This prospective, open-label study of 145 patients with advanced cancer assessed the efficacy and safety of methadone as a second-line opioid after rotation from other opioids. The main outcome measure, assessed with the Brief Pain Inventory and the CTCAE, was change in the variable “worst pain” at day 28. The median worst pain and average pain scores decreased significantly (30.3% and 47.5%, respectively) at day 28.
Keywords: Methadone, Neoplasms, Pain, Ambulatory care, Outpatient, Palliative medicine, Palliative care
Abstract
Introduction.
Most clinical reports on methadone rotation describe outcomes in hospitalized patients. The few studies that have included outpatients are retrospective. The aim of this study was to assess the efficacy and safety of methadone as a second-line opioid in adult patients with advanced cancer after rotation in routine clinical practice at a palliative care outpatient clinic.
Patients and Methods.
This was a prospective, open-label study of 145 patients whose treatment was rotated from other opioids to methadone. Informed consent was obtained in all cases. The main outcome measure was change in the variable “worst pain” at day 28. Pain and pain interference were assessed with the Brief Pain Inventory, with side effects evaluated according to the Common Terminology Criteria for Adverse Events version 3.0. Pain levels were evaluated at study entry and at days 3, 7, 9, 14, 21, and 28.
Results.
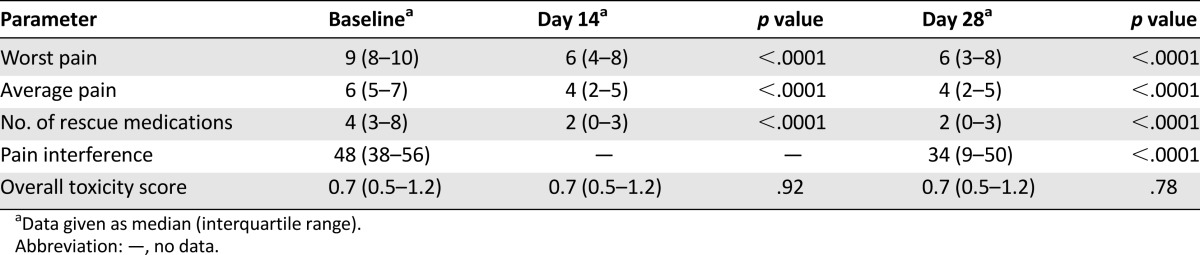
Rotation to methadone was performed for the following reasons: poor pain control (77.9%), opioid side effects (2.1%), or both (20%). The mean daily oral morphine equivalent dose before rotation was 193.7 mg. The median worst and average pain scores decreased significantly (p < .0001) from baseline to day 28: The median worst pain score decreased from 9 (interquartile range [IQR]: 8–10) to 6 (IQR: 3–8), and the median average pain score decreased from 6 (IQR: 5–7) to 4 (IQR: 2–5). The proportions of patients with moderate to severe worst and average pain decreased by 30.3% and 47.5%, respectively, by day 28. No increase in opioid toxicity was observed during the study.
Conclusion.
In outpatients with advanced cancer, rotation to methadone as a second-line opioid was efficacious and safe when using a tiered scheme with close follow-up by experienced health professionals.
Implications for Practice:
The results of this study, conducted prospectively under real clinical conditions, support the efficacy and safety of oral methadone as a second-line opioid in ambulatory patients with cancer. Moreover, these findings corroborate previously reported outcomes in retrospective outpatient studies and prospective studies that evaluated inpatient populations. Although more research into methadone rotation strategies is still needed, this study describes a successful tiered scheme of oral methadone rotation that was proven safe and effective during follow-up.
Introduction
Opioid rotation (OR) is a common procedure used in palliative care services to improve pain relief and manage the side effects of opioids. Although clinical experience and numerous published studies [1–5] suggest that rotation is beneficial, two systematic reviews concluded that the level of evidence to support OR is not strong [6, 7]. To date, it is still unclear which of the various strong opioids (i.e., fentanyl, oxycodone, hydromorphone, or methadone) is most suitable for use in first-line cancer pain analgesia. Moreover, none of these opioids [8–11] has proven more efficacious than morphine. Similarly, no internationally validated conversion table for opioids exists, thus making the switch from opioids to methadone complicated because of a lack of consensus on optimal conversion ratios [12, 13].
The use of methadone as part of an OR scheme is controversial. Recently published guidelines from the European Association for Palliative Care (EAPC) [14] on the use of opioid analgesics in the treatment of cancer pain recommend limiting the use of methadone to highly experienced teams because of its long, unpredictable half-life and substantial interindividual variability in its metabolism in the liver. In contrast to these EAPC recommendations, several studies have concluded that the pharmacokinetic properties and good safety profile of methadone make it an appealing alternative to other opioids [13–18]. However, one report raised doubts about the use of opioids for second-line therapy, reporting that patients switched to methadone are unlikely to benefit from any subsequent rotation to other opioids [19], a finding that suggests that methadone should probably be the final option in the rotation sequence. Nonetheless, several other studies have reported results that contradict that finding [20–22].
Given the considerable controversy surrounding the use of methadone as both a first- and second-line opioid, it is not surprising that most clinical experience with methadone rotation comes from hospitalized patients [5, 23], with only a few retrospective reports in outpatients [24–26] or home-care patients [27, 28]. To address this paucity of prospective data on methadone as part of an OR scheme in outpatients, our group conducted the present study in which we assessed the efficacy and safety of methadone as a second-line opioid in adult patients with advanced cancer in the context of routine clinical practice at a palliative care outpatient clinic.
Materials and Methods
This was a prospective, open-label study enrolling adult patients with advanced cancer at the palliative care outpatient clinic at our hospital (Catalan Institute of Oncology, Barcelona, Spain) from August 2009 to August 2012. The target patients were those who, despite increases in opioid doses, continued to present poor pain control and/or opioid side effects. All patients who, in our clinical opinion, could benefit from rotation to methadone as a second-line opioid (see inclusion criteria under Patients) were invited to participate. The study was approved by the hospital ethics committee, and all patients signed the informed consent form.
Patients
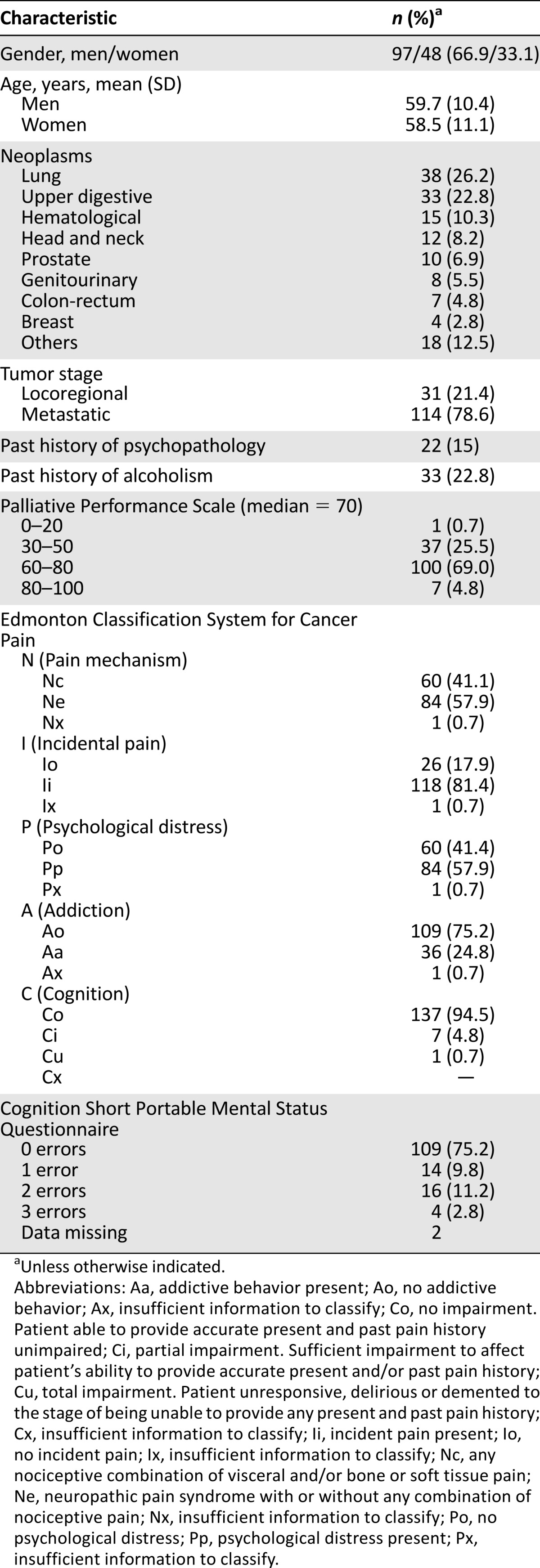
Inclusion criteria were as follows: (a) diagnosis of any cancer type (based on clinical records); (b) complaint of cancer-related pain after first-line treatment with any strong opioid, except methadone; (c) age 18 years or older; (d) no previous history of cardiac arrhythmia; (e) normal cognition at study entry as assessed by the Spanish version of the short portable mental status questionnaire (SPMSQ) [29]; (f) no diagnosis of any mental condition that would prevent pain scoring; (g) not treated with radiotherapy (RT) or chemotherapy in the 30 days before enrollment; and (h) agreement to participate and a signed informed consent form.
Criteria for withdrawal of patients from the study were as follows: (a) clinical impairment preventing pain assessment, (b) patient lost to follow-up, (c) voluntary withdrawal from study, (d) need to undergo an invasive analgesic technique or analgesic RT during the course of the study, (e) major changes in co-analgesics (e.g., steroids, anticonvulsants, or antidepressants), and (f) patient death.
Data Collection
The following variables were assessed: age, sex, cancer type, previous history of psychopathology or alcoholism (assessed with the CAGE test, Spanish version) [30], performance status (assessed with the Palliative Performance Scale) [31], and cancer pain prognosis as determined by the Edmonton Classification System for Cancer Pain (ECS-CP) [32]. The Spanish version of the Brief Pain Inventory (BPI) was used to assess pain and pain interference [33]. To determine pain intensity (average pain and worst pain in the last 24 hours), a verbal rating scale (VRS) (ranging from 0 to 10, with 0 = no pain and 10 = worst pain imaginable) was used [34]. Overall pain interference (OPI) was calculated by summing the seven interference items (general activity, mood, walking ability, normal work, relations with other people, sleep, and enjoyment of life) of the BPI. Each of these items was scored with a VRS (range 0 to 10, with 0 = does not interfere with pain and 10 = completely interferes with pain); total OPI scores ranged from 0 to 70. Breakthrough pain was considered any transient increase in pain to greater than moderate intensity over baseline pain.
Data about opioids and co-analgesic drugs used before and after rotation were recorded. Opioid side effects were assessed according to the Common Terminology Criteria for Adverse Events version 3.0. The following side effects were evaluated: absence or presence of somnolence and the degree thereof, confusion, hallucinations, myoclonus, hypoxia, nausea, vomiting, constipation, pruritus/itching, and dry mouth. An overall toxicity score (OTS) was calculated by averaging the scores of these 10 side effects. The OTS ranges from 0 to 4.6; to facilitate score interpretation, we converted the OTS scores to a scale ranging from 0 to 10 (0 = no side effect present, 10 = maximum intensity).
Study Procedure
All new patients who presented to the outpatient clinic were consecutively screened. Patients who met the study inclusion criteria were invited to participate and asked to sign an informed consent form. Participants were rotated to oral methadone (day 0; see Methadone Rotation Protocol) and instructed in its use. Patients were assessed by telephone on days 3, 9, and 21, and scheduled for an onsite consultation on days 7, 14, and 28.
Efficacy Assessment
The primary efficacy endpoint was reduction in the “worst pain” score at day 28 after rotation from baseline. Secondary efficacy parameters were as follows: reduction of worst pain at day 14 and decrease in mean rescue-medication use, and reduction of pain interference and average pain scores at days 14 and 28 after rotation. Worst pain score was selected as the primary efficacy endpoint because it is more strongly associated with pain interference and has clearly defined cut points in terms of its impact on functioning [34].
Safety Assessment
For the purpose of the study, subjects were allowed to continue taking any medications (at the same dose) that they had been taking before study enrollment if their condition allowed it. Adjustments in laxatives and other drugs (e.g., antihypertensives, antidiabetic medications, diuretics) unlikely to affect analgesia were permitted. Occasional intake of paracetamol or nonsteroidal anti-inflammatory drugs was allowed provided that the patient used the medication no more than three times per week. Other major changes in medications such as steroids, anticonvulsants, or antidepressants were considered justification for study withdrawal.
Methadone Rotation Protocol
Patients rotated to methadone followed the Palliative Care Service protocol [35]. The methadone rotation dose was calculated based on the oral morphine equivalent daily dose (MEDD) [36]. Then, the daily dose of oral methadone (DDOM) was calculated according to the ratio proposed by Ripamonti et al. [37]. Consequently, conversion ratios were as follows: for an MEDD between 30 and 90 mg, the conversion ratio was 4:1; between 90 and 300 mg, 8:1; and for MEDD greater than 300 mg, 12:1. The DDOM is divided into 3 doses administered every 8 hours [38]. The “stop-and-go” approach was used to implement rotation to methadone [39]. As a rescue medication, one-sixth of the DDOM was used but restricted to no more than 3 times per day (3 rescue doses represent a 33% increase in the DDOM, which would increase the risk of toxicity). If further analgesic rescue was required during the same day, an oral immediate release and intermediate short-acting opioid was used—in most cases, morphine, oxycodone, or oral transmucosal fentanyl citrate—depending on the opioid the patient had been taking before rotation (i.e., that opioid was not used for rescue analgesia). To calculate the rescue medication dose of morphine or oxycodone, we used one-sixth of the theoretical daily dose calculated for each drug based on the MEDD before rotation. For oral transmucosal fentanyl citrate, the dose was 200 μg [40].
Our protocol (based on the available pharmacological data [18]) stipulates that any patient rotated to methadone be assessed every 3 days (by telephone and/or site visit) [41] until day 15, after which less frequent evaluations are scheduled in accordance with the patient’s general condition. If the patient requires 3 methadone rescue doses (despite the use of other complementary rescue medications), a 33% increase in the DDOM is prescribed [38]. If any signs of methadone toxicity are evident, then the DDOM is divided into 2 daily doses (every 12 hours), or even 1 dose per day, if necessary [17].
Statistical Analysis
Efficacy and safety analyses were performed on the intent-to-treat (ITT) data set, which included patients who received at least one methadone dose. The last-observation-carried-forward (LOCF) method was used to impute missing and dropout data; consequently, the last observed nonmissing value was used to fill in missing values at a later point in the study.
Categorical variables are shown as percentages with 95% confidence intervals (CI), and continuous variables are presented as means and standard deviations (SDs), or as medians and interquartile range (IQR). Wilcoxon signed-rank tests were used to compare medians and ranges for non-normally distributed variables. A value of p < .05 was considered statistically significant. All analyses were performed with the SPSS package (version 20 for Windows; IBM Corp., Armonk, NY, http://www-01.ibm.com).
Results
Patients
During the study period, a total of 2,097 new patients were treated at the outpatient clinic. Of these, 635 had been previously rotated to an opioid other than methadone and, therefore, were excluded, and an additional 137 patients were not considered candidates for methadone rotation because of mental status or social factors (e.g., poor family support). Of the 184 patients considered eligible for OR to methadone, 39 refused to participate, thus leaving a sample of 145 patients who met inclusion criteria and agreed to participate. These patients were enrolled in the study after signing the informed consent. The demographic data of the sample are shown in Table 1. Most participants (137 patients; 94.5%) had at least 1 poor prognostic characteristic according to the ECS-CP.
Table 1.
Demographic characteristics of the sample
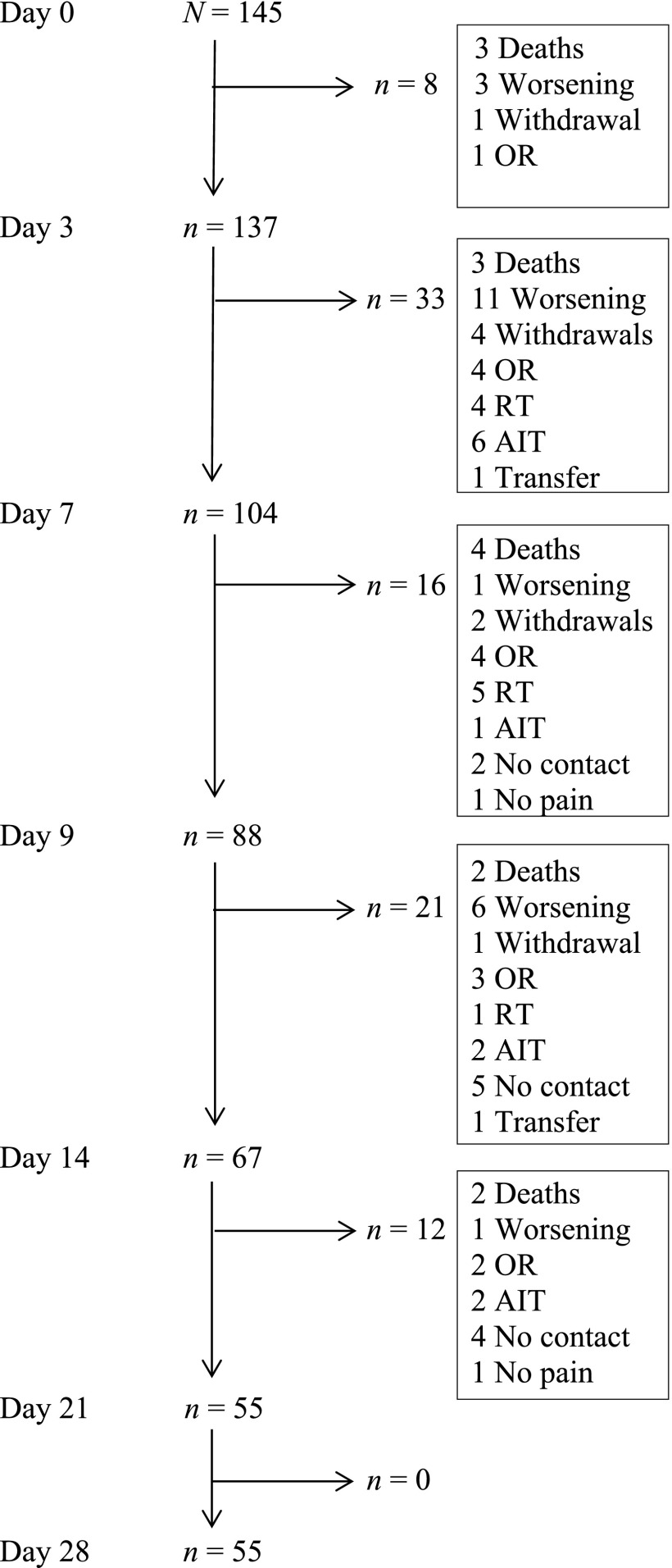
The percentage of patients completing the 28-day study period was 38%, with a median duration of 21 days (IQR: 10–28 days). The most frequent reasons for discontinuation were clinical worsening (24%), RT or analgesic invasive technique (23%), and death (15.5%), as shown in the ITT analysis in Figure 1. Indications for rotation to methadone were poor pain control in 113 patients (77.9%), opioid side effects in 3 (2.1%), and both indications in 29 (20%). The mean DDOM after rotation was 24.2 mg (SD: 9.5), for a mean conversion ratio of morphine to methadone of 8:1.
Figure 1.
Intention-to-treat analysis.
Abbreviations: AIT, analgesic invasive technique; OR, opioid rotation; RT, radiotherapy; Transfer, transfer to another hospital.
Before rotation to oral methadone, the following strong opioids were used: fentanyl patch in 81 patients (55.9%), oral morphine in 27 (18.6%), oral oxycodone in 23 (15.9%), and buprenorphine patch in 12 (8.3%), with 1 patient receiving oral hydromorphone and another receiving oral tapentadol. The mean MEDD before rotation was 193.7 mg (SD: 127.4 mg), ranging from 13 mg to 640 mg.
Rescue medications used before rotation included oral morphine for 43 patients (30.1%), transmucosal fentanyl for 79 (55.2%), oral oxycodone for 19 (13.3%), and oral hydromorphone for 2 (1.4%). The remaining two patients did not receive any rescue medications. Co-analgesics included corticosteroids in 75 cases (51.7%), antidepressants in 32 (22.1%), and anticonvulsants in 53 (36.6%).
Efficacy
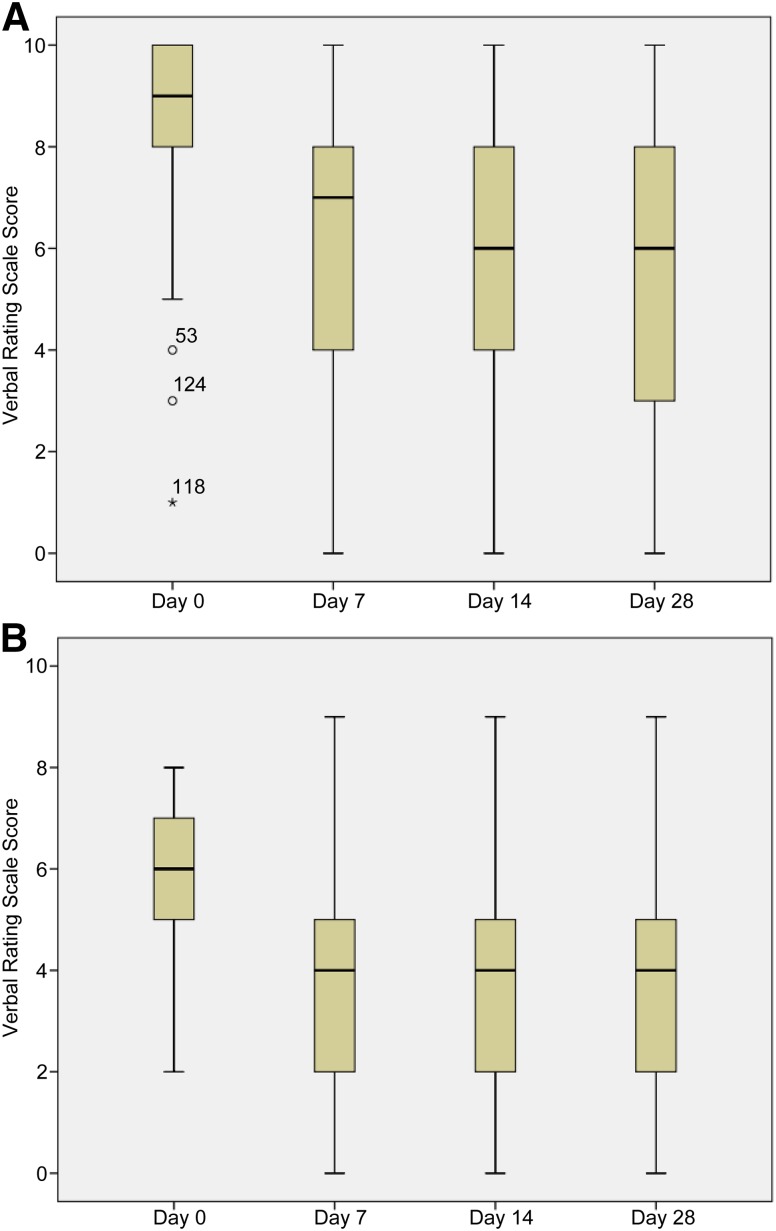
The main efficacy outcome, median worst pain score, decreased significantly from 9 (IQR: 8–10) at baseline to 6 (IQR: 3–8) at day 28 (p < .0001) (Fig. 2). Secondary efficacy outcomes also improved from baseline to days 14 and 28 (Table 2). Decreases in pain from baseline were significant for both worst and average pain at day 7, declining from 7 (IQR: 4–8; p < .0001) and 4 (IQR: 2–5; p < .0001), respectively. Similarly, the use of rescue medication also decreased significantly from baseline to day 3, from 4 (IQR: 3–8) and 2 (IQR: 0–4; p < .0001), respectively (Table 2).
Figure 2.
Changes in worst pain scores (A) and average pain scores (B) during the study period. *, °, outlier scores; numbers accompanying these symbols indicate patient number.
Table 2.
Median score variation at days 14 and 28 from baseline
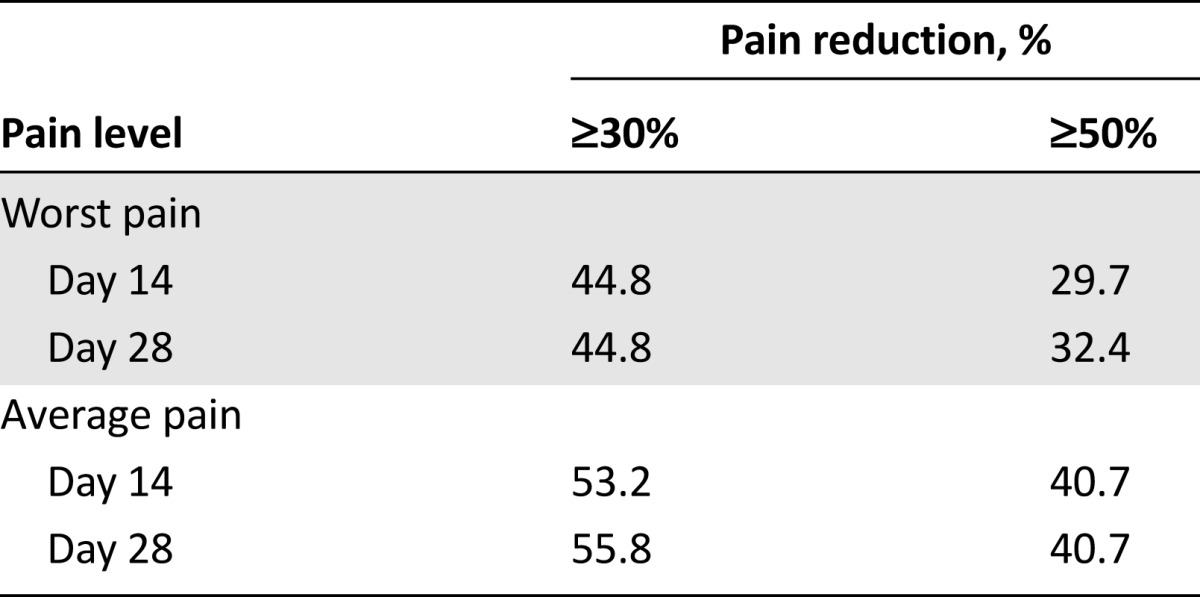
At the final study assessment (day 28), the proportions of patients with moderate to severe worst and average pain (visual analog scale score: 5 or higher) had decreased by 30.3% (95% CI: 20.7%–39.9%) and 47.5% (95% CI: 32.6%–62.4%), respectively. Similar reductions in the proportions of patients experiencing moderate to severe pain were observed at day 14 in both worst pain (28.9%; 95% CI: 19.5%–38.3%) and average pain (50.3%; 95% CI: 35.2%–65.4%). Table 3 shows the proportions of patients who obtained at least a 30% and a 50% pain reduction for the worst and average pain at days 14 and 28.
Table 3.
Percentage of patients obtaining at least a 30% and a 50% reduction in pain from baseline for worst and average pains at days 14 and 28
Safety
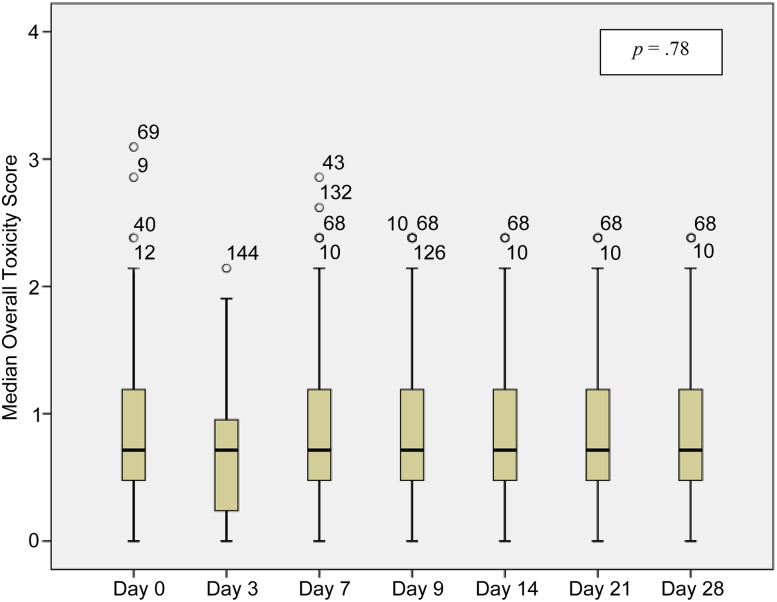
The most common opioid-related side effects before rotation were dry mouth (73.1%), somnolence (46.9%), constipation (43.8%), myoclonus (23.4%), confusion (21.4%), and nausea (19.3%). Other side effects included hallucinations (9.7%), vomiting (6.9%), and pruritus/itching (2.1%). No cases of respiratory depression were reported. The intensity of the side effects was mild in most cases. The median OTS at baseline for all patients was 0.7 (IQR: 0.5–1.2) without significant variation during the study (Fig. 3). The baseline OTS was higher in the subset of patients who were rotated to methadone because of opioid toxicity: 1.4 (IQR: 1.2–1.8); in addition, this score decreased progressively throughout the study period, reaching a median OTS of 0.7 (IQR: 0.5–1.4) by day 28 (p = .13).
Figure 3.
Changes in median overall toxicity scores (0–10) during the study period. *, °, outlier scores; numbers accompanying these symbols indicate patient number.
Discussion
In this prospective, open-label study carried out in patients with advanced cancer, we assessed the efficacy and safety of oral methadone as a second-line opioid in the context of normal clinical practice in a palliative care outpatient clinic. Very few studies have investigated methadone rotation in ambulatory patients with cancer [24–26]; moreover, to our knowledge, ours is the first to use a pragmatic approach to assess the efficacy and safety of methadone in an outpatient population with cancer. The main finding of the present study is that, under normal clinical conditions, rotation to oral methadone in an outpatient clinic appears to be safe and efficacious during the first 4 weeks after rotation, provided that a well-defined rotation and follow-up protocol are followed.
Our study fills an important knowledge gap in the literature, given that most studies of methadone rotation published to date have included only inpatients, and, as a recent systematic review reported, the reported safety results in those inpatient studies is not applicable to the outpatient setting [42]. Reports on methadone rotation in outpatients with cancer are scant and those that do exist are retrospective [24–26]. Despite the retrospective nature of those studies, it is worth noting that the findings they reported are generally in agreement with the results we present here.
De Conno et al. [24] performed a retrospective cohort study of 196 patients initiated or rotated from morphine and followed for 90 days. Based on the initial MEDD (60 mg or less, 70–90 mg, or 100 mg or more, respectively), patients were started on methadone at 5 mg t.i.d., one-fourth MEDD, and/or one-sixth MEDD. Outpatient rotation to methadone was successful in most cases, with only 6.6% of patients failing rotation because of side effects (drowsiness, constipation), and 11.2% because of insufficient pain relief. However, it should be noted that, before rotation, most of those patients had either been receiving low doses of opioids or no prior opioids, and, in patients who had been receiving slow-release morphine (18% of the sample), the mean daily dose was only 60 mg. In another retrospective study, Hagen and Wasylenko [25] reviewed charts from 29 patients with cancer-related pain who underwent rotation to methadone in an outpatient setting over an 8-year period. The mean MEDD in the sample was 1,024 mg and the rotation strategy was to begin 5 mg of oral methadone every 4 hours, increasing the dose by 5 mg every 3 days while patients continued to take all of their previous daily opioid until pain relief improved. The average daily methadone starting dose was 27.3 mg and rotation was considered successful in 17 patients (62%), despite the long period required to reach pain stability (32 days on average). The authors justified this extended titration period by arguing that faster titration may lead to worse toxicity and, therefore, titration should be undertaken with caution, particularly in the outpatient setting. Parsons et al. [26] reviewed the charts of 189 consecutive outpatients who were prescribed methadone (initiation or rotation from another opioid) at an initiation dose of 5 mg b.i.d. The methadone conversion ratios used in that study, based on the dose of the previous opioid, were as follows: previous MEDD of 90 mg/day or less, 5:1; MEDD of 91–300 mg/day, 8:1; and MEDD of 301 mg/day or more, 12:1. Methadone efficacy was assessed at the first follow-up consultation after rotation, at a median time of 13 days. In most patients (88%), the treatment successfully improved pain scores and reduced nausea and drowsiness without increasing symptoms associated with opioid toxicity.
A few authors have reported that rotation to methadone can be safely performed in the home-care setting when an experienced team is involved and when a minimum of twice-weekly visits are conducted during the first 2 weeks of follow-up. Mercadante et al. [27] carried out a prospective study in a consecutive sample of 45 patients with advanced cancer who were followed at home. These patients had never received other strong opioids for their pain. All study participants received 2–3 home visits per week until death (median: 55 days) by a team of doctors and nurses experienced in palliative care. The authors concluded that the risk with methadone was low with individually titrated doses, even in elderly patients. Hernansanz et al. [43] reported results based on their 6-year experience with 14 patients with advanced cancer who were rotated to methadone. Rotation was considered successful in 71% of the patients, with a median survival of 72 days. Another retrospective study in a home-care setting included 1,682 patients in Italy; 17.6% of patients underwent opioid rotation, mostly for convenience. However, rotation to methadone was rare, as the authors explained, because of difficulties in monitoring the patients in such a setting [28]. By contrast, our findings show that follow-up of outpatients is both feasible and successful after methadone rotation.
The efficacy of methadone rotation reported by Parsons et al. [26] and De Conno et al. [24] was 44% and 55%, respectively, when assessed as a decrease of at least 30% of average pain from baseline—similar figures to those found in our study. In our study, the worst and average pain scores clearly decreased over time after rotation. Perhaps more relevant was our finding that the percentage of patients experiencing moderate and severe pain also decreased considerably.
One of the main difficulties of assessing reported methadone rotation outcomes, in addition to the retrospective nature of most studies, is the diversity of protocols and assessment times. Weschules and Bain [42] carried out a systematic review of methadone rotation and concluded that the available evidence is insufficient to determine a superior rotation protocol. More recently, results were published from a phase II trial comparing the “stop-and-go” and “over 3 days” rotation strategies in patients on an MEDD of at least 900 mg/day. Those authors found no significant differences between the two methods in terms of efficacy and safety. Notably, however, serious adverse events were more frequent in the stop-and-go group, although not all of these events were clearly related to methadone [44]. Finally, a small retrospective study evaluated 10 patients on an MEDD of more than 1,200 mg/day, reporting that all patients were safely rotated to methadone, achieved primarily by using a fixed dose of 10 mg t.i.d. and a stop-and-go strategy [45].
In our study, methadone rotation was performed in accordance with the Palliative Care Service protocol [35] developed at our institution. This is an evidence-based protocol that also incorporates aspects gleaned from our long experience in the use of methadone. The main indication for rotation in our study was poor pain control, perhaps because, compared with other studies, the mean MEDD dose before rotation was only an intermediate dose (193.7 mg). This moderate dose level could well explain the low level of opioid toxicity in our sample before rotation. Moreover, we were able to minimize serious toxicity, including respiratory depression, by using a close follow-up strategy (every 3 days), an approach that enables early detection and management of toxicity. Toxicity can be further minimized, in our experience, by having experienced nurses who proactively contact patients and families.
Potential limitations of our study include the relatively large number of dropouts. However, this is common and expected in studies with patients with advanced cancer; for this reason, we used a pragmatic study approach with a defined method to manage missing data to minimize this drawback. The use of the LOCF method could favor the analgesic effect of methadone, although this potential bias would be limited because most of the dropouts are pooled at the end of the follow-up period. Another potential limitation is the lack of data about prolongation of the QTc interval during the study; although we cannot rule out any effect of prolongation in our sample, the available information does not support monitoring the QTc interval in every patient rotated to methadone [46, 47] unless the patient has a history of cardiac arrhythmia, or, as has been recently recommended, is elderly or receives a methadone dose of 100 mg/day or more [48].
Conclusion
In this open-label prospective study of outpatients with advanced cancer, rotation to methadone as a second-line opioid was efficacious and safe during the first month after rotation in the context of a tiered scheme with close follow-up by an experienced team of nurses and doctors during the first 2 weeks after rotation. Further studies would give us additional information about the use of methadone for longer periods in terms of efficacy and safety. However, these findings suggest that methadone merits more consideration in outpatient OR schemes.
Acknowledgments
We thank Rebeca Font, Luisa Aliste, and Judit Solà for providing statistical support, and Bradley Londres for his help in editing the manuscript. This study was funded by a grant from the Spanish Ministry of Health, FIS: EC08/00234. This study was accepted as oral communication (No. FC4) at the 8th World Research Congress of the Association for Palliative Care, held in Lleida, Spain, June 2014.
Author Contributions
Conception/Design: Josep Porta-Sales
Provision of study material or patients: Josep Porta-Sales, Cristina Garzón-Rodríguez, Christian Villavicencio-Chávez, Silvia Llorens-Torromé
Collection and/or assembly of data: Josep Porta-Sales, Silvia Llorens-Torromé, Jesús González-Barboteo
Data analysis and interpretation: Josep Porta-Sales
Manuscript writing: Josep Porta-Sales, Cristina Garzón-Rodríguez
Final approval of manuscript: Josep Porta-Sales, Cristina Garzón-Rodríguez, Christian Villavicencio-Chávez, Silvia Llorens-Torromé, Jesús González-Barboteo
Disclosures
The authors indicated no financial relationships.
References
- 1.de Stoutz ND, Bruera E, Suarez-Almazor M. Opioid rotation for toxicity reduction in terminal cancer patients. J Pain Symptom Manage. 1995;10:378–384. doi: 10.1016/0885-3924(95)90924-c. [DOI] [PubMed] [Google Scholar]
- 2.Ashby MA, Martin P, Jackson KA. Opioid substitution to reduce adverse effects in cancer pain management. Med J Aust. 1999;170:68–71. doi: 10.5694/j.1326-5377.1999.tb126885.x. [DOI] [PubMed] [Google Scholar]
- 3.Kloke M, Rapp M, Bosse B, et al. Toxicity and/or insufficient analgesia by opioid therapy: Risk factors and the impact of changing the opioid. A retrospective analysis of 273 patients observed at a single center. Support Care Cancer. 2000;8:479–486. doi: 10.1007/s005200000153. [DOI] [PubMed] [Google Scholar]
- 4.Müller-Busch HC, Lindena G, Tietze K, et al. Opioid switch in palliative care, opioid choice by clinical need and opioid availability. Eur J Pain. 2005;9:571–579. doi: 10.1016/j.ejpain.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Mercadante S, Ferrera P, Villari P, et al. Frequency, indications, outcomes, and predictive factors of opioid switching in an acute palliative care unit. J Pain Symptom Manage. 2009;37:632–641. doi: 10.1016/j.jpainsymman.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 6.Quigley C. WITHDRAWN: Opioid switching to improve pain relief and drug tolerability. Cochrane Database Syst Rev. 2013;10:CD004847. doi: 10.1002/14651858.CD004847.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dale O, Moksnes K, Kaasa S. European Palliative Care Research Collaborative pain guidelines: Opioid switching to improve analgesia or reduce side effects. A systematic review. Palliat Med. 2011;25:494–503. doi: 10.1177/0269216310384902. [DOI] [PubMed] [Google Scholar]
- 8.Shaheen PE, Walsh D, Lasheen W, et al. Opioid equianalgesic tables: Are they all equally dangerous? J Pain Symptom Manage. 2009;38:409–417. doi: 10.1016/j.jpainsymman.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Mercadante S, Caraceni A. Conversion ratios for opioid switching in the treatment of cancer pain: A systematic review. Palliat Med. 2011;25:504–515. doi: 10.1177/0269216311406577. [DOI] [PubMed] [Google Scholar]
- 10.Ahmedzai S, Brooks D. Transdermal fentanyl versus sustained-release oral morphine in cancer pain: Preference, efficacy, and quality of life. J Pain Symptom Manage. 1997;13:254–261. doi: 10.1016/s0885-3924(97)00082-1. [DOI] [PubMed] [Google Scholar]
- 11.Reid CM, Martin RM, Sterne JA, et al. Oxycodone for cancer-related pain: Meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166:837–843. doi: 10.1001/archinte.166.8.837. [DOI] [PubMed] [Google Scholar]
- 12.Pigni A, Brunelli C, Caraceni A. The role of hydromorphone in cancer pain treatment: A systematic review. Palliat Med. 2011;25:471–477. doi: 10.1177/0269216310387962. [DOI] [PubMed] [Google Scholar]
- 13.Bruera E, Palmer JL, Bosnjak S, et al. Methadone versus morphine as a first-line strong opioid for cancer pain: A randomized, double-blind study. J Clin Oncol. 2004;22:185–192. doi: 10.1200/JCO.2004.03.172. [DOI] [PubMed] [Google Scholar]
- 14.Caraceni A, Hanks G, Kaasa S, et al. Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol. 2012;13:e58–e68. doi: 10.1016/S1470-2045(12)70040-2. [DOI] [PubMed] [Google Scholar]
- 15.Säwe J. High-dose morphine and methadone in cancer patients. Clinical pharmacokinetic considerations of oral treatment. Clin Pharmacokinet. 1986;11:87–106. doi: 10.2165/00003088-198611020-00001. [DOI] [PubMed] [Google Scholar]
- 16.Ferrari A, Coccia CP, Bertolini A, et al. Methadone--metabolism, pharmacokinetics and interactions. Pharmacol Res. 2004;50:551–559. doi: 10.1016/j.phrs.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Bruera E, Sweeney C. Methadone use in cancer patients with pain: A review. J Palliat Med. 2002;5:127–138. doi: 10.1089/10966210252785097. [DOI] [PubMed] [Google Scholar]
- 18.Leppert W. The role of methadone in cancer pain treatment--a review. Int J Clin Pract. 2009;63:1095–1109. doi: 10.1111/j.1742-1241.2008.01990.x. [DOI] [PubMed] [Google Scholar]
- 19.Moryl N, Santiago-Palma J, Kornick C, et al. Pitfalls of opioid rotation: Substituting another opioid for methadone in patients with cancer pain. Pain. 2002;96:325–328. doi: 10.1016/S0304-3959(01)00465-1. [DOI] [PubMed] [Google Scholar]
- 20.Lawlor PG, Turner KS, Hanson J, et al. Dose ratio between morphine and methadone in patients with cancer pain: A retrospective study. Cancer. 1998;82:1167–1173. [PubMed] [Google Scholar]
- 21.Bhimji K. Opioid rotation from methadone: Fraught with difficulties. J Pain Symptom Manage. 2005;29:334–335. doi: 10.1016/j.jpainsymman.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Walker PW, Palla S, Pei BL, et al. Switching from methadone to a different opioid: What is the equianalgesic dose ratio? J Palliat Med. 2008;11:1103–1108. doi: 10.1089/jpm.2007.0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhondali W, Tremellat F, Ledoux M, et al. Methadone rotation for cancer patients with refractory pain in a palliative care unit: An observational study. J Palliat Med. 2013;16:1382–1387. doi: 10.1089/jpm.2013.0222. [DOI] [PubMed] [Google Scholar]
- 24.De Conno F, Groff L, Brunelli C, et al. Clinical experience with oral methadone administration in the treatment of pain in 196 advanced cancer patients. J Clin Oncol. 1996;14:2836–2842. doi: 10.1200/JCO.1996.14.10.2836. [DOI] [PubMed] [Google Scholar]
- 25.Hagen NA, Wasylenko E. Methadone: Outpatient titration and monitoring strategies in cancer patients. J Pain Symptom Manage. 1999;18:369–375. doi: 10.1016/s0885-3924(99)00083-4. [DOI] [PubMed] [Google Scholar]
- 26.Parsons HA, de la Cruz M, El Osta B, et al. Methadone initiation and rotation in the outpatient setting for patients with cancer pain. Cancer. 2010;116:520–528. doi: 10.1002/cncr.24754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mercadante S, Casuccio A, Agnello A, et al. Methadone response in advanced cancer patients with pain followed at home. J Pain Symptom Manage. 1999;18:188–192. doi: 10.1016/s0885-3924(99)00048-2. [DOI] [PubMed] [Google Scholar]
- 28.Mercadante S, Valle A, Porzio G, et al. Opioid switching in patients with advanced cancer followed at home. A retrospective analysis. J Pain Symptom Manage. 2013;45:298–304. doi: 10.1016/j.jpainsymman.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 29.Martínez de la Iglesia J, Dueñas Herrero R, Onís Vilches MC, et al. [Spanish language adaptation and validation of the Pfeiffer’s questionnaire (SPMSQ) to detect cognitive deterioration in people over 65 years of age] Med Clin (Barc) 2001;117:129–134. doi: 10.1016/s0025-7753(01)72040-4. [in Spanish] [DOI] [PubMed] [Google Scholar]
- 30.Rodríguez-Martos A, Navarro RM, Vecino C, et al. Validation of the KFA (CBA) and CAGE questionnaires for the diagnosis of alcoholism] Droga-alcohol. 1986;11:132–139. [in Spanish] [Google Scholar]
- 31.Anderson F, Downing GM, Hill J, et al. Palliative performance scale (PPS): A new tool. J Palliat Care. 1996;12:5–11. [PubMed] [Google Scholar]
- 32.Nekolaichuk CL, Fainsinger RL, Lawlor PG. A validation study of a pain classification system for advanced cancer patients using content experts: The Edmonton Classification System for Cancer Pain. Palliat Med. 2005;19:466–476. doi: 10.1191/0269216305pm1055oa. [DOI] [PubMed] [Google Scholar]
- 33.Badia X, Muriel C, Gracia A, et al. [Validation of the Spanish version of the Brief Pain Inventory in patients with oncological pain] Med Clin (Barc) 2003;120:52–59. doi: 10.1016/s0025-7753(03)73601-x. [in Spanish] [DOI] [PubMed] [Google Scholar]
- 34.Serlin RC, Mendoza TR, Nakamura Y, et al. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61:277–284. doi: 10.1016/0304-3959(94)00178-H. [DOI] [PubMed] [Google Scholar]
- 35.Porta J, Gómez-Batiste X, Tuca A. Handbook of symptoms in patients with advanced and terminal cancer. 2nd ed. Madrid, Spain: Arán; 2008. [in Spanish] [Google Scholar]
- 36.ICOPraxi for the medical and radiation treatment of cancer pain [in Spanish] 2nd ed. Barcelona: Institut Català d’Oncologia, 2012. Available at http://ico.gencat.cat/web/.content/minisite/ico/professionals/documents/arxius/icopraxi_dolor_2014.pdf
- 37.Ripamonti C, Groff L, Brunelli C, et al. Switching from morphine to oral methadone in treating cancer pain: What is the equianalgesic dose ratio? J Clin Oncol. 1998;16:3216–3221. doi: 10.1200/JCO.1998.16.10.3216. [DOI] [PubMed] [Google Scholar]
- 38.Mercadante S, Casuccio A, Fulfaro F, et al. Switching from morphine to methadone to improve analgesia and tolerability in cancer patients: A prospective study. J Clin Oncol. 2001;19:2898–2904. doi: 10.1200/JCO.2001.19.11.2898. [DOI] [PubMed] [Google Scholar]
- 39.Mercadante S, Casuccio A, Calderone L. Rapid switching from morphine to methadone in cancer patients with poor response to morphine. J Clin Oncol. 1999;17:3307–3312. doi: 10.1200/JCO.1999.17.10.3307. [DOI] [PubMed] [Google Scholar]
- 40.Mercadante S, Ferrera P, Arcuri E. The use of fentanyl buccal tablets as breakthrough medication in patients receiving chronic methadone therapy: An open label preliminary study. Support Care Cancer. 2011;19:435–438. doi: 10.1007/s00520-010-1015-6. [DOI] [PubMed] [Google Scholar]
- 41.Verebely K, Volavka J, Mulé S, et al. Methadone in man: Pharmacokinetic and excretion studies in acute and chronic treatment. Clin Pharmacol Ther. 1975;18:180–190. doi: 10.1002/cpt1975182180. [DOI] [PubMed] [Google Scholar]
- 42.Weschules DJ, Bain KT. A systematic review of opioid conversion ratios used with methadone for the treatment of pain. Pain Med. 2008;9:595–612. doi: 10.1111/j.1526-4637.2008.00461.x. [DOI] [PubMed] [Google Scholar]
- 43.Hernansanz S, Gutiérrez C, Rubiales AS, et al. Opioid rotation to methadone at home. J Pain Symptom Manage. 2006;31:2–4. doi: 10.1016/j.jpainsymman.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 44.Moksnes K, Dale O, Rosland JH, et al. How to switch from morphine or oxycodone to methadone in cancer patients? A randomised clinical phase II trial. Eur J Cancer. 2011;47:2463–2470. doi: 10.1016/j.ejca.2011.06.047. [DOI] [PubMed] [Google Scholar]
- 45.Chatham MS, Dodds Ashley ES, Svengsouk JS, et al. Dose ratios between high dose oral morphine or equivalents and oral methadone. J Palliat Med. 2013;16:947–950. doi: 10.1089/jpm.2012.0434. [DOI] [PubMed] [Google Scholar]
- 46.Cruciani RA, Sekine R, Homel P, et al. Measurement of QTc in patients receiving chronic methadone therapy. J Pain Symptom Manage. 2005;29:385–391. doi: 10.1016/j.jpainsymman.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 47.Reddy S, Hui D, El Osta B, et al. The effect of oral methadone on the QTc interval in advanced cancer patients: A prospective pilot study. J Palliat Med. 2010;13:33–38. doi: 10.1089/jpm.2009.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Price LC, Wobeter B, Delate T, et al. Methadone for pain and the risk of adverse cardiac outcomes. J Pain Symptom Manage. 2014;48:333–42.e1. doi: 10.1016/j.jpainsymman.2013.09.021. [DOI] [PubMed] [Google Scholar]