar injection of capsaicin (5 μl, 0.5% w/v; Fig. 1a) in the hind paw of mice to induce mechanical hyperalgesia that persisted for more than 6 hours (Fig. 1b) and was dependent on spinal protein synthesis (Supplementary Fig. 1A). Three hours after the first capsaicin injection, the sensitized pain pathways were reactivated with a second, identical capsaicin (or vehicle) injection combined with an intrathecal (i.t.) injection of the protein synthesis inhibitor, anisomycin (235 nmol), or vehicle. Anisomycin did not affect mechanical hyperalgesia when paired with intraplantar injection of vehicle, demonstrating that the hyperalgesia induced by the first capsaicin injection is well-established and insensitive to disruption by protein synthesis inhibition at that time. However, we observed a substantial reduction in hyperalgesia when the second injection of capsaicin was paired with anisomycin (Fig. 1b,c). The temporal window in which hyperalgesia is labile after the second injection of capsaicin was limited to less than two hours after treatment, since i.t. administration of anisomycin after this time did not reverse hyperalgesia (Supplementary Fig. 1b). Similarly, pairing a second plantar injection of capsaicin with the systemic administration of anisomycin (100 mg·kg−1 i.p.) also caused a robust inhibition of hyperalgesia (Supplementary Fig. 1c,d). Administration of another protein synthesis inhibitor, cycloheximide (175 nmol i.t.), also reversed hyperalgesia when paired with a second injection of capsaicin (Supplementary Fig. 1e). Thus, hyperalgesia is rendered labile and reversible after concurrent re-administration of capsaicin.
Figure 1.
Reactivation of sensitized pain pathways renders hyperalgesia labile and reversible. (a) Timeline of experimental protocol. (b) Changes in mechanical withdrawal thresholds induced by intraplantar injection of capsaicin (Cap) followed by a second ipsilateral intraplantar of Cap or vehicle (Veh) and intrathecal (i.t.) injection of anisomycin (Aniso) or saline (Sal). Injection times are indicated by arrows. (c) Summary of antihyperalgesia induced by the treatments in (a) expressed as percentage of maximum possible effect (MPE). n = 6 mice per group. (d) Capsaicin-induced hyperalgesia followed by low frequency (2 Hz) optical activation of anesthetized Nav1.8+-ChR2 mice (Light) or sham stimulation (Sham) and i.t. injection of Aniso or Veh. Treatment times are indicated by arrows. (e) Summary of results in (d) expressed as MPE. n = 6 mice per group, ** and *** indicate P < 0.05, P < 0.01, and P < 0.001, respectively, in (c,e). (f) Changes in mechanical withdrawal thresholds induced by intraplantar Complete Freund’s Adjuvant (CFA) followed by intraplantar Cap or Veh and intrathecal anisomycin or saline on Day 2. Veh + Veh, Veh + Aniso n = 8 mice; Cap + Veh, Cap+Aniso n = 9 mice. Injections indicated by arrows. (g) Summary of antihyperalgesia in (f) on Day 2, 3 hours after intrathecal injections and on Day 3. *, #, and ‡ in (f) indicate P < 0.05 for Cap + Aniso vs. Veh + Sal, Veh + Aniso, or Cap + Sal, respectively; *** in (g) indicates P < 0.001 vs all other groups within each time point. See also Supplementary table 1. All data are mean ± s.e.m.
We next asked whether intense nociceptor activity, such as that induced by sensitizing stimuli7, is sufficient to render capsaicin-induced hyperalgesia labile. For this, we used an optogenetic mouse model in which channelrhodopsin is expressed in Nav1.8+ nociceptors8, 9 (Nav1.8+-ChR2). 3 h after the induction of hyperalgesia by intraplantar capsaicin injection, the injected paw was exposed to low-frequency (2 Hz) optical stimulation that induces transient mechanical hyperalgesia9 (Supplementary Fig. 1f). The optogenetic reactivation of sensitized pain pathways rendered capsaicin-induced hyperalgesia labile and reversible, but did not modify mechanosensitivity in the absence of anisomycin administration (Fig. 1d,e). Notably the sensitivity of the Nav1.8+-ChR2 mice to light did not change throughout the experiment in any treatment group (Supplementary Fig. 1g).
To test whether the reversal of hyperalgesia is a permanent or temporary phenomenon, we examined whether hyperalgesia induced by Complete Freund’s Adjuvant (CFA) can also be rendered labile. Plantar injection of CFA (10 μl) induced mechanical hyperalgesia that lasted for up to 8 days after injection (Fig. 1f). On the second day after CFA administration, anisomycin (235 nmol i.t.) or vehicle was co-administered with intraplantar capsaicin or vehicle. The co-administration of anisomycin and capsaicin produced a persistent reduction of CFA-induced hyperalgesia that was evident 3 h after treatment (Fig. 1g). Furthermore, the mechanical withdrawal thresholds of mice that received capsaicin and anisomycin returned to baseline within 2 days post-injection. In contrast, the CFA-induced hyperalgesia was unaffected by vehicle + anisomycin, vehicle + vehicle or capsaicin + vehicle injection 3 h after drug administration, and in these treatment groups a significant degree of hyperalgesia was observed up to the 8th day post-injection of CFA (Supplementary Table 1). These data indicate that co-administration of capsaicin and anisomycin not only reversed CFA-induced hyperalgesia, but also significantly reduced the recovery time from hyperalgesia. Thus, well-established hyperalgesia can be rendered labile and permanently reduced following re-sensitization.
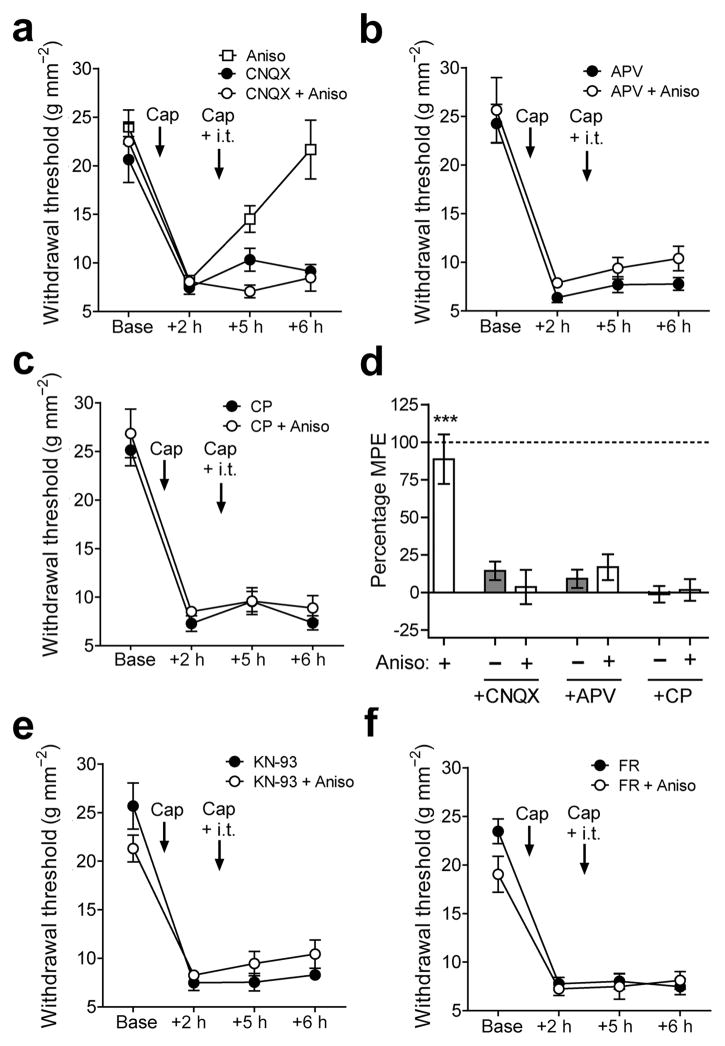
The activation of nociceptors by capsaicin induces the release of glutamate and substance P from C-fibers to induce hyperalgesia and sensitization of second-order neurons in the superficial dorsal horn (SDH)10. We thus tested whether the activation of spinal AMPA and NMDA receptors by glutamate, and NK-1 receptors by substance P are necessary to render hyperalgesia labile upon reactivation of the sensitized pain pathways. As before, the intrathecal administration of anisomycin paired with the plantar injection of capsaicin caused a reversal of capsaicin-induced hyperalgesia (Fig. 2a). However, the intrathecal co-administration of the AMPA, NMDA, and NK-1 receptor antagonists, CNQX (0.5 nmol; Fig. 2A), APV (1 nmol; Fig. 2b), and CP-99994 (5 nmol; Fig. 2c) respectively, all prevented the reversal of hyperalgesia by anisomycin after re-administration of capsaicin (Fig. 2d). The intrathecal administration of these antagonists without anisomycin had no effects on hyperalgesia (Fig. 2d). Thus, the activation of AMPA, NMDA and NK-1 receptors are necessary to render hyperalgesia labile following re-administration of intraplantar capsaicin. These data further suggest that the erasure of hyperalgesia seen here primarily involves synaptic activation of second-order neurons in the SDH.
Figure 2.
Requirement of glutamate, substance P, CaMKII and ERK signaling to render hyperalgesia labile. Changes in mechanical withdrawal thresholds induced by intraplantar injection of capsaicin followed by a second ipsilateral intraplantar of capsaicin (Cap) or vehicle (Veh) and intrathecal injection of: (a) CNQX ± Anisomycin (Aniso), (b) APV ± Aniso, (c) CP-99994 (CP) ± Aniso. Positive control with i.t. Aniso alone shown in (a). (d) Summary of antihyperalgesia induced by the treatments in (a–c) expressed as percentage of maximum possible effect (MPE). *** indicates P < 0.001 vs all other groups. (e,f) Changes in mechanical withdrawal thresholds induced by intraplantar injection of capsaicin followed by a second ipsilateral intraplantar of capsaicin and intrathecal injection of (e) the CaMKII inhibitor KN-93 or (f) the ERK inhibitor FR-180204 (FR), with or without Aniso. n = 6 mice per group except CP + Aniso in (c) n = 7 mice. All data are mean ± s.e.m.
We next tested whether the direct activation of spinal AMPA, NMDA or NK-1 receptors is sufficient to render capsaicin-induced hyperalgesia labile without capsaicin re-administration. The intrathecal administration of AMPA (375 pmol), NMDA (375 pmol), or Sar9,Met(O2)11-substance P (25 pmol) alone all produced nocifensive responses immediately after intrathecal injection but did not modulate capsaicin-induced hyperalgesia (Supplementary Figs. 1h–j). The co-administration of AMPA or NMDA with anisomycin reversed hyperalgesia, while anisomycin co-administered with Sar9,Met(O2)11-substance P did not (Supplementary Fig. 1k). These data further imply that “reconsolidation” of hyperagesia is triggered by the strong activation of second-order neurons in pain processing pathways, and thus may involve mechanisms of synaptic plasticity. In support of this, spinal inhibition of CaMKII or ERK, key mediators of sensitization and plasticity in pain pathways1, by KN-93 (50 pmol) or FR-180204 (25 pmol), respectively, prevented hyperalgesia from becoming labile after a second injection of capsaicin (Fig. 2e,f). The transformation of hyperalgesia to a labile state without nociceptor activation raises the intriguing possibility that drug-induced hyperalgesia (e.g., with opiates) may be similarly rendered labile or trigger lability despite the varied etiology of these pathologies11, 12.
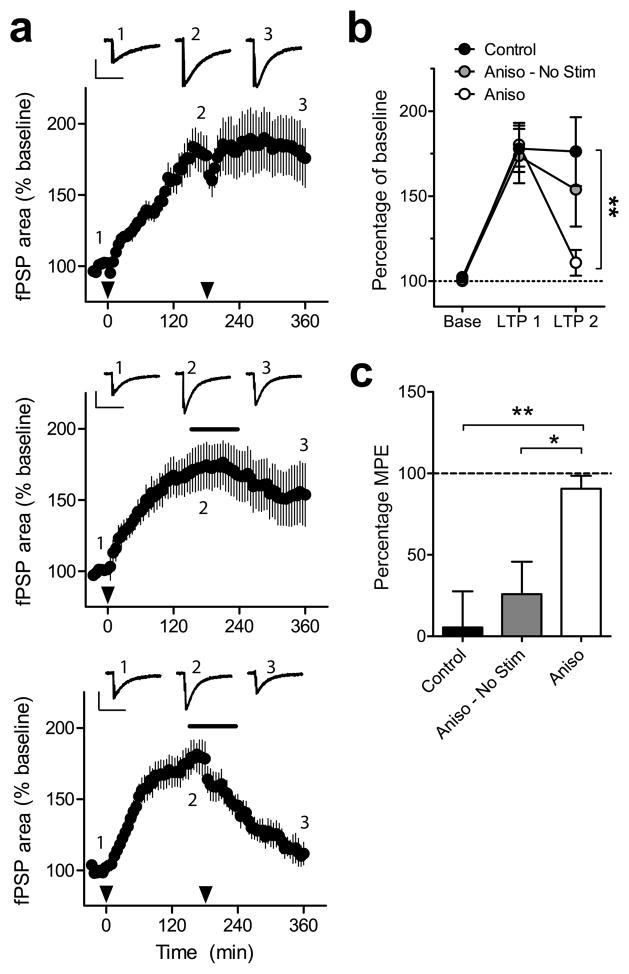
Synaptic plasticity is implicated in memory reconsolidation13. In a parallel to reconsolidation, late-phase synaptic long-term potentiation (LTP) in the in vitro hippocampal slice preparation can be disrupted by inhibiting protein synthesis only under conditions of ongoing synaptic activity14. We thus further explored the labile properties of synaptic plasticity in nociceptive pathways by inducing LTP in the SDH as an in vitro model of spinal sensitization7. The extracellularly-recorded post-synaptic field potentials (fPSPs) evoked in the SDH of whole spinal cord preparations reflect the summation of postsynaptic responses that are mainly monosynaptically evoked in laminae I and II15, 16. A slow-rising, NMDA-dependent LTP (Supplementary Fig. 1l) was induced by electrically stimulating dorsal roots at low frequency (2 Hz; Fig. 3a)17, and the stimulus was repeated 3 h after the initial induction of LTP. The second stimulation alone did not change the magnitude of LTP (Fig. 3b). However, the LTP was reversed when anisomycin was added to the bath for 2 h starting 30 minutes prior to the second stimulus (Fig. 3c). These results confirm that synaptic plasticity within the SDH can become labile upon reactivation in a manner similar to hyperalgesia.
Figure 3.
LTP in SDH rendered labile after repeated stimulation. (a) LTP of fPSPs induced by 2 Hz stimulation (2 min; black arrows) followed by either a second stimulation at 180 min (top), application of anisomycin (Aniso; black bar) without stimulation (middle), or stimulation and anisomycin (bottom). Insets show representative traces from one experiment at baseline (1), 180 min (2) and 360 min (3). Inset scale bars indicate 0.2 mV (vertical) and 500 ms (horizontal). (b) Summary of LTP results. (c) Reversal of LTP measured at 180 min and 360 min expressed as a percentage of maximum possible effect (MPE). n = 9 experiments from 5 mice per group, * and ** indicates P < 0.05 and P < 0.01, respectively. All data are mean ± s.e.m.
The demonstration that hyperalgesia and SDH LTP can be erased through the same paradigm provides evidence for a causal link between persistent pain and LTP of spinal nociceptive pathways3, 7. Taken together, these results show that hyperalgesia can be rendered labile at the spinal level and erased upon reactivation in a process analogous to memory reconsolidation, and forge a crucial link to define hyperalgesia as a pathological pain “memory”3, 4, 18. It remains to be tested whether spontaneous pain events per se are sufficient to trigger lability in certain chronic pain states. These data provide the first demonstration of a reconsolidation-like phenomenon in a sensory system that is removed from established memory pathways, suggesting that reconsolidation exists more broadly throughout the central nervous system than previously known. We propose that exploiting the ability to render hyperalgesia labile may eventually provide a novel therapeutic strategy for the treatment and erasure of established, persistent pain.
Materials and Methods
Mechanosensitivity assay
All behavioral experiments were conducted in accordance with the guidelines established by the Canadian Council for Animal Care. Adult (>12 weeks old) male C57Bl/6 mice were used for experiments except where indicated otherwise. Mice were kept on a 12:12 light:dark cycle in groups of 1 to 4 mice per cage with food and water provided ad libitum. All experiments were conducted on naïve mice and started prior to 10:00. Mechanosensitivity was measured using the SUDO up-down method with von Frey hairs to estimate the 50% withdrawal threshold in pressure units (g·mm−2)19. Care was taken to avoid the injection site when testing mechanosensitivity. Mechanical hyperalgesia was induced by intraplantar injection of capsaicin (5 μl, 0.5% w/v) and all intraplantar and intrathecal injections (5 μl) were performed under light (< 3 min) isoflurane anesthesia18. Animals were randomly assigned to treatment groups and the experimenter was blinded during testing and data analysis. Mice were excluded if they did not exhibit a reduction in withdrawal threshold greater than 10% after sensitization. One mouse was excluded from the study (substance P + anisomycin group) based on this criterion. Behavioral data were analyzed as percentage of maximum possible effect (MPE) using the formula 100%·(test 3 − test 1)·(baseline − test 1)−1. In behavioural experiments,
For optogenetic studies of hyperalgesia, mice expressing ChR2 in Nav1.8+ nociceptive afferents (Nav1.8-ChR2) were generated by crossing mice expressing Cre-recombinase in Nav1.8+ neurons (Nav1.8-Cre)8 with mice expressing a loxP-flanked STOP cassette upstream of a ChR2-EYFP fusion gene at the Rosa 26 locus (Rosa-CAG-LSL-ChR2(H134R)-EYFP-WPRE; stock number 012569, The Jackson Laboratory, Bar Harbor, ME, USA). In these mice, the vast majority (>90%) of Cre-expressing cells are unmyelinated C-fibers, but not exclusively8, 20. Notably, the behavioural responses associated with manipulation of Nav1.8+ afferents are consistent with a predominantly nociceptive role for Nav1.8+ afferents9, 21. Nocifensive responses were elicited from Nav1.8-ChR2 mice by focally applying 488 nm light (iBeam Smart PT, Toptica Inc, Germany) with an optic fiber (200 μm core diameter, 0.22 NA) placed approximately 0.5 mm from the plantar surface of the hind paw. Threshold responses were measured by increasing the light intensity until a sharp flinching response from the light was observed within 1 s of the light application. Light output power from the laser was measured with a power meter (PM100, Thor Labs, Germany) and divided by the cross sectional area of the fiber core to determine light intensity. Sensitization was induced in Nav1.8-ChR2 ice by stimulating the hind paw of anesthetized mice with 488 nm light at a low frequency (2 Hz, 10 ms pulses) for 20 minutes as previously described9. The light intensity used for sensitization was 4–5 times higher than threshold intensity. Anesthesia was induced with isoflurane at the minimum concentration required to prevent paw withdrawal from light stimulus (approximately 1.8%). Drugs were administered intrathecally just prior to stimulation. Mice in the sham stimulation group underwent the same anesthesia procedure but received no stimulation of the hind paw with light. The experimenter was blinded to treatment group.
6-cyano-7-nitroquinoxaline-2,3-dione disodium salt (CNQX), D-(—)-2-amino-5-phosphonovaleric acid (APV), (RS)-α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), N-Methyl-D-aspartic acid (NMDA), CP-99994 dihydrochloride, Sar9,Met(O2)11-Substance P, cycloheximide, FR-180204, KN-93 and anisomycin were dissolved in saline vehicle for intrathecal or intraperitoneal injection. Capsaicin was dissolved in a vehicle solution containing 10% ethanol, 10% Tween (Sigma-Aldrich Canada), and 80% saline. All drugs and chemicals used in behavioral experiments were purchased from Tocris Cookson (Ellisville, MO, USA) except where otherwise specified.
Electrophysiology
Electrophysiological recordings of dorsal root-evoked post-synaptic field potentials (fPSPs) were made using a whole spinal cord tissue preparation. Adult male C57Bl/6 mice were anesthetized with urethane (2 g·kg−1) and perfused with ice-cold sucrose-substituted artificial cerebral spinal fluid (aCSF; contains in mM: sucrose 252, KCl 2.5, CaCl2 1.5, MgCl2 6, D-glucose 10, bubbled with 95%:5% oxygen:CO2). The lumbar spinal column was removed and immersed in ice-cold sucrose aCSF after which the whole lumbar spinal cord was quickly removed via laminectomy. Ventral roots and connective tissue were removed from the spinal cord and the tissue was placed in room temperature aCSF (in mM: NaCl 126, KCl 2.5, CaCl2 2, MgCl2 2, D-glucose 10, bubbled with 95%:5% oxygen:CO2) for 1 h before experimentation. During experiments the tissue was perfused with aCSF at room temperature at a flow rate of 8 to 10 ml·min−1.
fPSPs were recorded via a borosilicate glass electrode inserted into the dorsal side of the spinal cord in the dorsal root entry zone. Electrodes were inserted superficially to a depth of no more than 125 μm from the dorsal surface of the spinal cord measured with an MPC-200 manipulator (Sutter Instrument Company, Novato, CA, USA). Electrodes had a tip resistance of 3–4 MOhm when filled with aCSF. fPSPs were evoked by electrical stimulation of the dorsal root using a suction electrode that is pulled from borosilicate glass and filled with aCSF, and placed near the cut end of the dorsal root. Field potentials were amplified with a Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA, USA), digitized with a Digidata 1322A (Molecular Devices), and recorded using pClamp 10 software (Molecular Devices). Data were filtered during acquisition with a low pass filter set at 1.6 kHz and sampled at 10 kHz.
Test stimuli were presented every 60 seconds to evoke fPSPs. The stimulus intensity was sufficient to activate C-fibers as indicated by the appearance of a third distinct fiber volley after the stimulus artifact, while a slightly (10–20%) higher intensity was used to induce long-term potentiation (LTP). Evidence that the fPSPs are largely dependent on C-fibers is given by the similarities between fPSPs evoked by electrical stimulation of the dorsal root or optogenetic activation of Nav1.8+ fibers9. Notably, optogenetic activation of Nav1.8+ afferents produces a similar LTP to that produced by electrical stimulation of dorsal roots9. LTP was induced by low frequency stimulation of the dorsal root (2 Hz, 2 min) as described previously17. This LTP likely reflects an increase in the net postsynaptic activation of superficial dorsal horn neurons since supraspinally projecting neurons only comprise a small percentage of the neurons in this area22. After a stable baseline recording (30 minutes), LTP stimuli were presented at time = 0 min and 180 min (in some experiments). Where indicated, anisomycin (100 μM) was added to the aCSF at 150 min and washed out at 270 min for a total application of 2 hours. In some experiments, APV was added to the perfusion solution 10 minutes prior to the induction of LTP and washed out immediately after the stimulation protocol. The 10-minute period immediately prior to the addition of APV was used for baseline in these experiments. Data were analyzed using ClampFit 10 software (Molecular Devices). The area of fPSPs relative to baseline was measured from 0–800 ms after the onset of the fPSP. All drugs and chemicals for electrophysiology experiments were purchased from Sigma-Aldrich (Oakville, ON, Canada).
Statistics
MPE was compared between groups using a one way ANOVA followed by Tukey’s multiple comparison post-hoc test or Student’s t-test as appropriate. All tests were two-sided. Withdrawal thresholds in the single capsaicin injection experiment, CFA hyperalgesia and sensitization induced optogenetic stimulation were compared on each day and time point, respectively, using a two-way repeated-measures ANOVA with Bonferroni post-hoc test. Recovery from CFA hyperalgesia for each treatment group was determined by comparing withdrawal thresholds to baseline using one-way repeated-measures ANOVA followed by Dunnett’s test (see Supplementary table 1). Average fPSP areas at baseline, 180 min, and 360 min were compared using a two-way repeated-measures ANOVA with Bonferroni post-hoc test. A D’Agostino-Pearson omnibus normality test was conducted on groups with n > 6, otherwise the Kolmogorov-Smirnov normality test was used. Sample sizes were not predetermined but reflect a balance between sample sizes generally employed in the field and a desire to minimize the use of animals in pain studies. A supplementary methods checklist is available.
Supplementary Material
Acknowledgments
We thank Mireille Desrochers-Couture and Dr. Loren J. Martin for their assistance and advice with the behavioral assays. This work was supported by a Pfizer - Fonds de recherche Québec – Santé (FRQS) Innovation Fund Award to YDK, an FRQS post-doctoral Fellowship to RPB, Canadian Institutes of Health Research grant MOP 12942 to YDK, and the Catherine Bushnell Pain Research Fellowship from the Louise and Alan Edwards foundation to RPB.
Footnotes
Contributions: RPB conducted all experiments and analyses. RPB and YDK designed the experiments and wrote the manuscript.
References
- 1.Latremoliere A, Woolf CJ. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sandkuhler J. Physiol Rev. 2009;89:707–758. doi: 10.1152/physrev.00025.2008. [DOI] [PubMed] [Google Scholar]
- 3.Ji RR, Kohno T, Moore KA, Woolf CJ. Trends Neurosci. 2003;26:696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 4.Sandkuhler J, Lee J. Trends Neurosci. 2013;36:343–352. doi: 10.1016/j.tins.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nader K, Schafe GE, Le Doux JE. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- 6.Debiec J, LeDoux JE, Nader K. Neuron. 2002;36:527–538. doi: 10.1016/s0896-6273(02)01001-2. [DOI] [PubMed] [Google Scholar]
- 7.Ruscheweyh R, Wilder-Smith O, Drdla R, Liu X-G, Sandkühler J. Mol Pain. 2011;7:20. doi: 10.1186/1744-8069-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agarwal N, Offermanns S, Kuner R. Genesis. 2004;38:122–129. doi: 10.1002/gene.20010. [DOI] [PubMed] [Google Scholar]
- 9.Daou I, et al. J Neurosci. 2013;33:18631–18640. doi: 10.1523/JNEUROSCI.2424-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Neill J, et al. Pharmacol Rev. 2012;64:939–971. doi: 10.1124/pr.112.006163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drdla R, Gassner M, Gingl E, Sandkuhler J. Science. 2009;325:207–210. doi: 10.1126/science.1171759. [DOI] [PubMed] [Google Scholar]
- 12.Ferrini F, et al. Nate Neurosci. 2013 [Google Scholar]
- 13.Tronson NC, Taylor JR. Nat Rev Neurosci. 2007;8:262–275. doi: 10.1038/nrn2090. [DOI] [PubMed] [Google Scholar]
- 14.Fonseca R, Nägerl UV, Bonhoeffer T. Nat Neurosci. 2006;9:478–480. doi: 10.1038/nn1667. [DOI] [PubMed] [Google Scholar]
- 15.Liu X, Sandkuhler J. J Neurophysiol. 1997;78:1973–1982. doi: 10.1152/jn.1997.78.4.1973. [DOI] [PubMed] [Google Scholar]
- 16.Schouenborg J. J Physiol. 1984;356:169–192. doi: 10.1113/jphysiol.1984.sp015459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikeda H, et al. Science. 2006;312:1659–1662. doi: 10.1126/science.1127233. [DOI] [PubMed] [Google Scholar]
- 18.Drdla-Schutting R, Benrath J, Wunderbaldinger G, Sandkuhler J. Science. 2012;335:235–238. doi: 10.1126/science.1211726. [DOI] [PubMed] [Google Scholar]
- 19.Bonin RP, Bories C, De Koninck Y. Mol Pain. 2014;10:26. doi: 10.1186/1744-8069-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shields SD, et al. Pain. 2012;153:2017–2030. doi: 10.1016/j.pain.2012.04.022. [DOI] [PubMed] [Google Scholar]
- 21.Chiu IM, et al. Nature. 2013;501:52–57. doi: 10.1038/nature12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Todd AJ. Nat Rev Neurosci. 2010;11:823–836. doi: 10.1038/nrn2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.