SUMMARY
SETTING
To assess the revised World Health Organization-recommended dose of 10–20 mg/kg rifampicin (RMP), we studied the steady state pharmacokinetics of RMP in South African children who received standard treatment for drug-susceptible tuberculosis (TB).
OBJECTIVE
To determine the formulation effect on the pharmacokinetics of RMP.
DESIGN
RMP plasma concentrations were characterised in 146 children (median age 1.4 years, range 0.2–10.2). The morning dose on the day of the pharmacokinetic evaluation was administered as one of two RMP single-drug oral suspensions.
RESULTS
While one formulation achieved 2 h concentrations in the range of those observed in adults (median 6.54 mg/l, interquartile range [IQR] 4.47–8.84), the other attained a median bioavailability of only 25% of this, with a median 2 h concentration of 1.59 mg/l (IQR 0.89–2.38).
CONCLUSION
RMP is a key drug for the treatment of TB. It is critical that the quality of RMP suspensions used to treat childhood TB is ensured.
Keywords: tuberculosis, first-line drug, quality assurance, children
Few studies have been conducted in children to support drug dosing for tuberculosis (TB) or to evaluate drug formulations.1 Instead, paediatric doses have generally been extrapolated from those used in adults. The World Health Organization (WHO) reviewed the evidence on first-line anti-tuberculosis drug formulation and dosage in children and found that rifampicin (RMP) concentrations were low and varied widely between and within studies.2,3 Although sampling processes and assay methods varied across studies, and forms of dosage, methods of administration and dosing schedules were not consistently described, experts concluded that the RMP dose in children needed to be increased to achieve concentrations comparable to those in adults. The WHO accordingly revised its dosing guidelines in 2010, recommending a 50% increase in the dose of RMP, from 10 (8–12) to 15 (10–20) mg/kg.4 We evaluated the pharmacokinetics of RMP, isoniazid (INH), pyrazinamide (PZA) and ethambutol (EMB) in children with TB at doses consistent with the revised WHO guidelines. Here we report findings regarding formulation effects on the pharmacokinetics of RMP.
STUDY POPULATION AND METHODS
Children aged < 12 years diagnosed with drug-susceptible TB who were receiving RMP, INH and PZA (with or without EMB or ethionamide) in daily doses at Red Cross Children’s Hospital, Tygerberg Hospital and Khayelitsha District Hospital, Cape Town, South Africa, were enrolled. The children were treated using fixed-dose combination (FDC) products available in the public health sector, with doses approximating the WHO’s 2010 guidelines.4 Children underwent pharmacokinetic evaluation after at least 2 weeks of anti-tuberculosis treatment. On the day of pharmacokinetic evaluation, single-drug formulations of registered products were administered by the study team, as FDC products providing doses in accordance with the revised WHO recommendations are not yet available. A granulate preparation of RMP for suspension (Eremfat®, RIEMSER Arzneimittel, Germany, registered for use in several European countries) was reconstituted to a concentration of 100 mg/5 ml and administered in accurately measured doses of 10–20 mg/kg using a syringe, or via a nasogastric tube in very young children. Due to an interruption in the supply of Eremfat, R-Cin® suspension (100 mg/5 ml; Aspen Pharmacare, Durban, South Africa, registered for use in South Africa) was used in subsequently enrolled children.
Serial 0.6 ml blood samples were drawn to determine the plasma pharmacokinetics of the anti-tuberculosis drugs. A pre-dose sample and samples at 2 and 4 h after the dose were drawn in all 146 children. An additional sample was drawn at 8 h in 86 children; in 20 children additional samples were taken at 1, 6 and 10 h; and in 40 children an additional sample was drawn at 1, 6 and 8 h after the dose. The samples were centrifuged to separate the plasma within 0.5 h of sampling and stored immediately at −70°C until analysis.
The study (NCT01637558) protocol was approved by the Human Research Ethics Committee of the Faculty of Health Sciences, University of Cape Town, Cape Town, and the South African Medicines Control Council, Pretoria, South Africa.
RMP plasma concentrations were determined using a liquid chromatography–mass spectrometry assay validated according to Federal Drug Administration (FDA) and European Medicines Agency (EMA) guidelines.4–6 The samples were processed with a protein precipitation extraction method using 20 μl plasma with 500 μl acetonitrile containing a stable isotope-labelled internal standard, RMP-D3. Five microliters of the supernatant were injected onto the high-performance liquid chromatography column. Chromatographic separation was achieved on a Discovery C18, 5 μm, 50 × 4.6 mm analytical column using acetonitrile, methanol and 0.1% formic acid in water (6:1:3, v/v/v) as the mobile phase, delivered at a constant flow rate of 400 μl/min. An AB Sciex API 3000 mass spectrometer (GenTech, Arcade, NY, USA) was operated at unit resolution in the multiple-reaction monitoring mode, monitoring the transition of the protonated molecular ions at m/z 823.4 to the product ions at m/z 791.4 for RMP and the protonated molecular ions at m/z 826.5 to the product ions at m/z 794.4 for the internal standard. Electrospray ionisation was used for ion production. The assay was validated over the concentration range of 0.117–30 μg/ml. The combined accuracy and precision statistics of the limit of quantification, low, medium and high quality controls (three validation batches, n=18) were between 101% and 107%, and 2.7% and 3.7%, respectively.
A truncated area under the RMP concentration-time curve to 4 h after the dose (AUC0–4) was computed as a measure of systemic RMP exposure using the concentrations from the sampling times common to all children (pre-dose, 2 and 4 h) in a non-compartmental analysis. Concentrations below the limit of quantification of the assay (respectively 95%, 0% and 2% for the pre-dose, 2 h and 4 h concentrations) were imputed a value of 0.06 mg/l. Differences between the groups of children receiving Eremfat and R-Cin RMP suspensions were compared using the Mann-Whitney U-test. Quantile regression was used as a robust approach to adjust for the effects of age, sex, dose per kg of body weight, human immunodeficiency virus (HIV) status and administration by nasogastric tube when evaluating the effect of formulation on AUC0–4. Each of these variables was tested separately, and then added to the base model describing the effect of formulation on the AUC0–4. Covariate effects with P < 0.2 were retained in the final model. Non-compartmental analysis and all statistical analyses were performed using Stata 13.1 (StataCorp LP, College Station, TX, USA).
The RMP content of the Eremfat and R-Cin formulations was compared using product batches used during the study. A fresh suspension of Eremfat was prepared as described in the product insert. The suspension was shaken well before 200 μl was added to 40 ml methanol. Similarly, 200 μl of the well-shaken R-Cin suspension from a freshly opened bottle was added to 40 ml methanol. The two suspensions were chromatographed with a gradient high-performance liquid chromatography-ultraviolet assay at a flow rate of 0.4 ml/min. Mobile phase A consisted of a mixture of 0.1% formic acid in water and acetonitrile (85:15, v/v), and mobile phase B consisted of 0.1% formic acid in acetonitrile. A mobile phase gradient was run from 100% A to 100% B over 3 min and remained at 100% B for another minute before returning to 100% over 0.5 min. Five microliters were injected onto a Phenomenex Max-RP 3 μ 50×2 mm column (Phenomenex, Torrance, CA, USA), and RMP was detected at a wavelength of 334 nm. A similar method was used to evaluate the effect of passage through a nasogastric tube on the RMP concentrations in a RMP suspension.
RESULTS
Among the 146 children included in the analysis, 92 received Eremfat and 54 received R-Cin formulations (Table 1). Children receiving R-Cin RMP suspension were younger (median 0.88 vs. 1.97 years, P < 0.001), and thus had lower body weight (median 8.37 vs. 11.48 kg, P < 0.001). They also received slightly higher doses of RMP in mg/kg (median 16.51 vs. 14.95). The children underwent pharmacokinetic sampling a median of 1.3 months (interquartile range [IQR] 1.1–1.6) after starting anti-tuberculosis treatment.
Table 1.
Characteristics of enrolled children, RMP dose per kg of body weight, and administration by nasogastric tube on the day of pharmacokinetic evaluation by RMP formulation
| Formulation | Eremfat® RMP (n = 92) median [IQR] |
R-Cin® RMP (n = 54) median [IQR] |
P value |
|---|---|---|---|
| Age, years | 1.97 [0.94–4.37] | 0.88 [0.5–2.47] | < 0.001 |
| Weight, kg | 11.48 [8.27–15.45] | 8.37 [5.7–13.3] | < 0.001 |
| HIV-infected, n (%)* | 5 (5) | 6 (11) | 0.217 |
| Female, n (%) | 43 (47) | 17 (31) | 0.070 |
| Dose, mg/kg | 14.95 [12.20–16.68] | 16.51 [14.85–18.67] | 0.002 |
| Nasogastric tube, n (%) | 14 (15) | 26 (48) | < 0.001 |
At the time of pharmacokinetic sampling, four children had not started antiretroviral treatment, one child was receiving an efavirenz-based regimen and the remaining children were on lopinavir/ritonavir with two nucleoside reverse transcriptase inhibitors.
RMP = rifampicin; IQR = interquartile range; HIV = human immunodeficiency virus.
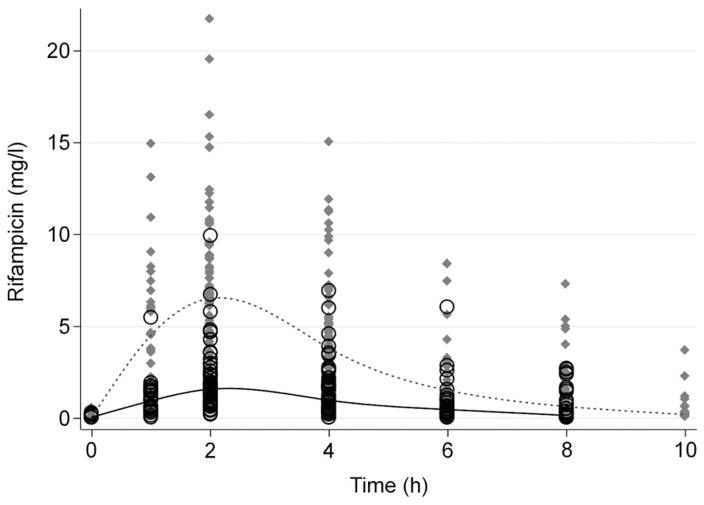
Plasma RMP concentrations by time after dose are shown in the Figure. The median 2 h concentration was 6.54 mg/l (IQR 4.47–8.84) in children receiving Eremfat, higher than the 1.59 mg/l (IQR 0.89–2.38) for those receiving R-Cin (Figure). The median RMP AUC0–4 was respectively 16.85 (IQR 11.80–23.24) and 4.19 (IQR 2.68–6.68) mg.h/l in the groups who received Eremfat and R-Cin RMP. When stratified by age, the RMP AUC0–4 remained consistently lower in children receiving R-Cin than in those who received Eremfat (Table 2, P < 0.001).
Figure.
Plasma RMP concentrations in 146 children during the pharmacokinetic sampling interval for children who received Eremfat® RMP (grey diamonds) and R-Cin® RMP (black circles). The dotted line and solid lines track the median splines for the concentrations after doses of Eremfat and R-Cin, respectively, over time. RMP = rifampicin.
Table 2.
Truncated RMP AUC (AUC0–4, mg.h/l) by age group and RMP formulation
| Age group years | Eremfat® RMP median [IQR] |
R-Cin® RMP median [IQR] |
P value |
|---|---|---|---|
| < 1 | 14.33 [8.31–18.48] [n = 26] | 4.65 [3.26–6.68] [n = 31] | < 0.001 |
| 1–< 2 | 18.49 [14.16–23.24] [n = 20] | 2.53 [2.32–4.69] [n = 8] | < 0.001 |
| 2–< 5 | 21.72 [15.90–30.15] [n = 25] | 4.23 [3.17–8.56] [n = 11] | < 0.001 |
| 5–< 12 | 15.65 [11.47–17.55] [n = 21] | 4.02 [2.50–8.60] [n = 4] | 0.014 |
RMP = rifampicin; AUC = area under the curve; IQR = interquartile range.
In univariate quantile regression, R-Cin was associated with a 12.81 mg.h/l (95% confidence interval [CI] −15.39 to −10.23) reduction in AUC0–4 compared to Eremfat RMP. Age, sex, HIV status or administration by nasogastric tube had no impact on the model describing the effect of formulation on AUC0–4. In the multivariate model adjusted for the effect of RMP dose per kg of body weight (AUC0–4 increased by 0.48 mg.h/l, 95%CI −0.03 to 0.10, for each 1 mg/kg increase in the dose), the strength of association slightly increased, with administration of R-Cin being associated with a 13.30 mg.h/l (95%CI −16.40 to −10.20) reduction in AUC0–4 compared to the administration of Eremfat RMP.
The RMP content of the two suspensions from the respective batches used during the study were equivalent, with RMP peak areas on the chromatogram for Eremfat and R-Cin solutions of respectively 2180 and 2047.
DISCUSSION
Under the National TB Control Programme, dispersible FDCs are used to treat the majority of South African children with TB. However, commercial suspensions may be useful in the most vulnerable of children requiring individualised care, including the very young or critically ill.
RMP exposures were dependent on the paediatric product used and were 76% lower among children who received the R-Cin suspension than in those who received Eremfat. While the children who received R-Cin were younger and weighed less, they received, on average, a higher dose of RMP per kg of body weight, and the differences in AUC0–4 between the two groups remained high (79%) even when adjusted for the effect of dose per kg of body weight.
For adults, a 2 h target RMP concentration of 8–20 mg/l has been proposed,7 and recent studies suggest that even higher exposures may be more effective.8–10 Despite the administration of a 15 mg/kg dose, as recommended in the most recent WHO guidelines, the median 2 h concentration achieved was only 1.59 mg/l (IQR 0.89–2.38) for children receiving R-Cin. Those receiving Eremfat RMP had a median 2 h concentration of 6.54 mg/l (IQR 4.47–8.84), in keeping with concentrations reported in adults with TB on standard anti-tuberculosis treatment,11 but lower than the proposed target of 8–20 mg/l for patients on standard treatment.
Although RMP may be adsorbed to certain plastics, the results of the multivariate analysis found that administration by nasogastric tube did not exert a detectable effect on RMP exposures in the children. We also measured RMP concentrations in an RMP suspension passed through a nasogastric tube. Aliquots of suspension left to stand for 30 min at room temperature in three nasogastric tubes were found to have similar RMP concentrations to a control aliquot of the suspension (data not shown).
Several reports of substandard and counterfeit RMP-containing products have been published. In most instances, these reports are based on in vitro tests such as the concentration of the active pharmaceutical ingredient and disintegration.12–14 An analysis of the RMP concentrations in the two formulations studied revealed almost identical RMP content. This indicates that the amount of RMP in R-Cin was adequate, and that the drug was stable in the suspension. We therefore hypothesise that the differences observed in bioavailability are due to the mixture of polymorphic forms of RMP in R-Cin that was not favourable for absorption. Low RMP concentrations attributed to formulation effects have been reported in adults with TB.15,16
Production of the active pharmaceutical ingredient is complex and can lead to forms of the molecule with variable solubility.17 RMP exists in anhydrous polymorphic forms (Form I and Form II), and also in amorphous form.18 As Form II is metastable, suspension my result in phase transition to a more stable form with subsequent crystal growth. The water solubility of RMP is reported to vary eight-fold depending on the crystalline state of the material,19 altered particle size affects solubility,20 and altered solubility is likely to affect bioavailability. However, the relationship between solid-state RMP, dissolution and bioavailability characteristics is poorly understood. Regulatory authorities, including the FDA and the EMA, would generally require in vivo bioequivalence testing for a suspension of a drug such as RMP.21,22 Likewise, in vivo bioequivalence studies are generally required for suspensions under the South African Medicines Control Council (MCC) guidelines. Although the guidelines state that waivers based on comparative dissolution studies may be acceptable,23 this condition should not be applied to a Biopharmaceutics Classification System Class II drug such as RMP.24 The methods used to test the active pharmaceutical ingredient and final product, R-Cin, and the results of such tests that were used to obtain MCC approval, are unknown to the investigators, as both the manufacturer and the regulatory authority regard this information as confidential.
Our study was not designed to compare the bioavailability of the formulations used, and the estimates of the effect of formulation type on the bioavailability of RMP were limited by a relatively small sample size for accurate adjustment for potentially confounding factors such as age, weight and nasogastric tube use. Nor were accurate measures of disease severity available for inclusion in the multivariate analysis. However, the massive impact of formulation on RMP exposures in these children is clear.
CONCLUSIONS
Our incidental findings reveal extremely low RMP concentrations in children as a result of very poor RMP bioavailability in a suspension licensed in South Africa. The findings raise important questions about the quality of the RMP-containing formulations available for children and the procedures in place to protect children from products that do not deliver adequate drug exposures.
Acknowledgments
The authors would like to acknowledge the contributions of N Kramer, M Prins, M Solomons and R Streicher. The research reported in this publication was supported by the National Institutes of Health (NIH; Bethesda, MD), Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD, Bethesda, MD) (R01HD069175) and TB Alliance [S003410]. In addition, grants from NIH’s National Institute of Allergy and Infectious Diseases (NIAID, Bethesda, MD) (UM1 AI068634, UM1 AI068636 and UM1 AI106701, U01 AI068632), and National Institute of Mental Health (NIMH, Bethesda, MD) (AI068632) supported the work. GM and HM were supported in part by the National Research Foundation of South Africa, Pretoria, South Africa (grants 85810 and 90729, respectively).
Footnotes
Conflicts of interest: none declared.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the any of the funders.
References
- 1.Duke T, Fuller D. Randomised controlled trials in child health in developing countries: trends and lessons over 11 years. Arch Dis Child. 2014;99:615–620. doi: 10.1136/archdischild-2013-305702. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Rapid advice: treatment of tuberculosis in children. Geneva, Switzerland: WHO; 2010. WHO/HTM/TB/2010.13. [PubMed] [Google Scholar]
- 3.Donald PR, Maritz JS, Diacon AH. The pharmacokinetics and pharmacodynamics of rifampicin in adults and children in relation to the dosage recommended for children. Tuberculosis. 2011;91:196–207. doi: 10.1016/j.tube.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Guidance for national tuberculosis programmes on the management of tuberculosis in children. 2. Geneva, Switzerland: WHO; 2014. WHO/HTM/TB/2014.03. [PubMed] [Google Scholar]
- 5.Center for Drug Evaluation and Research, Food & Drug Administration. Guidance for industry: bioanalytical method validation. WashingtonDC, USA: FDA; 2013. [Accessed March 2016]. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm368107.pdf. [Google Scholar]
- 6.European Medicines Agency. Guideline on bioanalytical method validation. London, UK: EMA; 2011. [Accessed March 2016]. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/08/WC500109686.pdf. [Google Scholar]
- 7.Alsultan A, Peloquin CA. Therapeutic drug monitoring in the treatment of tuberculosis: an update. Drugs. 2014;74:839–854. doi: 10.1007/s40265-014-0222-8. [DOI] [PubMed] [Google Scholar]
- 8.Chigutsa E, Pasipanodya JG, Visser ME, et al. Impact of nonlinear interactions of pharmacokinetics and MICs on sputum bacillary kill rates as a marker of sterilizing effect in tuberculosis. Antimicrob Agents Chemother. 2015;59:38–45. doi: 10.1128/AAC.03931-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruslami R, Ganiem AR, Dian S, et al. Intensified regimen containing rifampicin and moxifloxacin for tuberculous meningitis: an open-label, randomised controlled phase 2 trial. Lancet Infect Dis. 2013;13:27–35. doi: 10.1016/S1473-3099(12)70264-5. [DOI] [PubMed] [Google Scholar]
- 10.Boeree MJ, Hoelscher M. High-dose rifampin, SQ109 and moxifloxacin for treating TB: the PanACEA MAMS-TB Trial. 22nd Conference on Retroviruses and Opportunistic Infections; 23–26 February 2015; Seattle, WA, USA. [Accessed April 2016]. Abstract 95LB http://www.croiconference.org/sessions/high-dose-rifampin-sq109-and-moxifloxacin-treating-tb-panacea-mams-tb-trial. [Google Scholar]
- 11.McIlleron H, Wash P, Burger A, Norman J, Folb PI, Smith P. Determinants of rifampin, isoniazid, pyrazinamide, and ethambutol pharmacokinetics in a cohort of tuberculosis patients. Antimicrob Agents Chemother. 2006;50:1170–1177. doi: 10.1128/AAC.50.4.1170-1177.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bate R, Jensen P, Hess K, Mooney L, Milligan J. Substandard and falsified anti-tuberculosis drugs: a preliminary field analysis. Int J Tuberc Lung Dis. 2013;17:308–311. doi: 10.5588/ijtld.12.0355. [DOI] [PubMed] [Google Scholar]
- 13.Seear M, Gandhi D, Carr R, Dayal A, Raghavan D, Sharma N. The need for better data about counterfeit drugs in developing countries: a proposed standard research methodology tested in Chennai, India. J Clin Pharm Ther. 2011;36:488–495. doi: 10.1111/j.1365-2710.2010.01198.x. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. Counterfeit and substandard drugs in Myanmar and Viet Nam. Geneva, Switzerland: WHO; 1999. WHO/EDM/QSM/99.3. [Google Scholar]
- 15.McIlleron H, Wash P, Burger A, Folb P, Smith P. Widespread distribution of a single drug rifampicin formulation of inferior bioavailability in South Africa. Int J Tuberc Lung Dis. 2002;6:356–361. [PubMed] [Google Scholar]
- 16.van Crevel R, Alisjahbana B, de Lange WC, et al. Low plasma concentrations of rifampicin in tuberculosis patients in Indonesia. Int J Tuberc Lung Dis. 2002;6:497–502. doi: 10.5588/09640569513002. [DOI] [PubMed] [Google Scholar]
- 17.Cavenaghi R. Rifampicin raw material characteristics and their effect on bioavailability. Bull Int Union Tuberc Lung Dis. 1989;64(1):36–37. [PubMed] [Google Scholar]
- 18.Henwood SQ, de Villiers MM, Liebenberg W, Lötter AP. Solubility and dissolution properties of generic rifampicin raw materials. Drug Dev Ind Pharm. 2000;26:403–408. doi: 10.1081/ddc-100101246. [DOI] [PubMed] [Google Scholar]
- 19.Henwood SQ, Liebenberg W, Tiedt LR, Lötter AP, de Villiers MM. Characterization of the solubility and dissolution properties of several new rifampicin polymorphs, solvates, and hydrates. Drug Dev Ind Pharm. 2001;27:1017–1030. doi: 10.1081/ddc-100108364. [DOI] [PubMed] [Google Scholar]
- 20.Agrawal S, Ashokraj Y, Bharatam PV, Pillai O, Panchagnula R. Solid-state characterization of rifampicin samples and its biopharmaceutic relevance. Eur J Pharm Sci. 2004;22:127–144. doi: 10.1016/j.ejps.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 21.Food and Drug Administration. Draft guidance. US Department of Health and Human Services, FDA; 2013. [Accessed March 2016]. Bioequivalence studies with pharmacokinetic endpoints for drugs submitted under an ANDA. http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm. [Google Scholar]
- 22.European Medicines Agency. Note for guidance on the investigation of bioavailability and bioequivalence. London, UK: European Agency for the Evaluation of Medicinal Products; 2000. [Accessed March 2016]. CPMP/EWP/QWP/1401/98. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003519.pdf. [Google Scholar]
- 23.Medicines Control Council, Department of Health, Republic of South Africa. Biostudies. Pretoria, South Africa: MCC; 2105. [Accessed March 2016]. 2.06_Biostudies_Jun15_v6. http://www.mccza.com/documents/61de452d2.06_Biostudies_Jun15_v6.pdf. [Google Scholar]
- 24.Becker C, Dressman JB, Junginger HE, et al. Biowaiver monographs for immediate release solid oral dosage forms: rifampicin. J Pharm Sci. 2009;98:2252–2267. doi: 10.1002/jps.21624. [DOI] [PubMed] [Google Scholar]