Supplemental Digital Content is available in the text.
Key Words: survey development, psychometric testing, factor analysis, patient-reported outcomes
Abstract
Goals and Background:
Patients with Clostridium difficile infection (CDI) can experience long-term symptoms and poor quality of life due to the disease. Despite this, a health-related quality of life (HRQOL) instrument specific for patients with CDI does not exist. The aim of this study was to develop and validate a disease-specific instrument to assess HRQOL in patients with CDI.
Study:
A systematic literature review was conducted to identify HRQOL instruments and questions related to general health (n=3) or gastrointestinal disease (n=12) potentially related to CDI HRQOL. Removing duplicate questions and using direct patient or clinician interviews, a 36-item survey was developed. The survey was then tested using 98 patients with CDI and compared with the RAND Short-Form 36 (SF-36) Health Survey. Psychometric analysis techniques were used to identify domains and remove redundant items.
Results:
Exploratory factor analysis identified 3 major domains (physical, mental, and social) with 4 associated subdomains. Survey overall and domain scores displayed good internal consistency (Cronbach α coefficient >0.87) and concurrent validity evidenced by significant correlation with SF-36 scores. The C. difficile survey scores were better able than the SF-36 to discriminate quality-of-life score differences in patients with primary versus recurrent CDI and increasing time since last episode of CDI. The final version contained 32 items related to the physical, mental, and social health of CDI patients.
Conclusion:
The properties of the newly developed Cdiff32 should make it appropriate to assess changes over time in HRQOL in patients with CDI.
Clostridium difficile infection (CDI) is the most common cause of infectious diarrhea in hospitalized patients in the United States of America (USA) and is the most common health care–associated pathogen.1 The Centers for Disease Control and Prevention estimates there are approximately 453,000 cases of CDI per year, 29,300 deaths, and over one billion dollars in health care costs associated with CDI.2,3 One of the most common complications of CDI is recurrence, which occurs in 25% to 33% of patients with primary CDI treated with metronidazole or oral vancomycin.4,5 CDI also decreases a patient’s ability to function.6–8 In patients with chronic diarrhea, such as that associated with HIV infection9 or following kidney transplantation,10 intestinal symptoms may be associated with reduced quality of life including decreased general well-being, satisfaction, social, and physical functioning. The acute effects of antibiotic-associated diarrhea has been shown to impair functional capacity using a clinical estimate of the ability to perform activities of daily living.11 However, patient-specific health-related quality of life (HRQOL) changes including physical, mental, and social health because of CDI have not been studied. These changes may be especially important in patients with recurrent CDI, in whom persistent diarrhea or postinfectious irritable bowel syndrome may cause long-term reduced quality of life.12 An analysis of the literature did not show the existence of any CDI-specific HRQOL instrument. The objective of this study was to develop and validate a disease-specific instrument to assess HRQOL changes related to CDI with a focus on recurrent CDI.
MATERIALS AND METHODS
Phase I: Development of Candidate Items
The stepwise procedure for the elaboration of the C. difficile quality-of-life survey is shown in Table 1. To begin the process, a systematic literature review using a PubMed search was conducted on April 1, 2012 using the search terms “quality of life” and (diarrhea or gastrointestinal or bowel). Items identified from this search were aggregated and tabulated. Acceptability of the items and removal of redundant or unimportant items were done by direct interview of 10 clinicians, including physicians, nurses, infection preventionists, and pharmacists, with expertise in the treatment and care of patients with CDI. The remaining items were tested to assure that the questions did not exceed a sixth grade reading level using the Flesch-Kincaid scale,13 minimized ambiguity or cognitive difficulty; avoided multibarreled questions, were concisely and simply worded, and were easy to translate into other languages. Each survey item was scored on a 5-point Likert scale with a recall period of 7 days. Each item was categorized into one of 3 major domains (physical, mental, or social) based on the subjective theme of the question.
TABLE 1.
Stepwise Procedure for the Elaboration of the Clostridium difficile Quality of Life Survey
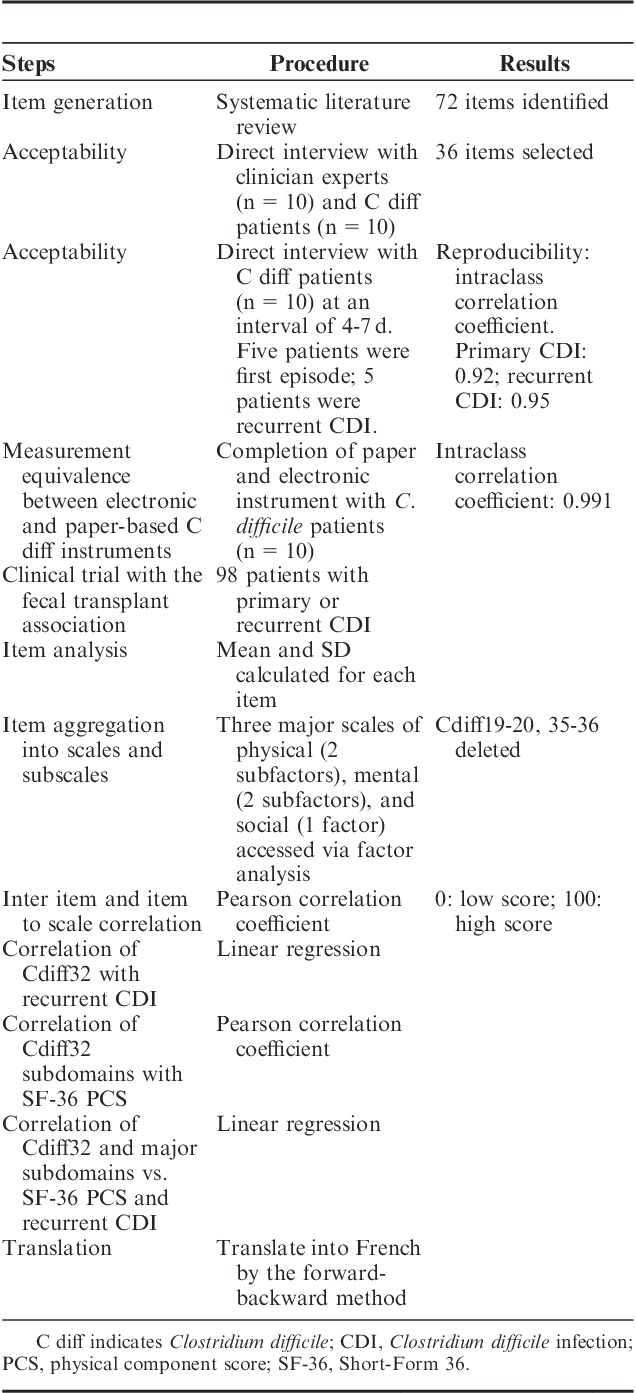
Structured feedback on the proposed questions was obtained by direct interviews of 5 hospitalized patients with CDI and 5 ambulatory patients with multiple episodes of CDI. An explicit, scripted interview guide was used to elicit feedback on the draft questions items based on the National Institute of Health’s Patient Reported Outcomes Measurement Information System (NIH PROMIS) suggested guidelines to evaluate respondent perceptions about language, comprehensibility, ambiguity, and relevance of each item.14,15 A standard set of probes was used as suggested by the PROMIS network. Items for enhancement were discussed after the completion of each set of 5 interviews. This iterative process resulted in stem and response item improvements culminating in a 36-item questionnaire for subsequent psychometric testing. The developed survey was then sent to the social media network (Facebook Inc., Menlo Park, CA) of the Fecal Transplant Foundation (http://thefecaltransplantfoundation.org/) for qualitative, anonymous feedback on the survey questions. Ten patients (5 with primary CDI and 5 with recurrent CDI) filled in the survey twice over a period of 4 to 7 days to assess for reproducibility of the survey answers. Ten patients with CDI also completed the survey in the paper format as well as the online survey format to assess the reliability of different survey formats. The online questionnaire was designed to be a minor modification and was not expected to change the content or meanings of items and response scales.16 Intraclass correlation coefficient was calculated for the test-retest procedure and the measurement equivalence between the paper and online form.
Phase II: Clinical Assessment
The questionnaire was administered along with the widely employed 36-item Short Form 36 (SF-36) Health Survey17 to a cohort of patients with CDI. Inclusion criteria included a positive toxin test for C. difficile, signs and symptoms of CDI, and a specific treatment for CDI prescribed by the treating physician. We purposely recruited patients with acute and recurrent CDI. Data collected included demographics, past medical history, number of episodes of CDI, time since last episode of CDI, and current use of antibiotics directed against CDI. Patients were recruited as part of an ongoing observational study in Houston, Texas as previously described.18 In addition, patients were also actively recruited from the social media network of the Fecal Transplant Foundation. The Fecal Transplant Foundation provides support for CDI patients seeking fecal microbiota transplantation. As part of this mission, the Foundation maintains an active social media presence with patients with recurrent CDI. All patients were nonhospitalized while completing the survey. All subjects were asked to answer the questions from the perspective of their prior 7 days of experiences. The study was approved by the Institutional Review Board of the University of Houston. The PI of the study (K.W.G.) had access to the study data and reviewed and approved the final manuscript.
Translation to Other Languages
The English version of the C. difficile questionnaire was translated to French using the forward-backward translation method.
Statistical Analysis
Each item was transformed to a score between 0 (worst QOL) and 100 (best QOL). Mean±SD was calculated for each item score. Exploratory factor analysis was used to identify item aggregation into subdomains from the 3 major domains (physical, mental, or social) and removal of redundant items. The number of factors chosen within each domain was based on the initial scree test with the percentage of common variance accounted for by a given factor.19 Factor number was chosen based on the Kaiser criteria including an eigenvalue >1, a break between factors observed on the scree plot, and factor loading >0.4.20 Item aggregation and reduction was chosen using a promax rotation as a measure of orthogonal rotation assuming correlation between items within subdomains. Final subdomains were chosen based on these analyses coupled with the conceptual meaning of each item. Interitem and item to scale correlation was measured using the Pearson correlation coefficient. Internal consistency was measured for each domain and total scale using Cronbach α reliability coefficient.
Demographics and CDI disease variables were compared between CDI patients stratified by primary CDI only compared with patients that experienced recurrent CDI. Variables were compared using the Pearson χ2 test for categorical variables and Student t test for continuous variables. Correlation between subdomains of the C. difficile questionnaire was compared with the corresponding SF-36 subscales. Mean differences in quality-of-life scores in patients with primary versus recurrent CDI were assessed using the Student t test. Finally, mean score parameter changes of the overall C. difficile quality-of-life score and the major domains along with the corresponding subscales of the SF-36 using multivariate linear regression. Any demographic or CDI disease variable that was different between patients with primary versus recurrent disease (defined as a P<0.2 in the univariate analysis) was included in the multivariate model. SAS version 9.3 (SAS Institute, Cary NC) or IBM SPSS Statistics version 22 (IBM, Armonk, NY) was used for all analyses. A P value <0.05 was considered significant for inferential statistics.
RESULTS
The literature search identified 12,380 citations which were reduced to 1364 citations using limits clinical trials, publications with 10 years, humans, and English language. The citations were screened for information about gastrointestinal or general quality-of-life questionnaires or rating scales that could contribute questions for a new QOL scale. Twenty-five relevant QOL surveys were identified of which 15 were available for review.21–33 From a review of these surveys, 72 separate items were identified and categorized as referring to physical, social, or mental QOL facets. Ten patients with CDI, 10 clinicians with expertise on the care of CDI patients, and 10 non-CDI healthy subjects were given the questionnaire on the relevance of the questions through a face-to-face interview. On the basis of the results of these interviews, some items were reformulated and the number of total items was reduced to 36 questions. The 36-item questionnaire was then given to 10 patients with CDI twice at an interval between 4 to 7 days. Intraclass correlation coefficient for test-retest was 0.92 for patients with primary CDI (n=5) and 0.95 for patients with recurrent CDI (n=5). Completion of the electronic survey and the paper survey was also completed by 10 patients with CDI. Intraclass correlation coefficient between the paper and electronic version was 0.99.
Clinical Assessment
Ninety-eight patients affected with CDI aged 52±16 years (female: 78%; white: 88%) were recruited and completed the C. difficile questionnaire of whom 76 completed the SF-36. Twenty-two patients (22%) were currently taking C. difficile antibiotics, most commonly oral vancomycin (16%), metronidazole (5%), or fidaxomicin (1%). Twenty-seven patients (28%) had experienced only a primary case of CDI. Patients had experienced an average of 2.6±2.6 recurrences (range, 0 to 14). The average time since CDI diagnosis was 4.2±1.8 months. Patients with recurrent CDI were more likely to be female (89%) as compared with patients with primary CDI (52%; P<0.001) and were less likely to have been hospitalized in the previous 3 months (P=0.026). There were no significant differences in age or past medical history between patients with primary or recurrent CDI.
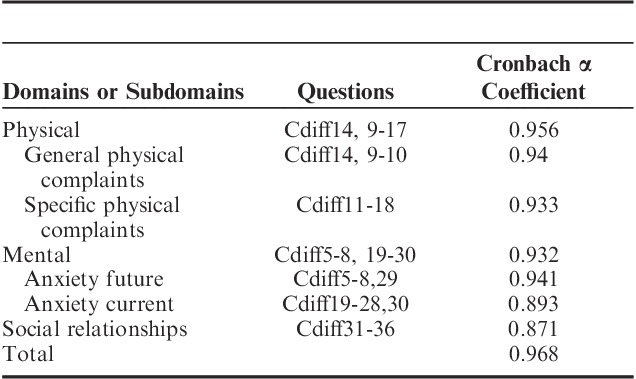
Domains, subdomains, questions, and item analysis for the survey are shown in Table 2. The scree test identified 2 factors in the physical and mental domains had an eigenvalue >1 with an associated drop in the scree plot after factor 2 (Fig. 1). For the social domain, 1 factor had an eigenvalue >1 with an associated drop in the scree plot after factor 1. For the physical domain, factors 1 and 2 explained 64.2% and 8.7% of the variance, respectively, resulting in 72.9% of the variance explained by both factors. For the mental domain, factors 1 and 2 explained 60.3% and 8.7% of the variance, respectively, resulting in 69.0% of the variance explained by both factors. For the social domain, factor 1 explained 61.8% of the variance. All items showed a factor loading of >0.5 to at least one of the factors. Factor analysis and domain structure is shown in Supplemental Table 1, Supplemental Digital Content 1, http://links.lww.com/JCG/A218. Results of the exploratory factorial analysis identified 2 subdomains for physical QOL categorized as general physical complaints or specific physical complaints related to the CDI. Two subdomains for mental QOL were also identified and categorized as anxiety of future complications of CDI and anxiety of current mental health related to CDI. One domain for social was identified and categorized as relationships. Scale reliability and content of the C. difficile questionnaire is shown in Table 3. Internal consistency measured with Cronbach α was ≥0.87 for all domains and subdomains. Interitem and item to scale correlation is shown in Supplemental Table 2, Supplemental Digital Content 1, http://links.lww.com/JCG/A218.
TABLE 2.
Clostridium difficile Survey Domains, Questions, and Item Analysis
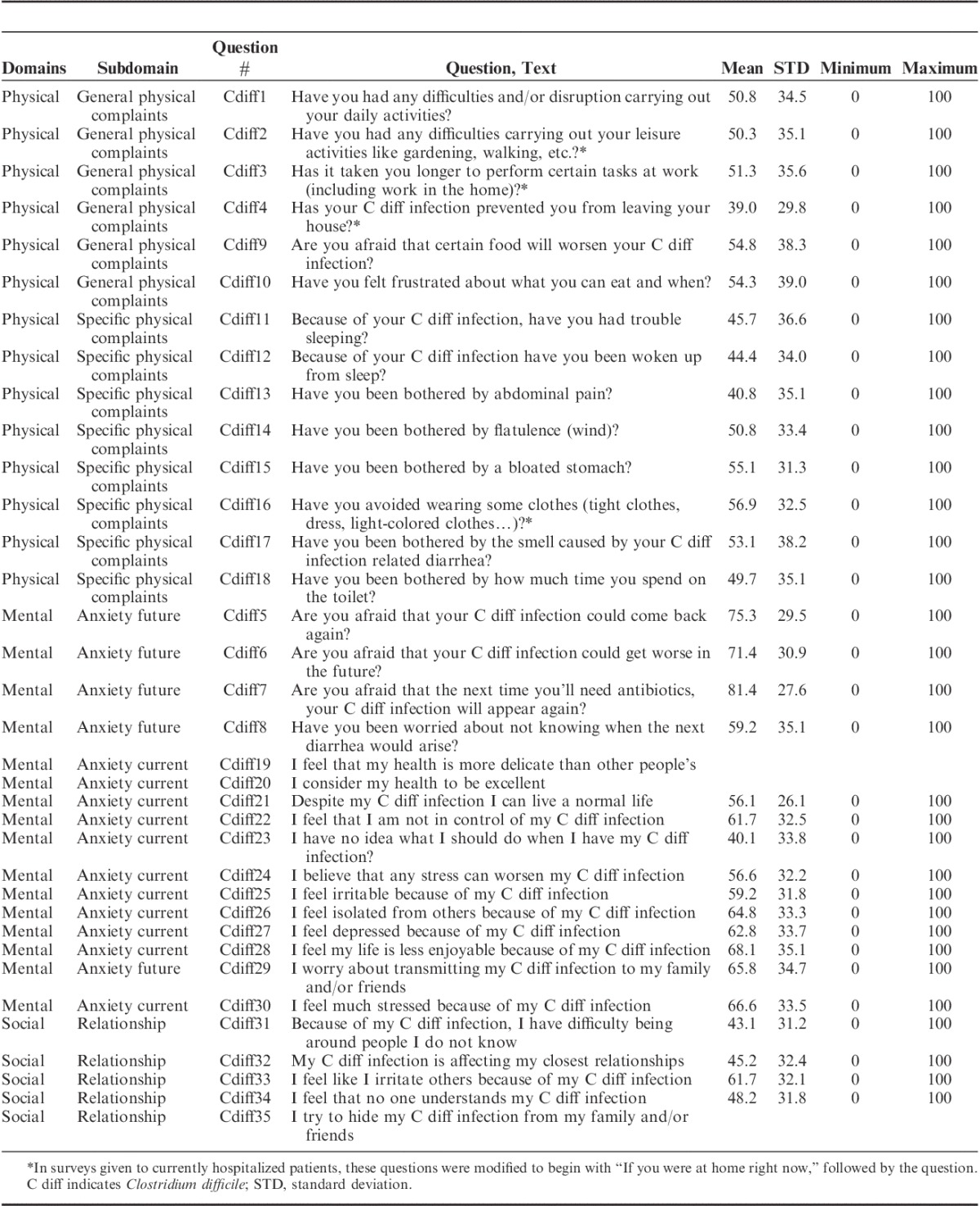
FIGURE 1.
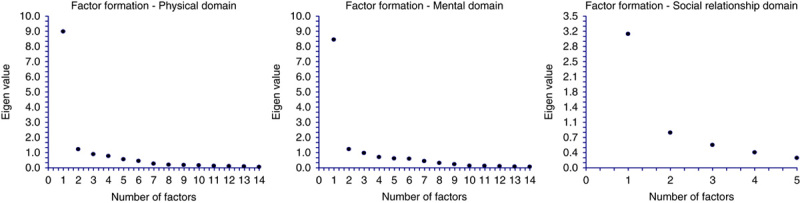
Factor formation-scree plots.
TABLE 3.
Scale Reliability and Clostridium difficile Survey Content (32 Items, 3 Major Domains, and 4 Subdomains)
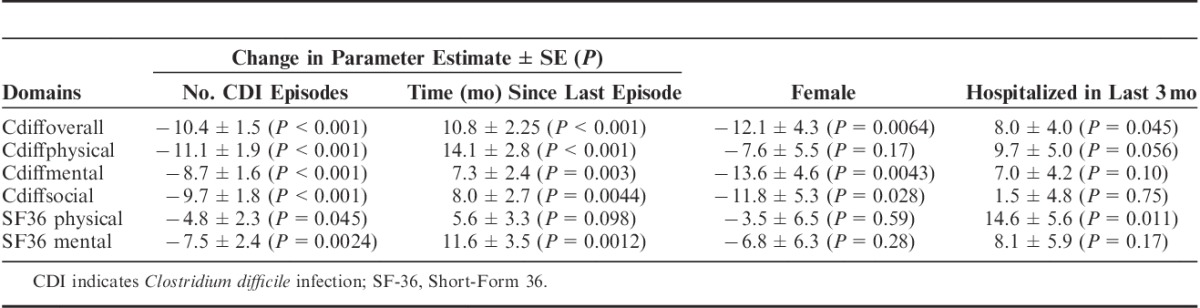
To measure concurrent validity, the overall, physical, and mental domains of the C. difficile questionnaire were compared with the physical and mental component summaries of the SF-36. All 5 of these domains were significantly correlated (P<0.001) using the Spearman rank-correlation coefficients (correlation coefficient range, 0.67 to 0.91). The overall C. difficile quality-of-life score was more highly correlated with the C. difficile physical (0.91) and C. difficile mental scores (0.91) than the SF-36 physical (0.63) or SF-36 mental (0.78) scores. Overall and major domains of the C. difficile questionnaire and the SF-36 physical and mental component summaries quality-of-life scores decreased in patients with recurrent CDI compared with patients with primary CDI (Fig. 2). All results were statistically significant (P<0.001) except for the SF-36 physical component scores (P=0.11). Separate multivariate linear regression models were built to assess parameter estimate changes for the overall and major domains of the C. difficile questionnaire and the SF-36 physical and mental component summaries quality-of-life scores (Table 4). Gender and previous hospitalization were included in all models based on the univariate analysis. Quality-of-life scores consistently decreased with increasing number of CDI episodes and improved with increasing time since last episode. C. difficile overall and major domains scores displayed greater mean changes in parameter estimates compared with the SF-36 physical or mental component scores. The French language translation for the Cdiff32 is shown in Supplemental Table 3, Supplemental Digital Content 1, http://links.lww.com/JCG/A218.
FIGURE 2.
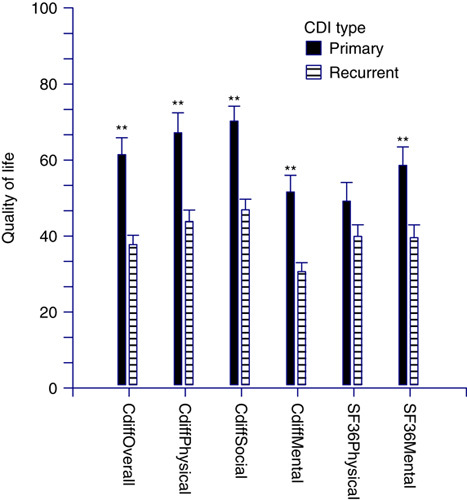
Distribution of mean quality-of-life (QOL) scores for Cdiff QOL and Short-Form 36 (SF36) stratified by primary versus recurrent Clostridium difficile infection (CDI). **P<0.001.
TABLE 4.
Multivariate Model to Assess Changes in Clostridium difficile Questionnaire and SF-36 Quality-of-Life Scores Stratified by Number of CDI Episodes, Time Since Last Episode, and Other Confounders
DISCUSSION
Patient-reported outcomes are increasingly recognized as important endpoints for clinical research. In Europe, >50% of the regulatory documents for approval of new pharmaceuticals include guidance for the use of patient-reported outcomes in the approval process.34 Although a number of gastrointestinal quality-of-life surveys exist, no equivalent HRQOL instrument exists for CDI. Therefore, the aim of this study was to develop a disease-specific scale able to measure quality-of-life differences in patients with CDI. In this study, we designed and validated the Cdiff32 HRQOL survey using an established method for scale development and validation.
In phase I of the study, we identified previous published QOL studies that had been applied to patients with gastrointestinal illness. All unique questions identified by this search were tabulated and categorized into physical, mental, and social domains to allow for future collaborative work with the NIH PROMIS initiative.35 All questions were reformulated onto a common 5-point Likert scale and assessed for comprehension and ease of use. Using focus group discussions with CDI patients and clinician experts, the number of items in the questionnaire was reduced to 36 questions. Test-retest and paper to electronic measurement equivalence were all acceptable.
Next, exploratory factor analysis was used to identify relevant subdomains and also reduced the total number of questions to 32. Two subscales were identified for the physical and mental domain. The overall survey, domains, and subdomains showed high internal consistency using Cronbach α coefficient analysis. Construct validity analysis showed a strong correlation with the SF-36 physical and mental components. Finally, the Cdiff32 and major subdomains were superior to the SF-36 at identifying differences in quality of life between CDI patients with primary compared with recurrent CDI.
Despite following scientifically appropriate psychometric methods, this study has certain limitations. This study was developed in the context of an observational trial and validated using an anonymous survey. The utility of the Cdiff32 to measure changes based on drug therapy or other targeted interventions will require future randomized-controlled studies, especially with larger sample sizes. The study was developed and validated only by English-speaking scientists and patients. Language translations and cultural nuances will need to be tested in the appropriate language and cultural populations. The study was not powered to identify differences in the subdomains identified in the development of the instrument. Future research will be needed to assess validity of these subdomains of the Cdiff32. Finally, although the major domains of the Cdiff32 were chosen to correlate with the PROMIS initiative, the correlation of the Cdiff32 domains scores with PRO questions from the PROMIS database will require further study.
In summary, this study developed and validated the Cdiff32, a multilanguage HRQOL instrument to be used in patients with CDI. The questionnaire should be evaluated for value in other forms of enteric infection and disease.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website, www.jcge.com.
Footnotes
Supported by a research grant from Merck & Co.
K.W.G.: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis; obtained funding. S.L.A.: study concept and design; acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content. L.G.: study concept and design; acquisition of data; critical revision of the manuscript for important intellectual content. S.G.: study concept and design; acquisition of data; critical revision of the manuscript for important intellectual content. Y.X.: acquisition of data; critical revision of the manuscript for important intellectual content. C.D.: acquisition of data; critical revision of the manuscript for important intellectual content. F.B.: study concept and design; acquisition of data; critical revision of the manuscript for important intellectual content. D.N.S.: study concept and design; acquisition of data; critical revision of the manuscript for important intellectual content. H.L.D.: study concept and design; acquisition of data; critical revision of the manuscript for important intellectual content.
D.N.S. has ongoing research support from Merck and Cubist; H.L.D. reports receiving funding from Actelion; K.W.G. reports receiving funding from Cubist, Summit, and Merck. The remaining authors declare that they have nothing to disclose.
REFERENCES
- 1.Miller BA, Chen LF, Sexton DJ, et al. Comparison of the burdens of hospital-onset, healthcare facility-associated Clostridium difficile infection and of healthcare-associated infection due to methicillin-resistant Staphylococcus aureus in community hospitals. Infect Control Hosp Epidemiol. 2011;32:387–390. [DOI] [PubMed] [Google Scholar]
- 2.Frieden T. Antibiotic resistance threats in the United States. 2013. Available at: http://www.cdc.gov/drugresistance/threat-report-2013/. editor. CDC.gov2013. Accessed January 6, 2016.
- 3.Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372:825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelly CP, LaMont JT. Clostridium difficile—more difficult than ever. N Engl J Med. 2008;359:1932–1940. [DOI] [PubMed] [Google Scholar]
- 5.Kelly CP. Can we identify patients at high risk of recurrent Clostridium difficile infection? Clin Microbiol Infect. 2012;18(suppl 6):21–27. [DOI] [PubMed] [Google Scholar]
- 6.Arora V, Kachroo S, Ghantoji SS, et al. High Horn’s index score predicts poor outcomes in patients with Clostridium difficile infection. J Hosp Infect. 2011;79:23–26. [DOI] [PubMed] [Google Scholar]
- 7.Ghantoji SS, Sail K, Lairson DR, et al. Economic healthcare costs of Clostridium difficile infection: a systematic review. J Hosp Infect. 2010;74:309–318. [DOI] [PubMed] [Google Scholar]
- 8.Salazar M, Baskin L, Garey KW, et al. Clostridium difficile-related death rates in Texas 1999-2005. J Infect. 2009;59:303–307. [DOI] [PubMed] [Google Scholar]
- 9.Siddiqui U, Bini EJ, Chandarana K, et al. Prevalence and impact of diarrhea on health-related quality of life in HIV-infected patients in the era of highly active antiretroviral therapy. J Clin Gastroenterol. 2007;41:484–490. [DOI] [PubMed] [Google Scholar]
- 10.Ekberg H, Kyllonen L, Madsen S, et al. Increased prevalence of gastrointestinal symptoms associated with impaired quality of life in renal transplant recipients. Transplantation. 2007;83:282–289. [DOI] [PubMed] [Google Scholar]
- 11.Peled N, Pitlik S, Samra Z, et al. Predicting Clostridium difficile toxin in hospitalized patients with antibiotic-associated diarrhea. Infect Control Hosp Epidemiol. 2007;28:377–381. [DOI] [PubMed] [Google Scholar]
- 12.Sethi S, Garey KW, Arora V, et al. Increased rate of irritable bowel syndrome and functional gastrointestinal disorders after Clostridium difficile infection. J Hosp Infect. 2011;77:172–173. [DOI] [PubMed] [Google Scholar]
- 13.Paasche-Orlow MK, Taylor HA, Brancati FL. Readability standards for informed-consent forms as compared with actual readability. New Eng J Med. 2003;348:721–726. [DOI] [PubMed] [Google Scholar]
- 14.Irwin DE, Varni JW, Yeatts K, et al. Cognitive interviewing methodology in the development of a pediatric item bank: a patient reported outcomes measurement information system (PROMIS) study. Health Qual Life Outcomes. 2009;7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeWalt DA, Rothrock N, Yount S, et al. Evaluation of item candidates: the PROMIS qualitative item review. Med Care. 2007;45:S12–S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coons SJ, Gwaltney CJ, Hays RD, et al. Recommendations on evidence needed to support measurement equivalence between electronic and paper-based patient-reported outcome (PRO) measures: ISPOR ePRO Good Research Practices Task Force report. Value Health. 2009;12:419–429. [DOI] [PubMed] [Google Scholar]
- 17.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 18.Garey KW, Jiang ZD, Ghantoji S, et al. A common polymorphism in the interleukin-8 gene promoter is associated with an increased risk for recurrent Clostridium difficile infection. Clin Infect Dis. 2010;51:1406–1410. [DOI] [PubMed] [Google Scholar]
- 19.Cappelleri JC, Gerber RA, Kourides IA, et al. Development and factor analysis of a questionnaire to measure patient satisfaction with injected and inhaled insulin for type 1 diabetes. Diabetes care. 2000;23:1799–1803. [DOI] [PubMed] [Google Scholar]
- 20.Bailey KD. Methods of Social Research, 3rd ed New York: Press Press; 1987:533. [Google Scholar]
- 21.Aaronson NK, Acquadro C, Alonso J, et al. International Quality of Life Assessment (IQOLA) Project. Qual Life Res. 1992;1:349–351. [DOI] [PubMed] [Google Scholar]
- 22.Onega LL. Helping those who help others: the Modified Caregiver Strain Index. Am J Nurs. 2008;108:62–69. Quiz 69-70. [DOI] [PubMed] [Google Scholar]
- 23.Chassany O, Marquis P, Scherrer B, et al. Validation of a specific quality of life questionnaire for functional digestive disorders. Gut. 1999;44:527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patrick DL, Drossman DA, Frederick IO, et al. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci. 1998;43:400–411. [DOI] [PubMed] [Google Scholar]
- 25.Hahn BA, Kirchdoerfer LJ, Fullerton S, et al. Evaluation of a new quality of life questionnaire for patients with irritable bowel syndrome. Aliment Pharmacol Ther. 1997;11:547–552. [DOI] [PubMed] [Google Scholar]
- 26.Svedlund J, Sjodin I, Dotevall G. GSRS—a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis Sci. 1988;33:129–134. [DOI] [PubMed] [Google Scholar]
- 27.Guyatt G, Mitchell A, Irvine EJ, et al. A new measure of health status for clinical trials in inflammatory bowel disease. Gastoenterology. 1989;96:804–810. [PubMed] [Google Scholar]
- 28.Irvine EJ, Zhou Q, Thompson AK. The Short Inflammatory Bowel Disease Questionnaire: a quality of life instrument for community physicians managing inflammatory bowel disease. CCRPT Investigators. Canadian Crohn’s Relapse Prevention Trial. Am J Gastroenterol. 1996;91:1571–1578. [PubMed] [Google Scholar]
- 29.Drossman DA, Leserman J, Li ZM, et al. The rating form of IBD patient concerns: a new measure of health status. Psychosom Med. 1991;53:701–712. [DOI] [PubMed] [Google Scholar]
- 30.Ortega F, Bravo J, Cantarell C, et al. Development and validation of a specific questionnaire for evaluating the impact of gastrointestinal symptoms on the health-related quality of life of transplant patients. Transplant Proc. 2012;44:1281–1286. [DOI] [PubMed] [Google Scholar]
- 31.Eypasch E, Williams JI, Wood-Dauphinee S, et al. Gastrointestinal Quality of Life Index: development, validation and application of a new instrument. Br J Surg. 1995;82:216–222. [DOI] [PubMed] [Google Scholar]
- 32.Berghofer P, Fragkos KC, Baxter JP, et al. Development and validation of the disease-specific Short Bowel Syndrome-Quality of Life (SBS-QoL) scale. Clin Nutr. 2013;32:789–796. [DOI] [PubMed] [Google Scholar]
- 33.Holtmann G, Chassany O, Devault KR, et al. International validation of a health-related quality of life questionnaire in patients with erosive gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2009;29:615–625. [DOI] [PubMed] [Google Scholar]
- 34.Szende A, Leidy NK, Revicki D. Health-related quality of life and other patient-reported outcomes in the European centralized drug regulatory process: a review of guidance documents and performed authorizations of medicinal products 1995 to 2003. Value Health. 2005;8:534–548. [DOI] [PubMed] [Google Scholar]
- 35.Alonso J, Bartlett SJ, Rose M, et al. The case for an international patient-reported outcomes measurement information system (PROMIS(R)) initiative. Health Qual Life Outcomes. 2013;11:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website, www.jcge.com.


