Differences in tumor response and patient outcome were studied between patients with right-sided colorectal tumors treated with anti-epidermal growth factor receptor (EGFR) antibodies and those with left-sided tumors. Patients with RAS and BRAF wild-type metastatic colorectal cancer who were treated with single-agent anti-EGFRs or with cetuximab-irinotecan (if refractory to previous irinotecan) were included. Those with right-sided tumor did not seem to benefit from single-agent anti-EGFR treatment.
Keywords: Metastatic colorectal cancer, Right-sided tumor, Left-sided tumor, Anti-EGFR monoclonal antibodies
Abstract
Introduction.
Right- and left-sided colorectal cancers (CRCs) differ in clinical and molecular characteristics. Some retrospective analyses suggested that patients with right-sided tumors derive less benefit from anti-epidermal growth factor receptor (EGFR) antibodies; however, molecular selection in those studies was not extensive.
Patients and Methods.
Patients with RAS and BRAF wild-type metastatic CRC (mCRC) who were treated with single-agent anti-EGFRs or with cetuximab-irinotecan (if refractory to previous irinotecan) were included in the study. Differences in outcome between patients with right- and left-sided tumors were investigated.
Results.
Of 75 patients, 14 and 61 had right- and left-sided tumors, respectively. None of the right-sided tumors responded according to RECIST, compared with 24 left-sided tumors (overall response rate: 0% vs. 41%; p = .0032), and only 2 patients with right-sided tumors (15%) versus 47 patients with left-sided tumors (80%) achieved disease control (p < .0001). The median duration of progression-free survival was 2.3 and 6.6 months in patients with right-sided and left-sided tumors, respectively (hazard ratio: 3.97; 95% confidence interval: 2.09–7.53; p < .0001).
Conclusion.
Patients with right-sided RAS and BRAF wild-type mCRC seemed to derive no benefit from single-agent anti-EGFRs.
Implications for Practice:
Right- and left-sided colorectal tumors have peculiar epidemiological and clinicopathological characteristics, distinct gene expression profiles and genetic alterations, and different prognoses. This study assessed the potential predictive impact of primary tumor site with regard to anti-epidermal growth factor receptor (EGFR) monoclonal antibody treatment in patients with RAS and BRAF wild-type metastatic colorectal cancer. The results demonstrated the lack of activity of anti-EGFRs in RAS and BRAF wild-type, right-sided tumors, thus suggesting a potential role for primary tumor location in driving treatment choices.
Abstract
摘要
引言. 右侧和左侧结直肠癌 (CRC) 的临床特征和分子特征都不相同。一些回顾性分析提示右侧肿瘤患者从抗表皮生长因子受体 (EGFR) 抗体治疗的获益较少, 然而这些研究并未广泛开展分子选择。
患者与方法. 本研究纳入了接受抗 EGFR 单药或西妥昔单抗+伊立替康 (既往伊立替康治疗后复发患者) 治疗的 RAS 和 BRAF 野生型转移性 CRC (mCRC) 患者。观察左侧肿瘤和右侧肿瘤患者转归的差异。
结果. 75 例患者中, 14 例为右侧肿瘤, 61 例为左侧肿瘤。根据 RECIST 标准, 无一例右侧肿瘤患者达到缓解, 而 24 例左侧肿瘤患者达到缓解 (总缓解率0% vs. 41%, P=0.003 2); 仅 2 例 (15%) 右侧肿瘤患者达到疾病控制, 而左侧肿瘤患者中有 47 例 (80%) 达到疾病控制 (P<0.000 1)。右侧和左侧肿瘤患者的中位无进展生存分别为 2.3 个月和 6.6 个月 (风险比: 3.97, 95%置信区间: 2.09∼7.53, P<0.000 1)。
结论. 抗 EGFR 单药治疗对于右侧 RAS 和 BRAF 野生型 mCRC 患者似乎没有获益。The Oncologist 2016;21:988–994
对临床实践的提示: 右侧和左侧结直肠癌的流行病学和临床病理学特征各异, 基因表达谱及变异截然不同, 预后也不一样。本研究评估了原发肿瘤部位对 RAS 和 BRAF 野生型转移性结直肠癌患者对抗表皮生长因子受体 (EGFR) 单克隆抗体治疗的潜在预测作用。结果证实抗 EGFR 治疗对 RAS 和 BRAF 野生型右侧肿瘤患者疗效有限, 提示了原发肿瘤部位在治疗选择中具有潜在作用。
Introduction
The proximal and distal colon differ in terms of embryological origin, microbial flora, and exposure to environmental mutagens. As a consequence, colorectal carcinomas (CRCs) show heterogeneous epidemiological and clinicopathological characteristics based on their anatomical location [1–3].
A growing amount of evidence has unveiled distinct gene expression profiles and genetic alterations in right- and left-sided CRCs. Whereas right-sided tumors (i.e., those originating from cecum to transverse colon) are more likely diploid, hypermutated, and CpG-island methylated, exhibit microsatellite instability, and contain BRAF mutations, left-sided tumors (i.e., those originating from splenic flexure to rectum) frequently present chromosomal instability, EGFR and HER2-neu amplifications, and gene expression patterns associated with epidermal growth factor receptor (EGFR) pathway activation [1–6].
From a clinical perspective, it has been clearly demonstrated that the anatomical location also affects prognosis in patients with metastatic CRC (mCRC). Indeed, right-sided primary tumors are associated with shorter survival when compared with left-sided primary tumors [7, 8]. A relevant question is whether the primary tumor site may also predict differential benefit from available treatments.
Although the effect of the antiangiogenic bevacizumab is independent of tumor location [9], different retrospective analyses seem to suggest that patients with right-sided tumors derive less benefit from anti-EGFR monoclonal antibodies (moAbs) than those with left-sided tumors [6, 10, 11]. Moreover, in a subgroup analysis of patients with KRAS exon 2 wild-type mCRC included in CO.17, a phase III trial of cetuximab versus best supportive care in chemorefractory mCRC patients, primary tumor location showed a significant interaction with the outcome (p for interaction = .002) [12]. In particular, unlike patients with left-sided tumors, those with right-sided mCRCs seemed to derive no benefit from cetuximab monotherapy in terms of progression-free survival (PFS) [12]. A major limitation of this study was that extended RAS and BRAF mutation analyses were not taken into account; thus, independent of the primary tumor site, the study included patients with KRAS exon 3 and 4 and NRAS exon 2, 3, and 4 mutations, who do not derive benefit from anti-EGFR moAbs [13], and patients with BRAF mutation, who derive minimal benefit from the use of anti-EGFR moAbs [13, 14]. As recently confirmed in the new classification of mCRC molecular subtypes, RAS and BRAF mutations tend to occur more often in right-sided tumors [15]; therefore, the negative predictive impact of the proximal location with regard to the efficacy of cetuximab may be confounded by the higher percentage of mutations in this group.
Drawing from these considerations, we analyzed the potential predictive impact of primary tumor site with regard to the efficacy of anti-EGFR moAbs in a homogeneous population of patients with RAS and BRAF wild-type mCRC treated with anti-EGFR moAb monotherapy or in combination with irinotecan, if clearly refractory to irinotecan.
Patients and Methods
Patient Population
Consecutive patients with RAS and BRAF wild-type mCRC who were referred to three Italian institutions (Azienda Ospedaliero-Universitaria Pisana, Pisa; National Cancer Institute, Milan; and Veneto Institute of Oncology, Padua) from 2008 to 2015 and treated with panitumumab, cetuximab, or cetuximab plus irinotecan (only if refractory to irinotecan) were included. Refractoriness to irinotecan was defined as documented disease progression during or within 3 months from the last irinotecan-containing therapy. Only patients not previously treated with anti-EGFRs, with measurable disease according to RECIST version 1.1, and who underwent tumor reassessments during the treatment every 8 weeks were eligible.
Objectives and Definitions
The objective of this analysis was to examine potential differences in response and survival parameters between patients with right- and left-sided tumors (i.e., proximal or distal to the splenic flexure). Overall response rate (ORR) was defined as the proportion of patients achieving partial or complete response according to RECIST version 1.1. Disease control rate (DCR) was defined as the proportion of patients achieving partial or complete response or stable disease according to RECIST version 1.1. PFS was defined as the time from the first administration of anti-EGFR MoAbs to the evidence of disease progression according to RECIST version 1.1, or death from any cause. Postprogression survival (PPS) was defined as the time from evidence of disease progression to anti-EGFR MoAb treatment to death from any cause. Overall survival (OS) was defined as the time from the first administration of anti-EGFR moAbs to death from any cause.
RAS and BRAF Analyses
DNA was extracted from formalin-fixed, paraffin-embedded blocks. Hematoxylin- and eosin-stained slides were reviewed by expert pathologists who macrodissected proper representative areas of tumor tissue to obtain an amount containing at least 50% neoplastic cells. Genomic DNA was extracted using the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany, https://www.qiagen.com) with overnight proteinase K digestion, and DNA concentration was determined by the NanoDrop 2000c spectrophotometer (Thermo Fisher Scientific Life Sciences, Waltham, MA, http://www.thermofisher.com). KRAS (exons 2, 3, and 4), NRAS (exons 2, 3, and 4), and BRAFV600E mutational status were tested by pyrosequencing on the PyroMarkQ96 ID instrument (Qiagen) with commercially available kits (Diatech Pharmacogenetics, Jesi, Italy, http://www.diatechpharmacogenetics.com) or by mass spectrometry using the matrix-assisted laser desorption ionization-time of flight MassARRAY system (Sequenom, San Diego, CA, https://www.sequenom.com). The sensitivity (detectable percentage of mutant alleles) of pyrosequencing and mass spectrometry techniques is approximately 5%.
Statistical Analysis
The chi-square test, Wilcoxon test, and Fisher exact test were used, when appropriate, to compare clinical and biological features, ORR, and DCR between right- and left-sided tumor groups. PFS, PPS, and OS analyses were determined according to the Kaplan-Meier method, and survival curves were compared using the log-rank test. Statistical significance was set at p = .05 for a bilateral test. All analyses were carried out with GraphPad Software (La Jolla, CA, http://www.graphpad.com).
Results
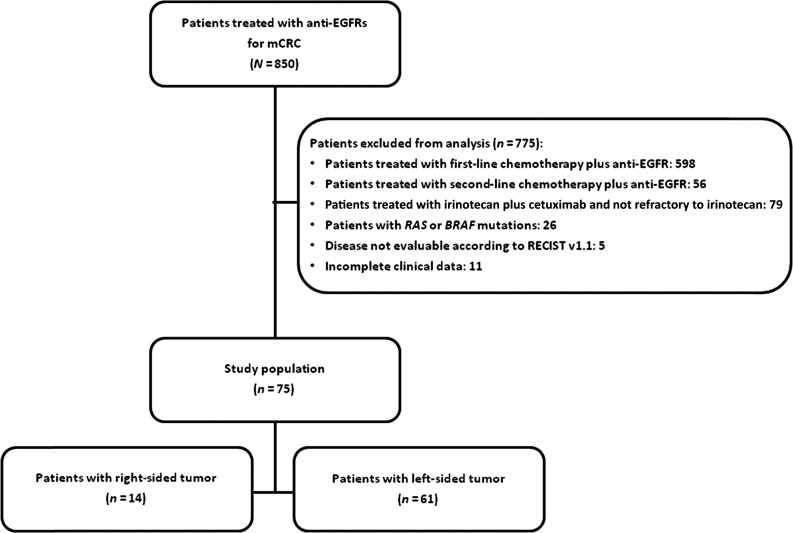
From a common data set including 850 mCRC patients treated with anti-EGFRs, we extracted 75 with RAS and BRAF wild-type mCRC who fulfilled the inclusion criteria (Fig. 1). Of these, 14 (18.7%) and 61 (81.3%) had right- and left-sided tumors, respectively.
Figure 1.
CONSORT diagram.
Abbreviations: EGFR, epidermal growth factor receptor; mCRC, metastatic colorectal cancer.
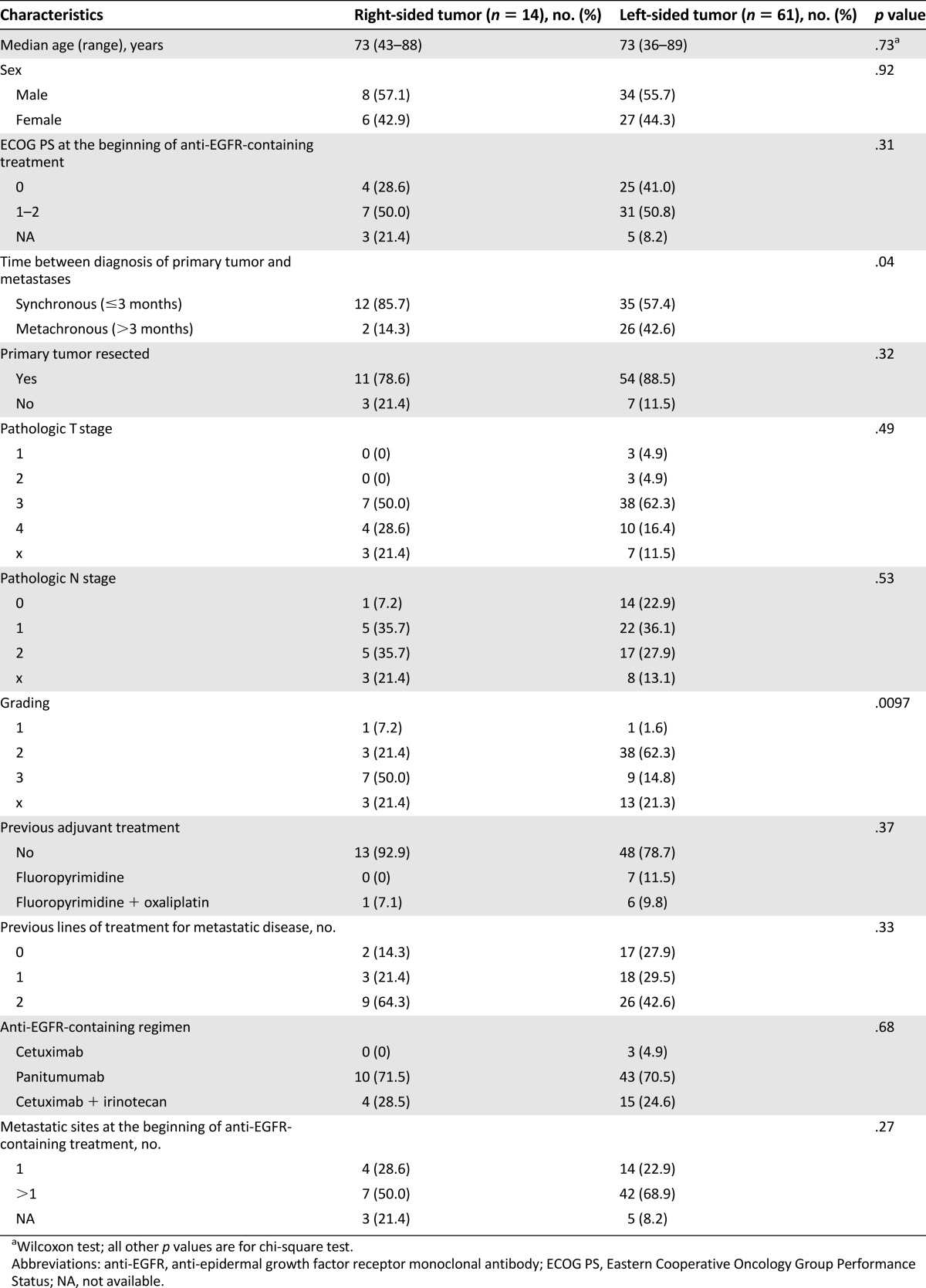
Clinical and pathological characteristics at baseline are summarized in Table 1. No significant differences between groups were observed in terms of sex (p = .92), Eastern Cooperative Oncology Group Performance Status 1–2 (p = .31), median age (p = .73), pathologic stage (pT: p = .49; pN: p = .53), number of metastatic sites at the beginning of the anti-EGFR-containing treatment (p = .27), and resection of the primary tumor (p = .32). Patients in the right-sided tumor group more frequently had synchronous metastases (p = .04) and poorly differentiated tumors when compared with patients in the left-sided tumor group (p = .0097). No significant differences were found with regard to prior adjuvant treatment (p = .37), the number of previous lines of treatment received for metastatic disease (p = .33), and which anti-EGFR-containing regimen was administered (p = .68).
Table 1.
Patients’ characteristics
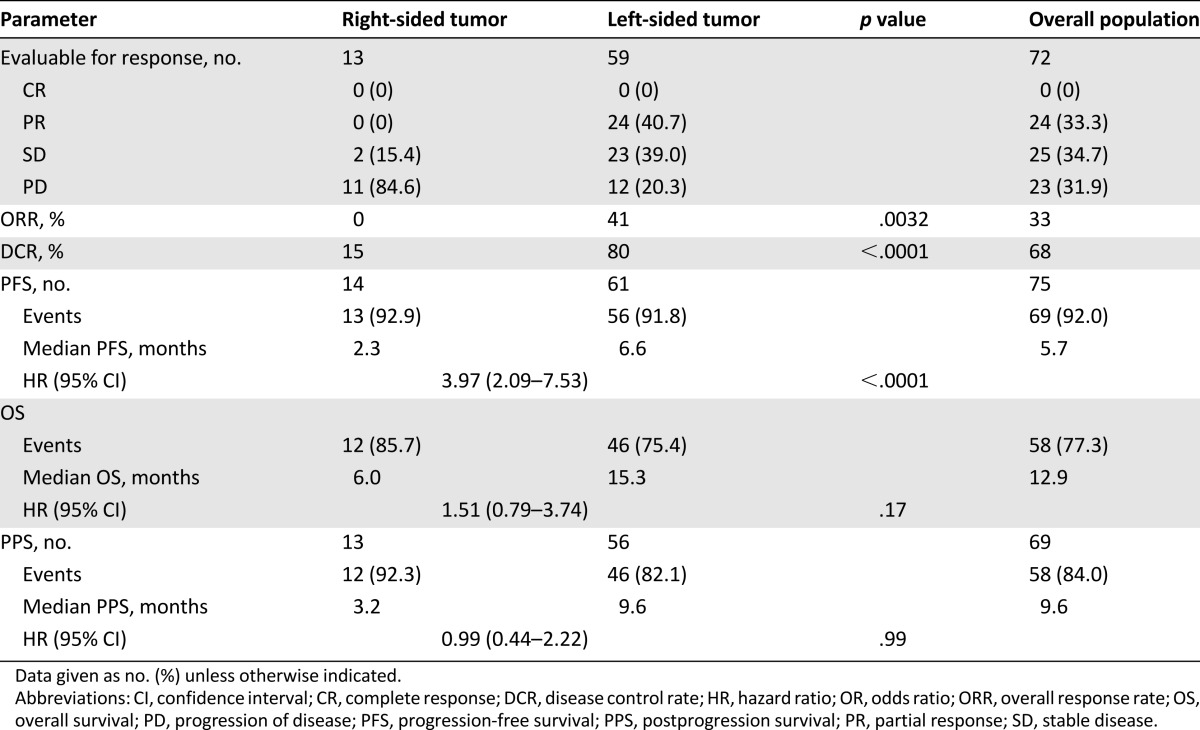
Response and survival parameters are listed in Table 2. Of 72 evaluable patients, 24 (33%) achieved RECIST response. All 24 had a left-sided tumor. Therefore, the ORR in left-sided tumors was 41% compared with 0% in right-sided tumors (p = .0032). Of the patients with left-sided tumors, 47 (80%) achieved disease control compared with 2 patients (15%) with right-sided primary tumors (p < .0001).
Table 2.
Response and survival parameters
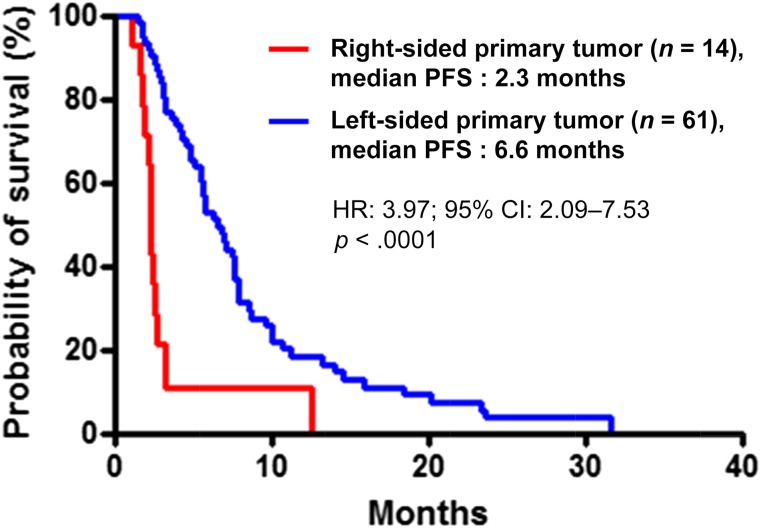
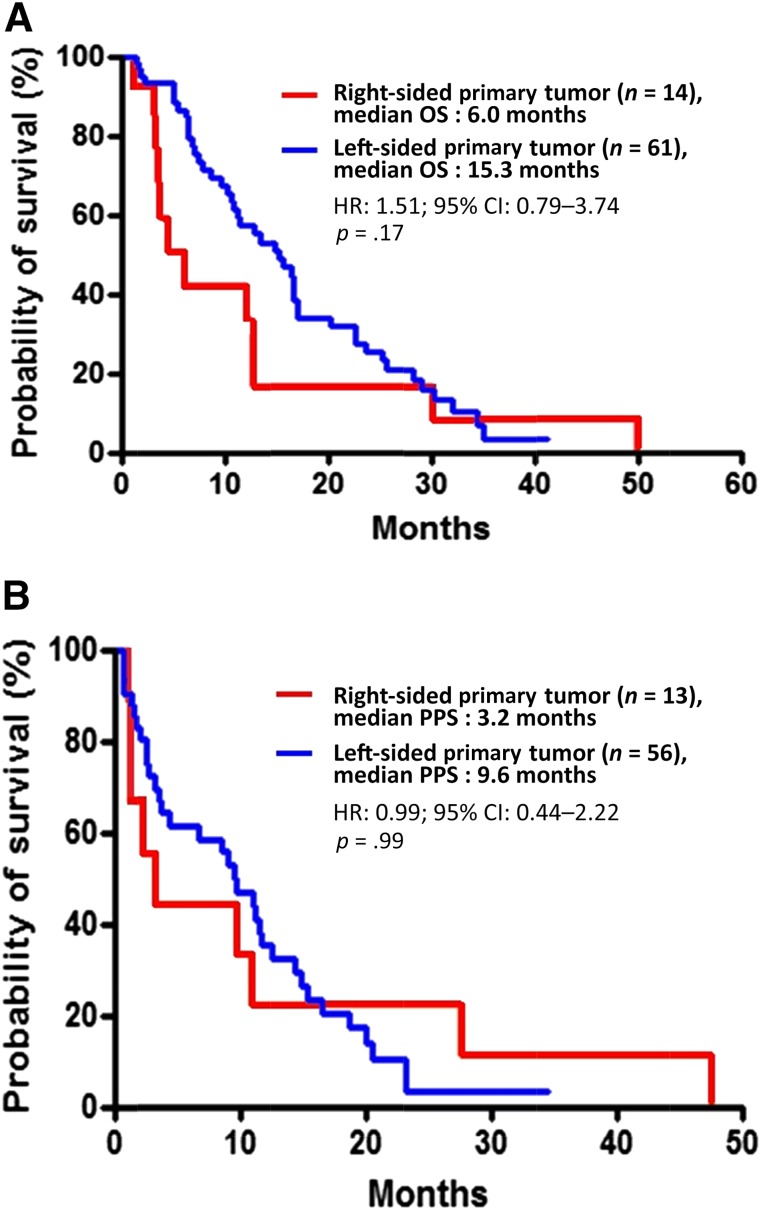
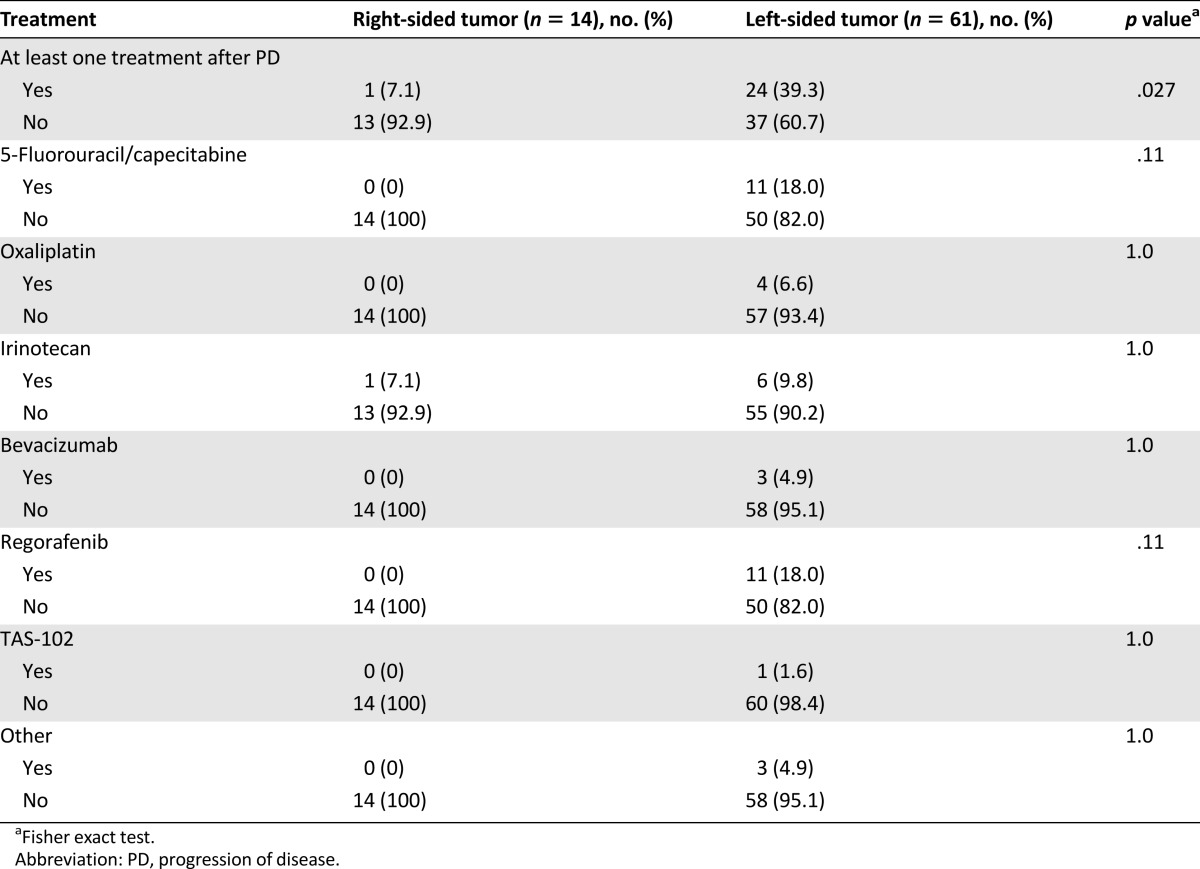
In the overall population, disease progression and death occurred in 69 patients (92%) and 58 patients (77%), respectively. Median PFS was 2.3 months in the right-sided tumor group and 6.6 months in the left-sided tumor group (hazard ratio [HR]: 3.97; 95% CI: 2.09–7.53; p < .0001) (Fig. 2). Patients with right-sided tumors also had shorter OS, although not significantly so (6.0 vs. 15.3 months; HR: 1.51; 95% CI: 0.79–3.74; p = .17), whereas no difference was shown in PPS (3.2 vs. 9.6 months; HR: 0.99; 95% CI: 0.44–2.22; p = .99) (Fig. 3). OS from the diagnosis of metastatic disease was 21.0 and 35.4 months for patients with right-sided and left-sided tumors, respectively (HR: 0.99; 95% CI: 0.49–1.98; p = .97). A higher percentage of patients with left-sided tumors received at least one more treatment after disease progression (Table 3).
Figure 2.
Kaplan-Meier analyses of PFS comparing patients with right-sided (red line) and left-sided (blue line) tumors.
Abbreviations: CI, confidence interval; HR, hazard ratio; PFS, progression-free survival.
Figure 3.
Kaplan-Meier analyses of (A) overall survival and (B) PPS, comparing patients with right-sided (red line) and left-sided (blue line) tumors.
Abbreviations: CI, confidence interval; HR, hazard ratio; PPS, postprogression survival.
Table 3.
Treatments after therapy with anti-epidermal growth factor receptor monoclonal antibody
Discussion
This series underlines a significant difference in clinical outcome among patients with right- and left-sided primary tumors treated with anti-EGFR moAbs, thus confirming and reinforcing results of the retrospective subgroup analysis of the phase III randomized CO.17 study of cetuximab versus best supportive care [12].
A crucial question remained when interpreting results provided by Brulé et al. [12]: was the significant interaction observed in that subgroup analysis due to the higher incidence of BRAF and RAS mutated tumors in the right-side colon? To answer this question, our analysis was restricted to patients with extended RAS and BRAF wild-type mutations, as assessed on archived tissue samples collected before any treatment. Based on our results, we can conclude that the lack of activity of anti-EGFRs in right-sided tumors cannot be attributed to the negative predictive impact of these mutations.
In our opinion, a point of strength of this study that makes it different from previous analyses [7, 11] lies in the choice to restrict the analysis to patients receiving anti-EGFR moAbs as single agents or in combination with irinotecan, only in strictly defined irinotecan-refractory patients. By excluding patients with potentially chemosensitive disease, we were able to focus on the true interaction of tumor site and outcome of anti-EGFR agents, at least in terms of response and PFS.
A clear limitation is the lack of a control arm including untreated patients. This prevented us from drawing definitive conclusions about the predictive role of the primary location. Nevertheless, the evidence of a significant difference in terms of response rate and PFS (i.e., outcome parameters more tightly related to the activity and efficacy of the study treatment, and not in terms of PPS and OS) might suggest a predictive, rather than prognostic, impact of the primary tumor site.
The reasons for such a different efficacy of anti-EGFRs in right- and left-sided mCRCs likely should be sought in a different molecular phenotype underlying these two groups. In fact, translational studies showed distinct and specific genetic features and expression profiles according to primary tumor location [16]. Not only are left-sided tumors often present with gene signatures associated with EGFR and MAPK activation [6] but they are also characterized by higher levels of epiregulin and amphiregulin expression when compared with right-sided primary tumors. High levels of EGFR endogenous ligands have been associated with response to anti-EGFRs, whereas low levels have been related to resistance to EGFR inhibition [17].
CpG island methylation is an epigenetic mechanism of gene silencing more frequently observed in right- than left-sided tumors and the methylation of the EGFR promoter may be responsible for the loss of EGFR expression [18] and, thus, for inefficacy of anti-EGFRs. On the other hand, the “canonical” CMS2 subtype, characterized by epithelial activation, and, therefore, potentially more sensitive to EGFR inhibition, is highly represented among left-sided tumors [15].
Conclusion
Our results support the importance of considering the primary tumor site in tailoring the best treatment for every patient with mCRC. To this purpose, the data from this study deserve confirmation in subgroup analyses of clinical studies randomizing patients to receive, or not receive, an anti-EGFR moAb and they underline the importance of collecting this information in ongoing and future trials.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Acknowledgment
We thank the nonprofit ARCO Foundation for supporting the molecular and statistical analyses in this study.
Author Contributions
Conception and Design: Roberto Moretto, Chiara Cremolini, Daniele Rossini, Filippo Pietrantonio, Fotios Loupakis, Gianluca Masi, Gabriella Fontanini, Sara Lonardi, Filippo De Braud, Alfredo Falcone
Provision of study material or patients: Roberto Moretto, Chiara Cremolini, Daniele Rossini, Filippo Pietrantonio, Fotios Loupakis, Gianluca Masi, Gabriella Fontanini, Sara Lonardi, Filippo De Braud, Alfredo Falcone
Collection and/or assembly of data: Roberto Moretto, Chiara Cremolini, Daniele Rossini, Filippo Pietrantonio, Francesca Battaglin, Alessia Mennitto, Francesca Bergamo, Federica Marmorino, Rosa Berenato, Valentina Angela Marsico, Marta Caporale, Carlotta Antoniotti, Lisa Salvatore, Beatrice Borelli
Data analysis and interpretation: Roberto Moretto, Chiara Cremolini, Daniele Rossini, Filippo Pietrantonio, Fotios Loupakis, Gianluca Masi, Gabriella Fontanini, Sara Lonardi, Filippo De Braud, Alfredo Falcone
Manuscript writing: Roberto Moretto, Chiara Cremolini, Daniele Rossini, Filippo Pietrantonio, Fotios Loupakis, Alfredo Falcone
Final approval of manuscript: Roberto Moretto, Chiara Cremolini, Daniele Rossini, Filippo Pietrantonio, Francesca Battaglin, Alessia Mennitto, Francesca Bergamo, Fotios Loupakis, Federica Marmorino, Rosa Berenato, Valentina Angela Marsico, Marta Caporale, Carlotta Antoniotti, Gianluca Masi, Lisa Salvatore, Beatrice Borelli, Gabriella Fontanini, Sara Lonardi, Filippo De Braud, Alfredo Falcone
Disclosures
Sara Lonardi: Roche, Sanofi, Amgen, Eli Lilly, Bayer (C/A), Roche, Amgen (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Bufill JA. Colorectal cancer: Evidence for distinct genetic categories based on proximal or distal tumor location. Ann Intern Med. 1990;113:779–788. doi: 10.7326/0003-4819-113-10-779. [DOI] [PubMed] [Google Scholar]
- 2.Distler P, Holt PR. Are right- and left-sided colon neoplasms distinct tumors? Dig Dis. 1997;15:302–311. doi: 10.1159/000171605. [DOI] [PubMed] [Google Scholar]
- 3.Iacopetta B. Are there two sides to colorectal cancer? Int J Cancer. 2002;101:403–408. doi: 10.1002/ijc.10635. [DOI] [PubMed] [Google Scholar]
- 4.Tran B, Kopetz S, Tie J, et al. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer. 2011;117:4623–4632. doi: 10.1002/cncr.26086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hutchins G, Southward K, Handley K, et al. Value of mismatch repair, KRAS, and BRAF mutations in predicting recurrence and benefits from chemotherapy in colorectal cancer. J Clin Oncol. 2011;29:1261–1270. doi: 10.1200/JCO.2010.30.1366. [DOI] [PubMed] [Google Scholar]
- 6.Missiaglia E, Jacobs B, D'Ario G, et al. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol. 2014;25:1995–2001. doi: 10.1093/annonc/mdu275. [DOI] [PubMed] [Google Scholar]
- 7.Sunakawa Y, Ichikawa W, Tsuji A, et al. Prognostic impact of primary tumor location on survival time in patients (pts) with metastatic colorectal cancer (mCRC) treated with cetuximab plus oxaliplatin-based chemotherapy: A subgroup analysis of the JACCRO CC-05/06. J Clin Oncol. 2016;34(suppl 4S):613a. doi: 10.1016/j.clcc.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Matos I, Ortiz C, Elez E, et al. Prognostic impact of primary tumor site location in metastatic colorectal cancer (mCRC) J Clin Oncol. 2016;34(suppl 4S):578a. [Google Scholar]
- 9.Loupakis F, Yang D, Yau L, et al. Primary tumor location as a prognostic factor in metastatic colorectal cancer. J Natl Cancer Inst. 2015;107:107. doi: 10.1093/jnci/dju427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Einem JC, Heinemann V, von Weikersthal LF, et al. Left-sided primary tumors are associated with favorable prognosis in patients with KRAS codon 12/13 wild-type metastatic colorectal cancer treated with cetuximab plus chemotherapy: An analysis of the AIO KRK-0104 trial. J Cancer Res Clin Oncol. 2014;140:1607–1614. doi: 10.1007/s00432-014-1678-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heinemann V, Modest DP, von Weikerstahl LF, et al. Gender and tumor location as predictors for efficacy: Influence on endpoints in first-line treatment with FOLFIRI in combination with cetuximab or bevacizumab in the AIO KRK 0306 (FIRE3) trial. J Clin Oncol. 2014;32(suppl 5S):3600a. [Google Scholar]
- 12.Brulé SY, Jonker DJ, Karapetis CS, et al. Location of colon cancer (right-sided versus left-sided) as a prognostic factor and a predictor of benefit from cetuximab in NCIC CO.17. Eur J Cancer. 2015;51:1405–1414. doi: 10.1016/j.ejca.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 13.Sorich MJ, Wiese MD, Rowland A, et al. Extended RAS mutations and anti-EGFR monoclonal antibody survival benefit in metastatic colorectal cancer: A meta-analysis of randomized, controlled trials. Ann Oncol. 2015;26:13–21. doi: 10.1093/annonc/mdu378. [DOI] [PubMed] [Google Scholar]
- 14.Pietrantonio F, Petrelli F, Coinu A, et al. Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab: A meta-analysis. Eur J Cancer. 2015;51:587–594. doi: 10.1016/j.ejca.2015.01.054. [DOI] [PubMed] [Google Scholar]
- 15.Guinney J, Dienstmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21:1350–1356. doi: 10.1038/nm.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grimminger PP, Shi M, Barrett C, et al. TS and ERCC-1 mRNA expressions and clinical outcome in patients with metastatic colon cancer in CONFIRM-1 and -2 clinical trials. Pharmacogenomics J. 2012;12:404–411. doi: 10.1038/tpj.2011.29. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs B, De Roock W, Piessevaux H, et al. Amphiregulin and epiregulin mRNA expression in primary tumors predicts outcome in metastatic colorectal cancer treated with cetuximab. J Clin Oncol. 2009;27:5068–5074. doi: 10.1200/JCO.2008.21.3744. [DOI] [PubMed] [Google Scholar]
- 18.Scartozzi M, Bearzi I, Mandolesi A, et al. Epidermal growth factor receptor (EGFR) gene promoter methylation and cetuximab treatment in colorectal cancer patients. Br J Cancer. 2011;104:1786–1790. doi: 10.1038/bjc.2011.161. [DOI] [PMC free article] [PubMed] [Google Scholar]