Abstract
AIM
To investigate evidence of clinical protection in infants after one dose of 7-valent pneumococcal conjugate vaccine (7vPCV) owing to carrier priming.
METHODS
Using Australian National Notifiable Diseases Surveillance System data, we conducted a descriptive analysis of cases of vaccine type invasive pneumococcal disease (VT-IPD) during “catch-up” years, when 7vPCV was carrier primed by prior administration of DTPa vaccine. We compared the number of VT-IPD cases occurring 2-9 wk after a single dose of 7vPCV (carrier primed), with those < 2 wk post vaccination, when no protection from 7vPCV was expected yet. Further comparison was conducted to compare the occurrence of VT-IPD cases vs non-VT-IPD cases after a single carrier-primed dose of 7vPCV.
RESULTS
We found four VT-IPD cases occurring < 2 wk after one carrier primed dose of 7vPCV while only one case occurred 2-9 wk later. Upon further comparison with the non-VT-IPD cases that occurred after one carrier primed dose of 7vPCV, two cases were detected within 2 wk, whereas seven occurred within 2-9 wk later; suggesting a substantial level of protection from VT-IPD occurring from 2 wk after carrier-primed dose of 7vPCV.
CONCLUSION
This data suggest that infants may benefit from just one dose of 7vPCV, likely through enhanced immunity from carrier priming effect. If this is proven, an adjusted 2-dose schedule (where the first dose of PCV is not given until after DTPa) may be sufficient and more cost-effective.
Keywords: Carrier priming, Conjugate vaccine, Infant, Invasive pneumococcal disease
Core tip: With the inclusion of newer conjugate vaccines with higher number of serotypes in the immunisation schedule, literature suggests that prior immunisation with tetanus/diphtheria-containing vaccines could enhance the immunogenicity of subsequently administered glycoconjugate vaccine, a phenomenon known as “carrier priming”. This analysis provides evidence of substantial clinical protection ensued after one dose of 7-valent pneumococcal conjugate vaccine as result of carrier priming. This phenomenon could be implemented to enhance the immunogenicity of conjugate vaccines among vulnerable populations such as infants in resource-poor settings, travellers, immigrants and refugees.
INTRODUCTION
Streptococcus pneumoniae (SPn) is responsible for 33% of childhood mortalities due to pneumonia worldwide[1]. Invasive pneumococcal disease (IPD) is caused by SPn and is defined as an infection confirmed by the isolation of pneumococci from a normally sterile body site, such as the blood stream and cerebrospinal fluid whereas non-invasive disease includes otitis media, sinusitis and bronchitis[2]. The incidence of IPD is often used as a measure of pneumococcal disease burden[3]. The 7-valent pneumococcal conjugate vaccine (7vPCV) was introduced to the Australian National Immunisation Program (NIP) for vaccination against SPn for medically at-risk and Indigenous children in 2001 and for all children from January 2005[4]. The dosage schedule used was three doses at 2, 4 and 6 mo of age along with other vaccines such as diphtheria, tetanus and acellular pertussis (DTPa). A concurrent catch up vaccination program was implemented for two years targeting children up to two years of age, many of whom would have received DTPa vaccine prior to their first catch up dose of 7vPCV. The use of the 3 + 0 schedule is strongly supported by a systematic review of several randomized controlled clinical trials (RCTs) of pneumonia and IPD in developing country settings[5]. A 3-dose 2-4-6 mo schedule result in the optimum antibody levels after the primary series for many serotypes. However, interestingly, the 2-dose 3-5 mo schedule demonstrated higher antibody levels for five serotypes than the 3-dose schedule (at 2-3-4 mo) and equivalent antibody responses for serotypes 6B and 23F suggesting that the optimal timing of doses is perhaps more important than the number of doses[6].
The PCV is currently available in less than 60% of countries across the world[7] as the cost of the vaccine is an important barrier. Affordability of the vaccine could be improved through adoption of schedules with reduced doses by taking the advantage of a phenomenon called “carrier priming” when PCV is administered after DTPa vaccination[8]. Carrier priming is defined as an enhanced antibody response to a glycoconjugate vaccine when an individual has been previously primed with the carrier protein[9].
PCVs utilise carrier proteins such as tetanus toxoid, diphtheria toxoid or cross-reacting material 197 of diphtheria toxin. It is apparent that there is a high resemblance between these carrier proteins and the contents of DTPa vaccine. The carrier priming effect is attributed to the development of carrier-specific T-cells in response to a preceding immunisation with a vaccine (such as DTPa) that contains antigens similar to the carrier proteins in conjugate vaccines; this has been demonstrated in various studies[8].
The catch-up vaccination program implemented in 2005 accompanying the introduction of universal 7vPCV vaccination program in Australia provides a unique opportunity to examine the potential protective effect of carrier priming on IPD. We hypothesise that due to the effect of carrier priming, the number of vaccine type-IPD (VT-IPD) cases 2-9 wk following the administration of the first dose of 7vPCV (through catch up program in those children primed with previous dose of DTPa), would be less frequent than that of the VT-IPD cases within the first two weeks post-vaccination (where no protection is expected yet). In this analysis, we compared the number of IPD occurring after the 2nd week post-vaccination until the 9th week (the time of the next dose) to that occurring two weeks post-vaccination.
MATERIALS AND METHODS
Data source and case definition
We conducted a retrospective descriptive analysis by obtaining data from the National Notifiable Diseases Surveillance System (NNDSS), Australia. IPD has been a notifiable disease in Australia since the year 2001. Laboratories, medical practitioners and allied health providers are required to report IPD cases to the health authorities. De-identified data on notified cases are reported by authorities electronically to NNDSS.
A case of IPD is defined as an identification of SPn through culture or nucleic acid testing from any normally sterile body site. The onset date is considered as the date of diagnosis. Demographic and clinical information including Indigenous status, age, vaccination status and serotype of the isolated pneumococci were collected from each case of IPD. According to the Australian NIP, all cases ≥ 2 mo old were presumed to receive dose one of DTPa. The VT-IDP was defined as the isolation of one of the serotypes contained in 7vPCV (4, 6B, 9V, 14, 18C, 19F and 23F). Isolation of other serotypes was defined as non-vaccine type-IPD (NVT-IDP). Eligibility criteria of IPD cases for analysis were non-Indigenous, infant (aged ≤ 12 mo) of both genders with no documented underlying pre-existing medical conditions.
We undertook the following comparisons: (1) the first analysis in this paper compares the number of VT-IPD within two weeks after a single carrier primed dose of 7vPCV with that occurred during 2-9 wk; (2) the second analysis compares the number of VT-IPD cases vs NVT-IPD cases after a single carrier primed dose of 7vPCV during and two weeks after vaccination; (3) the third analysis compares the number of VT-IPD cases after a single carrier primed dose of 7vPCV with the number of VT-IPD cases after non-carrier-primed dose of 7vPCV during and two weeks after vaccination; and (4) the final analysis explores herd immunity after the introduction of 7vPCV to assess the herd effect during the transitional period (when most of the analysed cases occurred). Herd immunity was explored by detecting numbers of VT-IPD among infants < 2 mo before, during and after the introduction of 7vPCV.
Ethics approval
Permission to access NNDSS data for the study was granted by the data custodian Communicable Disease Network Australia of the Australian Department of Health. Australian Capital Territory Health Human Research Ethics Committee approval was obtained as a prerequisite for data access (Reference number ETHLR.13.318).
Statistical analysis
Statistical Package for the Social Sciences (SPSS) version 22 software program (SPSS, Inc., Chicago, IL, United States) was used to carry out descriptive data analyses. Categorical variables were compared by using the one-sided fisher’s exact test. A P value ≤ 0.05 was considered statistically significant.
RESULTS
A total of 23632 IPD cases were identified and scanned for study eligibility. The number of cases included in the study was 184 among non-Indigenous Australian children who developed IPD after at least one dose of 7vPCV and were born between 1st January 2001 (when 7vPCV became available in the private market) until 31st December 2006 (when the 7vPCV catch up program ended). Of these, 108 (58.7%) were males. The majority of cases were from New South Wales [61 (33.2%)], Victoria [50 (27.2%)] and Queensland [42 (22.3%)]. Serotype was determined in 174 cases (94.5%). Final analysis in this study included 22 IPD cases with median ages as shown in Figure 1.
Figure 1.
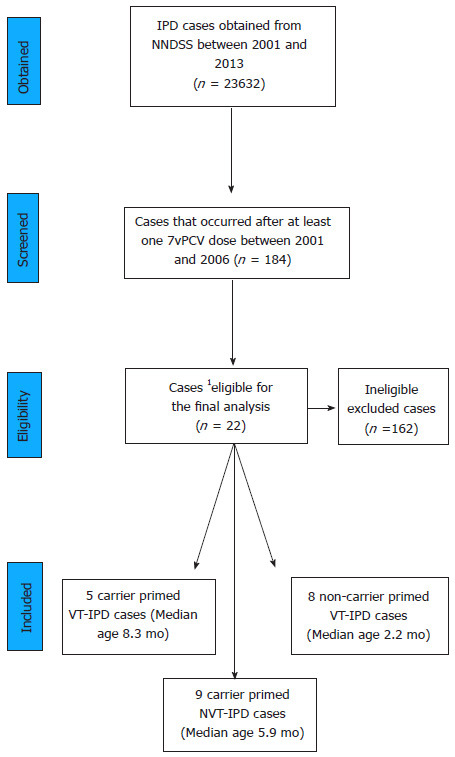
Flowchart showing the selection process of analysed cases and their median ages. 1Australian non-Indigenous immunocompetent infants with receipt of first dose of 7vPCV after diphtheria, tetanus and acellular pertussis vaccine. NNDSS: National Notifiable Diseases Surveillance System; 7vPCV: 7-valent pneumococcal conjugate vaccine; VT-IPD: Vaccine type invasive pneumococcal disease; NVT-IPD: Non-vaccine type invasive pneumococcal disease.
While examining a carrier-priming protective effect after one dose of 7vPCV, we found that four VT-IPD cases (serotypes: 23F, 4, 14 and 19F) occurred within two weeks after one carrier primed dose of 7vPCV. We did not expect the vaccine to work effectively for two weeks; we found that only one case (serotype 19F) occurred 2-9 wk later (Figure 2), indicating that one carrier primed dose could provide a substantial protection after two weeks.
Figure 2.
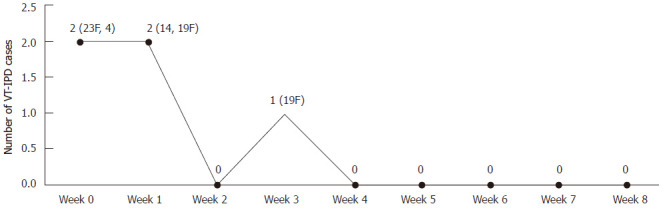
Number of vaccine type invasive pneumococcal disease cases 9 wk after a single carrier primed dose of the 7-valent pneumococcal conjugate vaccine among non-Indigenous Australian infants (2001-2006). VT-IPD: Vaccine type invasive pneumococcal disease.
Further analysis revealed that two NVT-IPD cases (serotypes: 6C and 22F) occurred within two weeks after the first carrier primed dose of 7vPCV whereas seven NVT-IPD cases (serotypes: 35B, 38 and five 10A) were reported 2-9 wk after vaccination (Figure 3). Compared to the number of VT-IPD cases after the carrier primed dose, this suggests a protective effect (P = 0.06) against VT-IPD occurring after only one carrier primed dose (Table 1).
Figure 3.
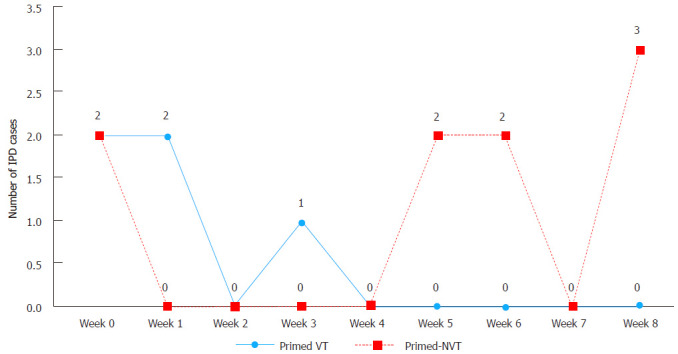
Number of vaccine type invasive pneumococcal disease compared to non-vaccine type invasive pneumococcal disease cases 9 wk after single carrier primed dose of 7-valent pneumococcal conjugate vaccine among non-Indigenous Australian infants (2001-2006). IPD: Invasive pneumococcal disease; VT: Vaccine type; NVT: Non VT.
Table 1.
Contingency table comparing the numbers of vaccine type invasive pneumococcal disease and non-vaccine type invasive pneumococcal disease cases 9 wk after single carrier primed dose of the 7-valent pneumococcal conjugate vaccine among non-Indigenous Australian infants (2001-2006)
| Two weeks after carrier primed dose of 7vPCV | 2-9 wk after carrier primed dose of 7vPCV | |
| Number of VT-IPD cases | 4 | 1 |
| Number of NVT-IPD cases | 2 | 7 |
| 1P value | 0.06 | |
One-sided fisher’s exact test. 7vPCV: 7-valent pneumococcal conjugate vaccine; VT-IPD: Vaccine type invasive pneumococcal disease; NVT-IPD: Non-vaccine type invasive pneumococcal disease.
Upon further comparison with the non-carrier primed VT-IPD cases, two VT-IPD cases (serotypes: 18C and 14) occurred within two weeks after the first dose of 7vPCV while six VT-IPD cases (serotypes: 18C, 14, 23F, 6B and two 19F) occurred 2-9 wk after vaccination (Figure 4); which although not quite significant, may indicate that protection ensued (P = 0.08) (Table 2). However, age would be a confounder in the latter comparison as the primed cases were older with possibly more mature immunity.
Figure 4.
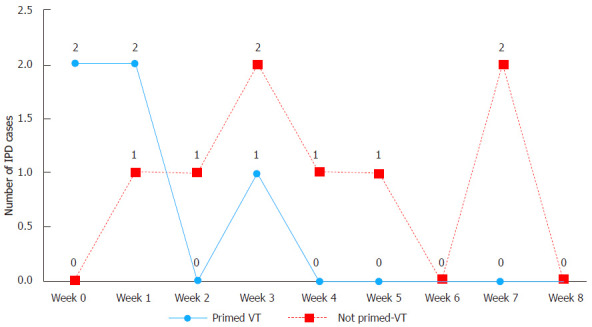
Number of vaccine type invasive pneumococcal disease cases 9 wk after a single carrier primed dose of 7-valent pneumococcal conjugate vaccine compared to the vaccine type invasive pneumococcal disease cases after non-carrier primed single 7-valent pneumococcal conjugate vaccine dose among non-Indigenous Australian infants (2001-2006). IPD: Invasive pneumococcal disease; VT: Vaccine type.
Table 2.
Contingency table comparing the numbers of vaccine type invasive pneumococcal disease cases 9 wk after carrier primed and non-carrier primed dose of the 7-valent pneumococcal conjugate vaccine among non-Indigenous Australian infants (2001-2006)
| Two weeks after dose of 7vPCV | 2-9 wk after dose of 7vPCV | |
| Number of VT-IPD cases after carrier primed 7vPCV | 4 | 1 |
| Number of VT-IPD cases after non-carrier primed 7vPCV | 2 | 6 |
| 1P value | 0.08 | |
One-sided fisher’s exact test. 7vPCV: 7-valent pneumococcal conjugate vaccine; VT-IPD: Vaccine type invasive pneumococcal disease.
Considering the fact that most of the cases included in our analysis took place during the transitional years of 2005-2006, we explored herd immunity during this transitional period to evaluate its effect. The trends shown in Table 3 and Figure 5 demonstrate little evidence of clinical protection (herd immunity) among young infants aged < 2 mo (before first vaccine dose). This suggests that herd immunity was unlikely to have contributed to the protection of young infants against IPD during observation period of our study. Therefore, the explanation for protection is likely to be the direct effect of one PCV dose enhanced by prior carrier priming.
Table 3.
Invasive pneumococcal disease cases among non-Indigenous Australian children < 6 mo (2001-2010)
|
Year |
2001-2002 |
2003-2004 |
2005-2006 |
2007-2008 |
2009-2010 |
|||||||||||
| Age | < 2 mo | 2 mo-< 4 mo | 4 mo-< 6 mo | < 2 mo | 2 mo-< 4 mo | 4 mo-< 6 mo | < 2 mo | 2 mo-< 4 mo | 4 mo-< 6 mo | < 2 mo | 2 mo-< 4 mo | 4 mo-< 6 mo | < 2 mo | 2 mo-< 4 mo | 4 mo-< 6 mo | |
| VT-IPD | Vaccinated | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 7 | 2 | 1 | 0 | 0 | 0 | 1 | 4 |
| Not vaccinated | 7 | 14 | 26 | 7 | 19 | 37 | 12 | 2 | 0 | 3 | 2 | 1 | 1 | 2 | 0 | |
| NVT-IPD | Vaccinated | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 8 | 2 | 9 | 14 | 2 | 8 | 18 |
| Not vaccinated | 8 | 7 | 13 | 14 | 6 | 13 | 13 | 3 | 3 | 7 | 3 | 5 | 14 | 3 | 4 | |
VT-IPD: Vaccine type invasive pneumococcal disease; NVT-IPD: Non-vaccine type invasive pneumococcal disease.
Figure 5.
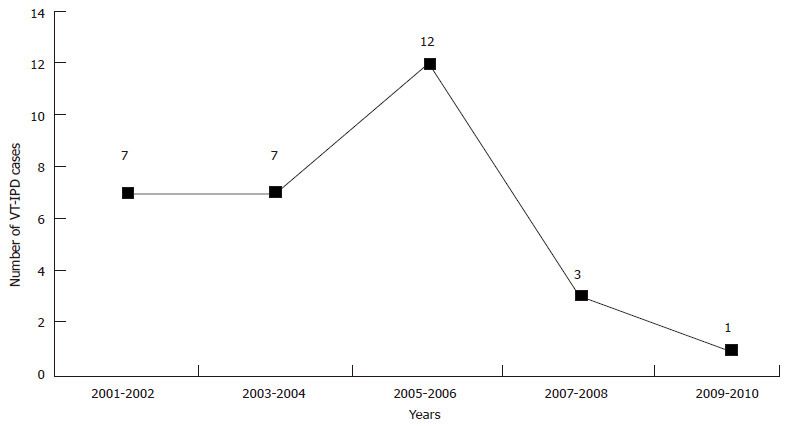
Number of vaccine type invasive pneumococcal disease cases among non-Indigenous Australian in children aged < 2 mo (2001-2010). VT-IPD: Vaccine type invasive pneumococcal disease.
DISCUSSION
Our analysis suggests that infants may receive some protection even from a single dose of 7vPCV if conjugate vaccines are offered after DTPa vaccination; this could be attributed to enhanced protection through a carrier priming effect even after one dose of vaccine. This is consistent with other incidental findings among infants, adults and even in animal models where prior exposure to DTPa or one of its components was shown to enhance the immunogenicity of subsequent PCV[6,10-13]. There is evidence from other settings that children who had not carrier primed would still be susceptible to IPD at 2-8 wk after one dose of 7vPCV (unpublished Danish IPD data, Z Harboe personal communication). It has been shown elsewhere that one dose of 7vPCV provides no significant protection to young infants in the absence of carrier priming effect[14].
Our limited data suggested that herd protection in infants was not prominent in the first two years of vaccine introduction which is not surprising as the impact on carriage takes some years, and the proportion of infants and children that were vaccinated was still low[15].
PCV is highly effective, but it is also one of the most expensive vaccines on the routine paediatric schedule, at about USD $100/dose[16]. Among Australian children < 5 years of age there were approximately 700 cases of IPD and 16 associated deaths in the year prior to universal 7vPCV introduction. In 5 years of 7vPCV use IPD due to VT declined by 97% and total IPD by 68% in these children[17]. The percentage of the world’s birth cohort living in countries with PCV in their NIPs rose from 1% in 2000 to 58% in 2014[7]. This suggests that efforts to increase PCV use globally are succeeding; however, important gaps in PCV introduction remain, notably in the World Health Organization South-East Asia Region that includes several countries with large birth cohorts but limited financial capacities to purchase these costly vaccines[18].
The implication of carrier priming raises hope for developing countries where IPD is still a major cause of morbidity and mortality[19,20]. The serotypes in the current PCV formulations account for 49%-88% of deaths in Africa, Asia and Latin America where IPD morbidity and mortality are the highest, yet many children do not have access to these vaccines[21]. Achieving sufficient immunity against pneumococcal disease in spite of sparing a dose of vaccine could be of great value to these countries.
Simply minimizing the number of doses of PCV would not likely to be beneficial unless the carrier priming effect is also harnessed. A study in Fiji by Russell et al[22,23] was conducted to explore the immunogenicity of the reduced dose schedule of 7vPCV in order to determine optimal pneumococcal vaccination strategy for poor settings. They found that the immunogenicity of three PCV doses is better than two doses with potentials for a two dose PCV primary series to offer similar protection as provided by three doses for most serotypes. They also noted that a significant protection from one dose of PCV would not continue for children throughout the highest risk period for IPD and an early booster at 6 or 9 mo of age (“1 + 1” schedule) deserves a further investigation for use in the developing world[22,23]. However, in their variable schedules/methods, they only administered DTPa with the first dose of PCV, and missed the chance to examine the effect of carrier priming.
This paper sheds light on the need for further RCTs designed specifically to detect/provide conclusive evidence of the positive impact of carrier priming. If priming occurs, there is a possibility in the third world that if DTPa vaccine is given first, at anywhere between 2 and 6 wk of age, a subsequent single dose of PCV may be at least partially protective, and a second dose 4 wk later highly protective so that a third dose may not be required, either making a substantial saving in vaccine cost or allowing the third dose of PCV to be used at a more strategic time, e.g., between 9 and 12 mo of age. We believe that this theory is applicable to other conjugate vaccines, e.g., Hib and meningococcal conjugate vaccines, irrespective of the carrier protein. Furthermore, carrier-priming phenomenon could be implemented to reinforce immunisation schedules in resource-poor settings.
We are currently investigating this innovative concept of carrier priming by RCT among adult travellers to mass gathering, where we offer DTPa before, with and after conjugate vaccines to examine the effect.
A limitation of the study is that it is retrospective and observational with a limited number of cases included in the final analysis. Additionally, the exact dates of receipt of DTPa were not accessible as the NDDS system registers only the vaccines related to the disease, in this case PCV. However, the Australian surveillance data indicated that the coverage of DTPa was ≥ 90% during that time[24]. This is the first established descriptive analysis looking at clinical evidence of carrier priming for prevention of pneumococcal disease.
In conclusion, these data suggest a favourable level of evidence of the effectiveness of one dose of PCV; this could be attributed to enhanced immunity through a carrier priming effect. If priming really occurs, an adjusted 2-dose schedule (where the first PCV is given following DTPa) may be sufficient and more cost-effective for vulnerable populations, particularly those that have used PCV for several years so that herd immunity is also operating.
COMMENTS
Background
Conjugate vaccines such as pneumococcal conjugate vaccine (PCV) have a carrier protein to enhance its immunogenicity. These carrier proteins have some similar antigens to the contents of diphtheria, tetanus and pertussis vaccine (DTP). This similarity may bring a potential interaction between PCV and DTP. This occurs as upon administering DTP before PCV which leads to development of carrier-specific T-cells resulting in an enhance immunogenicity of PCV, a phenomenon called carrier priming.
Research frontiers
Invasive pneumococcal diseases carry substantial morbidity and mortality particularly in developing countries and among vulnerable populations. Currently, infants are required to receive at least three doses of (the expensive) PCV. In this analysis, the authors propose investigating the use of carrier priming to enhance the immunogenicity of PCV in order to spare one of the three doses.
Innovations and breakthroughs
Most studies exploring conjugate vaccine interactions, examine concurrent co-administration. This unique analysis examines sequential administration and its effect on the protectiveness conjugate vaccines.
Applications
If carrier priming used judicially to enhance the immunogenicity of PCV, an adjusted 2-dose schedule (where the first PCV is given after DTPa) may be sufficient and cost-effective.
Terminology
Invasive pneumococcal disease (IPD): Infection confirmed by the isolation of pneumococci from a normally sterile body site, such as the blood stream, cerebrospinal fluid and joint fluid. Vaccine type invasive pneumococcal disease (VT-IPD): IPD caused by one of the pneumococcal serotype that is included in the pneumococcal vaccine. Non-vaccine type invasive pneumococcal disease (NVT-IPD): IPD caused by one of the pneumococcal serotype that is not included in the pneumococcal vaccine. Carrier priming: Enhanced antibody response to a glycoconjugate vaccine when an individual has been previously primed with the carrier protein. Carrier primed IPD case: IPD case that occurred after one dose of PCV that was administered at least one dose of DTP vaccine. Non-carrier primed VT-IPD cases: IPD case that occurred after one dose of PCV without previous exposure to at least one dose of DTP vaccine.
Peer-review
This is an interesting descriptive analysis investigating evidence for clinical protection in infants after one dose of the 7-valent PCV as a result of possible prior carrier priming from Tdap vaccine administration. It provides evidence for efficacy of reduced PCV schedule if administered following Tdap vaccination. This is valuable especially for developing countries as saving cost.
Footnotes
Institutional review board statement: Access to data was obtained by a formal permission of the Department of Health and Ageing and the Australian Capital Territory Health Human Research Ethics Committee, approval reference number (ETHLR.13.318).
Informed consent statement: Patients were not required to give informed consent to the study because the analysis used anonymous clinical data.
Conflict-of-interest statement: Professor Robert Booy has received funding from Baxter, CSL, GSK, Merck, Novartis, Pfizer, Roche, Romark and Sanofi Pasteur for the conduct of sponsored research, travel to present at conferences or consultancy work; all funding received is directed to research accounts at the Children’s Hospital at Westmead; Dr Harunor Rashid has received fees from Pfizer and Novartis for consulting or serving on an advisory board; the other authors have declared no conflict of interest in relation to this work.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Pediatrics
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: May 20, 2016
First decision: June 6, 2016
Article in press: July 22, 2016
P- Reviewer: Krishnan T, Moschovi MA, Pourshafie MR S- Editor: Gong XM L- Editor: A E- Editor: Wu HL
References
- 1.Rudan I, O’Brien KL, Nair H, Liu L, Theodoratou E, Qazi S, Lukšić I, Fischer Walker CL, Black RE, Campbell H. Epidemiology and etiology of childhood pneumonia in 2010: estimates of incidence, severe morbidity, mortality, underlying risk factors and causative pathogens for 192 countries. J Glob Health. 2013;3:010401. doi: 10.7189/jogh.03.010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Randle E, Ninis N, Inwald D. Invasive pneumococcal disease. Arch Dis Child Educ Pract Ed. 2011;96:183–190. doi: 10.1136/adc.2010.191718. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organisation. Pneumococcal vaccines WHO position paper - 2012. Available from: http: //www.who.int/wer/2012/wer8714.pdf.
- 4.Australian Government Department of Health. The Australian Immunisation Handbook 2013 (10th ed [updated 2015 Jun]) Available from: http://www.immunise.health.gov.au/internet/immunise/publishing.nsf/Content/7B28E87511E08905CA257D4D001DB1F8/$File/Aus-Imm-Handbook.pdf.
- 5.Conklin L, Knoll MD, Loo J, Fleming-Dutra K, Park D, Johnson TS, Kirk J, Goldblatt D, O’Brien KL, Whitney CG. Landscape analysis of pneumococcal conjugate vaccine dosing schedules: A systematic review (Sub-report on the 3-dose schedules. A project of the AVI Technical Assistance Consortium (AVI-TAC) Final Report 1. 0. 2011) Available from: http: //www.who.int/immunization/sage/3_Conklin_L_PCV_Dosing_Landscape_Report_Oct_17_2011_FINAL_nov11.pdf.
- 6.Spijkerman J, Veenhoven RH, Wijmenga-Monsuur AJ, Elberse KE, van Gageldonk PG, Knol MJ, de Melker HE, Sanders EA, Schouls LM, Berbers GA. Immunogenicity of 13-valent pneumococcal conjugate vaccine administered according to 4 different primary immunization schedules in infants: a randomized clinical trial. JAMA. 2013;310:930–937. doi: 10.1001/jama.2013.228052. [DOI] [PubMed] [Google Scholar]
- 7.Murray J, Agócs M, Serhan F, Singh S, Deloria-Knoll M, O’Brien K, Mwenda JM, Mihigo R, Oliveira L, Teleb N, et al. Global invasive bacterial vaccine-preventable diseases surveillance--2008-2014. MMWR Morb Mortal Wkly Rep. 2014;63:1159–1162. [PMC free article] [PubMed] [Google Scholar]
- 8.Pobre K, Tashani M, Ridda I, Rashid H, Wong M, Booy R. Carrier priming or suppression: understanding carrier priming enhancement of anti-polysaccharide antibody response to conjugate vaccines. Vaccine. 2014;32:1423–1430. doi: 10.1016/j.vaccine.2014.01.047. [DOI] [PubMed] [Google Scholar]
- 9.Kurikka S. Priming with diphtheria-tetanus-pertussis vaccine enhances the response to the Haemophilus influenzae type b tetanus conjugate vaccine in infancy. Vaccine. 1996;14:1239–1242. doi: 10.1016/s0264-410x(96)00025-4. [DOI] [PubMed] [Google Scholar]
- 10.Goldblatt D, Southern J, Andrews N, Ashton L, Burbidge P, Woodgate S, Pebody R, Miller E. The immunogenicity of 7-valent pneumococcal conjugate vaccine versus 23-valent polysaccharide vaccine in adults aged 50-80 years. Clin Infect Dis. 2009;49:1318–1325. doi: 10.1086/606046. [DOI] [PubMed] [Google Scholar]
- 11.Lucero MG, Puumalainen T, Ugpo JM, Williams G, Käyhty H, Nohynek H. Similar antibody concentrations in Filipino infants at age 9 months, after 1 or 3 doses of an adjuvanted, 11-valent pneumococcal diphtheria/tetanus-conjugated vaccine: a randomized controlled trial. J Infect Dis. 2004;189:2077–2084. doi: 10.1086/420849. [DOI] [PubMed] [Google Scholar]
- 12.Peeters CC, Tenbergen-Meekes AM, Poolman JT, Beurret M, Zegers BJ, Rijkers GT. Effect of carrier priming on immunogenicity of saccharide-protein conjugate vaccines. Infect Immun. 1991;59:3504–3510. doi: 10.1128/iai.59.10.3504-3510.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shelly MA, Pichichero ME, Treanor JJ. Low baseline antibody level to diphtheria is associated with poor response to conjugated pneumococcal vaccine in adults. Scand J Infect Dis. 2001;33:542–544. doi: 10.1080/00365540110026502. [DOI] [PubMed] [Google Scholar]
- 14.Mahon BE, Hsu K, Karumuri S, Kaplan SL, Mason EO, Pelton SI. Effectiveness of abbreviated and delayed 7-valent pneumococcal conjugate vaccine dosing regimens. Vaccine. 2006;24:2514–2520. doi: 10.1016/j.vaccine.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 15.van Hoek AJ, Sheppard CL, Andrews NJ, Waight PA, Slack MP, Harrison TG, Ladhani SN, Miller E. Pneumococcal carriage in children and adults two years after introduction of the thirteen valent pneumococcal conjugate vaccine in England. Vaccine. 2014;32:4349–4355. doi: 10.1016/j.vaccine.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 16.Centers of Disease Control and Prevention. CDC vaccine price list (web page) (2013) Available from: http://www.cdc.gov/vaccines/programs/vfc/awardees/vaccine-management/price-list/2013/2013-07-01.html.
- 17.Jayasinghe S, Chiu C, Menzies R, Lehmann D, Cook H, Giele C, Krause V, McIntyre P. Evaluation of impact of 23 valent pneumococcal polysaccharide vaccine following 7 valent pneumococcal conjugate vaccine in Australian Indigenous children. Vaccine. 2015;33:6666–6674. doi: 10.1016/j.vaccine.2015.10.089. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention (CDC) Progress in introduction of pneumococcal conjugate vaccine - worldwide, 2000-2012. MMWR Morb Mortal Wkly Rep. 2013;62:308–311. [PMC free article] [PubMed] [Google Scholar]
- 19.Berkley JA, Lowe BS, Mwangi I, Williams T, Bauni E, Mwarumba S, Ngetsa C, Slack MP, Njenga S, Hart CA, et al. Bacteremia among children admitted to a rural hospital in Kenya. N Engl J Med. 2005;352:39–47. doi: 10.1056/NEJMoa040275. [DOI] [PubMed] [Google Scholar]
- 20.Brent AJ, Ahmed I, Ndiritu M, Lewa P, Ngetsa C, Lowe B, Bauni E, English M, Berkley JA, Scott JA. Incidence of clinically significant bacteraemia in children who present to hospital in Kenya: community-based observational study. Lancet. 2006;367:482–488. doi: 10.1016/S0140-6736(06)68180-4. [DOI] [PubMed] [Google Scholar]
- 21.Johnson HL, Deloria-Knoll M, Levine OS, Stoszek SK, Freimanis Hance L, Reithinger R, Muenz LR, O’Brien KL. Systematic evaluation of serotypes causing invasive pneumococcal disease among children under five: the pneumococcal global serotype project. PLoS Med. 2010;7:pii: e1000348. doi: 10.1371/journal.pmed.1000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russell FM, Carapetis JR, Burton RL, Lin J, Licciardi PV, Balloch A, Tikoduadua L, Waqatakirewa L, Cheung YB, Tang ML, et al. Opsonophagocytic activity following a reduced dose 7-valent pneumococcal conjugate vaccine infant primary series and 23-valent pneumococcal polysaccharide vaccine at 12 months of age. Vaccine. 2011;29:535–544. doi: 10.1016/j.vaccine.2010.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russell FM, Balloch A, Tang ML, Carapetis JR, Licciardi P, Nelson J, Jenney AW, Tikoduadua L, Waqatakirewa L, Pryor J, et al. Immunogenicity following one, two, or three doses of the 7-valent pneumococcal conjugate vaccine. Vaccine. 2009;27:5685–5691. doi: 10.1016/j.vaccine.2009.06.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hull B, Deeks S, Menzies R, McIntyre P. Immunisation coverage annual report, 2007. Commun Dis Intell Q Rep. 2009;33:170–187. [PubMed] [Google Scholar]


