Abstract
We aim to provide an up-to-date summary of infantile hepatic hemangioma (IHH) and its misnomers and to dialectically present the differential diagnosis of these rare entities of the liver. Eligible peer-reviewed articles on hepatic infantile hemangiomas, published between 2000 and 2015, were reviewed for this study. IHH is the most common hepatic vascular tumor in children. Once a liver mass is identified in an infant, the differential diagnosis ranges from vascular malformations to benign and malignant tumors including mesenchymal hamartoma, hepatoblastoma, metastatic neuroblastoma, so careful physical examination, imaging studies, and, if indicated, tumor markers and biopsy, are of pivotal importance to ascertain the correct diagnosis. Despite the benign nature of IHHs, some of these lesions may demand medical and/or surgical intervention, especially for multiple and diffuse IHH. Complications can include hepatomegaly, hypothyroidism and cardiac failure. Therefore, a close follow-up is required until complete involution of the lesions. We propose an algorithm to guide the physicians towards the proper management of hepatic lesions.
Keywords: Hepatic hemangiomas, Infant, Children, Vascular tumors
Core tip: Differential diagnosis of pediatric liver lesions ranges from vascular malformations to benign and malignant tumors. Infantile hepatic hemangioma (IHH) is the most common, benign, hepatic vascular tumor in infants. They are sub-classified in focal, multiple and diffuse lesions, based on degree of unaffected liver parenchyma. Despite the benign nature of IHHs, multiple and diffuse lesions can present with life-threatening complications including severe hypothyroidism and cardiac failure, requiring prompt medical intervention. Therefore, a proper diagnosis is of pivotal importance. Including severe hypothyroidism and cardiac failure, requiring prompt medical intervention, therefore, a proper diagnosis is of pivotal importance.
INTRODUCTION
Infantile hemangiomas (IHs) are the most common benign tumor of infancy, affecting up to 10% of the pediatric population with a higher incidence in female (3:1), preterm infants, and Caucasian population. Most IHs are not present at birth but become apparent a few days to a few weeks after birth. IHs are characterized by a rapid proliferative phase in the first 6-10 mo, followed by a slow involution, which can last up to 10 years[1].
Despite their benign nature, IHs can cause severe morbidities and therefore sometimes require medical intervention[2]. IHs can range from asymptomatic to life threatening. Vital functions such as breathing, vision, and feeding can be impaired, depending on the location of the lesion.
IHs are be confirmed by positive immunostaining for glucose transporter-1 (GLUT-1), which is pathognomonic for the diagnosis of IHs, and therefore helps to distinguish IHs from other vascular anomalies[3]. While most IHs are present in the skin, IHs can occur in the viscera, with or without cutaneous manifestations. The liver is the most common site of visceral IHs, followed by the gastrointestinal system[4].
Screening for liver IHs (IHH) by ultrasonography (USG) is recommended when 5 or more cutaneous IHs are noted. However, the majority of IHHs are discovered as incidental findings during routine imaging.
IHHs are classified in three different subtypes, focal, multifocal and diffuse IHH, based on the remaining unaffected liver parenchyma.
Singular or focal lesions will often involute rapidly after birth without any complications. Multifocal lesions tend to involute in a similar pattern to cutaneous IHs, over a 6-10 year period. Diffuse lesions tend to replace almost the entire liver parenchyma, with severe complications.
These can include cardiac failure[5], high volume arteriovenous shunting[6], hypothyroidism secondary to overproduction of type III iodothyronine deiodinase[7], bleeding and abdominal compartment syndrome[8].
Therefore, once diagnosed, patients with IHHs usually require close monitoring until complete involution of the lesions.
Before the modern classification system developed by Mulliken in 1982[9], and the more recent subtyping of liver IHs by Christison-Lagay et al[10] in 2007, there was widespread confusion. Terminology for IHHs has been varied, a fact which can propagate the confusion and delay in the correct diagnosis and proper treatment of the affected patients. Moreover, there are several other hepatic lesions that may mimic different types of IHHs. Solitary hepatic lesions in an infant can also include, hepatoblastoma, mesenchymal hamartoma, congenital cysts (such as ciliated foregut duplication cysts) or kaposiform hemangioendotheliomas (KHEs). IHHs have also been historically called “hemangiomaendothelioma”, regardless of the type of lesion.
It is therefore imperative to distinguish all 3 subtypes of true IHHs from other benign and malignant liver lesions, as this can deeply impact the management of these conditions. For this reason, we systematically review the literature in order to provide an up-to-date understanding of IHHs and their misnomers and to dialectical present the differential diagnosis of these rare entities of the liver.
RESEARCH AND LITERATURE
Eligible articles were identified thorough search of the PubMed bibliographical database extending from January 2000 to 2015. Two investigators working independently executed the search using the following keywords in all the possible combinations: Hepatic hemangioma, infantile hepatic hemangioma, liver hemangioma and visceral hemangioma. In addition, we checked all the references of relevant reviews and eligible articles that our search retrieved. Search of the literature was restricted to those articles published in English, based on the following criteria: (1) original clinical series and case reports describe infants with hepatic hemangiomas; and (2) reviews of the literature on infantile hepatic hemangiomas. The selection-process excluded at the same time: (1) studies that describe malignant lesions alone; (2) lesions that are mistakenly categorized under the definition of infantile hepatic hemangiomas; (3) studies that do not contain the main outcomes of interest as described below.
In addition, historical evaluations of the failings of certain clinical treatments from beyond that timeline have been considered and included in order to present the story of IHHs as it has evolved.
CLINICAL PRESENTATION
A complete description of the clinical, radiological, histological findings of the different subtypes of IHH, and recommended treatment, can be found in Table 1. IHH lesions typically present and evolve in three different categorized patterns: Focal lesions, multifocal lesions, and diffuse lesions[11].
Table 1.
Description of the clinical, radiological, histological findings of the different subtypes of infantile hepatic hemangiomas and recommended treatment for the three different subgroups of infantile hepatic hemangiomas
| IHH | Focal | Multifocal | Diffuse |
| Onset | Prenatal development | Postnatal (few weeks after birth) | Postnatal (few weeks after birth) |
| Association with cutaneous IH | Rarely | Frequently | Frequently |
| MRI | Solitary tumor; robust enhancement; often with Ca2+ and central cystic change | Hypointense to liver on T1, hyperintense on T2. Rapid enhancement. May have central flow voids on T2 spin echo sequence | Near-total replacement of the hepatic parenchyma with many lesions |
| CT | Rapid enhancement. Often with Ca2+ and central cystic changes | Homogenously; uniform or centripetal | Innumerable centripetally but rapidly enhancing lesions |
| Glut-1 staining | Negative | Positive | Positive |
| Comorbidities | Possible anemia and relatively mild thrombocytopenia; AV shunting; High-output cardiac state | High-flow shunting resulting in high-output; Cardiac failure | High-output cardiac failure; Abdominal compartment syndrome; Severe hypothyroidism |
| Treatment | Observation; embolization for problematic shunting | Observation; propranolol/embolization for problematic shunting, possibly propranolol; hypothyroidism | Propranolol, thyroid hormone replacement, embolization in the cases of severe arteriovenous shunting (rare in diffuse IHHs), transplantation evaluation for the most extreme cases |
IHH: Infantile hepatic hemangioma; CT: Computed tomography; MRI: Magnetic resonance imaging.
Focal lesions
Focal lesions are completely formed at birth and are mainly detected prenatally during routine USG. They fully involute soon after birth, sharing a similar evolution of their cutaneous counterpart, rapidly involuting congenital hemangiomas (RICHs)[10]. Similar to RICHs, focal hepatic hemangiomas stain negative for GLUT-1. Magnetic resonance imaging (MRI) shows these lesions as single hypointense areas relative to the surrounding liver parenchyma on T1-weighted sequences, and hyperintense on T2-weighted sequences (Figure 1)[12]. They often demonstrate central cystic areas that may be interpreted as central necrosis. In over 15% of cases, focal liver lesions are associated with cutaneous IHs[13]. Despite being usually asymptomatic, focal lesions can be accompanied by mild thrombocytopenia and arteriovenous shunting, which may require medical intervention if present after involution of the IHH. The presence of mild thrombocytopenia has to be distinguished from Kasabach Merritt Phenomenon (KMP) in which the symptoms of severe thrombocytopenia and coagulopathy are seen in combination with a rapidly growing vascular lesion. KMP is associated with KHE and Tufted Angiomas, rare and locally aggressive vascular infantile tumors which are part of the same neoplastic spectrum[14]. Mild coagulopathy can also occur in venous malformations and can be defined as Localized Intravascular Coagulopathy.
Figure 1.
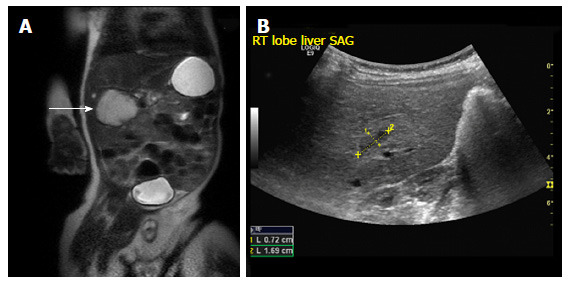
Focal infantile hepatic hemangiomas. A: Coronal T2 weighted MRI image through the abdomen of an 8-wk-old boy revealing a large hyperintense mass arising from the liver (arrow); B: Abdominal USG of the same patient at 17-mo-old shows a minimal residual scar (demarcated by calipers). USG: Ultrasonography; MRI: Magnetic resonance imaging.
Multifocal lesions
Multifocal lesions share a similar clinical course with cutaneous IHs. As for the cutaneous counterpart, multiple IHHs develop postnatally and exhibit a proliferating phase of around 9-12 mo in length, followed by a slow involution phase. They are more prevalent in females and Caucasians, and stain positive for GLUT-1. Multifocal IHHs can be detected on MRI as intensely enhancing spherical masses that are hypointense relative to the liver on T1-weighted sequences and hyperintense on T2-weighted sequences. They often present with a stellate (star shaped) central flow void on T2 spin echo sequences (Figure 2). Unlike focal lesions, multifocal IHHs can lead, in some cases, to moderate cardiomegaly and high-output heart failure due to arteriovenous and portovenous shunting. In over 60% of the cases, multifocal lesions are accompanied by cutaneous counterparts[5].
Figure 2.
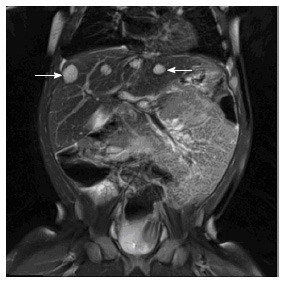
Multiple infantile hepatic hemangiomas. Coronal T2 weighted MRI image through the upper abdomen in a 5-mo-old girl depicts multiple well-defined, T2 hyperintense masses in the liver (arrows). This was consistent with multifocal infantile hepatic hemangiomas. MRI: Magnetic resonance imaging.
Diffuse lesions
Diffuse lesions are characterized by massive replacement of the hepatic parenchyma with various proliferating lesions with hyper enhancement on MRI (Figure 3). They have similar demographics with multifocal lesions, and also stain positive for GLUT-1. Association of cutaneous IH, may be present. Aortovenos, aortoportal, and venoportal shunting lead to high output cardiac failure[15]. Severe hepatomegaly may lead to compression of the systemic veins and thoracic cavity, leading to respiratory distress, abdominal compartment syndrome and multi-organ system failure. Diffuse lesions may also lead to severe hypothyroidism due to massive overproduction of type III iodothyronine deiodinase[7] (an enzyme involved in converting thyroxine into an inactive form) and leads to acquired hypothyroidism. Therefore, it is mandatory that when diffuse lesions are suspected, thyroid hormone levels be closely monitored, as undetected hypothyroidism can cause permanent neurologic damage, impaired hemostasis, and low-flow cardiac depression[16].
Figure 3.
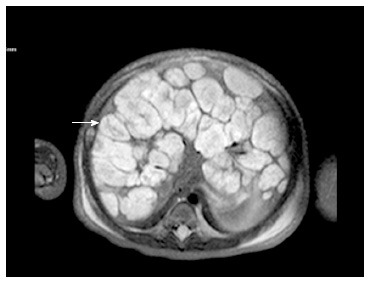
Diffuse infantile hepatic hemangiomas. T2 weighted axial MRI image of a 7-d-old with diffuse hemangiomas. Note the innumerable T2 hyperintense masses throughout the liver with central hypo-intense central regions (arrow). MRI: Magnetic resonance imaging.
DIFFERENTIAL DIAGNOSIS
Since IHHs are benign lesions, more aggressive liver malignancies need to be excluded at the time of diagnosis that at times may be challenging. Radiological imaging should be the first-line diagnostic analysis for physicians. A schematic algorithm to guide physicians through correct diagnosis and management of hepatic lesion can be seen in Figure 4.
Figure 4.
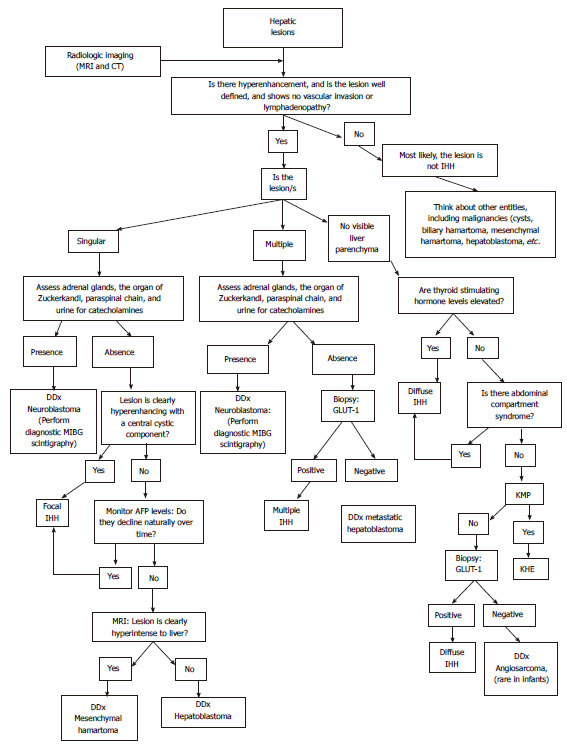
A schematic algorithm to guide physicians through correct diagnosis and management of hepatic lesions. IHH: Infantile hepatic hemangioma; CT: Computed tomography; MRI: Magnetic resonance imaging; DDx: Differential diagnosis; GLUT-1: Glucose transporter-1; AFP: α-fetoprotein; KHE: Kaposiform Hemangioendothelioma.
Single lesions
Focal hepatic hemangiomas have been shown to be biologically identical to RICH. They may present with central calcifications, a finding amenable to detection by ultrasound or CT scan. Posterior acoustic shadowing and high density are the hallmarks of calcification on ultrasound and CT scan, respectively. The primary differential diagnoses include metastatic neuroblastoma, hepatoblastoma (Figure 5) and mesenchymal hamartoma (Figure 6). Primary sites of neuroblastoma (adrenal glands, organ of Zuckerkandel and paraspinal chain) should be evaluated and the urine samples should be screened for the presence of catecholamines. If the above results are unremarkable, the lesion demonstrates hyper enhancement (either on multiphase CT with iodinated contrast or MRI with gadolinium based contrast material) and there is no invasion of other structures, then a presumptive focal IHHs may be diagnosed but should still be monitored to ensure there is no further growth.
Figure 5.
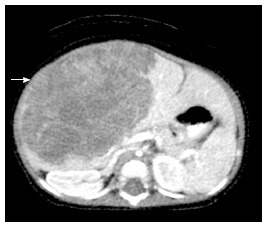
Hepatoblastoma. Axial computed tomography scan after the injection of IV contrast material in a 7-mo-old girl demonstrates a poorly enhancing large mass within the liver (arrow).
Figure 6.
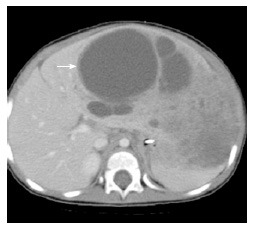
Mesenchymal hamartoma. 15-mo-old with abdominal distention: Axial computed tomography scan after the administration of IV contrast material demonstrates a large multi-cystic mass arising from the liver (arrow). Additional mixed solid and cystic elements are present laterally in the expanded left hepatic lobe.
Unlike IHHs, hepatoblastomas typically demonstrate a more heterogenic signal on T2 weighted MRI. The enhancement pattern is variable after the administration of contrast. Hepatoblastomas often enhance less than the surrounding hepatic parenchyma, as opposed to the typical hyper enhancement of IHHs. When hepatoblastoma is considered in the differential diagnosis of IHH, α-fetoprotein (AFP) levels should be monitored over time. AFP is a major plasma protein produced by the yolk sac and later on from the liver during fetal development. At birth, normal infants have high AFP levels that decreases to a normal range over the first year of life. A baseline measurement in an infant with a focal hepatic lesion can be useful to be certain AFP is appropriately trending down towards the normal range, especially in a patient for whom the diagnosis of focal IHHs is later questioned. In case of hepatoblastomas, AFP levels do not decline over time.
Persistent mild elevation of AFP can also be observed in mesenchymal hamartoma. Mesenchymal hamartoma lesions typically appear distinct from IHHs, as they have predominant cystic components. However, the more mass-like variants can have some imaging overlap with IHHs. Both IHHs and mesenchymal hamartomas are benign lesions. In the literature evidence of association between these two entities has been reported[17].
Multiple and diffuse lesions
When multiple well-demarked hepatic lesions are present - particularly if they demonstrate avid enhancement at MRI, and all appear similar on each sequence - a multifocal IH is the most likely diagnosis, although an atypical presentation of neuroblastoma is still possible. When the hepatic parenchyma appears entirely or nearly entirely replaced with similar appearing lesions as previously described without other abdominal masses, diffuse IHH is likely. The diagnosis can be more confidently made in the context of supporting evidence such as profoundly suppressed thyroid function (or can say elevated TSH), bleeding or compartment syndrome.
For a presumptive diagnosis of IHHs, regardless of the subtype, the enhancement pattern must be hyperacute (i.e., arterial), the lesion(s) must be well defined and there must not be any vascular invasion or lymphadenopathy. If any of these criteria are not met, an alternative diagnosis should be considered. The lesion may warrant tissue sampling or resection.
Other rare tumors that can potentially mimic IHHs include the KHE. This tumor is more infiltrative appearing (less well-defined) and Kasabach-Merrit phenomenon often accompanies it. Undifferentiated embryonal sarcoma is a primary hepatic tumor, which can be included in the differential diagnosis (DDx), however it typically presents later in childhood. Though it is typically rare in infants, angiosarcoma can mimic IHH on CT and MRI. In this case of suspected malignancy, a biopsy staining for GLUT-1 positivity can rule out or confirm the diagnosis of IHHs.
Radiological imaging can prove extremely helpful to further characterize the lesion(s) and define the anatomic extent of liver involvement. For instance, radiologically IHHs can be differentiated from hepatoblastomas. Hepatoblastomas tend to appear heterogeneous on a T2 weighted MRI sequence and enhance heterogeneosly as opposed to the homogeneous and rapid enhancement of IHHs. If there are atypical features, percutaneous biopsy and staining for GLUT-1 positivity is indicated, despite the high risk of bleeding[4]. DDx includes also other benign lesions such as cysts, biliary hamartomas and arteriovenous malformations. These entities, however, present imaging characteristics in the neonates that do not overlap with IHH.
MANAGEMENT OF COMPLICATIONS
Despite the benign nature of IHs, a careful follow-up should be planned in case of hepatic lesions. Unlike focal IHHs, multifocal and diffuse IHHs may lead to severe complications and possibly death[18].
Multifocal and diffuse lesions are often associated with arteriovenus shunting. In this case, frequent echocardiograms and close cardiologic follow-ups should be recommended until complete regression of the lesion, due to increased risk of congestive heart failure secondary to high-cardiac output. Congestive heart failure represents the main cause of mortality in these patients.
In case of severe high-flow arteriovenous shunting, embolization should be considered. Shunt embolization should be attempted however only in refractory lesions and those with a worsening clinical course. Potential complications of this procedure include hepatic infarction, necrosis, cirrhosis, and sepsis. Therefore, embolization has to be performed only when expert interventional radiologists, skilled in performing intrahepatic infant embolization, are available.
As previously mentioned, another possible complication of multifocal and diffuse IHHs is severe consumptive hypothyroidism. For this reason, TSH, T3, and T4 levels should be closely monitored by specialized pediatric endocrinologists. Thyroid hormone replacement should be considered if thyroid hormone levels are low. These patients, however, require much higher doses of thyroxin to achieve a stabilizing euthyroid status than required in patients with congenital hypothyroidism due to continuous catabolism of the exogenous thyroid hormone by the deiodinase 3. As IHHs undergo involution, the hypothyroidism resolves[19]. Therefore, thyroid hormone levels represent excellent biomarkers of tumor response to IHHs treatment.
When the lesions occupy a significant portion of the liver parenchyma, hepatic transaminases, bilirubin and coagulation factors should also be included in the laboratory follow-up to monitor liver and coagulation function.
TREATMENT
Following the validated treatment algorithm developed by the Fishman’s group at Boston Children’s Hospital[10], focal hemangioma mostly do not require medical intervention, since they mostly involute before or soon after birth. In the rare cases associated with arteriovenous shunting, embolization should be considered. Multifocal and diffuse IHHs may require medical intervention and/or therapy. A recent study confirmed that the mortality rate is greater in patients with diffuse IHHs than in those with multifocal lesions[10]. In 2008 there was a serendipitous discovery of the effectiveness of treatment of cutaneous IH with propranolol, a non-selective ß-blocker, revolutionizing the treatment of hemangiomas by accelerating IH involution compared to other therapies[20].
IHHs have been shown to successfully respond to the propranolol, as well as the cutaneous counterpart[21-23]. Despite this, many studies published after 2008 still indicated interferon-α (INF-α) and corticosteroids for the treatment of IHHs. It is estimated that 2.5% of children who received INF-α for the treatment of vascular anomalies developed spastic diplegia (SD), while an additional 4.1% were diagnosed with a motor developmental disturbance other than SD[24].
Before propranolol was established as the mainstay therapy, corticosteroids were considered the gold-standard treatment for problematic multifocal or diffuse IHHs[25]. However failure rate was as high as 20%-30% and in 40% of cases there was only a stabilization of the lesion growth more than acceleration in the involution[26]. Moreover corticosteroids lead to significant side effects. These include growth retardation, hyperglycemia, Cushinoid syndrome, hypertension and immunosuppression[27]. It has to be mentioned, however, that even propranolol is not free from side effects and include hypotension, hypoglycemia and bradycardia and exacerbation of bronchospasm, that are much less severe than the above medications[22].
Before pharmacotherapy proved successful for the treatment of IHH lesions, and when the benign nature of IHHs had not been clearly established, surgical resection and embolization was considered the mainstay treatment[28]. Surgery for hepatic hemangiomas is rarely performed, mainly only in cases that are refractory to medical management cases. Surgery complications include internal bleeding and hepatic necrosis[29]. In rare case, patients may presents with acute IHH complications such as compartment syndrome. This represents a negative prognostic factor and if medical treatment is not effective decompressed laparotomy up to hepatic transplant should be considered.
CONCLUSION
The literature of infantile hepatic hemangiomas has been greatly confusing in the past. Recent acceptance of IHH classification and subsequent treatment algorithms have proven an advancement in the diagnosis and management of these vascular lesions. Treatment of IHHs has evolved rapidly in the past decade, especially in the studies on the efficacy of propranolol as opposed to the efficacy and problems with long-term corticosteroid treatment. The understanding of IHHs is likely nearing the tipping point into a new revolution of clinical knowledge and treatment. Based on the new classification of IHHs, we propose an algorithm to guide the physicians towards the proper management of hepatic lesions.
Footnotes
Conflict-of-interest statement: All the authors declare that they have no competing interests.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Pediatrics
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: January 19, 2016
First decision: March 24, 2016
Article in press: June 3, 2016
P- Reviewer: Ji Y, Sirli R S- Editor: Qiu S L- Editor: A E- Editor: Wu HL
References
- 1.Itinteang T, Withers AH, Davis PF, Tan ST. Biology of infantile hemangioma. Front Surg. 2014;1:38. doi: 10.3389/fsurg.2014.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drolet BA, Swanson EA, Frieden IJ. Infantile hemangiomas: an emerging health issue linked to an increased rate of low birth weight infants. J Pediatr. 2008;153:712–715, 715.e1. doi: 10.1016/j.jpeds.2008.05.043. [DOI] [PubMed] [Google Scholar]
- 3.Patiño-Seijas B, Lorenzo-Franco F, Rey-Sanjurjo JL, González-Cuesta M, López-Cedrún Cembranos JL. Vascular Lesions: GLUT-1 expression as a diagnostic tool to discriminate tumors from malformations. J Oral Maxillofac Surg. 2012;70:2333–2342. doi: 10.1016/j.joms.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 4.Dickie B, Dasgupta R, Nair R, Alonso MH, Ryckman FC, Tiao GM, Adams DM, Azizkhan RG. Spectrum of hepatic hemangiomas: management and outcome. J Pediatr Surg. 2009;44:125–133. doi: 10.1016/j.jpedsurg.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 5.Lu CC, Ko SF, Liang CD, Kuo HW, Tiao MM. Infantile hepatic hemangioendothelioma presenting as early heart failure: report of two cases. Chang Gung Med J. 2002;25:405–410. [PubMed] [Google Scholar]
- 6.Gallego C, Miralles M, Marín C, Muyor P, González G, García-Hidalgo E. Congenital hepatic shunts. Radiographics. 2004;24:755–772. doi: 10.1148/rg.243035046. [DOI] [PubMed] [Google Scholar]
- 7.Huang SA, Tu HM, Harney JW, Venihaki M, Butte AJ, Kozakewich HP, Fishman SJ, Larsen PR. Severe hypothyroidism caused by type 3 iodothyronine deiodinase in infantile hemangiomas. N Engl J Med. 2000;343:185–189. doi: 10.1056/NEJM200007203430305. [DOI] [PubMed] [Google Scholar]
- 8.Zenzen W, Perez-Atayde AR, Elisofon SA, Kim HB, Alomari AI. Hepatic failure in a rapidly involuting congenital hemangioma of the liver: failure of embolotherapy. Pediatr Radiol. 2009;39:1118–1123. doi: 10.1007/s00247-009-1346-y. [DOI] [PubMed] [Google Scholar]
- 9.Mulliken JB, Glowacki J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg. 1982;69:412–422. doi: 10.1097/00006534-198203000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Christison-Lagay ER, Burrows PE, Alomari A, Dubois J, Kozakewich HP, Lane TS, Paltiel HJ, Klement G, Mulliken JB, Fishman SJ. Hepatic hemangiomas: subtype classification and development of a clinical practice algorithm and registry. J Pediatr Surg. 2007;42:62–67; discussion 67-68. doi: 10.1016/j.jpedsurg.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 11.Meyers RL. Tumors of the liver in children. Surg Oncol. 2007;16:195–203. doi: 10.1016/j.suronc.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Kassarjian A, Zurakowski D, Dubois J, Paltiel HJ, Fishman SJ, Burrows PE. Infantile hepatic hemangiomas: clinical and imaging findings and their correlation with therapy. AJR Am J Roentgenol. 2004;182:785–795. doi: 10.2214/ajr.182.3.1820785. [DOI] [PubMed] [Google Scholar]
- 13.Kulungowski AM, Alomari AI, Chawla A, Christison-Lagay ER, Fishman SJ. Lessons from a liver hemangioma registry: subtype classification. J Pediatr Surg. 2012;47:165–170. doi: 10.1016/j.jpedsurg.2011.10.037. [DOI] [PubMed] [Google Scholar]
- 14.Arai E, Kuramochi A, Tsuchida T, Tsuneyoshi M, Kage M, Fukunaga M, Ito T, Tada T, Izumi M, Shimizu K, et al. Usefulness of D2-40 immunohistochemistry for differentiation between kaposiform hemangioendothelioma and tufted angioma. J Cutan Pathol. 2006;33:492–497. doi: 10.1111/j.1600-0560.2006.00461.x. [DOI] [PubMed] [Google Scholar]
- 15.Smith AA, Nelson M. High-Output Heart Failure from a Hepatic Hemangioma With Exertion-Induced Hypoxia. Am J Cardiol. 2016;117:157–158. doi: 10.1016/j.amjcard.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 16.Fisher DA. Clinical review 19: Management of congenital hypothyroidism. J Clin Endocrinol Metab. 1991;72:523–529. doi: 10.1210/jcem-72-3-523. [DOI] [PubMed] [Google Scholar]
- 17.Behr GG, Fishman SJ, Caty MG, Kulungowski AM, Paltiel HJ, Alomari AI. Hepatic mesenchymal hamartoma and infantile hemangioma: a rare association. J Pediatr Surg. 2012;47:448–452. doi: 10.1016/j.jpedsurg.2011.10.049. [DOI] [PubMed] [Google Scholar]
- 18.Rialon KL, Murillo R, Fevurly RD, Kulungowski AM, Christison-Lagay ER, Zurakowski D, Kozakewich HP, Alomari AI, Fishman SJ. Risk factors for mortality in patients with multifocal and diffuse hepatic hemangiomas. J Pediatr Surg. 2015;50:837–841. doi: 10.1016/j.jpedsurg.2014.09.056. [DOI] [PubMed] [Google Scholar]
- 19.Konrad D, Ellis G, Perlman K. Spontaneous regression of severe acquired infantile hypothyroidism associated with multiple liver hemangiomas. Pediatrics. 2003;112:1424–1426. doi: 10.1542/peds.112.6.1424. [DOI] [PubMed] [Google Scholar]
- 20.Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, Boralevi F, Thambo JB, Taïeb A. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649–2651. doi: 10.1056/NEJMc0708819. [DOI] [PubMed] [Google Scholar]
- 21.Marsciani A, Pericoli R, Alaggio R, Brisigotti M, Vergine G. Massive response of severe infantile hepatic hemangioma to propanolol. Pediatr Blood Cancer. 2010;54:176. doi: 10.1002/pbc.22262. [DOI] [PubMed] [Google Scholar]
- 22.Mazereeuw-Hautier J, Hoeger PH, Benlahrech S, Ammour A, Broue P, Vial J, Ohanessian G, Léauté-Labrèze C, Labenne M, Vabres P, et al. Efficacy of propranolol in hepatic infantile hemangiomas with diffuse neonatal hemangiomatosis. J Pediatr. 2010;157:340–342. doi: 10.1016/j.jpeds.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Sarialioglu F, Erbay A, Demir S. Response of infantile hepatic hemangioma to propranolol resistant to high-dose methylprednisolone and interferon-α therapy. Pediatr Blood Cancer. 2010;55:1433–1434. doi: 10.1002/pbc.22691. [DOI] [PubMed] [Google Scholar]
- 24.Barlow CF, Priebe CJ, Mulliken JB, Barnes PD, Mac Donald D, Folkman J, Ezekowitz RA. Spastic diplegia as a complication of interferon Alfa-2a treatment of hemangiomas of infancy. J Pediatr. 1998;132:527–530. doi: 10.1016/s0022-3476(98)70034-4. [DOI] [PubMed] [Google Scholar]
- 25.Bertrand J, McCuaig C, Dubois J, Hatami A, Ondrejchak S, Powell J. Propranolol versus prednisone in the treatment of infantile hemangiomas: a retrospective comparative study. Pediatr Dermatol. 2011;28:649–654. doi: 10.1111/j.1525-1470.2011.01551.x. [DOI] [PubMed] [Google Scholar]
- 26.Enjolras O, Riche MC, Merland JJ, Escande JP. Management of alarming hemangiomas in infancy: a review of 25 cases. Pediatrics. 1990;85:491–498. [PubMed] [Google Scholar]
- 27.Boon LM, MacDonald DM, Mulliken JB. Complications of systemic corticosteroid therapy for problematic hemangioma. Plast Reconstr Surg. 1999;104:1616–1623. doi: 10.1097/00006534-199911000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Markiewicz-Kijewska M, Kasprzyk W, Broniszczak D, Bacewicz L, Ostoja-Chyzynska A, Ismail H, Kosciesza A, Dembowska-Baginska B, Teisseyre J, Kluge P, et al. Hemodynamic failure as an indication to urgent liver transplantation in infants with giant hepatic hemangiomas or vascular malformations--report of four cases. Pediatr Transplant. 2009;13:906–912. doi: 10.1111/j.1399-3046.2008.01050.x. [DOI] [PubMed] [Google Scholar]
- 29.Draper H, Diamond IR, Temple M, John P, Ng V, Fecteau A. Multimodal management of endangering hepatic hemangioma: impact on transplant avoidance: a descriptive case series. J Pediatr Surg. 2008;43:120–125; discussion 126. doi: 10.1016/j.jpedsurg.2007.09.030. [DOI] [PubMed] [Google Scholar]


