It is suggested the physical properties of the motor unit (MU) influence firing rate characteristics; however, no study has examined firing rate characteristics in relation to the physical properties of the MU in vivo. In this study, there was a strong relationship between MU firing rate characteristics, particularly of higher-threshold MUs, at a target force level and type I percent myosin heavy chain isoform content of the vastus lateralis during a moderate-intensity contraction.
Keywords: motor unit firing rates, myosin heavy chain, onion skin control scheme, surface EMG decomposition, vastus lateralis
Abstract
It is suggested that firing rate characteristics of motor units (MUs) are influenced by the physical properties of the muscle. However, no study has correlated MU firing rates at recruitment, targeted force, or derecruitment with the contractile properties of the muscle in vivo. Twelve participants (age = 20.67 ± 2.35 yr) performed a 40% isometric maximal voluntary contraction of the leg extensors that included linearly increasing, steady force, and decreasing segments. Muscle biopsies were collected with myosin heavy chain (MHC) content quantified, and surface electromyography (EMG) was recorded from the vastus lateralis. The EMG signal was decomposed into the firing events of single MUs. Slopes and y-intercepts were calculated for 1) firing rates at recruitment vs. recruitment threshold, 2) mean firing rates at steady force vs. recruitment threshold, and 3) firing rates at derecruitment vs. derecruitment threshold relationships for each subject. Correlations among type I %MHC isoform content and the slopes and y-intercepts from the three relationships were examined. Type I %MHC isoform content was correlated with MU firing rates at recruitment (y-intercepts: r = −0.577; slopes: r = 0.741) and targeted force (slopes: r = 0.853) vs. recruitment threshold and MU firing rates at derecruitment (y-intercept: r = −0.597; slopes: r = 0.701) vs. derecruitment threshold relationships. However, the majority of the individual MU firing rates vs. recruitment and derecruitment relationships were not significant (P > 0.05) and, thus, revealed no systematic pattern. In contrast, MU firing rates during the steady force demonstrated a systematic pattern with higher firing rates for the lower- than higher-threshold MUs and were correlated with the physical properties of MUs in vivo.
NEW & NOTEWORTHY
It is suggested the physical properties of the motor unit (MU) influence firing rate characteristics; however, no study has examined firing rate characteristics in relation to the physical properties of the MU in vivo. In this study, there was a strong relationship between MU firing rate characteristics, particularly of higher-threshold MUs, at a target force level and type I percent myosin heavy chain isoform content of the vastus lateralis during a moderate-intensity contraction.
it is well understood that as excitation increases to the motoneuron pool to increment force, motor units (MUs) are recruited in order of increasing size (Henneman 1957; Milner-Brown et al. 1973) with a concurrent increase in the firing rates of MUs (De Luca and Contessa 2012; De Luca and Hostage 2010; Kiehn and Eken 1997; Milner-Brown et al. 1973; Monster and Chan 1977). It has been reported that higher-threshold, larger-diameter MUs have greater firing rates than lower-threshold, smaller-diameter MUs in electrically stimulated anesthetized, decerebrate cats (Eccles et al. 1958; Kernell 1965) and in humans when analysis was performed on aggregated data from multiple contractions and subjects (Barry et al. 2007; Gydikov and Kosarov 1974; Moritz et al. 2005; Tracy et al. 2005). However, when observations of MU firing characteristics are made separately for each contraction and subject during voluntary isometric efforts in humans, there is an inverse relationship between the firing rates and recruitment thresholds of MUs regardless of force level (De Luca et al. 1982b; De Luca and Contessa 2012; De Luca and Hostage 2010; Kanosue et al. 1979; Masakado 1991, 1994; Masakado et al. 1995; McGill et al. 2005; Monster and Chan 1977; Person and Kudina 1972; Rose and McGill 2001; Stashuk and De Bruin 1988; Tanji and Kato 1973). Therefore, the time- and force-related firing rates of earlier-recruited lower-threshold MUs are reported to be greater than later-recruited higher-threshold MUs. It has been suggested that this control scheme is not designed to produce maximal force but to optimize the relationship between force production and duration of MU activity (De Luca et al. 1982b; De Luca and Contessa 2015; De Luca and Hostage 2010).
Previous research suggests that the central nervous system does not control the firing rates of individual MUs but rather provides the motoneuron pool with a common synaptic input during voluntary contractions (De Luca et al. 1982a; De Luca and Erim 1994; Farina et al. 2014; Farmer et al. 1993). Subsequently, in response to the twitch amplitudes of MUs, it is suggested that the common synaptic input to the motoneuron pool adjusts to match the target force level (De Luca et al. 1996). Furthermore, it is well understood that twitch forces of MUs vary as a function of fiber type (Garnett et al. 1979). Thus it has been proposed that the physical properties of the MU pool, particularly the ratio of fast-twitch to slow-twitch fibers (Beck et al. 2007; De Luca et al. 1982b; Herda et al. 2010; Orizio and Veicsteinas 1992; Trevino and Herda 2016) and their influence on MU twitch forces, play a prominent role in firing rate characteristics at steady force (Beck et al. 2007; De Luca and Contessa 2012; Orizio and Veicsteinas 1992). In support of this, Herda et al. (2015) reported that differences in the firing rates of lower- and higher-threshold MUs at a target force level can exist within the vastus lateralis (VL) as a function of chronic exercise training status and/or type I % myosin heavy chain (MHC) isoform content. Therefore, it is hypothesized that interindividual differences in the physical properties of the MU may be correlated with the firing rate characteristics at a target force. Specifically, a relationship may exist between the MHC isoform content of the muscle and the firing rate vs. recruitment threshold relationship that depicts the firing rate characteristics of the lower- and higher-threshold MUs. This hypothesis has not been tested in vivo. In addition, it is unknown if the physical properties of the MU influences firing rate characteristics at recruitment and derecruitment.
MHC isoform content influences twitch forces (Meijer et al. 2015) and contractile speed (Bottinelli 2001; Pette et al. 1999) and is associated with the fatigability of the muscle fiber (Swallow et al. 2007; Taylor et al. 1997). Therefore, the MHC isoforms have been regarded as an important factor in the physical properties of the MU (Booth et al. 2010; Pette and Staron 2000) and are the most appropriate markers for fiber type delineation (Pette and Staron 2000). It is speculated that the firing rates of MUs will be altered as a function of type I MHC isoform content, such that individuals with greater amounts of type I MHC isoform content will have lower MU twitch forces, particularly higher-threshold MUs, which will result in an increase in the firing rates of those MUs to match a moderate-intensity targeted force. Therefore, it would be expected that the slope from the firing rate vs. recruitment threshold relationship would be less negative for muscles with a greater amount of type I MHC isoform content as the firing rates of the higher-threshold MUs would be greater compared with a muscle with a lower percentage of type I MHC isoform content. This phenomenon would simultaneously increase resistance to fatigue in muscles with lower percentages of type I MHC isoform content.
Therefore, the purpose of the present study was to examine the possible relationships between MHC isoform content of the VL and MU firing rates during an isometric leg extensor trapezoidal muscle action performed at 40% maximal voluntary contraction (MVC). We hypothesized that at recruitment, targeted force, and derecruitment, firing rate characteristics, particularly higher-threshold MUs, will be influenced by type I %MHC isoform content.
METHODS
Subjects.
Six healthy men (mean ± SD age = 21.67 ± 2.80 yr, height = 166.60 ± 32.40 cm, weight = 80.28 ± 10.88 kg) and six healthy women (age = 19.67 ± 1.37 yr, height = 164.83 ± 5.44 cm, weight = 58.73 kg ± 7.97 kg) volunteered for this investigation. None of the participants reported any history of current or ongoing neuromuscular diseases or musculoskeletal injuries specific to the ankle, knee, or hip joints. This study was approved by the University's institutional review board for human subjects research. Each subject read a signed an informed consent form and completed a preexercise health status questionnaire.
Isometric testing.
Each participant was seated with restraining straps over the pelvis, trunk, and contralateral thigh, and the lateral condyle of the femur was aligned with the input axis of the Biodex System 3 isokinetic dynamometer (Biodex Medical Systems, Shirley, NY) in accordance with the Biodex User's Guide (Biodex Pro Manual, Applications/Operations, 1998). All isometric leg extensor strength assessments were performed on the right leg at a flexion of 90°. Isometric strength for the right leg extensor muscles was measured using the force signal from a load cell (LC, Omegadyne, Sunbury, OH) that was fitted to the Biodex System 3 isokinetic dynamometer.
Each participant visited the laboratory for one experimental trial. During the experimental trial participants performed three isometric MVCs with strong verbal encouragement for motivation followed by a submaximal isometric trapezoid muscle actions at 40%. The highest force output from the three MVCs was used to determine the maximal torque output for each participant and the force level for the 40% isometric trapezoid muscle actions. For the isometric trapezoid muscle action, the force was increased at 10% MVC/s to the desired force level, where it was held for 12 s and then decreased to baseline at a rate of 10% MVC/s (Fig. 1). Therefore, the duration of each contraction lasted 20 s. Three to five minutes of rest was given between each muscle action. Participants practiced the isometric trapezoid muscle action at 20% MVC prior to the 40% MVC. Participants were instructed to maintain their force output as close as possible to the target force presented digitally in real time on a computer monitor. Participants were given a second attempt if unable to maintain the targeted force during the initial trial.
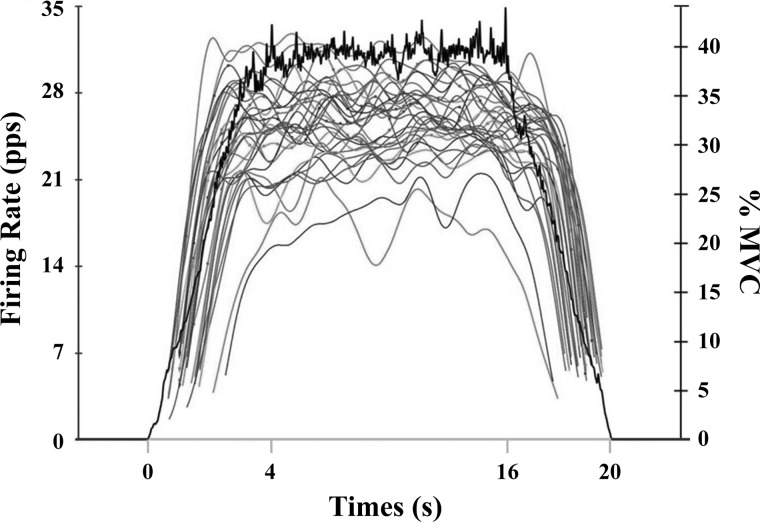
Fig. 1.
An example of the average firing rate plots of detected motor units recorded from the 5-pin surface array sensor for the vastus lateralis during isometric trapezoidal contraction at 40% maximal voluntary contraction (MVC) for 1 participant. The black line shows the force signal as it appeared to the participant during the trial. The gray curves represent the average firing rates in pulses per second (pps) across time for each motor unit.
Electromyographic recording.
During the trapezoid muscle actions, surface electromyographic (EMG) signals were recorded from the VL using a 5-pin surface array sensor (Delsys, Boston, MA). The pins had a diameter of 0.5 mm and were positioned at the corners of a 5 × 5 mm square, with the fifth pin in the center. Prior to sensor placement, the surface of the skin was prepared by shaving, removing superficial dead skin with adhesive tape (3M, St Paul, MN), and sterilizing with an alcohol swab. The sensor was placed over the VL muscle at 50% of the distance between the greater trochanter and lateral condyle of the femur with adhesive tape. The reference electrode was placed over the left patella. The signals from four pairs of the sensor electrodes were differentially amplified and filtered with a bandwidth of 20 Hz to 9.5 kHz. The signals were sampled at 20 kHz and stored on a computer for off-line analysis.
EMG decomposition.
For detailed information regarding the signal processing of the EMG signals, refer to De Luca et al. (2006) and Nawab et al. (2010). Action potentials were extracted into firing events of single MUs from the four separate EMG signals via the Precision Decomposition (PD) III algorithm as described by De Luca et al. (2006). This algorithm is designed for decomposing EMG signals into their constituent MU action potential trains. The accuracy of the decomposed firing instances was tested with the reconstruct-and-test procedure (Nawab et al. 2010). Only MUs with >90% accuracies were used for further analysis. In addition, the firing rate curve of each MU was computed by low-pass filtering the impulse train with a unit area Hanning window of 1-s duration (De Luca et al. 2006; Defreitas et al. 2014; Herda et al. 2015; Stock et al. 2012). For each MU, five parameters were extracted from the firing rate data: 1) the recruitment threshold (REC Thresh) [expressed relative to MVC (%MVC)], 2) firing rate at recruitment (FR-REC) [pulses per second (pps)], 3) mean firing rate (MFR) at the targeted contraction level (40% MVC; pps), 4) the derecruitment threshold (DEREC Thresh) (expressed relative to MVC), and 5) firing rate at derecruitment (FR-DEREC, pps). The mean firing rate was calculated as the average value of the mean firing rate trajectory during the entire 12-s steady force (Contessa and De Luca 2013; Herda et al. 2015; Trevino et al. 2014). The FR-REC and FR-DEREC were estimated with the inverse of the average of the first and last three interpulse intervals. However, if the coefficient of variation (CoV) of the first three interpulse intervals was >50% (Jesunathadas et al. 2010), interpulse intervals 2, 3, and 4 were selected to calculate FR. This procedure continued until the CoV of the interpulse intervals was <50% to minimize the influence of unstable firings at REC and DEREC Thresh (appendix a). An average 0.10-ms epoch of force that began at the first (REC) and last discharge (DEREC) used to calculate firing rate was selected as the recruitment and derecruitment threshold for the MU, respectively.
Muscle biopsy.
After the experimental testing session, muscle biopsies were taken from the VL at the midpoint of the thigh (similar positon of EMG electrode), midway between the inguinal ligament and the patella on the right leg, with the percutaneous needle biopsy methods of Bergstrom (Bergstrom 1962). After careful cleaning of the sample site, a local anesthetic (2% lidocaine) was injected cutaneously, and a small incision was made through the skin and deep fascia with a no. 11 scalpel. The sample was taken with a traditional Bergstrom needle (Pelomi Medicals, Albertslund, Denmark), utilizing the double chop and suction method (Evans et al. 1981; Staron et al. 1990). The subjects were required to return to the laboratory 24–28 h after the biopsy procedures to ensure that the incision was healing properly. Muscle samples were mounted with fibers parallel and on end in tragacanth gum and frozen with isopentane cooled in liquid nitrogen for later analysis of MHC isoform content. MHC isoform content was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Carraro and Catani 1983; Perrie and Bumford 1986). Muscle samples were extracted with a cryostat (MC1800; Leica) taking eight to twelve 40-μm thick serial sections. Sections were then lysed with 500 μl of lysing buffer [10% (wt/vol) glycerol, 5% (vol/vol) β-mercaptoethanol, 2.3% (wt/vol) SDS in 62.5 mmol/l Tris·HCl, pH 6.8] and heated for 10 min at 60°C (Fry et al. 1994). To determine MHC isoform content, small amounts of extracts (3–5 μl) were loaded on 4–8% gradient gels with 4% stacking gels, run overnight (18–24 h) at 120 V, and stained with Coomassie blue. The MHC isoforms (types I and II) were identified according to their molecular masses (Staron and Hikida 1992; Staron and Johnson 1993) (Fig. 2). The percentage of type I MHC isoform content (type I %MHC) was used for the correlations with the data derived from the EMG signals. It has been reported that the fiber type distribution of the whole VL muscle in humans is not different along the axis of the muscle (Lexell et al. 1983) and that the differences in variation with single-fiber measurements from muscle biopsies account for <2% of a change in fiber type proportion (Williamson et al. 2001).
Fig. 2.
Results of the type I myosin heavy chain (MHC) isoform content analysis for subjects 2 (S2) and 5 (S5). Percent type I MHC isoform content was 65.3% and 30.7% for subjects 2 and 5, respectively.
Statistical analysis.
For each subject, linear regressions were performed on the following relationships: 1) FR-REC (pps) vs. REC Thresh (%MVC), 2) MFR (pps) vs. REC Thresh (%MVC), and 3) FR-DEREC (pps) vs. DEREC Thresh (%MVC). Linear regressions were performed on each of the three relationships with the inclusion of MUs with accuracies > 90%, > 92%, and > 95%, and thus three linear regressions were performed on each of the three previously mentioned relationships. Slopes and y-intercepts were calculated for each linear regression model, and values from significant and nonsignificant relationships were used for further analysis. For all relationships, the y-intercept reflects the maximal firing rate sustainable by the lowest threshold MUs, whereas the slope of the regressed line characterizes the overall MU control scheme. There were minimal differences in the y-intercept and slope coefficients among the different accuracy criteria (appendix b) and, furthermore, the number of subjects was reduced from 12 to 7 with the 95% accuracy condition because of fewer MUs used for calculations. Therefore, only the results for the 90% accuracy criterion are reported. Pearson's product moment correlation coefficients were calculated comparing the percentage of type I MHC isoform content of the VL with the slopes and y-intercepts from each relationship. In addition, Pearson's product moment correlation coefficients were calculated comparing the percentage of type I MHC isoform content of the VL and the maximum REC and DEREC thresholds and the minimum and maximal MU firing rates at REC, target force, and DEREC. Slopes and y-intercepts were calculated using Microsoft Excel version 2013 (Microsoft, Redmond, WA). The level of significance was set at P ≤ 0.05, and all statistical analyses were performed with SPSS 22 (IBM, Armonk, NY).
RESULTS
Table 1 contains the number of MUs, REC and DEREC Thresh ranges, and MU firing rate ranges at REC, target force, and DEREC, slopes and y-intercepts for all linear regressions performed, and type I %MHC isoform content for each participant. Correlations among type I %MHC isoform content and the maximum REC and DEREC thresholds, minimum and maximal MU firing rates at REC, target force, and DEREC were not significant (P = 0.098–0.760, r = −0.134–0.499). The lack of significant relationships may be due to the decomposition algorithm or because of the moderate-intensity contraction. For example, the maximum REC and DEREC Thresh and corresponding MU firing rate ranges only reflect the observed MUs. Consequently, there were other MUs contributing to force production that were not recorded either because they were outside of the area recorded by the decomposition sensor or because, due to influence of the characteristics of the EMG signal, the signal-to-noise ratio of the collected signals, and the shapes of the action potentials on the decomposition algorithm, they were not observed (De Luca and Contessa 2012). However, using the slopes and y-intercepts for the individual relationships for each subject provides insight on the firing rate characteristics of the recorded and unrecorded lower- and higher-threshold MUs (i.e., MU control scheme) and thus allows for comparison of MU characteristics across individuals (Herda et al. 2015; Stock et al. 2012; Trevino et al. 2014). In addition, it is plausible that maximal recruitment ranges could differ as a function of type I %MHC isoform content during a higher-intensity contraction (>80% MVC). It would be expected, however, that MUs would be recruited throughout the force spectrum during a 40% MVC, as recruitment of MUs is typically reported up to 90% MVC for the VL (De Luca and Hostage 2010).
Table 1.
Number of MUs, type I %MHC isoform content, recruitment threshold, derecruitment threshold, and firing rate ranges, and slope and y-intercept values for FR-REC vs. REC, MFR vs, REC, and FR-DEREC vs. DEREC relationships
| FR-REC vs. REC |
MFR vs. REC |
FR-DEREC vs. DEREC |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject | No. of MUs | Type I %MHC | Slope | y-Int | RT Range | FR Range | Slope | y-Int | FR Range | Slope | y-Int | DT Range | FR Range |
| 1 | 29 | 25.91 | −0.152 | 12.856 | 9.24–24.71 | 9.24–24.71 | −0.615* | 35.633 | 22.95–32.52 | −0.388* | 14.361 | 4.66–19.51 | 5.86–16.73 |
| 2 | 37 | 65.25 | 0.131 | 7.647 | 0.44–30.71 | 3.95–20.10 | −0.307* | 28.414 | 19.45–29.69 | −0.03 | 9.446 | 0.37–31.49 | 4.77–14.18 |
| 3 | 28 | 33.87 | 0.140 | 8.172 | 1.33–22.92 | 2.97–20.13 | −0.489* | 29.796 | 18.56–30.66 | −0.153 | 12.729 | 8.96–20.47 | 6.15–16.71 |
| 4 | 10 | 35.23 | −0.252 | 9.456 | 3.45–20.88 | 3.27–11.39 | −0.503* | 22.835 | 13.52–23.33 | 0.130 | 5.712 | 4.64–27.74 | 4.53–12.01 |
| 5 | 27 | 49.25 | 0.049 | 10.418 | 1.62–14.70 | 7.00–15.92 | −0.403* | 29.938 | 23.47–31.13 | −0.019 | 10.418 | 7.45–24.66 | 6.35–12.58 |
| 6 | 10 | 51.68 | 0.033 | 13.412 | 4.96–17.00 | 7.21–18.66 | −0.317* | 31.191 | 25.77–30.54 | 0.445 | 6.796 | 12.52–19.71 | 8.73–24.79 |
| 7 | 16 | 42.81 | 0.099 | 6.592 | 0.92–24.43 | 4.19–15.73 | −0.259* | 24.185 | 15.96–23.81 | −0.085 | 9.854 | 7.63–28.48 | 3.76–14.64 |
| 8 | 26 | 42.23 | −0.165 | 11.642 | 0.76–21.63 | 3.55–17.56 | −0.467* | 29.408 | 18.21–30.22 | −0.153 | 13.787 | 12.39–26.61 | 8.10–16.71 |
| 9 | 14 | 67.91 | 0.506 | 3.235 | 9.61–16.79 | 7.63–16.63 | −0.211 | 29.292 | 23.78–29.36 | 0.467* | 2.376 | 13.00–32.31 | 9.74–22.97 |
| 10 | 12 | 58.27 | 0.757 | −3.227 | 13.04–23.89 | 5.37–19.27 | −0.379* | 33.529 | 22.90–29.41 | 0.315 | 6.303 | 5.11–18.27 | 5.16–15.60 |
| 11 | 21 | 30.73 | −0.320 | 11.152 | 3.79–17.18 | 3.79–14.80 | −0.653* | 27.952 | 16.61–26.86 | −0.031 | 8.173 | 3.66–25.61 | 5.14–12.03 |
| 12 | 27 | 34.27 | −0.186* | 10.715 | 12.62–37.08 | 3.20–11.44 | −0.557* | 29.289 | 9.91–26.73 | −0.161* | 10.822 | 6.95–31.80 | 4.36–11.77 |
| Mean | 0.053 | 8.506 | 5.15–22.66 | 5.11–17.19 | −0.430 | 29.289 | 19.26–28.69 | 0.028 | 9.231 | 7.28–25.56 | 6.05–15.89 | ||
| SD | 0.316 | 4.663 | 4.73–6.37 | 2.12–3.78 | 0.141 | 3.474 | 4.74–2.89 | 0.262 | 3.551 | 3.92–5.09 | 1.88–4.20 | ||
Values are no. of motor units (MUs), type I % myosin heavy chain (%MHC) isoform content, recruitment threshold (RT) and derecruitment threshold (DT) ranges (expressed as % of maximal voluntary contraction), firing rate (FR) range (expressed as pulses per second), and slope and y-intercept (y-Int) values for the MU firing rate at recruitment (FR-REC) vs. recruitment (REC), mean firing rate (MFR) vs. REC, and firing rate at derecruitment (FR-DEREC) vs. derecruitment (DEREC) threshold relationships.
Significant relationship.
FR-REC vs. REC Thresh relationships.
For the FR-REC vs. REC Thresh relationships, 1 of the 12 relationships (8%) was significant (P ≤ 0.05; R range = −0.686 to 0.557). For the y-intercept the mean ± SD was 8.51 ± 4.66 pps, whereas the slope was −0.05 ± 0.32 pps/%MVC. In addition, the firing rates of the lowest-threshold MUs were 8.5 pps at recruitment (y-intercepts) (Table 1).
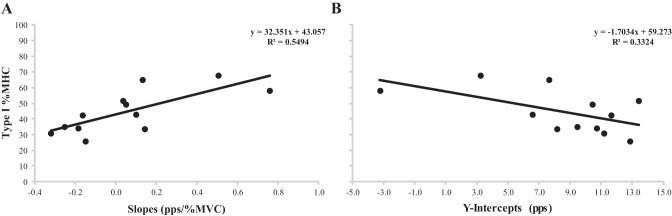
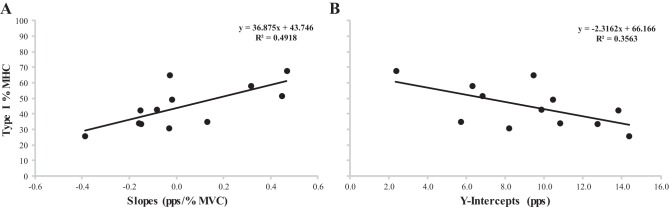
Pearson's product moment correlations were significant among type I %MHC isoform content and the slopes (P = 0.006, r = 0.741) and the y-intercepts (P = 0.050, r = −0.577) from the FR-REC vs. REC Thresh relationships (Fig. 3).
Fig. 3.
Plotted relationships between type I % myosin heavy chain (%MHC) isoform content and the slopes (A) and y-intercepts (B) for the firing rate at recruitment vs. recruitment threshold relationships.
MFR vs. REC Thresh relationships.
For the MFR vs. REC Thresh relationships, 11 of the 12 relationships (92%) were significant (P ≤ 0.05; R range = −0.917 to −0.257). For the y-intercept the mean ± SD was 29.29 ± 3.47 pps, whereas the slope was −0.430 ± 0.141 pps/%MVC (Table 1). The slopes were negative for all subjects, indicating that MUs recruited at higher thresholds tended to sustain lower average firing rates at the target force level (Fig. 4). In addition, the lowest-threshold MUs achieved firing rates of 29.29 pps at the target force (y-intercepts) (Table 1).
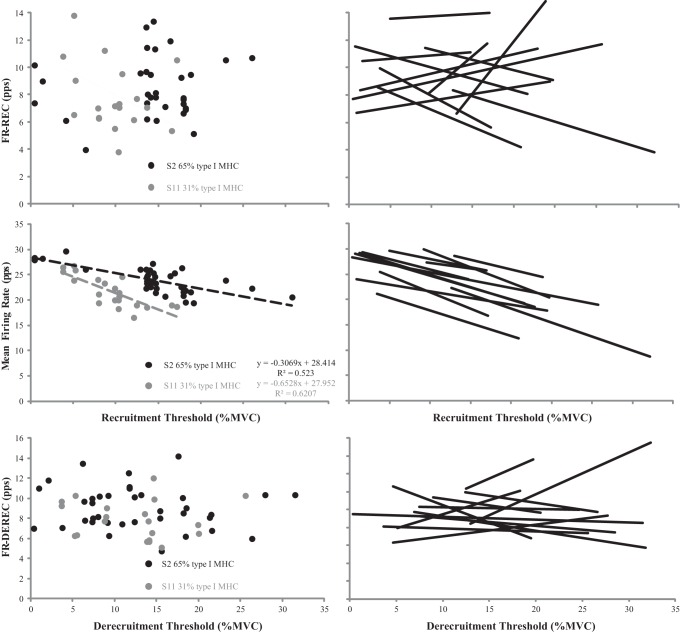
Fig. 4.
Plotted motor unit firing rates at recruitment (FR-REC) [pulses per second (pps)] vs. recruitment [% maximal voluntary contraction (%MVC)] (top), mean firing rate (pps) vs. recruitment (middle), and firing rates at derecruitment (FR-DEREC) (pps) vs. derecruitment threshold relationships (bottom) with linear regressions applied to the significant relationships for subjects 2 [65% type I myosin heavy chain (MHC)] and 11 (30% type I MHC) (left) and all subjects (black lines) (right).
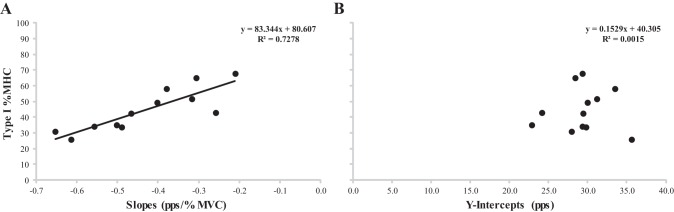
Pearson's product moment correlations were significant between the type I %MHC isoform content and the slopes from the MFR vs. REC Thresh relationships (P < 0.001, r = 0.853). However, there was no significant correlation when comparing type I %MHC isoform content with the y-intercepts (P = 0.905, r = 0.039) (Fig. 5).
Fig. 5.
Plotted relationships between type I % myosin heavy chain (%MHC) isoform content and the slopes (A) and y-intercepts (B) for the mean firing rate vs. recruitment threshold relationships.
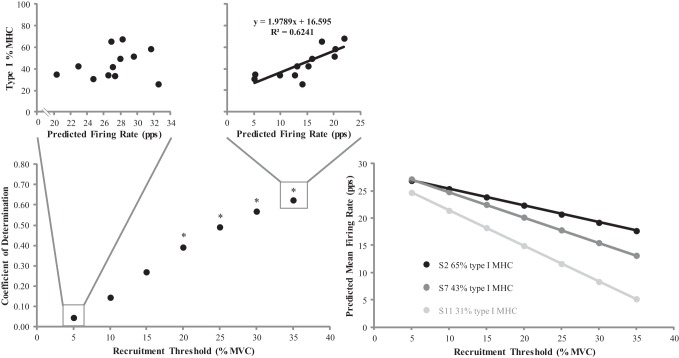
To further determine the influence of type I MHC isoform content on firing rate characteristics of MUs, predicted firing rates for MUs recruited at 5–35% MVC (in 5% MVC increments) were calculated from the linear regression equations for each subject. Subsequently, the linear regression equation for each subject's MFR vs. REC Thresh relationship was used to predict firing rates at the target force level (i.e., 40% MVC) for lower- and higher-threshold MUs. For each REC Thresh examined, correlations were performed between predicted firing rates and type I %MHC isoform content. There were no significant correlations when comparing type I %MHC isoform content with predicted firing rates at the target force level for MUs recruited at 5%, 10%, and 15% MVC (P = 0.083–0.502; r range = 0.215–0.520). In contrast, Pearson's product moment correlations were significant among type I %MHC isoform content and the predicted firing rates at the target force level for MUs recruited at 20%, 25%, 30%, and 35% MVC (P = 0.002–0.030; r = 0.626–0.790) (Fig. 6). Firing rates of the higher-threshold MUs (REC Thresh > 20% MVC) were greater for individuals with higher percentages of type I MHC isoform content.
Fig. 6.
Bottom left: plotted relationships between the recruitment threshold [expressed as percent maximal voluntary contraction (%MVC)] and the coefficient of determination for the predicted firing rate [pulses per second (pps)] vs. type I % myosin heavy chain isoform content (%MHC) relationships. Top: predicted firing rates vs. type I %MHC relationships for MUs recruited at 5% (left) and 35% (right) MVC. Bottom right: plotted predicted mean firing rate vs. recruitment threshold relationships for subjects 2 (65% type I MHC), 7 (43% type I MHC), and 11 (31% type I MHC). *Significant relationship between the predicted firing rate and type I %MHC for the respective recruitment threshold (P ≤ 0.05).
FR-DEREC vs. DEREC Thresh relationships.
For the FR-DEREC vs. DEREC Thresh relationships, 3 of the 12 relationships (25%) were significant (P ≤ 0.05; R range = −0.636 to 0.744). For the y-intercepts the mean ± SD was 9.23 ± 3.55 pps, whereas the slope was 0.03 ± 0.26 pps/%MVC (Table 1). The positive slope indicated that the firing rates of the higher-threshold MUs were greater than the lower-threshold MUs at derecruitment. In addition, the firing rates of the lowest-threshold MUs at derecruitment (y-intercepts) were 9 pps (Table 1).
Pearson's product moment correlations were significant among the type I %MHC isoform content and the slopes (P = 0.011, r = 0.701) and y-intercepts (P = 0.040, r = −0.597) from the FR-DEREC vs. DEREC Thresh relationships (Fig. 7).
Fig. 7.
Plotted relationships between type I % myosin heavy chain (%MHC) isoform content and the slopes (A) and y-intercepts (B) for the firing rate at derecruitment vs. derecruitment threshold relationships.
DISCUSSION
The main finding from this study was that MU firing rate characteristics during a 40% isometric trapezoidal contraction was correlated with the contractile properties of the muscle in vivo. Although there were significant correlations among type I %MHC isoform content and the slopes and y-intercepts for the FR-REC vs. REC and the FR-DEREC vs. DEREC Thresh relationships, few (16%) of the individual subject relationships for these parameters were significant. Therefore, the MFR vs. REC Thresh relationships may be the most appropriate measurement for physiological interpretation and/or to monitor changes in type I %MHC isoform content since the majority of the individual relationships were significant (11 of 12) and the slope coefficient accounted for the greatest percentage of type I MHC isoform content.
MFR vs. REC Thresh relationships.
Previous studies have reported large interindividual variability in these relationships (De Luca and Contessa 2012; De Luca and Hostage 2010). Herda et al. (2015) reported within-muscle differences of the y-intercept values for the VL between chronic (>3 yr) aerobic- and resistance-trained individuals. The results indicated greater firing rates for the aerobically trained than resistance-trained individuals during 40% and 70% MVCs. A closer examination of Fig. 4 presented in Herda et al. (2015) indicated that the difference in the mean MU firing rates at the 40% MVC targeted force level were becoming progressively larger with increments in recruitment threshold between the chronic training groups, which was also reflected in the mean slope values (aerobically trained had less negative slopes). However, there was no significant difference between the slope values, which may have been the result of the small sample size (n = 5) or the research design (multiple contraction intensities and statistical procedures). Nonetheless, the authors suggested the findings were due to training status-induced differences in MHC isoform content, and therefore differences in the physical properties of the motoneuron pool may explain the interindividual differences often reported in the literature.
Considering the results of Herda et al. (2015), it was expected that the y-intercept values would be correlated with type I %MHC isoform content, which was unfounded in the present study. It is plausible that aerobic capacity more so than MHC isoform content influences the y-intercept values from the MFR vs. REC Thresh relationships. Herda et al. (2015) reported greater y-intercepts for the aerobically trained, and a biopsy of a small number of subjects indicated a difference in MHC isoform content between training groups. In the present study, there were subjects who had similar percentages of type I MHC isoform content despite differences in exercise training status. For example, subjects 7 and 8 had similar type I %MHC isoform contents (49% and 51%) but reported vastly different exercise statuses, such as 12–20 total hours a week of aerobic and resistance training vs. zero hours a week of physical activity. Therefore, it is plausible that the operating point of the MU control scheme (y-intercept from the MFR vs. REC Thresh relationships) may be influenced by other factors more so than the physical properties of the muscle, such as metabolic conditions (i.e., creating, storing, and utilizing energy). In contrast, there was a strong relationship between type I %MHC isoform content and the slope values from the MFR vs. REC Thresh relationships (Fig. 6). Individuals with a greater percentage of type I %MHC isoform content had less negative slopes than individuals with a greater percentage of type II MHC isoform content. Therefore, the difference between the firing rates for the lower- and higher-threshold MUs at the target force level was less for the individuals with a greater percentage of type I MHC isoform content.
To further describe the influence of type I MHC isoform content on firing rate characteristics of MUs, predicted firing rates at the target force for lower- and higher-threshold MUs were calculated from the linear regression equations for each subject (Fig. 6). The correlations performed between the predicted firing rates and type I %MHC isoform content indicated that lower-threshold MUs (Rec Thresh < 20% MVC) were not correlated, unlike, for higher-threshold MUs (Rec Thresh > 20% MVC). Individuals with greater type I %MHC isoform content displayed higher firing rates at the target force level for MUs recruited beyond 20% MVC. In addition, this phenomenon became more evident with increases in REC Thresh as indicated by the R2 values. Therefore, the less negative slopes from the MFR vs. REC Thresh relationships for individuals with a greater percentage of type I MHC isoform content were primarily the result of higher-threshold MUs (REC Thresh >20% MVC) firing at greater rates at the targeted force. In addition, this analysis supports the lack of relationships among the y-intercepts and type I %MHC isoform content as demonstrated by the firing rates of lower-threshold MUs having no relationship with type I %MHC isoform content. Although individuals may differ in overall expressions of MHC isoform content, it is speculated that the lower-threshold MUs may display similar physical properties. Therefore, changes in firing rate characteristics of lower-threshold MUs (Herda et al. 2015) are likely influenced more by other properties of the MU.
It is understood that MUs comprised of a greater area of type II MHC isoform content have greater twitch forces (Aagaard et al. 2001; Bottinelli et al. 1999; Dons et al. 1979). In addition, it has been suggested that force production is regulated by adjustments in common synaptic input due to MU twitch forces. Thus it is plausible that the firing rates of the higher-threshold MUs are lower in individuals with greater percentages of type II MHC isoform content as a result of greater twitch forces generated by the MU. Therefore, MU force twitches, which are largely dictated by the physical properties (i.e., %MHC isoform content) of the MU pool (Meijer et al. 2015), play a significant factor in firing rate characteristics (Contessa and De Luca 2013). In addition, a principle of the onion skin control scheme is minimizing energy expenditure with greater reliance on lower-threshold MUs. For example, lower-threshold MUs are less fatigable (Burke 1981; Garnett et al. 1979), making them more suitable for maintaining a target force level (De Luca and Contessa 2015; Monster and Chan 1977). Our data would fit this principle, as less energy would be expended by the higher-threshold MUs in individuals with a greater percentage of type II MHC isoform content and thus would be a means to increase the resistance to fatigue.
FR-REC vs. REC and FR-DEREC vs. DEREC Thresh relationships.
For the FR-REC vs. REC Thresh relationships the y-intercept represents the firing rate at REC for the lowest-threshold MUs and the slope values represent the firing rate at REC for the low- and high threshold MUs, whereas for the FR-DEREC vs DEREC Thresh relationships the y-intercept represents the firing rate at DEREC for the lowest-threshold MUs and the slope values represent the firing rate at DEREC for the low- and high-threshold MUs. Although there were relationships among type I %MHC isoform content and the y-intercepts (r = −0.577; −0.597) and the slopes (r = 0.741; 0.701) from both relationships, only 1 and 3 of the 12 individual relationships were significant, respectively.
The lack of significant relationships at REC may be due to the technical difficulty of measuring REC thresholds at low force levels (Erim et al. 1996). In addition, measuring REC thresholds for MUs of the VL, which is one of several muscles that produces force during leg extension, may increase error in the measurement. Another possible explanation is unstable firings near REC during linearly increasing muscle actions (Erim et al. 1996; Kudina 1974; Milner-Brown et al. 1973; Person and Kudina 1972) resulting in MU firing rate underestimations (Erim et al. 1996). Although precautions were taken to reduce the variability of the firing intervals on FR-REC and FR-DEREC, the majority of the individual relationships (83%) were not significant and thus the physiological interpretation between the coefficients and type I %MHC isoform content is unclear. Our data, however, cannot rule out the possibility that there is a lack of systematic behavior of firing rates at recruitment (De Luca and Erim 1994; Monster and Chan 1977; Person and Kudina 1972). In contrast, Pope et al. (2016) recently reported strong correlations (R2 > 0.90) between REC Threshs and MU action potential sizes for the VL and therefore would suggest that there were minimal errors in quantifying force at MU recruitment and tentatively provide further support that the poor relationships between REC Threshs and FR-REC may be due to the lack of systematic firing rate patterns at REC rather than measurement error. Nonetheless, there were no systematic firing rate characteristics at recruitment or derecruitment in the present study, unlike firing rate characteristics at the targeted force.
Lower firing rates at DEREC compared with REC have been reported for the first dorsal interosseous and deltoid muscles during triangular force contractions at 40% and 80% MVC, with lower-threshold MUs exhibiting lower firing rates at DEREC than higher-threshold MUs (De Luca et al. 1982a). Recently, Herda et al. (2016) reported that the change in MU firing rates at DEREC relative to REC was associated with the type I %MHC isoform content of the VL, such that individuals with a greater amount of type I %MHC isoform content were more likely to have greater MU firing rates at REC than DEREC. The authors suggested that the lower firing rates at DEREC relative to recruitment may be an indication of greater resistance to fatigue. It is hypothesized that MU twitch forces of the muscle regulate the amount of excitation sent to the motoneuron pool and thus determine the MU firing rates at DEREC (Adam and De Luca 2005; De Luca et al. 1982b; Dorfman et al. 1990). Subsequently, potentiation-related increases in MU twitch forces are associated with decreases in MU firing rates, whereas fatigue-related decreases in twitch forces are associated with increased MU firing rates and eventual recruitment of additional MUs necessary to match forces. In the present study, FR-DEREC was greater than FR-REC for the majority of the observed motoneuron pool, as indicated by Fig. 8, therefore tentatively suggesting that fatigue-related decline in MU twitch forces was occurring as excitation to the motoneuron pool was decreasing. It should be noted that potentiation- and fatigue-related responses in MU twitch forces occur simultaneously in a given contraction. The contradiction between results of the present study and previous findings is likely a function of the muscle tested and the duration and intensity of the contraction (De Luca et al. 1982b).
Fig. 8.
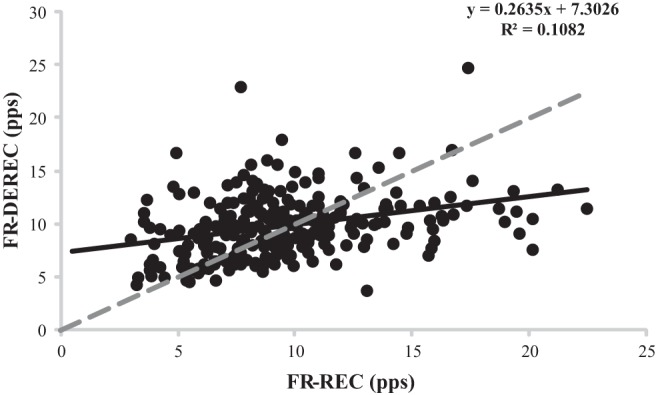
Plotted motor unit (MU) firing rates at derecruitment (FR-DEREC) vs. firing rates at recruitment (FR-REC) for all subjects. The dashed line represents a perfect relationship (y-intercept = 0, slope = 1). MUs above the dashed line had a greater FR-DEREC than FR-REC, while MUs below the dashed line exhibited the opposite behavior.
A limitation of our speculation regarding potentiation- or fatigue-related responses is that the FR-DEREC vs. DEREC Thresh relationship is only one component needed to indirectly examine MU twitch forces as the amount of excitation to the motoneuron pool is decreasing. The focus of this work was not necessarily potentiation or fatigue but rather the influence of MHC isoform content of the VL on MU firing rate characteristics of the muscle to match a targeted force. Future research is needed to fully understand the mechanisms of MU twitch potentiation. In addition, it should be noted that MU characteristics and the contractile properties of the VL were assessed but the entire leg extensors contribute to the force output. Consequently, this could have introduced error in quantifying forces at recruitment and derecruitment and reduced the correlations.
Furthermore, studies have reported that persistent inward currents (PIC) result in lower FR-DEREC than REC and MUs being DEREC at lower force value than REC due to sustained depolarization via PIC initiated at REC (Gorassini et al. 2002). This phenomenon is hypothesized to influence lower-threshold MUs more so than higher-threshold MUs. The effect of PIC on lower-threshold MU characteristics of the VL during a moderate-intensity contraction is unclear; however, it is possible that the FR-DEREC and DEREC threshold values for the lowest-threshold MUs were influenced by PIC in addition to the MHC isoform content. It should also be stated that the analyses for the slope and y-intercept values were obtained from a single contraction. Future research should examine whether the average of multiple contractions would improve the correlations with MHC isoform content.
Numerous studies have hypothesized that the physical properties of the motoneuron pool play a prominent role in MU firing rate characteristics. Results from the present study would tentatively support this hypothesis, as there was a relationship between type I %MHC isoform content and firing rate characteristics, particularly the higher-threshold MUs (REC Thresh > 20% MVC). Subsequently, this is the first study to support this hypothesis with in vivo measurements made possible by muscle biopsies and EMG decomposition techniques. Future research should examine possible systematic changes in these relationships (slopes and y-intercepts) across targeted forces (low to high intensity) in relation to %MHC isoform content to better understand the influence of the contractile properties of muscle on overall MU characteristics (recruitment vs. firing rate patterns).
GRANTS
This study was supported by the General Research Fund from the University of Kansas, Lawrence, KS.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.A.T., T.J.H., A.C.F., P.M.G., J.P.V., and E.M.M. performed experiments; M.A.T., T.J.H., E.M.M., and J.D.M. analyzed data; M.A.T., T.J.H., E.M.M., and J.D.M. interpreted results of experiments; M.A.T. and T.J.H. prepared figures; M.A.T. drafted manuscript; M.A.T., T.J.H., A.C.F., P.M.G., J.P.V., E.M.M., and J.D.M. approved final version of manuscript; T.J.H. conception and design of research; T.J.H., A.C.F., P.M.G., and J.P.V. edited and revised manuscript.
ACKNOWLEDGMENTS
We are grateful for the contributions of the undergraduate research assistants in the Neuromechanics Laboratory for their assistance in performing the experiments.
Appendix A
Firing Interval Variability at Recruitment vs. Derecruitment
MUs have previously been reported to fire in an unstable manner at recruitment, such as starting to fire, stopping, and firing again when the force output of a muscle fluctuates near the REC threshold during linearly increasing muscle actions (Erim et al. 1996; Kudina 1974; Milner-Brown et al. 1973). In addition, the unstable firings occur less frequently at DEREC than at REC (Kudina 1974; Milner-Brown et al. 1973; Person and Kudina 1972). In support, a paired-samples t-test indicated that the coefficient of variation (CoV) of the interpulse intervals was greater for the FR-REC (46.3 ± 10.7%) compared with the FR-DEREC (37.2 ± 6.9%; P = 0.001). After accounting for the variability of the firings as described in EMG decomposition, the CoVs were not different (P = 0.435) between the FR-REC (27.6 ± 4.5%) and FR-DEREC (28.7 ± 4.1%). For the FR-REC, the mean ± SD number of firing intervals removed when accounting for unstable firings was 1.80 ± 0.24 and the change between the REC Threshs (uncorrected vs. corrected) was 1.92 ± 1.13%. For the FR-DEREC, the number of firing intervals removed when accounting for variability due to unstable firings was 1.47 ± 0.31 and the change between the DEREC Threshs (uncorrected vs. corrected) was 0.78 ± 0.65%.
Appendix B
Surface Electromyographic Signal Decomposition Accuracy Criteria
Multiple studies have utilized surface electromyographic signal decomposition technology (De Luca and Hostage 2010; Defreitas et al. 2014; Herda et al. 2015, 2016); however, it is unclear which accuracy criteria are appropriate to investigate MU characteristics in relation to recruitment and derecruitment thresholds, as studies have used 90%, 92%, and 95% (Defreitas et al. 2014; Herda et al. 2015; Stock et al. 2012). Therefore, we sought to identify an accuracy criterion (i.e., ≥90%, ≥92%, and 95%) that would be suitable to correlate with type I percent myosin heavy chain (%MHC) isoform content. First, we examined whether the slopes and y-intercepts from the firing rate at recruitment (FR-REC) vs. recruitment threshold (REC Thresh), the mean firing rate (MFR) vs. REC Thresh, and the firing rate at derecruitment (FR-DEREC) vs. derecruitment threshold (DEREC Thresh) relationships would be altered as a function of accuracy level. A paired-samples t-test indicated that the slopes and y-intercepts for the 90% accuracy criterion were not significantly different from the 92% accuracy criterion for all relationships investigated (P = 0.157–0.707). The 95% accuracy criterion resulted in a loss of four subjects because of a lack of usable MUs (≤2). A fifth subject had seven usable MUs; however, there was a 775% and a 65% difference in slopes from the MFR vs. REC Thresh and FR-DEREC vs. DEREC Thresh relationships between the 95% and 90% accuracy criteria. Thus the fifth subject's data were not included in the 95% accuracy criterion analyses. Therefore, a one-way repeated-measures ANOVA was performed on the seven subjects that had relationships for each accuracy criterion. The analyses indicated no significant differences among the slopes and y-intercepts for the FR-REC vs. REC Thresh and MFR vs. REC Thresh relationships (P = 0.671–0.957) among accuracy criteria. In addition, there were no significant differences (P = 0.058) among the y-intercepts from the FR-DEREC vs. DEREC Thresh relationships; however, there was a significant difference for the slopes from the FR-DEREC vs. DEREC Thresh relationships (P = 0.025). The slopes for the 92% accuracy criterion (−0.107 ± 0.161 pps/%MVC) were less than those for the 95% accuracy criterion (−0.057 ± 0.187 pps/%MVC; P = 0.043). The reduced number of MUs for the 95% accuracy criterion may have altered the slopes from one of the three tested relationships (FR-DEREC vs. DEREC Thresh); however, the majority of the regression coefficients for the relationships were similar across accuracy criteria (Table B1). In addition, the r values among type I MHC isoform content and the relationships were similar across accuracy criteria (Table B1). Therefore, using the coefficients from the regressions utilizing the 90% accuracy criterion is appropriate for describing the parameters of the onion skin control scheme and their relation with type I MHC isoform content.
Table B1.
Pearson correlation coefficients between type I %MHC isoform content and slope and y-intercept for FR-REC vs. REC, MFR vs. REC, and FR-DEREC vs. DEREC relationships for 90%, 92%, and 95% accuracy criteria
| FR-REC vs. REC Threshold |
MFR vs. REC Threshold |
FR-DEREC vs. DEREC Threshold |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Accuracy | n | Slope | r | y-Int | r | Slope | r | y-Int | r | Slope | r | y-Int | r |
| 90% | 12 | 0.053 (0.316) | 0.741* | 8.506 (4.663) | −0.577* | −0.430 (0.141) | 0.853* | 29.289 (3.474) | 0.039 | 0.028 (0.262) | 0.701* | 9.231 (3.551) | −0.597* |
| 92% | 12 | 0.002 (0.250) | 0.628* | 9.303 (3.865) | −0.398 | −0.421 (0.151) | 0.828* | 29.229 (3.720) | 0.035 | 0.007 (0.220) | 0.764* | 9.713 (3.361) | −0.523 |
| 95% | 7 | −0.109 (0.312) | 0.669 | 9.883 (2.840) | −0.848* | −0.544 (0.294) | 0.812* | 29.027 (3.718) | −0.661 | −0.057 (0.187) | 0.500 | 9.732 (3.043) | −0.386 |
Mean (SD) values, sample size (n), and Pearson correlation coefficients (r) between type I %MHC isoform content and slope and y-intercept values for motor unit firing rate at recruitment (FR-REC) vs. recruitment threshold (REC), mean firing rate (MFR) vs. REC, and firing rate at derecruitment (FR-DEREC) vs. derecruitment threshold (DEREC) relationships for the 90%, 92%, and 95% accuracy criteria.
Significant relationship (P ≤ 0.05).
REFERENCES
- Aagaard P, Andersen JL, Dyhre-Poulsen P, Leffers AM, Wagner A, Magnusson SP, Halkjaer-Kristensen J, Simonsen EB. A mechanism for increased contractile strength of human pennate muscle in response to strength training: changes in muscle architecture. J Physiol 534: 613–623, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam A, De Luca CJ. Firing rates of motor units in human vastus lateralis muscle during fatiguing isometric contractions. J Appl Physiol (1985) 99: 268–280, 2005. [DOI] [PubMed] [Google Scholar]
- Barry BK, Pascoe MA, Jesunathadas M, Enoka RM. Rate coding is compressed but variability is unaltered for motor units in a hand muscle of old adults. J Neurophysiol 97: 3206–3218, 2007. [DOI] [PubMed] [Google Scholar]
- Beck TW, Housh TJ, Fry AC, Cramer JT, Weir JP, Schilling BK, Falvo MJ, Moore CA. The influence of muscle fiber type composition on the patterns of responses for electromyographic and mechanomyographic amplitude and mean power frequency during a fatiguing submaximal isometric muscle action. Electromyogr Clin Neurophysiol 47: 221–232, 2007. [PubMed] [Google Scholar]
- Bergstrom J. Muscle electrolytes in man determined by neutron activation analysis on needle biopsy specimens. Scand J Clin Lab Invest 14: 1–110, 1962. [Google Scholar]
- Booth FW, Laye MJ, Spangenburg EE. Gold standards for scientists who are conducting animal-based exercise studies. J Appl Physiol (1985) 108: 219–221, 2010. [DOI] [PubMed] [Google Scholar]
- Bottinelli R. Functional heterogeneity of mammalian single muscle fibres: do myosin isoforms tell the whole story? Pflügers Arch 443: 6–17, 2001. [DOI] [PubMed] [Google Scholar]
- Bottinelli R, Pellegrino MA, Canepari M, Rossi R, Reggiani C. Specific contributions of various muscle fibre types to human muscle performance: an in vitro study. J Electromyogr Kinesiol 9: 87–95, 1999. [DOI] [PubMed] [Google Scholar]
- Burke R. Motor units: anatomy, physiology, and functional organization. Handbook of Physiology. The Nervous System. Motor Control. Bethesda, MD: Am. Physiol. Soc, 1981, sect. 1, vol. II, p. 345–422. [Google Scholar]
- Carraro U, Catani C. A sensitive SDS-PAGE method separating myosin heavy chain isoforms of rat skeletal muscles reveals the heterogeneous nature of the embryonic myosin. Biochem Biophys Res Commun 116: 793–802, 1983. [DOI] [PubMed] [Google Scholar]
- Contessa P, De Luca CJ. Neural control of muscle force: indications from a simulation model. J Neurophysiol 109: 1548–1570, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca C, LeFever R, McCue M, Xenakis A. Control scheme governing concurrently active human motor units during voluntary contractions. J Physiol 329: 129–142, 1982a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca CJ, Adam A, Wotiz R, Gilmore LD, Nawab SH. Decomposition of surface EMG signals. J Neurophysiol 96: 1646–1657, 2006. [DOI] [PubMed] [Google Scholar]
- De Luca CJ, Contessa P. Hierarchical control of motor units in voluntary contractions. J Neurophysiol 107: 178–195, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca CJ, Contessa P. Biomechanical benefits of the onion-skin motor unit control scheme. J Biomech 48: 195–203, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca CJ, Erim Z. Common drive of motor units in regulation of muscle force. Trends Neurosci 17: 299–305, 1994. [DOI] [PubMed] [Google Scholar]
- De Luca CJ, Foley PJ, Erim Z. Motor unit control properties in constant-force isometric contractions. J Neurophysiol 76: 1503–1516, 1996. [DOI] [PubMed] [Google Scholar]
- De Luca CJ, Hostage EC. Relationship between firing rate and recruitment threshold of motoneurons in voluntary isometric contractions. J Neurophysiol 104: 1034–1046, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca CJ, LeFever RS, McCue MP, Xenakis AP. Behaviour of human motor units in different muscles during linearly varying contractions. J Physiol 329: 113–128, 1982b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defreitas JM, Beck TW, Ye X, Stock MS. Synchronization of low- and high-threshold motor units. Muscle Nerve 49: 575–583, 2014. [DOI] [PubMed] [Google Scholar]
- Dons B, Bollerup K, Bonde-Petersen F, Hancke S. The effect of weight-lifting exercise related to muscle fiber composition and muscle cross-sectional area in humans. Eur J Appl Physiol Occup Physiol 40: 95–106, 1979. [DOI] [PubMed] [Google Scholar]
- Dorfman LJ, Howard JE, McGill KC. Triphasic behavioral response of motor units to submaximal fatiguing exercise. Muscle Nerve 13: 621–628, 1990. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Eccles RM, Lundberg A. The action potentials of the alpha motoneurones supplying fast and slow muscles. J Physiol 142: 275, 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erim Z, De Luca CJ, Mineo K, Aoki T. Rank-ordered regulation of motor units. Muscle Nerve 19: 563–573, 1996. [DOI] [PubMed] [Google Scholar]
- Evans W, Phinney S, Young V. Suction applied to a muscle biopsy maximizes sample size. Med Sci Sports Exerc 14: 101–102, 1981. [PubMed] [Google Scholar]
- Farina D, Negro F, Dideriksen JL. The effective neural drive to muscles is the common synaptic input to motor neurons. J Physiol 592: 3427–3441, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer S, Bremner F, Halliday D, Rosenberg J, Stephens J. The frequency content of common synaptic inputs to motoneurones studied during voluntary isometric contraction in man. J Physiol 470: 127, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry AC, Allemeier CA, Staron RS. Correlation between percentage fiber type area and myosin heavy chain content in human skeletal muscle. Eur J Appl Physiol Occup Physiol 68: 246–251, 1994. [DOI] [PubMed] [Google Scholar]
- Garnett R, O'Donovan M, Stephens J, Taylor A. Motor unit organization of human medial gastrocnemius. J Physiol 287: 33–43, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorassini M, Yang JF, Siu M, Bennett DJ. Intrinsic activation of human motoneurons: possible contribution to motor unit excitation. J Neurophysiol 87: 1850–1858, 2002. [DOI] [PubMed] [Google Scholar]
- Gydikov A, Kosarov D. Some features of different motor units in human biceps brachii. Pflügers Arch 347: 75–88, 1974. [DOI] [PubMed] [Google Scholar]
- Henneman E. Relation between size of neurons and their susceptibility to discharge. Science 126: 1345–1347, 1957. [DOI] [PubMed] [Google Scholar]
- Herda TJ, Housh TJ, Fry AC, Weir JP, Schilling BK, Ryan ED, Cramer JT. A noninvasive, log-transform method for fiber type discrimination using mechanomyography. J Electromyogr Kinesiol 20: 787–794, 2010. [DOI] [PubMed] [Google Scholar]
- Herda TJ, Miller JD, Trevino MA, Mosier EM, Gallagher PM, Fry AC, Vardiman JP. The change in motor unit firing rates at de-recruitment relative to recruitment is correlated with type I myosin heavy chain isoform content of the vastus lateralis in vivo. Acta Physiol (Oxf) 216: 454–463, 2016. [DOI] [PubMed] [Google Scholar]
- Herda TJ, Siedlik J, Trevino MA, Cooper MA, Weir JP. Motor unit control strategies of endurance- versus resistance-trained individuals. Muscle Nerve. 52: 832–843, 2015. [DOI] [PubMed] [Google Scholar]
- Jesunathadas M, Marmon AR, Gibb JM, Enoka RM. Recruitment and derecruitment characteristics of motor units in a hand muscle of young and old adults. J Appl Physiol 108: 1659–1667, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanosue K, Yoshida M, Akazawa K, Fuji K. The number of active motor units and their firing rates in voluntary contraction of human brachialis muscle. Jpn J Physiol 29: 427–443, 1979. [DOI] [PubMed] [Google Scholar]
- Kernell D. The limits of firing frequency in cat lumbosacral motoneurones possessing different time course of afterhyperpolarization. Acta Physiol Scand 65: 87–100, 1965. [Google Scholar]
- Kiehn O, Eken T. Prolonged firing in motor units: evidence of plateau potentials in human motoneurons? J Neurophysiol 78: 3061–3068, 1997. [DOI] [PubMed] [Google Scholar]
- Kudina LP. [Double discharges of human motor neurons.] Neirofiziologiia 6: 152–160, 1974. [PubMed] [Google Scholar]
- Lexell J, Henriksson-Larsén K, Sjöström M. Distribution of different fibre types in human skeletal muscles 2. A study of cross-sections of whole m. vastus lateralis. Acta Physiol Scand 117: 115–122, 1983. [DOI] [PubMed] [Google Scholar]
- Masakado Y. The firing pattern of motor units in the mono-and multidirectional muscle. Jpn Rehabil Med 28: 703–712, 1991. [Google Scholar]
- Masakado Y. Motor unit firing behavior in man. Keio J Med 43: 137–142, 1994. [DOI] [PubMed] [Google Scholar]
- Masakado Y, Akaboshi K, Nagata Ma Kimura A, Chino N. Motor unit firing behavior in slow and fast contractions of the first dorsal interosseous muscle of healthy men. Electroencephalogr Clin Neurophysiol 97: 290–295, 1995. [DOI] [PubMed] [Google Scholar]
- McGill KC, Lateva ZC, Marateb HR. EMGLAB: an interactive EMG decomposition program. J Neurosci Methods 149: 121–133, 2005. [DOI] [PubMed] [Google Scholar]
- Meijer J, Jaspers R, Rittweger J, Seynnes O, Kamandulis S, Brazaitis M, Skurvydas A, Pišot R, Šimunič B, Narici M. Single muscle fibre contractile properties differ between body-builders, power athletes and control subjects. Exp Physiol 100: 1331–1341, 2015. [DOI] [PubMed] [Google Scholar]
- Milner-Brown HS, Stein RB, Yemm R. Changes in firing rate of human motor units during linearly changing voluntary contractions. J Physiol 230: 371–390, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monster AW, Chan H. Isometric force production by motor units of extensor digitorum communis muscle in man. J Neurophysiol 40: 1432–1443, 1977. [DOI] [PubMed] [Google Scholar]
- Moritz CT, Barry BK, Pascoe MA, Enoka RM. Discharge rate variability influences the variation in force fluctuations across the working range of a hand muscle. J Neurophysiol 93: 2449–2459, 2005. [DOI] [PubMed] [Google Scholar]
- Nawab SH, Chang SS, De Luca CJ. High-yield decomposition of surface EMG signals. Clin Neurophysiol 121: 1602–1615, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orizio C, Veicsteinas A. Soundmyogram analysis during sustained maximal voluntary contraction in sprinters and long distance runners. Int J Sports Med 13: 594–599, 1992. [DOI] [PubMed] [Google Scholar]
- Perrie W, Bumford S. Electrophoretic separation of myosin isoenzymes: implications for the histochemical demonstration of fibre types in biopsy specimens of human skeletal muscle. J Neurol Sci 73: 89–96, 1986. [DOI] [PubMed] [Google Scholar]
- Person RS, Kudina LP. Discharge frequency and discharge pattern of human motor units during voluntary contraction of muscle. Electroencephalogr Clin Neurophysiol 32: 471–483, 1972. [DOI] [PubMed] [Google Scholar]
- Pette D, Peuker H, Staron RS. The impact of biochemical methods for single muscle fibre analysis. Acta Physiol Scand 166: 261–277, 1999. [DOI] [PubMed] [Google Scholar]
- Pette D, Staron RS. Myosin isoforms, muscle fiber types, and transitions. Microsc Res Tech 50: 500–509, 2000. [DOI] [PubMed] [Google Scholar]
- Pope ZK, Hester GM, Benik FM, DeFreitas JM. Action potential amplitude as a noninvasive indicator of motor unit-specific hypertrophy. J Neurophysiol 115: 2608–2614, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J, McGill K. Muscle activation and motor unit-firing characteristics in cerebral palsy. Gait Posture 13: 285–286, 2001. [DOI] [PubMed] [Google Scholar]
- Staron R, Malicky E, Leonardi M, Falkel J, Hagerman F, Dudley G. Muscle hypertrophy and fast fiber type conversions in heavy resistance-trained women. Eur J Appl Physiol Occup Physiol 60: 71–79, 1990. [DOI] [PubMed] [Google Scholar]
- Staron RS, Hikida RS. Histochemical, biochemical, and ultrastructural analyses of single human muscle fibers, with special reference to the C-fiber population. J Histochem Cytochem 40: 563–568, 1992. [DOI] [PubMed] [Google Scholar]
- Staron RS, Johnson P. Myosin polymorphism and differential expression in adult human skeletal muscle. Comp Biochem Physiol B 106: 463–475, 1993. [DOI] [PubMed] [Google Scholar]
- Stashuk D, De Bruin H. Automatic decomposition of selective needle-detected myoelectric signals. IEEE Trans Biomed Eng 35: 1–10, 1988. [DOI] [PubMed] [Google Scholar]
- Stock MS, Beck TW, Defreitas JM. Effects of fatigue on motor unit firing rate versus recruitment threshold relationships. Muscle Nerve 45: 100–109, 2012. [DOI] [PubMed] [Google Scholar]
- Swallow EB, Gosker HR, Ward KA, Moore AJ, Dayer MJ, Hopkinson NS, Schols AM, Moxham J, Polkey MI. A novel technique for nonvolitional assessment of quadriceps muscle endurance in humans. J Appl Physiol 103: 739–746, 2007. [DOI] [PubMed] [Google Scholar]
- Tanji J, Kato M. Firing rate of individual motor units in voluntary contraction of abductor digiti minimi muscle in man. Exp Neurol 40: 771–783, 1973. [DOI] [PubMed] [Google Scholar]
- Taylor AD, Bronks R, Smith P, Humphries B. Myoelectric evidence of peripheral muscle fatigue during exercise in severe hypoxia: some references to m. vastus lateralis myosin heavy chain composition. Eur J Appl Physiol Occup Physiol 75: 151–159, 1997. [DOI] [PubMed] [Google Scholar]
- Tracy BL, Maluf KS, Stephenson JL, Hunter SK, Enoka RM. Variability of motor unit discharge and force fluctuations across a range of muscle forces in older adults. Muscle Nerve 32: 533–540, 2005. [DOI] [PubMed] [Google Scholar]
- Trevino MA, Herda TJ. The effects of chronic exercise training status on motor unit activation and deactivation control strategies. J Sports Sci 34: 199–208, 2016. [DOI] [PubMed] [Google Scholar]
- Trevino MA, Herda TJ, Cooper MA. The effects of poliomyelitis on motor unit behavior during repetitive muscle actions: a case report. BMC Res Notes 7: 611, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D, Gallagher P, Carroll C, Raue U, Trappe S. Reduction in hybrid single muscle fiber proportions with resistance training in humans. J Appl Physiol 91: 1955–1961, 2001. [DOI] [PubMed] [Google Scholar]