The dorsal cochlear nucleus contains a population of GABAergic Golgi cells that are proposed to control processing of multisensory signals in the auditory system. In this study, Golgi cells are shown to form a highly interconnected interneuron network using gap junctions but not chemical synapses.
Keywords: auditory system, brain stem, cerebellum, inhibition
Abstract
The mossy fiber-granule cell-parallel fiber system conveys proprioceptive and corollary discharge information to principal cells in cerebellum-like systems. In the dorsal cochlear nucleus (DCN), Golgi cells inhibit granule cells and thus regulate information transfer along the mossy fiber-granule cell-parallel fiber pathway. Whereas excitatory synaptic inputs to Golgi cells are well understood, inhibitory and electrical synaptic inputs to Golgi cells have not been examined. Using paired recordings in a mouse brain slice preparation, we find that Golgi cells of the cochlear nucleus reliably form electrical synapses onto one another. Golgi cells were only rarely electrically coupled to superficial stellate cells, which form a separate network of electrically coupled interneurons in the DCN. Spikelets had a biphasic effect on the excitability of postjunctional Golgi cells, with a brief excitatory phase and a prolonged inhibitory phase due to the propagation of the prejunctional afterhyperpolarization through gap junctions. Golgi cells and stellate cells made weak inhibitory chemical synapses onto Golgi cells with low probability. Electrical synapses are therefore the predominant form of synaptic communication between auditory Golgi cells. We propose that electrical synapses between Golgi cells may function to regulate the synchrony of Golgi cell firing when electrically coupled Golgi cells receive temporally correlated excitatory synaptic input.
NEW & NOTEWORTHY
The dorsal cochlear nucleus contains a population of GABAergic Golgi cells that are proposed to control processing of multisensory signals in the auditory system. In this study, Golgi cells are shown to form a highly interconnected interneuron network using gap junctions but not chemical synapses.
while all vertebrates have a cerebellum, many have an additional brain structure with cerebellum-like cellular and synaptic architecture (Bell et al. 2008). In all such cerebellum-like structures, principal cells integrate two sources of information: a primary sensory modality and diverse input from a mossy fiber-granule cell-parallel fiber (MGP) system. The MGP system conveys proprioceptive and corollary discharge that may be used by principal cells to distinguish between self-generated and externally sourced sensory signals (Oertel and Young 2004; Requarth and Sawtell 2011). In the mammalian dorsal cochlear nucleus (DCN) and the electrosensory lobe of mormyrid fish, two cerebellum-like structures, anti-Hebbian spike timing-dependent plasticity occurs at parallel fiber synapses onto principal cells (Bell et al. 1993; Han et al. 2000; Zhao and Tzounopoulos 2011), which may act as a mechanism for filtering out self-generated sensory input.
However, the MGP system does not act as a simple relay of proprioceptive and corollary discharge signals. In addition to requiring temporal summation of mossy fiber excitatory postsynaptic potentials to reach spike threshold (Balakrishnan and Trussell 2008; Chadderton et al. 2014; Requarth et al. 2014; Sawtell 2010), granule cells receive inhibitory input from Golgi cells (Yaeger and Trussell 2015; Zhang et al. 2007; see Fig. 1A for circuit diagram). While Golgi cells may thus act as gatekeepers of information transmission along the MGP system, understanding the role of Golgi cells in cerebellum-like systems is limited by uncertainty over their synaptic inputs. Although sources of glutamatergic input to DCN Golgi cells have been studied (Ferragamo et al. 1998; Irie et al. 2006; Yaeger and Trussell 2015), the sources of other types of synaptic input to Golgi cells are still unknown.
Fig. 1.
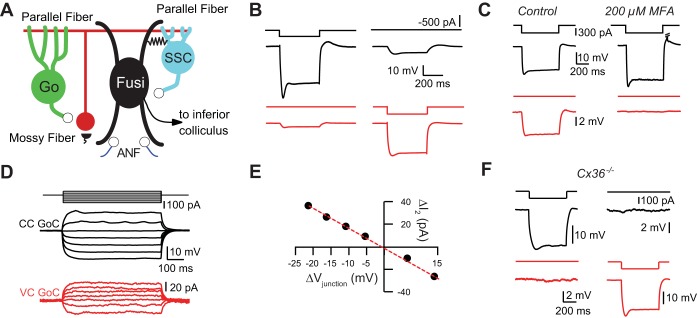
Golgi cells are connected by electrical synapses. A: simplified circuit diagram of the dorsal cochlear nucleus. Granule cells (red circle) receive glutamatergic mossy fiber input (black semicircles). Golgi cells (Go) provide inhibitory GABA/glycinergic inhibition to granule cells. Parallel fibers, the axons of granule cells (red horizontal line), provide glutamatergic input to Golgi cells, inhibitory superficial stellate cells (SSC), and fusiform cells (Fusi). Fusiform cells and superficial stellate cells are connected by electrical synapses (resistor), and superficial stellate cells provide chemical inhibition to fusiform cells. Fusiform cells receive glutamatergic input from auditory nerve fibers (ANF; purple lines at bottom) and project to the inferior colliculus. B: bidirectional electrical coupling between Golgi cells. Hyperpolarizing current injection into the black cell triggers a large hyperpolarization in the black cell and a smaller hyperpolarization of the red cell. Similarly, hyperpolarizing current injection into the red cell triggers a voltage deflection in the black cell. C: electrical coupling in a Golgi cell pair was blocked by bath application of the gap junction blocker meclofenamic acid at 200 μM. D: current injection into black Golgi cell (GoC) evokes currents in red Golgi cell. Black Golgi cell recorded with CsCl-based intracellular solution containing QX-314. CC, current clamp; VC, voltage clamp. E: transjunctional voltage (Vjunction)-current (I) relationship for pair in D. Red dashed line is linear fit to black points, indicating lack of voltage-dependent rectification (r2 value for linear fit = 0.998). F: electrical coupling was absent in dual recordings between IG17/Cx36−/− mice.
Cerebellar Golgi cells are known to form electrical and GABAergic synapses with one another (Dugué et al. 2009; Hull and Regehr 2012; Vervaeke et al. 2010, 2012). Early anatomical studies have suggested that cochlear nucleus Golgi cells are not connected by gap junctions (Wouterlood et al. 1984) but may be connected by inhibitory chemical synapses (Mugnaini et al. 1980). However, recent physiological experiments in DCN have uncovered electrical coupling between principal cells and associated interneurons, superficial stellate cells, a connection that was not previously identified with anatomical methods (Apostolides and Trussell 2013). Thus physiological approaches may reveal new synaptic relationships between cells in the DCN.
Using acute slices of mouse DCN, we show that the majority of Golgi cells are electrically coupled by connexin 36-containing gap junctions, which mediate both excitatory and inhibitory signals within a Golgi cell network. Furthermore, unlike superficial stellate cells, Golgi cells are nearly exclusively coupled to other Golgi cells and not to other cell types. In contrast to the exclusivity of electrical coupling, Golgi cells receive sparse chemical inhibitory inputs from both superficial stellate cells and other Golgi cells. Thus synaptic communication between cochlear nucleus Golgi cells is primarily mediated by gap junctions, with chemical synapses playing a minor role.
METHODS
Animals.
All experimental procedures using animals were approved by the Oregon Health and Science University Institutional Animal Care and Use Committee. Postnatal day (P)16–P24 homozygous or heterozygous IG17 mice were used for all experiments (except for experiments in Fig. 1F), affording identification of Golgi cells in brain slices. In the IG17 mouse line, GFP fused to the human interleukin-2 receptor α-subunit is expressed under the control of the promoter for metabotropic glutamate receptor (mGluR) subtype 2 (Watanabe et al. 1998; Watanabe and Nakanishi 2003). Cochlear nucleus Golgi cells and unipolar brush cells express GFP in the IG17 mouse line (Borges-Merjane and Trussell 2015; Irie et al. 2006; Yaeger and Trussell 2015). For the experiments in Fig. 1F, P16–P24 IG17/Cx36−/− mice were used. These mice were generated by crossing IG17 and Cx36−/− mice (Hormuzdi et al. 2001). IG17/Cx36+/− mice were subsequently crossed to obtain IG17/Cx36−/− mice. Cx36−/− mice were genotyped by polymerase chain reaction. Both copies of the gene coding for the gap junction protein Connexin 36 are deleted in Cx36−/− mice (Hormuzdi et al. 2001). Male and female mice were used in all experiments.
Slice preparation.
Coronal brain slices (300 μm) containing cochlear nucleus were cut in a solution that contained (in mM) 87 NaCl, 25 NaHCO3, 25 glucose, 75 sucrose, 2.5 KCl, 1.25 NaH2PO4, 0.5 CaCl2, and 7 MgCl2 (bubbled with 95% O2-5% CO2; ∼320 mosM; 4°C). The N-methyl-d-aspartic acid receptor antagonists 3-((R)-2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid (R-CPP) and MK-801 were routinely added to the cutting solution to improve slice viability (2–5 μM). Slices were incubated in 34°C standard artificial cerebrospinal fluid (ACSF) for 15–30 min after slicing and then stored at room temperature until recording. Standard ACSF contained (in mM) 130 NaCl, 2.1 KCl, 1.2 KH2PO4, 3–5 HEPES, 1 MgSO4, 1.7 CaCl2, 10 glucose, and 20 NaHCO3 (bubbled with 95% O2-5% CO2; ∼305 mosM). All recordings were performed in standard ACSF.
Electrophysiological recording.
Slices were transferred to a recording chamber on the stage of an upright microscope (Zeiss Examiner.D1) and perfused continuously with ACSF with a peristaltic pump (Gilson Minipulse 3). Bath temperature was maintained at 34–36°C by an inline heater (Warner Instrument TC-324B). Cells were visualized with a ×40 objective lens with Dodt gradient contrast optics using an infrared camera (Sony XC-ST30). GFP-positive cells were visualized with epifluorescence optics (excitation 469/35, dichroic FF497, emission 525/39; Semrock) and a custom-built LED excitation source.
Current- and voltage-clamp recordings from Golgi cells and superficial stellate cells were made with either a K-gluconate-based or a KMeSO3-based internal solution. All recordings from granule cells were made with the K-gluconate-based solution. The KMeSO3-based internal solution was used mainly for the experiments in Fig. 6 to reduce rundown of K+ currents mediating the afterhyperpolarization during prolonged whole cell recordings (Zhang et al. 1994). The K-gluconate-based solution was composed of (in mM) 113 K-gluconate, 2.75 MgCl2, 1.75 MgSO4, 9 HEPES, 0.1 EGTA, 14 Tris2-phosphocreatine, 4 Na2-ATP, and 0.3 Tris-GTP (osmolarity adjusted to ∼295 mosM with sucrose and pH adjusted to 7.25 with KOH). All reported membrane values recorded with the K-gluconate-based internal solution were corrected off-line for a −10-mV junction potential. The KMeSO3 solution was composed of (in mM) 120 KMeSO3, 10 KCl, 10 HEPES, 0.03 EGTA, 10 Na2-phosphocreatine, 4 Mg-ATP, and 0.5 Na2-GTP (∼295 mosM and pH adjusted to 7.25 with KOH). All reported membrane values recorded with the KMeSO3-based internal solution were corrected off-line for a −6-mV junction potential.
Fig. 6.
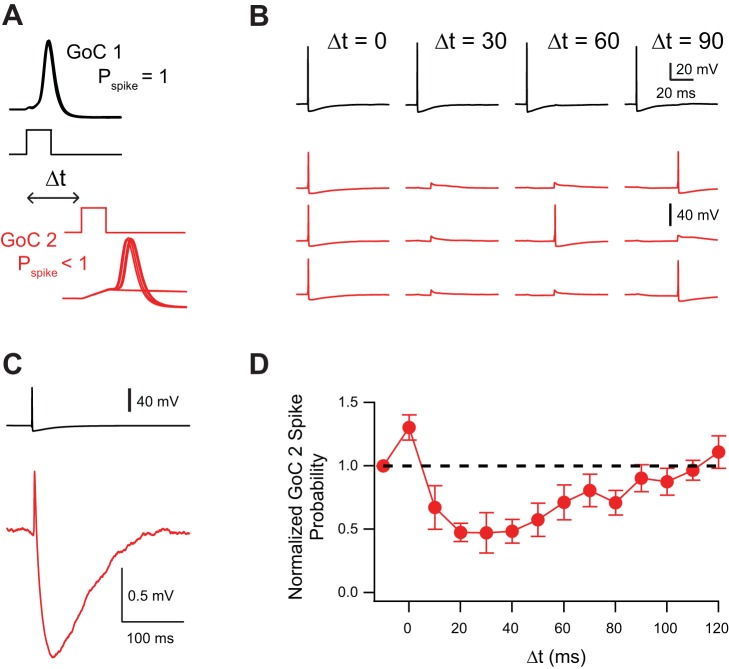
Spikelets have a biphasic effect on Golgi cell excitability. A: 1-ms current injection into Golgi cell 1 was strong enough to trigger spiking with no failures [spike probability (Pspike) = 1.0]. Current injection into Golgi cell 2 was set such that occasional spike failures occurred (spike probability < 1.0). The delay between the current injection into the 2 Golgi cells (Δt) was varied. All experiments performed in NBQX, R-CPP, SR, and strychnine. B: example trials in which AP in Golgi cell 1 did not inhibit firing of Golgi cell 2 at Δt = 0 ms but inhibited firing at Δt = 30, 60, and 90 ms. Actual normalized spike probability for this pair was 1.1 at Δt = 0 ms, 0.2 at Δt = 30 ms, 0.5 at Δt = 60 ms, and 0.9 at Δt = 90 ms. C: spikelet from same pair as in B showing brief depolarization and long-lasting hyperpolarization. D: influence of Δt on spike probability of Golgi cell 2 (n = 6). Error bars show SE.
A CsCl-based intracellular solution was used for voltage-clamp experiments in which voltage steps were delivered to Golgi cells, for some paired recordings between Golgi cells, and for paired recordings between Golgi cells and superficial stellate cells (see Fig. 3, Fig. 4, and Fig. 7). The CsCl-based internal solution was composed of (in mM) 115 CsCl, 4.5 MgCl2, 8 QX-314-Cl, 10 HEPES, 10 EGTA, 4 Na2-ATP, and 0.5 Tris-GTP (osmolarity ∼295 mosM and pH adjusted to 7.25 with CsOH). The CsCl-based internal solution had a small junction potential (∼2 mV), for which no correction was made. A CsMeSO3-based intracellular solution was used for the experiments in Fig. 8 and was composed of (in mM) 110 CsMeSO3, 40 HEPES, 1 KCl, 4 NaCl, 10 Na2-phosphocreatine, 4 Mg-ATP, 0.4 Tris-GTP. Recordings made with the CsMeSO3-based intracellular solution were corrected off-line for a −10-mV junction potential. Patch pipettes were pulled from borosilicate glass (WPI), and open-tip resistances were 3–7 MΩ when filled with internal solution when recording from Golgi cells and 5–11 MΩ when recording from granule cells and superficial stellate cells.
Fig. 3.
Depolarization triggers spikelets in recordings from single Golgi cells. A: depolarization of voltage-clamped Golgi cell from −60 mV to +20 mV triggered the sudden appearance of spikelets. Note that spikelets were not present prior to, or following, depolarizing step. All recordings were performed with an intracellular solution containing QX-314 to block sodium channels. Strychnine, NBQX, SR 95531, and R-CPP/MK-801 were present in all recordings to block fast chemical synaptic transmission. B: spikelets in paired Golgi-Golgi cell recordings resemble depolarization-evoked spikelets. Top: spikelet from paired Golgi-Golgi cell recording. Bottom: spikelet triggered by depolarization of a different Golgi cell from −60 mV to −20 mV. C: portions of 3 consecutive sweeps in which a Golgi cell was depolarized from −60 mV to −20 mV. Downward deflections indicate depolarizing junctional currents. Inset: expanded portion of the trace. D: bath application of the sodium channel blocker TTX at 1 μM blocked the depolarization-evoked spikelets. Same cell as in C. Inset as in C. E: portions of 3 consecutive sweeps in which a Golgi cell was depolarized from −60 mV to +40 mV. Downward deflections indicate depolarizing junctional currents. Inset as in C. F: bath application of the gap junction blocker MFA at 200 μM blocked the depolarization-evoked spikelets. Same cell as in E. Inset as in C. Note that MFA decreased membrane conductance.
Fig. 4.
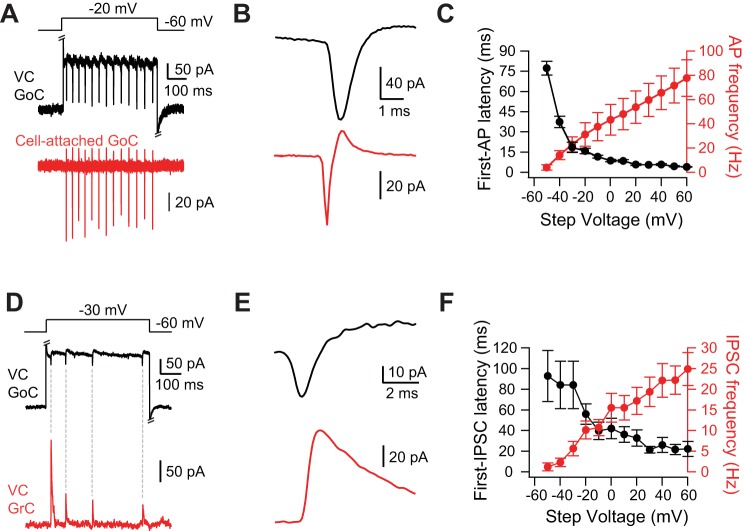
Depolarization-triggered spikelets originate in Golgi cells. A: step to −20 mV from a holding potential of −60 mV in the voltage-clamped Golgi cell evoked fast inward currents (spikelets) in the voltage-clamped Golgi cell (black trace) and spikes in the Golgi cell (red) simultaneously recorded in the cell-attached configuration. Fast chemical synaptic transmission blocked by strychnine, SR 95531, NBQX, and R-CPP. Intracellular solution for black Golgi cell was CsCl based and contained QX-314. B: spike-triggered average of spikelets from same pair as in A. C: latency to first extracellularly recorded AP from the beginning of the voltage step (black) and frequency of extracellularly recorded APs (red) as a function of the voltage step (n = 4). D: step to −20 mV from a holding potential of −60 mV evoked spikelets in the Golgi cell (black) and IPSCs onto the simultaneously recorded voltage-clamped granule cell (red). Excitatory synaptic transmission blocked by NBQX and R-CPP. Intracellular solution for Golgi cell was CsCl based and contained QX-314. E: IPSC-triggered average of spikelets from same pair as in D. Note that depolarizing junctional current precedes IPSC. F: latency to first IPSC onto the granule cell from the beginning of the voltage step (black) and frequency of IPSCs onto granule cells (red) as a function of the voltage step (n = 18). Error bars show SE.
Fig. 7.
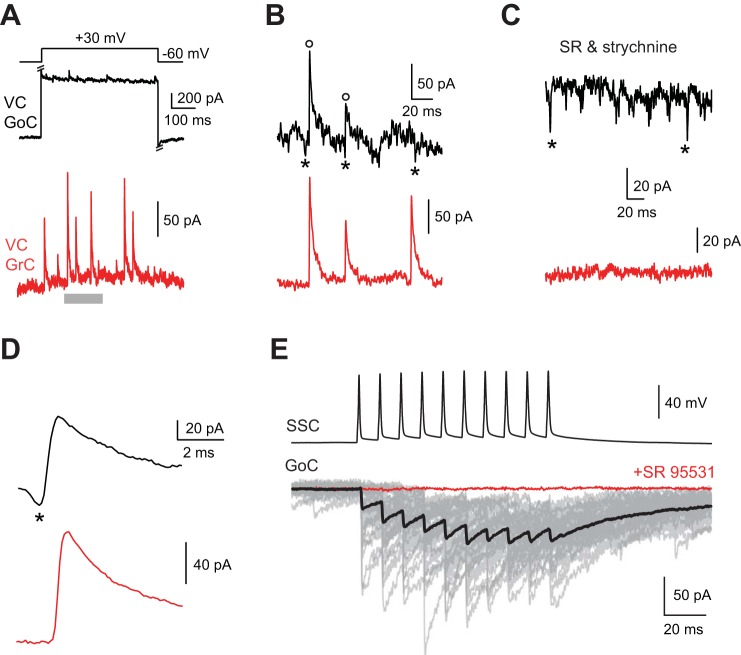
Golgi cells receive rare chemical synaptic input from other Golgi cells and from superficial stellate cells. A: step to +30 mV from a holding potential of −60 mV in black voltage-clamped Golgi cell evoked synchronous IPSCs in simultaneously recorded red granule cell. Synchronous voltage step-evoked IPSCs in Golgi and granule cells occurred in 1 of 23 dual Golgi-granule cell recordings. B: time block denoted in A by gray bar displayed at higher temporal resolution. Open circles indicate synchronous IPSCs in Golgi and granule cell, and asterisks indicate putative spikelets preceding IPSCs. C: 10 μM SR95531 and 0.5 μM strychnine blocked IPSCs and revealed putative spikelets (indicated by asterisks; Golgi cell holding voltage of +30 mV). The other smaller-amplitude fast current deflections may also be spikelets. D: IPSC-triggered average revealed synchronous IPSCs preceded by spikelet in Golgi cell (indicated by asterisk). E: 100-Hz train of APs in a superficial stellate cell (SSC) evoked IPSCs onto voltage-clamped Golgi cell (GoC; gray, individual trials; black, average of trials) that were completely blocked by 10 μM SR95531 (red trace).
Fig. 8.
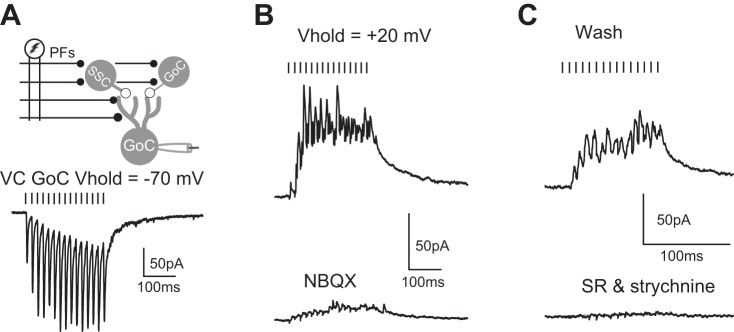
Stimulation of parallel fibers evokes disynaptic IPSCs onto Golgi cells. A: extracellular stimulation of parallel fibers (PFs) evokes EPSCs onto Golgi cell voltage-clamped at −70 mV. Black bars indicate the timing of parallel fiber stimulation. Golgi cells were recorded with a CsMeSO3-based intracellular solution (see methods). Stimulus artifacts blanked for clarity. Vhold, holding potential. B: holding Golgi cell at +20 mV (previously determined reversal potential for EPSCs) reveals IPSCs (top) that were partially blocked by 2.5 μM NBQX (bottom). Same cell as in A. C: after washout of NBQX, IPSCs partially recovered (top) and were completely blocked by 10 μM SR95531 and 0.5 μM strychnine (bottom). Same cell as in B.
Data acquisition and analysis.
Single and dual whole cell patch-clamp recordings were made with a MultiClamp 700B amplifier using Clampex 9.2 (Axon Instruments, Union City, CA). Golgi cells were identified on the basis of GFP expression in IG17 mice, multipolar appearance, and medium- to large-sized somas (≥15 μm; Irie et al. 2006). Golgi cells were found most often in the granule cell lamina between ventral cochlear nucleus and DCN but were also recorded in the cell body layer of the DCN (Irie et al. 2006; Yaeger and Trussell 2015). Granule cells were identified on the basis of their small soma size (≤10 μm), characteristic intrinsic properties (Balakrishnan and Trussell 2008), and lack of GFP expression in IG17 mice. Superficial stellate cells were identified on the basis of the position of their soma in the molecular layer, small- to medium-sized somas (≤15 μm; Wouterlood et al. 1984), characteristic membrane properties (Apostolides and Trussell 2014), and lack of GFP expression in IG17 mice. Dual recordings were only attempted if the somas of the two neurons were within 120 μm of each other. Whole cell access resistance was 6–25 MΩ in voltage-clamp recordings from Golgi cells and 12–35 MΩ in voltage-clamp recordings from granule cells. Access resistance was compensated by 70% online. Recordings were acquired at 50 kHz and low-pass filtered at 10 kHz with a Digidata 1322A (Axon Instruments).
The coupling coefficient in Golgi-Golgi cell pairs was defined as the amplitude of the voltage deflection in the postjunctional cell (into which current was not injected) over the amplitude of the voltage deflection in the prejunctional cell (into which current was injected). Golgi cells were considered to be coupled if the coupling coefficient in both cells was >0.005. All reported spikelet parameters were measured in the absence of prejunctional bias current, as spikelet waveforms are sensitive to prejunctional holding potential (Bennett and Zukin 2004; Gibson et al. 2005). In paired recordings between Golgi cells action potentials (APs) were evoked with a 1-ms 1.0- to 1.8-nA current injection, and postjunctional Golgi cells were voltage clamped at −50 to −60 mV or recorded in current clamp.
In dual voltage-clamp recordings from Golgi cells and granule cells, Golgi cells were held at −60 mV when using the CsCl-based solution. Granule cells were held at 0 mV. For the experiments in Fig. 3 and Fig. 4, Golgi cell voltage was stepped from −60 to +60 mV in 10-mV increments for 500 ms. Inhibitory postsynaptic currents (IPSCs) onto granule cells were detected with a template-matching algorithm in Axograph X (Clements and Bekkers 1997). Voltage steps in the Golgi cell were considered to have evoked IPSCs in the granule cell by depolarization of a coupled Golgi cell if a granule cell IPSC-triggered average returned a spikelet in the Golgi cell. Furthermore, we required that the peak of the returned spikelet precede the peak of the first derivative of the averaged granule cell IPSC in order for the experiment to be interpreted as the Golgi voltage step recruiting a coupled Golgi cell. In 2 of 47 dual Golgi-granule recordings, voltage steps in the Golgi cell evoked a burst of IPSCs in the granule cell that were not preceded by spikelets but occurred with the voltage-dependent onset of an inward, putative Ca2+ current in the Golgi cell; these recordings were not included in the data set for Fig. 4.
Spikelet-to-IPSC latency was calculated as the time between the peak of the depolarizing junctional current (DJC) and the first derivative of the IPSC (Crowley et al. 2009). To determine whether IPSCs occurred synchronously in the granule cell and the Golgi cell (see Fig. 4), IPSCs were detected and averaged during Golgi cell steps to potentials +20 mV and above to allow for adequate driving force for Cl− in the Golgi cell. In one pair, the granule cell IPSC-triggered average for positive Golgi cell steps returned both an IPSC and a spikelet in the Golgi cell (see Fig. 7).
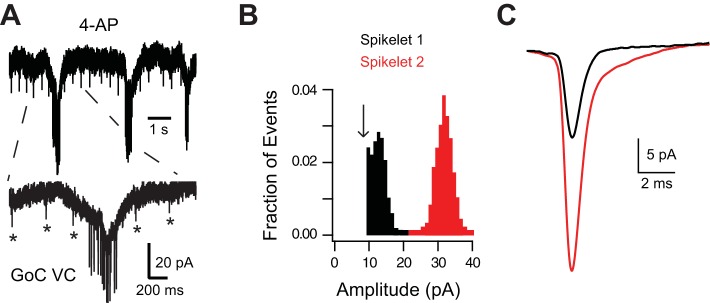
Spikelets were detected with a template-matching algorithm using a fast rising and decaying inward current as the template (similar to the DJC component of the spikelet) in Axograph X. Spikelets were rarely recorded in the absence of Golgi cell voltage steps or before bath application of 4-aminopyridine (4-AP). The scarcity of spontaneous spikelets is consistent with reports that Golgi cells do not fire spontaneously in cochlear nucleus (Irie et al. 2006) and with our conclusion that Golgi cells are only electrically coupled to other Golgi cells. Spikelets sometimes occurred in bursts in the presence of 4-AP but never occurred in bursts in the absence of 4-AP. When spikelets induced by bath application of 4-AP were detected in recordings from single Golgi cells (see Fig. 5), detected events were rejected if their amplitude was <10 pA. Golgi cells were considered to have two separate spikelet amplitudes if there were two separate peaks in the amplitude histograms.
Fig. 5.
Electrically coupled Golgi cells converge onto one another. A: application of 200 μM 4-AP in the presence of NBQX, R-CPP, SR 95531, and strychnine evoked putative spikelets of 2 different amplitudes: 1 small, regularly occurring spikelet (indicated by asterisks) and a larger-amplitude spikelet occurring in bursts. B: events detected with a template-matching algorithm from cell in A with an amplitude cutoff (arrow) of 10 pA. C: averaging events with amplitudes specified by the red or black distribution results in events with spikeletlike waveforms. The red, larger spikelet does not have a HJC component because these events occurred during slow inward currents (see A) likely due to burst firing by the prejunctional Golgi cell.
Statistics.
All averages are reported in text as means ± SD unless otherwise noted. Statistical significance was tested with the Student's t-test.
RESULTS
Golgi cells are electrically coupled through connexin 36-containing gap junctions.
To determine whether auditory Golgi cells are electrically coupled, we made paired recordings between GFP+ Golgi cells in brain slices from the IG17 mouse line, in which GFP-tagged human interleukin-2 receptor α-subunit is expressed under the control of the mGluR2 promoter (Watanabe et al. 1998; Watanabe and Nakanishi 2003). In 68 of 76 (90%) dual recordings made in brain slices from 51 mice, injection of a pulse of hyperpolarizing current into one Golgi cell resulted in a measurable voltage change in the other Golgi cell. As predicted for gap junction-mediated electrical coupling (Bennett and Zukin 2004), electrical coupling between Golgi cells was bidirectional (Fig. 1B). The average coupling constant was 0.11 ± 0.10 (n = 68). Coupling was blocked by the gap junction blocker meclofenamic acid (MFA, 200 μM; 88 ± 10% block of coupling coefficient, n = 3 pairs from 2 mice; Fig. 1C), suggesting that coupling was mediated by connexin-containing gap junctions (Pan et al. 2007).
What subtype of connexin mediates electrical coupling between Golgi cells? Connexin 36 is the most common connexin forming gap junctions between neurons in the mammalian brain (Connors and Long 2004) and was shown to be necessary for electrical coupling between cerebellar Golgi cells (Vervaeke et al. 2010). Gap junctions formed by connexin 36 show only weak transjunctional voltage-dependent rectification (Alcami and Marty 2013; Moreno et al. 2005; Srinivas et al. 1999), in contrast to other connexins (Palacios-Prado et al. 2013). Coupling conductance, measured by injecting current into one Golgi cell and recording the junctional current in a coupled Golgi cell, was well-fit by a straight line (Fig. 1, D and E; coupling conductance = 0.64 ± 0.51 nS, n = 10 cells from 9 mice; r2 values for linear fit of transjunctional voltage-current relations range: 0.95–1.00). Thus coupling conductance in Golgi-Golgi cell pairs does not depend on transjunctional voltage, suggesting that connexin 36 mediates electrical coupling between Golgi cells. To confirm that connexin 36 mediates electrical coupling, recordings were made in slices from IG17/Cx36−/− mice. No instances of electrical coupling were found in Golgi-Golgi dual recordings in slices from IG17/Cx36−/− mice (Fig. 1F; coupling coefficient = 0.003 ± 0.002, n = 9 pairs from 3 mice).
Properties of prejunctional action potentials.
APs in prejunctional Golgi cells resulted in a small, brief depolarization [depolarizing junctional potential (DJP); 0.6 ± 0.4 mV depolarization from rest; half-width: 3.2 ± 0.9 ms; n = 6 pairs from 2 mice] and long-lasting hyperpolarization due to propagation of the prejunctional afterhyperpolarization [hyperpolarizing junctional potential (HJP); −0.8 ± 0.4 mV hyperpolarization from rest; half-width: 79 ± 32 ms; Fig. 2A]. The DJP peaked 1.0 ± 0.4 ms after the peak of the prejunctional spike, and the HJP peaked 40 ± 10 ms after the prejunctional spike. The coupling coefficient of the DJP to the spike was strongly reduced compared with the coupling coefficient determined by direct current injection (0.16 ± 0.11 with direct current injection compared with 0.009 ± 0.006 for spike, n = 6), as expected given the low-pass filtering characteristics of electrical synapses (Dugué et al. 2009; Gibson et al. 2005).
Fig. 2.
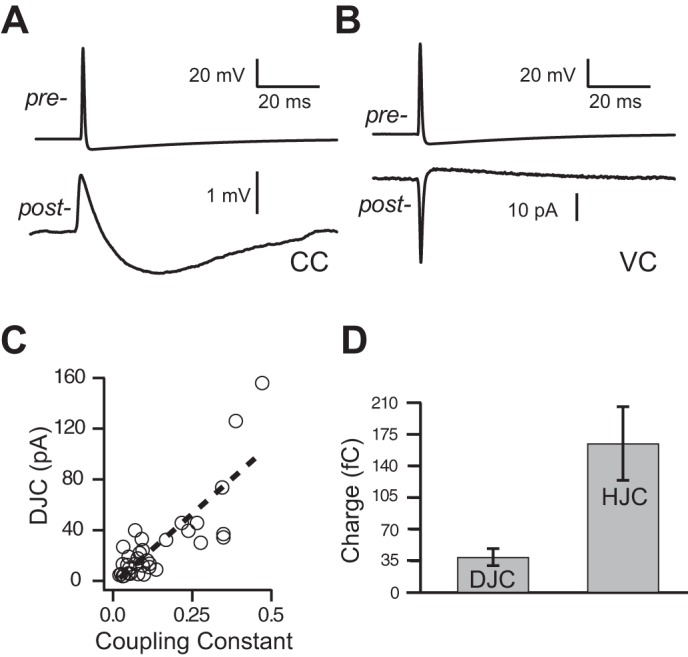
Properties of spikelets in paired Golgi-Golgi cell recordings. A: spike in Golgi cell evoked spikelet in postjunctional Golgi cell recorded in current clamp consisting of brief depolarization and a longer-lasting hyperpolarization. B: spike in Golgi cell evoked brief inward current (DJC) and longer-lasting outward current in voltage-clamped Golgi cell (HJC). Same pair on left and right. NBQX, R-CPP, SR95531, and strychnine in the bath. C: amplitude DJC is correlated with the coupling constant in paired Golgi-Golgi cell recordings (n = 33; r2 = 0.68, P < 0.01), indicating that spikelet amplitudes are a measure of coupling strength. Dashed line is a linear fit. D: excitatory charge carried by DJC component of spikelet and inhibitory charge carried by HJC component of spikelet (n = 17). Error bars show SE.
When the postjunctional cell was recorded in voltage clamp, prejunctional spikes resulted in brief inward currents [depolarizing junctional current (DJC); 38 ± 43 pA, n = 17 pairs from 9 mice] and sustained hyperpolarizing currents [hyperpolarizing junctional current (HJC); 4 ± 3 pA; Fig. 2B]. The amplitude of the DJC of the spikelet was significantly correlated with the coupling coefficient (Fig. 2C, P < 0.01; Long et al. 2004; Zsiros and Maccaferri 2005), and spikelets were typically undetectable in electrically connected Golgi cell pairs with coupling coefficients < 0.03. The latency between the peak of the prejunctional spike and the peak of the postjunctional DJC was 0.4 ± 0.3 ms, and the latency from prejunctional spike peak to postjunctional HJC peak was 16 ± 11 ms. Despite the small amplitude of the HJC, its charge (time integral of current) was significantly greater than that of the DJC (Fig. 2D; P < 0.01), indicating that spikelets are mainly inhibitory to postjunctional cells.
Depolarization evokes spikelets in single Golgi cell recordings.
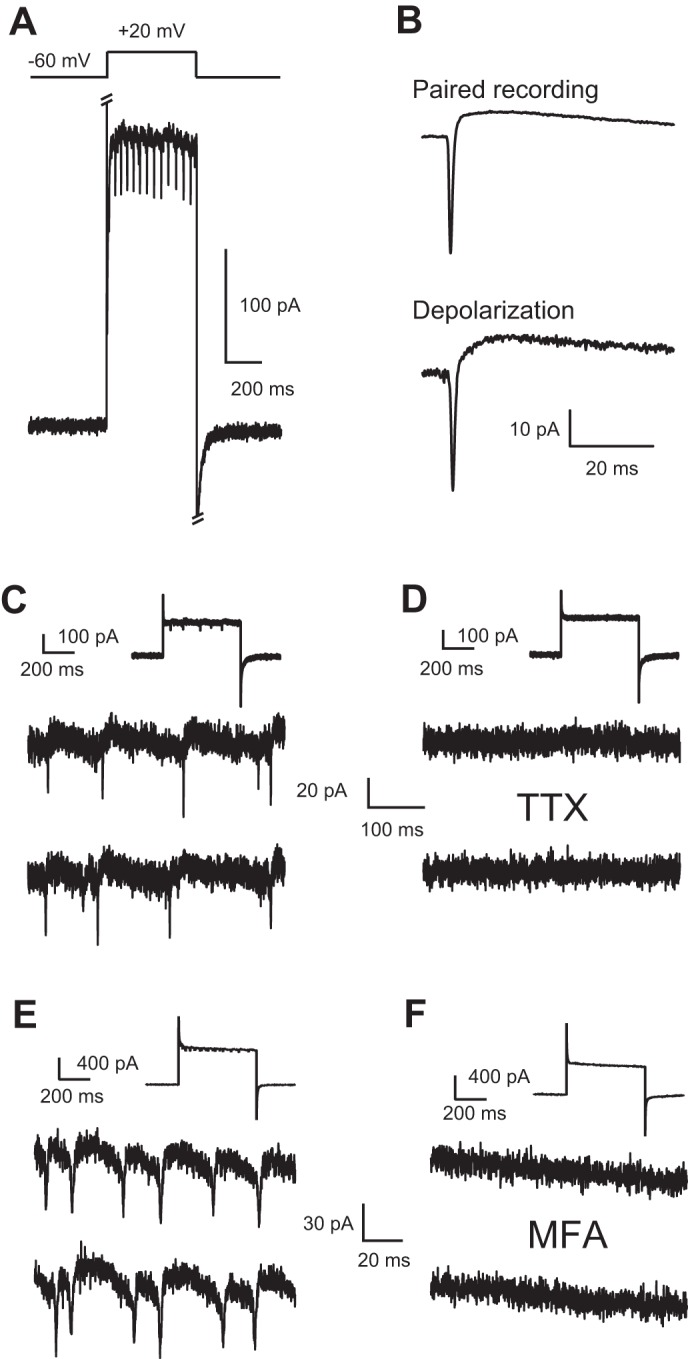
In 28 of 48 (58%) recordings from single voltage-clamped Golgi cells patched with an intracellular solution containing QX-314 to block Na+ channels in slices from 24 mice, depolarization of the Golgi cell resulted in the sudden appearance of repetitive fast inward currents (Fig. 3A). These events were not due to chemical synaptic activity, as they were evoked in the presence of fast chemical synaptic blockers. The depolarization-evoked events had a waveform similar to spikelets recorded in paired Golgi-Golgi cell recordings. Figure 3B, top, shows the spikelet recorded in a paired Golgi-Golgi cell recording, and Fig. 3B, bottom, shows the waveform recorded in a different Golgi cell in response to depolarization of the Golgi cell. Depolarization-evoked events were blocked by 1 μM TTX (n = 5 cells from 3 mice; Fig. 3, C and D), suggesting that they were mediated by sodium channels. MFA (200 μM) also blocked the spikeletlike event (n = 4 cells from 2 mice; Fig. 3, E and F). Together, these data indicate that depolarization of a Golgi cell triggers APs in a coupled neuron.
Spikelets originate in other Golgi cells.
Did the spikelets evoked by depolarization of single Golgi cells originate just in Golgi cells or is an additional class of neuron electrically coupled to Golgi cells? To answer this question, single Golgi cells were depolarized in voltage clamp to evoke spikelets while the spiking of the nearest visually identified neighboring Golgi cell was monitored in a cell-attached recording. In four of five attempts in slices from five mice, we were able to elicit spiking of a Golgi cell by delivering depolarizing voltage steps to the neighboring Golgi cell in voltage clamp (Fig. 4A). The voltage-clamped cell was loaded with a Cs-based solution and contained QX-314 to block spikes (see methods). Every cell-attached spike had a corresponding spikelet in the voltage-clamped Golgi cell. The negative peak of spikes recorded in cell-attached mode preceded the peak DJC of the spikelet by 0.7 ± 0.2 ms (Fig. 4B). Differentiation of the spikelet waveform generated a waveform similar to the cell-attached spike waveform (data not shown; Trenholm et al. 2013), and the peak of the cell-attached spike preceded the peak of the differentiated spikelet by 0.3 ± 0.2 ms, similar to the spike-to-spikelet latency observed in paired whole cell Golgi cell recordings. The latency to the first cell-attached spike from the beginning of the step in the Golgi cell decreased as a function of voltage, and the frequency of Golgi cell APs increased as a function of voltage (Fig. 4C).
Although Golgi cells were electrically connected to one another in paired recordings, this does not exclude the possibility that Golgi cells are electrically coupled to other types of neurons. Inhibitory interneurons couple promiscuously with other classes of interneurons in several brain regions, including the neocortex (Gibson et al. 1999; Simon et al. 2005) and hippocampus (Zsiros and Maccaferri 2005). Superficial stellate cells are the only other electrically coupled interneurons in DCN (Wouterlood et al. 1984), and so we performed paired recordings between Golgi cells and superficial stellate cells to determine whether the two interneuron types were electrically coupled. Injection of direct current revealed electrical coupling in only 1 of the 53 superficial stellate cell-Golgi cell dual recordings from slices from 15 mice (connection probability = 0.02; superficial stellate cell-to-Golgi cell coupling coefficient in uncoupled pairs: 0.002 ± 0.004), and coupling in this pair was too weak for prejunctional superficial stellate cell APs to propagate into the Golgi cell (coupling coefficient in coupled pair: 0.02; data not shown).
As a further test of whether spikelets originate in Golgi cells, we tested the effect of the mGluR2/3 agonist LY 354740 on depolarization-evoked spikelets. As Golgi cells, but not superficial stellate cells, are immunopositive for mGluR2/3 (Petralia et al. 1996, 2000), and mGluR2/3 agonists activate G protein-coupled inward-rectifier K+ currents that dampen Golgi cell excitability (Irie et al. 2006), we predicted that LY 354740 application would reduce the number of spikelets evoked by a depolarizing voltage step. Bath application of 300 nM LY 354740 significantly reduced the number of spikelets evoked by a 500-ms voltage step from −60 to −20 mV in recordings from single Golgi cells performed in slices from two mice (6.1 ± 3.8 spikelets per step during control, 0.5 ± 0.8 in LY 354740; P = 0.04, n = 4; data not shown). The number of spikelets evoked by the step recovered after washout of LY 354740 (5.2 ± 3.8 spikelets). The reduction in depolarization-evoked spikelets by LY 354740 application is consistent with the idea that spikelets originate solely in other Golgi cells.
As a final test of whether spikelets originate in Golgi cells, dual recordings were made between Golgi cells and granule cells. In 21 of 45 (47%) dual Golgi-granule cell recordings made in slices from 30 mice, Golgi cell voltage steps evoked spikelets in Golgi cells (DJC: range 37 to 3 pA, average = 17 ± 10 pA) that preceded IPSCs onto granule cells (Fig. 4, D–F). Voltage steps evoked spikelets in only 73% (33 of 45) of dual Golgi-granule cell recordings, and, considering only this subset of recordings, Golgi cell voltage steps evoked granule cell IPSCs in 64% (21 of 33) of cases. Granule cell IPSCs were evoked by Golgi cell voltage steps in the absence of spikelets in only two dual recordings; in these cases, granule cell IPSCs appeared to be evoked by a putative Ca2+ current in the Golgi cell (see methods). Moreover, the relationships between first-IPSC latency and IPSC frequency to the step voltage (Fig. 4F) were similar to those observed for Golgi cell APs in Fig. 4C. As Golgi cells are the only known source of inhibition onto granule cells (Yaeger and Trussell 2015), these results further suggest that spikelets originate solely in other Golgi cells.
Convergence of electrical synapses onto Golgi cells.
Multiple spikelet amplitudes were sometimes seen in recordings from Golgi cells, suggesting that Golgi cells may be electrically coupled to multiple Golgi cells. Additionally, as the amplitude of the DJC was linearly correlated with the coupling coefficient (Fig. 2C) in paired Golgi-Golgi recordings, multiple spikelet amplitudes may be observed if Golgi cells converge onto one another with different coupling coefficients, as do inferior olivary neurons (Hoge et al. 2011). Thus we attempted to detect multiple spikelet amplitudes in voltage-clamp recordings from Golgi cells in order to estimate the number of coupled partners. It is important to note that this method is biased toward strongly coupled Golgi cells, as weakly coupled Golgi cells have small or negligible spikelet amplitudes.
Golgi cells in the DCN only rarely fire spontaneously (Irie et al. 2006), and thus the K+ channel blocker 4-AP was used to promote spontaneous firing (Roberts and Trussell 2010). With fast chemical synaptic transmission blocked, bath application of 100–200 μM 4-AP induced spikelets in 14 of 17 Golgi cells recorded in slices from 11 mice (82%). Spikelets were never observed after 4-AP application in slices from IG17/Cx36−/− mice (0 of 7 cells in slices from 2 mice). Application of 4-AP increased the frequency of IPSCs in granule cells, also indicating that this drug increases the frequency of Golgi cell APs (data not shown). Spikelets (DJC: 30 ± 18 pA) were detected with a template-matching algorithm (see methods). In 11 cells one spikelet amplitude was detected, whereas in 3 others two spikelet amplitudes were detected. Figure 5A shows a portion of an exemplary recording in which bath application of 100 μM 4-AP induced spikelets of two different amplitudes. A spikelet with an amplitude of ∼30 pA occurred in bursts, whereas a spikelet with an amplitude of ∼10 pA (indicated by asterisks in Fig. 5A) occurred at regular intervals. In Fig. 5B, an amplitude histogram is shown for the detected events from the recording shown in Fig. 5A with an amplitude cutoff of 10 pA. Averaging events with amplitudes in the blue distribution resulted in a spikelet waveform in Fig. 5C, as did averaging events in the red distribution. The larger red events lacked an HJC component because they tended to occur during slow inward currents (see Fig. 5A). Based on spikelets as an indicator of coupling and assuming that all Golgi cells fire in the presence of 4-AP, ∼18% (3 of 17) of Golgi cells were not coupled to another Golgi cell, ∼65% (11 of 17) were coupled to one other Golgi cell, and ∼18% were coupled to two Golgi cells (3 of 17). This estimate of the fraction of Golgi cells electrically coupled to one another is likely an underestimate because electrically coupled Golgi cell pairs with DJCs < 10 pA represented 27% of Golgi cell pairs (9 of 33) in which the spikelet was measured, and spikelets with amplitudes < 10 pA fell below detection threshold.
Spikelets have a biphasic effect on excitability of Golgi cells.
As prejunctional spikes resulted mainly in hyperpolarizing current injection into the postjunctional cell (Fig. 2D), we hypothesized that the predominant functional effect of spikelets would be a reduction in excitability of the postjunctional cell. With an experimental design similar to a study on cerebellar Golgi cells (Vervaeke et al. 2010), a 1-ms, suprathreshold current was injected into one Golgi cell. A perithreshold 1-ms current was injected into a second Golgi cell such that the current injection failed to evoke APs on roughly half of trials, and the timing between the current injections into the two cells was then varied (Fig. 6A). Figure 6 shows representative trials for one Golgi cell pair; AP probability was slightly enhanced when the current injections were made simultaneously into the two Golgi cells (Δt = 0) but fell below control and then slowly recovered when current injection into Golgi cell 2 followed current injection into Golgi cell 1 (Fig. 6B). The results of altering the timing of current injections into the two Golgi cells is shown in Fig. 6D for six Golgi-Golgi cell pairs with coupling coefficients ranging between 0.05 and 0.22 (average = 0.14 ± 0.07). The average time course of AP probability changes in Golgi cell 2 (Fig. 6D) resembled the time course of the spikelet (Fig. 6C), suggesting that the AP probability changes in Golgi cell 2 were due to the voltage changes induced by the spikelet. Although the spikelet has a brief excitatory component, the time course of inhibition is considerably longer. Thus, unless electrically coupled Golgi cells are close to firing threshold at the same time, a spikelet will tend to inhibit the postjunctional cell.
Golgi cells receive rare chemical connections from other Golgi cells and superficial stellate cells.
Cerebellar Golgi cells make weak GABAergic synapses onto one another (∼0.3 nS; Hull and Regehr 2012). In the present study no chemical connections were found in 10 electrically coupled Golgi-Golgi cell pairs or in 4 uncoupled cell pairs with a K-based intracellular solution. We recorded from eight additional electrically coupled pairs in which the prejunctional cell was recorded with a K-based intracellular solution and the postjunctional cell was recorded with a CsCl-based intracellular solution to allow for a larger driving force for Cl− when holding postjunctional Golgi cells at −50 to −60 mV. Addition of GABAA receptor antagonist SR95531 at 10 μM and the glycine receptor antagonist strychnine at 1 μM decreased the peak DJC in individual cells by ≤3 pA and changed the peak HJC in individual cells by ≤1 pA (data not shown). If these small blocker-induced changes in junctional current were due to IPSCs, the average IPSC conductance was ≤50 pS, which is equivalent to the single-channel conductance of a glycine receptor or twice the single-channel conductance of a GABAA receptor (Dieudonné 1995). Thus it is unlikely that the blocker-induced changes in junctional current were due to block of chemical synaptic transmission. In summary, paired recordings between Golgi cells showed no evidence of chemical connections (32 directions tested in 21 Golgi-Golgi dual recordings in slices from 18 mice). One chemically connected Golgi-Golgi cell pair was observed in slices from an IG17/Cx36−/− mouse (9 directions tested in 7 Golgi-Golgi dual recordings in slices from 2 mice).
Despite the apparent lack of chemical connections among Golgi cells in paired recordings in IG17/Cx36+/+ mice, we obtained indirect evidence for chemical inhibition onto Golgi cells in the Golgi-granule cell dual recording data set. In 1 of the 21 recordings in which Golgi cell voltage steps evoked IPSCs onto granule cells, voltage steps also evoked IPSCs onto the Golgi cell that coincided with granule cell IPSCs (Fig. 7, A and B). Golgi cell IPSCs at positive step potentials (at which IPSCs were outward with the CsCl-based intracellular solution; see methods) appeared to be preceded by brief inward currents similar to DJCs (denoted by asterisks in Fig. 7B). Synchronous IPSCs in the Golgi and granule cells remained after application of 10 μM SR95531 (data not shown) but were blocked fully by the subsequent addition of 1 μM strychnine, revealing putative spikelets in the Golgi cell (Fig. 7C). Granule cell IPSC-triggered averaging at Golgi cell potentials +20 mV and above revealed a spikelet preceding the granule and Golgi cell IPSCs (Fig. 7D). As Golgi cells are the only source of inhibition onto granule cells (Yaeger and Trussell 2015), the results of the experiment shown in Fig. 7, A–D, suggest that Golgi cells make infrequent chemical synapses onto one another.
In an additional two Golgi-granule cell recordings, voltage steps did not evoke IPSCs onto the granule cell but did evoke IPSCs onto the Golgi cell whose latency decreased as step voltage became more depolarized (data not shown), as for Golgi voltage step-evoked APs (Fig. 4C). Taken together, evidence for chemical inhibition among Golgi cells occurred in 3 of 65 recordings in which the voltage step protocol was applied to a single Golgi cell. If on average each Golgi cell is coupled to one other Golgi cell (see above), then we estimate the chemical connection probability among Golgi cells to be 5%, which is ∼18 times lower than the connection probability for electrical synapses between Golgi cells (∼90%).
In the course of performing paired recordings between superficial stellate cells and Golgi cells, APs in presynaptic superficial stellate cells evoked IPSCs onto Golgi cells in 3 of 53 pairs in slices from 15 mice (6% connection probability; Fig. 7E). IPSCs were completely blocked by 10 μM SR 95531 in two of two recordings. Thus both superficial stellate cells and Golgi cells are sources of chemical inhibition onto Golgi cells, but connections from these two sources are infrequent.
Feedforward chemical inhibition onto Golgi cells.
Both Golgi cells and superficial stellate cells receive glutamatergic parallel fiber input (Apostolides and Trussell 2014; Irie et al. 2006; Mugnaini et al. 1980; Wouterlood et al. 1984). Thus stimulation of parallel fibers at intensities sufficient to excite these two interneuron types would be predicted to evoke chemical inhibition onto Golgi cells. While coupling between individual cells is uncommon, it is possible that the aggregate of all chemical connections could be more potent. We therefore stimulated parallel fibers extracellularly and recorded from Golgi cells in voltage clamp, using a low-Cl− intracellular solution. As reported previously (Yaeger and Trussell 2015), stimulation of parallel fibers at 100 Hz evoked facilitating excitatory postsynaptic currents (EPSCs) (Fig. 8A). Holding the Golgi cell at the empirically determined reversal potential for EPSCs in this cell (+20 mV; Fig. 8B) revealed putative IPSCs that were partially blocked by the AMPA receptor blocker NBQX at 2.5–5 μM (81 ± 10% block of IPSC integral; n = 4 cells in slices from 3 mice). Washout of NBQX resulted in recovery of putative IPSCs, which were then blocked by 10 μM SR 95531 and 1 μM strychnine (88 ± 2% block of IPSC integral, n = 3 cells in slices from 3 mice), confirming that the events recorded at a holding potential of +20 mV were disynaptic IPSCs (Fig. 8C).
DISCUSSION
Synaptic contacts between Golgi cells in cerebellum-like structures.
Cochlear nucleus Golgi cells are electrically coupled through connexin 36-containing gap junctions, as are cerebellar Golgi cells (Vervaeke et al. 2010, 2012). We report that Golgi cells of the cochlear nucleus are electrically coupled to superficial stellate cells only rarely (2% connection probability), similar to reports in the cerebellum that molecular layer interneurons and Golgi cells are not electrically coupled (Hull and Regehr 2012). Furthermore, as we suggest below, connections of such rarity may be “accidents of neural wiring” and may reflect occasional mistakes in a developmentally regulated neural wiring program. We did not examine whether Golgi cells and fusiform cells are electrically coupled in DCN, as is the case for superficial stellate cells and fusiform cells (Apostolides and Trussell 2013), but this seems unlikely given that spikelets were rarely observed in Golgi cells without depolarization or bath application of 4-AP and that fusiform cells fire spontaneously (Leao et al. 2012; Roberts and Trussell 2010). Thus cerebellar and cochlear nucleus Golgi cells appear to be electrically coupled nearly exclusively to other Golgi cells.
Although Golgi cells in the cerebellum and the DCN make chemical synapses onto one another (Hull and Regehr 2012; this study), Golgi cells in the cerebellum make GABAergic synapses onto one another with a higher connection probability (20%) than found in cochlear nucleus (∼5%). Electrical synapses appear to be the predominant form of synaptic contact among Golgi cells in the cerebellum and cochlear nucleus, structures in which the electrical connection probability for Golgi cells within 100 μm of one another is considerably greater than the chemical connection probability (this study; Hull and Regehr 2012; Vervaeke et al. 2010).
Sources of chemical inhibitory input to cochlear nucleus Golgi cells.
We showed that cochlear nucleus Golgi cells receive sparse inhibitory chemical inputs from Golgi cells and from superficial stellate cells, a type of molecular layer interneuron similar to stellate cells in cerebellum (Apostolides and Trussell 2014; Wouterlood et al. 1984). Molecular layer interneurons do not synapse onto cerebellar Golgi cells (Hull and Regehr 2012; but see Barmack and Yakhnitsa 2008; Dumoulin et al. 2001; Eccles et al. 1967). However, the connection probabilities for inhibitory chemical connections from superficial stellate cells and Golgi cells onto Golgi cells are so low (5–6%) that they may constitute “accidents of neural wiring,” similar to reports of rare electrical connections between spiny stellate cells and GABAergic fusiform cells in layer 4 of somatosensory cortex (Bennett and Zukin 2004; Venance et al. 2000). Nonetheless, stimulation of parallel fibers triggers disynaptic IPSCs onto Golgi cells in DCN (Fig. 8), indicating that Golgi cells receive weak inhibitory chemical input from parallel fiber target neurons, apparently including superficial stellate cells and Golgi cells. Additional sources of chemical inhibitory input to cochlear nucleus Golgi cells are unknown. Cerebellar Golgi cells receive additional mixed GABAergic/glycinergic input from Lugaro cells (Dieudonné and Dumoulin 2000; Dumoulin et al. 2001) and from neurons in the cerebellar nuclei (Ankri et al. 2015), but these sources are not present in DCN.
Electrical synapses may synchronize or desynchronize Golgi cell spiking.
Previous studies of electrical synapses have focused on the ability of these connections to synchronize the firing of coupled cells (Beierlein et al. 2000; Dugué et al. 2009; Haas and Landisman 2011; Mann-Metzer and Yarom 1999;). However, in most of these studies tonic current was injected into neurons to induce spiking, neuromodulators were applied to provide tonic depolarization, or chemical synaptic transmission was blocked. As has been pointed out recently (Trenholm et al. 2014; Vervaeke et al. 2010), these are not physiological situations, and with intact network chemical synaptic activity spiking among electrically coupled interneurons may be uncorrelated (Sippy and Yuste 2013; Vervaeke et al. 2010). Alternately, correlated spiking by electrically coupled cells may require shared chemical synaptic input (Trenholm et al. 2014).
In agreement with studies on cerebellar Golgi cells (Dugué et al. 2009; Vervaeke et al. 2010), we find that spikelets produce a very brief and small excitation of cells but a prolonged inhibition. The DJP of the spikelet is too weak (≤1 mV) to directly evoke spikes in postjunctional cells at typical resting membrane potentials (Trenholm et al. 2013) and is so brief (half-width of ∼3 ms) that coupled Golgi cells would need to be close to threshold at the same time in order to take advantage of the DJP. The HJP is considerably longer than the DJP (half-width ∼80 ms) and thus could act on longer timescales than the DJP to inhibit firing of coupled Golgi cells. In principle, excitatory synaptic input that brought electrically coupled cells close to threshold nearly simultaneously could act to synchronize firing (Galarreta and Hestrin 2001; Hestrin and Galarreta 2005; Otsuka and Kawaguchi 2013). Shared parallel fiber input, which could promote Golgi cell spike synchrony, is rare among cartwheel cells and fusiform cells in DCN (Roberts and Trussell 2010), but this issue has not been studied in other neuron types in DCN. Shared parallel fiber input has been proposed to explain the loose synchrony of cerebellar Golgi cell spiking in vivo (Maex et al. 2000). Golgi cells may also receive excitatory synaptic input from extrinsic mossy fibers (Mugnaini et al. 1980; but see Mugnaini et al. 2011; Weedman et al. 1996) or from auditory nerve fibers (Ferragamo et al. 1998; Irie et al. 2006), but the extent to which these inputs are shared among Golgi cells is not known.
Implications for dorsal cochlear nucleus circuit function.
Modeling and experimental studies in the cerebellum and cerebellum-like electrosensory lobe of weakly electric fish have focused on the fact that granule cells receive three to seven mossy fiber inputs and thus different combinations of mossy fiber input may evoke different patterns of granule cell firing in terms of spike timing and spike frequency and thus encode different sensory stimuli (Billings et al. 2014; Chabrol et al. 2015; Huang et al. 2013; Kennedy et al. 2014; Liu and Regehr 2014; Sawtell 2010). Curiously, cochlear nucleus granule cells receive only one to three mossy fiber/small bouton inputs (Balakrishnan and Trussell 2008; Mugnaini et al. 1980), and thus from a theoretical standpoint their pattern storage abilities may be more restricted compared with cerebellar granule cells receiving an average of four mossy fiber inputs (Billings et al. 2014).
However, four or five Golgi cells converge onto individual cochlear nucleus granule cells (Balakrishnan et al. 2009; Yaeger and Trussell 2015), and thus patterns of activity in Golgi cells may also contribute to the output of granule cells and encoding of sensory inputs. A correlation was found between the size of inhibitory postsynaptic potentials (IPSPs) in cochlear nucleus granule cells and the length of pauses in their firing, which was attributed to repriming of an A-type K+ current during the IPSP (Balakrishnan et al. 2009). Synchronous IPSPs onto granule cells from two or more synchronously firing Golgi cells would likely result in a larger IPSP and a longer pause in granule cell firing. Thus correlations in the spike trains of coupled Golgi cells could be encoded in the length of pauses in granule cell spike trains. Indeed, we observed that electrically coupled Golgi cells projected to the same granule cell in 4 of 10 recordings (40%; data not shown). Alternatively, electrical coupling may also act to synchronize or desynchronize inhibition onto sets of granule cells innervated by different Golgi cells depending on the temporal correlations in excitatory input to electrically coupled Golgi cells. The electrically coupled network of Golgi cells may therefore allow the MGP pathway to encode patterns in the durations of pauses in activity imposed by Golgi cell-mediated inhibition.
GRANTS
This work was supported by the National Institute on Deafness and Other Communication Disorders [DC-004450 (L. O. Trussell), F31 DC-013223 (D. B. Yaeger)] and by a Tartar Trust fellowship (D. B. Yaeger).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
D.Y. and L.O.T. conception and design of research; D.Y. performed experiments; D.Y. analyzed data; D.Y. and L.O.T. interpreted results of experiments; D.Y. and L.O.T. prepared figures; D.Y. and L.O.T. drafted manuscript; D.Y. and L.O.T. edited and revised manuscript; D.Y. and L.O.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. Robert Duvoisin and Gary Westbrook for donation of the IG17 and Cx36−/− mice, respectively. We thank Dr. Emma Coddington and members of the Trussell Lab for comments on the manuscript.
REFERENCES
- Alcami P, Marty A. Estimating functional connectivity in an electrically coupled interneuron network. Proc Natl Acad Sci USA 110: E4798–E4807, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankri L, Husson Z, Pietrajtis K, Proville R, Léna C, Yarom Y, Dieudonné S, Uusisaari MY. A novel inhibitory nucleo-cortical circuit controls cerebellar Golgi cell activity. eLife 4: 06262, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolides PF, Trussell LO. Regulation of interneuron excitability by gap junction coupling with principal cells. Nat Neurosci 16: 1764–1772, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolides PF, Trussell LO. Superficial stellate cells of the dorsal cochlear nucleus. Front Neural Circuits 8: 63, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishnan V, Kuo SP, Roberts PD, Trussell LO. Slow glycinergic transmission mediated by transmitter pooling. Nat Neurosci 12: 286–294, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishnan V, Trussell LO. Synaptic inputs to granule cells of the dorsal cochlear nucleus. J Neurophysiol 99: 208–219, 2008. [DOI] [PubMed] [Google Scholar]
- Barmack NH, Yakhnitsa V. Functions of interneurons in mouse cerebellum. J Neurosci 28: 1140–1152, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beierlein M, Gibson JR, Connors BW. A network of electrically coupled interneurons drives synchronized inhibition in neocortex. Nat Neurosci 3: 904–910, 2000. [DOI] [PubMed] [Google Scholar]
- Bell CC, Caputi A, Grant K, Serrier J. Storage of a sensory pattern by anti-Hebbian synaptic plasticity in an electric fish. Proc Natl Acad Sci USA 90: 4650–4654, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell CC, Han V, Sawtell NB. Cerebellum-like structures and their implications for cerebellar function. Annu Rev Neurosci 31: 1–24, 2008. [DOI] [PubMed] [Google Scholar]
- Bennett MV, Zukin RS. Electrical coupling and neuronal synchronization in the mammalian brain. Neuron 41: 495–511, 2004. [DOI] [PubMed] [Google Scholar]
- Billings G, Piasini E, Lorincz A, Nusser Z, Silver RA. Network structure within the cerebellar input layer enables lossless sparse encoding. Neuron 83: 960–974, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges-Merjane C, Trussell LO. ON and OFF unipolar brush cells transform multisensory inputs to the auditory system. Neuron 85: 1029–1042, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabrol FP, Arenz A, Wiechert MT, Margrie TW, DiGregorio DA. Synaptic diversity enables temporal coding of coincident multisensory inputs in single neurons. Nat Neurosci 18: 718–727, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadderton P, Schaefer AT, Williams SR, Margrie TW. Sensory-evoked synaptic integration in cerebellar and cerebral cortical neurons. Nat Rev Neurosci 15: 71–83, 2014. [DOI] [PubMed] [Google Scholar]
- Clements JD, Bekkers JM. Detection of spontaneous synaptic events with an optimally scaled template. Biophys J 73: 220–229, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors BW, Long MA. Electrical synapses in the mammalian brain. Annu Rev Neurosci 27: 393–418, 2004. [DOI] [PubMed] [Google Scholar]
- Crowley JJ, Fioravante D, Regehr WG. Dynamics of fast and slow inhibition from cerebellar Golgi cells allow flexible control of synaptic integration. Neuron 63: 843–853, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieudonné S. Glycinergic synaptic currents in Golgi cells of the rat cerebellum. Proc Natl Acad Sci USA 92: 1441–1445, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieudonné S, Dumoulin A. Serotonin-driven long-range inhibitory connections in the cerebellar cortex. J Neurosci 20: 1837–1848, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugué GP, Brunel N, Hakim V, Schwartz E, Chat M, Lévesque M, Courtemanche R, Léna C, Dieudonné S. Electrical coupling mediates tunable low-frequency oscillations and resonance in the cerebellar Golgi cell network. Neuron 61: 126–139, 2009. [DOI] [PubMed] [Google Scholar]
- Dumoulin A, Triller A, Dieudonné S. IPSC kinetics at identified GABAergic and mixed GABAergic and glycinergic synapses onto cerebellar Golgi cells. J Neurosci 21: 6045–6057, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC, Ito M, Szentagothai J. The Cerebellum as a Neuronal Machine. Berlin: Springer, 1967. [Google Scholar]
- Ferragamo MJ, Golding NL, Gardner SM, Oertel D. Golgi cells in the superficial granule cell domain overlying the ventral cochlear nucleus: morphology and electrophysiology in slices. J Comp Neurol 400: 519–528, 1998. [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. Spike transmission and synchrony detection in networks of GABAergic interneurons. Science 292: 2295–2299, 2001. [DOI] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature 402: 75–79, 1999. [DOI] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Functional properties of electrical synapses between inhibitory interneurons of neocortical layer 4. J Neurophysiol 93: 467–480, 2005. [DOI] [PubMed] [Google Scholar]
- Haas JS, Landisman CE. State-dependent modulation of gap junction signaling by the persistent sodium current. Front Cell Neurosci 5: 31, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han VZ, Grant K, Bell CC. Reversible associative depression and nonassociative potentiation at a parallel fiber synapse. Neuron 27: 611–622, 2000. [DOI] [PubMed] [Google Scholar]
- Hestrin S, Galarreta M. Electrical synapses define networks of neocortical GABAergic neurons. Trends Neurosci 28: 304–309, 2005. [DOI] [PubMed] [Google Scholar]
- Hoge GJ, Davidson KG, Yasumura T, Castillo PE, Rash JE, Pereda AE. The extent and strength of electrical coupling between inferior olivary neurons is heterogeneous. J Neurophysiol 105: 1089–1101, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hormuzdi SG, Pais I, LeBeau FE, Towers SK, Rozov A, Buhl EH, Whittington MA, Monyer H. Impaired electrical signaling disrupts gamma frequency oscillations in connexin 36-deficient mice. Neuron 31: 487–495, 2001. [DOI] [PubMed] [Google Scholar]
- Huang CC, Sugino K, Shima Y, Guo C, Bai S, Mensh BD, Nelson SB, Hantman AW. Convergence of pontine and proprioceptive streams onto multimodal cerebellar granule cells. eLife 2: e00400, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull C, Regehr WG. Identification of an inhibitory circuit that regulates cerebellar Golgi cell activity. Neuron 73: 149–158, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie T, Fukui I, Ohmori H. Activation of GIRK channels by muscarinic receptors and group II metabotropic glutamate receptors suppresses Golgi cell activity in the cochlear nucleus of mice. J Neurophysiol 96: 2633–2644, 2006. [DOI] [PubMed] [Google Scholar]
- Kennedy A, Wayne G, Kaifosh P, Alviña K, Abbott LF, Sawtell NB. A temporal basis for predicting the sensory consequences of motor commands in an electric fish. Nat Neurosci 17: 416–422, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leao RM, Li S, Doiron B, Tzounopoulos T. Diverse levels of an inwardly rectifying potassium conductance generate heterogeneous neuronal behavior in a population of dorsal cochlear nucleus pyramidal neurons. J Neurophysiol 107: 3008–3019, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, Regehr WG. Normalization of input patterns in an associative network. J Neurophysiol 111: 544–551, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long MA, Jutras MJ, Connors BW, Burwell RD. Electrical synapses coordinate activity in the suprachiasmatic nucleus. Nat Neurosci 8: 61–66, 2004. [DOI] [PubMed] [Google Scholar]
- Maex R, Vos BP, De Schutter E. Weak common parallel fibre synapses explain the loose synchrony observed between rat cerebellar Golgi cells. J Physiol 523: 175–192, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann-Metzer P, Yarom Y. Electrotonic coupling interacts with intrinsic properties to generate synchronized activity in cerebellar networks of inhibitory interneurons. J Neurosci 19: 298–3306, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno AP, Berthoud VM, Perez-Palacios G, Perez-Armendariz EM. Biophysical evidence that connexin-36 forms functional gap junction channels between pancreatic mouse beta-cells. Am J Physiol Endocrinol Metab 288: E948–E956, 2005. [DOI] [PubMed] [Google Scholar]
- Mugnaini E, Osen KK, Dahl AL, Friedrich VLJ, Korte G. Fine structure of granule cells and related interneurons (termed Golgi cells) in the cochlear nuclear complex of cat, rat and mouse. J Neurocytol 9: 537–570, 1980. [DOI] [PubMed] [Google Scholar]
- Mugnaini E, Sekerkova G, Martina M. The unipolar brush cell: a remarkable neuron finally receiving deserved attention. Brain Res Rev 66: 220–245, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel D, Young ED. What's a cerebellar circuit doing in the auditory system? Trends Neurosci 27: 104–110, 2004. [DOI] [PubMed] [Google Scholar]
- Otsuka T, Kawaguchi Y. Common excitatory synaptic inputs to electrically connected cortical fast-spiking cell networks. J Neurophysiol 110: 795–806, 2013. [DOI] [PubMed] [Google Scholar]
- Palacios-Prado N, Hoge G, Marandykina A, Rimkute L, Chapuis S, Paulauskas N, Skeberdis VA, O'Brien J, Pereda AE, Bennett MV, Bukauskas FF. Intracellular magnesium-dependent modulation of gap junction channels formed by neuronal connexin36. J Neurosci 33: 4741–4753, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan F, Mills SL, Massey SC. Screening of gap junction antagonists on dye coupling in the rabbit retina. Vis Neurosci 24: 609–618, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Rubio ME, Wang YX, Wenthold RJ. Differential distribution of glutamate receptors in the cochlear nuclei. Hear Res 147: 59–69, 2000. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Zhao HM, Wenthold RJ. Ionotropic and metabotropic glutamate receptors show unique postsynaptic, presynaptic, and glial localizations in the dorsal cochlear nucleus. J Comp Neurol 372: 356–383, 1996. [DOI] [PubMed] [Google Scholar]
- Requarth T, Kaifosh P, Sawtell NB. A role for mixed corollary discharge and proprioceptive signals in predicting the sensory consequences of movements. J Neurosci 34: 16103–16116, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Requarth T, Sawtell NB. Neural mechanisms for filtering self-generated sensory signals in cerebellum-like circuits. Curr Opin Neurobiol 21: 602–608, 2011. [DOI] [PubMed] [Google Scholar]
- Roberts MT, Trussell LO. Molecular layer inhibitory interneurons provide feedforward and lateral inhibition in the dorsal cochlear nucleus. J Neurophysiol 104: 2462–2473, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawtell NB. Multimodal integration in granule cells as a basis for associative plasticity and sensory prediction in a cerebellum-like circuit. Neuron 66: 573–584, 2010. [DOI] [PubMed] [Google Scholar]
- Simon A, Olah S, Molnar G, Szabadics J, Tamas G. Gap-junctional coupling between neurogliaform cells and various interneuron types in the neocortex. J Neurosci 25: 6278–6285, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sippy T, Yuste R. Decorrelating action of inhibition in neocortical networks. J Neurosci 33: 9813–9830, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas M, Rozental R, Kojima T, Dermietzel R, Mehler M, Condorelli DF, Kessler JA, Spray DC. Functional properties of channels formed by the neuronal gap junction protein connexin36. J Neurosci 19: 9848–9855, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenholm S, McLaughlin AJ, Schwab DJ, Awatramani GB. Dynamic tuning of electrical and chemical synaptic transmission in a network of motion coding retinal neurons. J Neurosci 33: 14927–14938, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenholm S, McLaughlin AJ, Schwab DJ, Turner MH, Smith RG, Rieke F, Awatramani GB. Nonlinear dendritic integration of electrical and chemical synaptic inputs drives fine-scale correlations. Nat Neurosci 17: 1759–1766, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venance L, Rozov A, Blatow M, Burnashev N, Feldmeyer D, Monyer H. Connexin expression in electrically coupled postnatal rat brain neurons. Proc Natl Acad Sci USA 97: 10260–10265, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vervaeke K, Lorincz A, Gleeson P, Farinella M, Nusser Z, Silver RA. Rapid desynchronization of an electrically coupled interneuron network with sparse excitatory synaptic input. Neuron 67: 435–451, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vervaeke K, Lorincz A, Nusser Z, Silver RA. Gap junctions compensate for sublinear dendritic integration in an inhibitory network. Science 335: 1624–1628, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe D, Inokawa H, Hashimoto K, Suzuki N, Kano M, Shigemoto R, Hirano T, Toyama K, Kaneko S, Yokoi M, Moriyoshi K, Suzuki M, Kobayashi K, Nagatsu T, Kreitman RJ, Pastan I, Nakanishi S. Ablation of cerebellar Golgi cells disrupts synaptic integration involving GABA inhibition and NMDA receptor activation in motor coordination. Cell 95: 17–27, 1998. [DOI] [PubMed] [Google Scholar]
- Watanabe D, Nakanishi S. mGluR2 postsynaptically senses granule cell inputs at Golgi cell synapses. Neuron 39: 821–829, 2003. [DOI] [PubMed] [Google Scholar]
- Weedman DL, Pongstaporn T, Ryugo DK. Ultrastructural study of the granule cell domain of the cochlear nucleus in rats: mossy fiber endings and their targets. J Comp Neurol 369: 345–360, 1996. [DOI] [PubMed] [Google Scholar]
- Wouterlood FG, Mugnaini E, Osen KK, Dahl AL. Stellate neurons in rat dorsal cochlear nucleus studies with combined Golgi impregnation and electron microscopy: synaptic connections and mutual coupling by gap junctions. J Neurocytol 13: 639–664, 1984. [DOI] [PubMed] [Google Scholar]
- Yaeger DB, Trussell LO. Single granule cells excite Golgi cells and evoke feedback inhibition in the cochlear nucleus. J Neurosci 35: 4741–4750, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Han VZ, Meek J, Bell CC. Granular cells of the mormyrid electrosensory lobe and postsynaptic control over presynaptic spike occurrence and amplitude through an electrical synapse. J Neurophysiol 97: 2191–2203, 2007. [DOI] [PubMed] [Google Scholar]
- Zhang L, Weiner JL, Valiante TA, Velumian AA, Watson PL, Jahromi SS, Schertzer S, Pennefather P, Carlen PL. Whole-cell recording of the Ca2+-dependent slow afterhyperpolarization in hippocampal neurones: effects of internally applied anions. Pflügers Arch 426: 247–253, 1994. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Tzounopoulos T. Physiological activation of cholinergic inputs controls associative synaptic plasticity via modulation of endocannabinoid signaling. J Neurosci 31: 3158–3168, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsiros V, Maccaferri G. Electrical coupling between interneurons with different excitable properties in the stratum lacunosum-moleculare of the juvenile CA1 rat hippocampus. J Neurosci 25: 8686–8695, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]