We have explored how transplanted interneurons, which have potential therapeutic value for treating a variety of neurological and psychiatric diseases, integrate into the neural circuitry of the recipient brain. We found that exogenous cells develop rapidly to exhibit intrinsic properties and send and receive synapses appropriate to their specific cell subtype. These findings indicate that transplanted cells are fully able to contribute to neural information processing while therapeutically increasing inhibitory synaptic tone.
Keywords: cell therapy, interneuron, medial ganglionic eminence, neural transplant, synaptic integration
Abstract
Interneuron-based cell transplantation is a powerful method to modify network function in a variety of neurological disorders, including epilepsy. Whether new interneurons integrate into native neural networks in a subtype-specific manner is not well understood, and the therapeutic mechanisms underlying interneuron-based cell therapy, including the role of synaptic inhibition, are debated. In this study, we tested subtype-specific integration of transplanted interneurons using acute cortical brain slices and visualized patch-clamp recordings to measure excitatory synaptic inputs, intrinsic properties, and inhibitory synaptic outputs. Fluorescently labeled progenitor cells from the embryonic medial ganglionic eminence (MGE) were used for transplantation. At 5 wk after transplantation, MGE-derived parvalbumin-positive (PV+) interneurons received excitatory synaptic inputs, exhibited mature interneuron firing properties, and made functional synaptic inhibitory connections to native pyramidal cells that were comparable to those of native PV+ interneurons. These findings demonstrate that MGE-derived PV+ interneurons functionally integrate into subtype-appropriate physiological niches within host networks following transplantation.
NEW & NOTEWORTHY
We have explored how transplanted interneurons, which have potential therapeutic value for treating a variety of neurological and psychiatric diseases, integrate into the neural circuitry of the recipient brain. We found that exogenous cells develop rapidly to exhibit intrinsic properties and send and receive synapses appropriate to their specific cell subtype. These findings indicate that transplanted cells are fully able to contribute to neural information processing while therapeutically increasing inhibitory synaptic tone.
neuronal networks include a diverse population of inhibitory interneurons responsible for regulating neural activity (Le Magueresse and Monyer 2013). Alterations in network excitability, often associated with interneuron dysfunction, underlie a variety of neurological disorders, e.g., epilepsy (Powell et al. 2003), Alzheimer's disease (Verret et al. 2012), and schizophrenia (Gonzalez-Burgos et al. 2015). In many of these “interneuronopathies” there is a reduction in GABA-mediated synaptic inhibition in hippocampal or cortical networks (Rossignol 2011). Increasing inhibitory synaptic tone within neural circuits via interneuron-based cell transplantation has emerged as a potential treatment for these network disorders (Baraban et al. 2009; Hunt et al. 2013; Perez and Lodge 2013; Tong et al. 2014; Tyson and Anderson 2014). For synaptic inhibition to be restored, these approaches require a population of cells fated to differentiate into specific interneuron subtypes and the capacity for these interneurons to functionally integrate in the host brain following transplantation.
The first of these requirements was met with the discovery of GABA progenitor cell populations within embryonic medial, caudal, and lateral ganglionic eminences (MGE, CGE, and LGE, respectively; Anderson et al. 1997; Lavdas et al. 1999). MGE progenitors give rise to the majority of forebrain interneurons and primarily comprise fast-spiking, parvalbumin (PV)- or regular-spiking nonpyramidal, somatostatin (Sst)-expressing subtypes (Xu et al. 2004). In neonatal or adult rodents, transplanted embryonic MGE progenitors maintain a unique ability to migrate widely in the host brain where they differentiate to PV+ or Sst+ neurons that receive and project synapses with endogenous neurons (Wichterle et al. 1999). Beginning at 30 days after transplantation (DAT), MGE-derived interneurons, in hippocampus or cortex, exhibit intrinsic firing properties identical to mature endogenous PV+ or Sst+ interneurons. These interneurons enhance synaptic inhibition in the host brain, satisfying the second requirement (Alvarez-Dolado et al. 2006; Baraban et al. 2009; Calcagnotto et al. 2010; Howard et al. 2014). Consistent with these findings, a near complete suppression of spontaneous seizures is observed with MGE progenitor cell transplantation as early as 30 DAT in genetic or acquired mouse models of chronic epilepsy (Baraban et al. 2009; Henderson et al. 2014; Hunt et al. 2013). However, suppression of acutely evoked or absence-like seizure events was also reported as early as 7 to 17 DAT (De la Cruz et al. 2011; Hammad et al. 2015), e.g., a time when only immature MGE-derived interneurons with migratory morphology are present (Alvarez-Dolado et al. 2006).
To modify host network excitability in a nondisruptive but therapeutic manner, transplanted MGE-derived interneurons must receive appropriate excitatory input from the host brain, filter this input through mature intrinsic properties, and elicit a synaptic inhibitory output onto native excitatory cells. In this study we address these issues by comparing the physiology of transplanted MGE-derived fast-spiking PV+ interneurons with endogenous PV+ interneurons. We found that MGE-derived PV+ interneurons are functionally immature between 7 and 21 DAT but attain mature excitatory synaptic inputs and intrinsic properties at 35 DAT that are almost indistinguishable from those of endogenous fast-spiking PV+ interneurons. In dual whole cell patch-clamp recordings, MGE-derived PV+ interneurons make functional inhibitory synapses onto native pyramidal neurons that are indistinguishable from those formed by native PV+ interneurons. These data are consistent with our hypothesis that therapeutic actions of MGE transplantation coincide with the full, subtype-appropriate integration of mature interneurons into native networks.
METHODS
Progenitor cell transplantation.
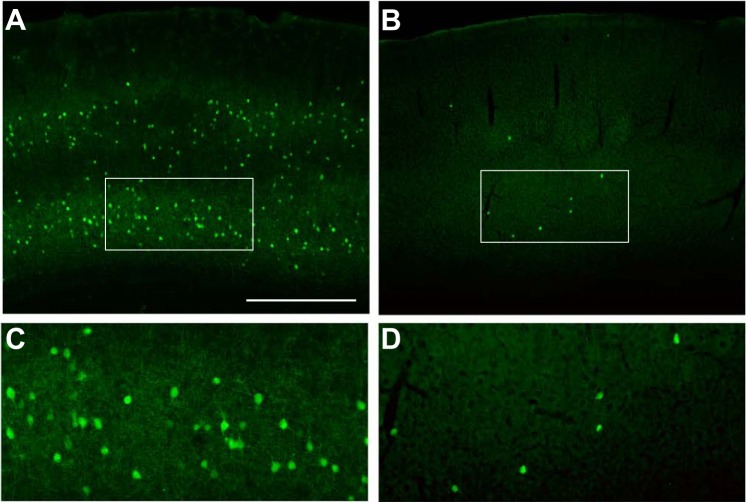
All procedures described were approved by the University of California, San Francisco Institutional Animal Care and Use Committee (AN09993-03D). Donor MGE progenitors were obtained by crossing CD1 females with green fluorescent protein-expressing (GFP+) male mice of the G42 line, in which PV+ fast-spiking interneurons express GFP (Chattopadhyaya et al. 2004), back-crossed for more than five generations onto a CD1 background. At embryonic day 13.5, MGEs were dissected from embryos, and cells were dissociated and concentrated in cell culture media. Cells were loaded into glass needles and stereotactically transplanted into a single cortical site in each hemisphere of wild-type CD1 pups, postnatal day 1–2 (P1-2). We made relatively small injections (∼10,000 cells) at single sites such that, after migration, transplanted cells would only populate the cortex sparsely and have minimal impact on normal cortical circuit processing. Examination of fixed sections of P35 cortex revealed large numbers of GFP+ cells in the wild-type G42 brain (Fig. 1, A and C), whereas transplanted brains had larger numbers of GFP+ cells closest to the transplant site but progressively fewer over hundreds of microns anterior and posterior to the site as shown in Fig. 1, B and D. It should be noted that MGE progenitors also differentiated into other cell types (primarily Sst+ interneurons) that did not express GFP and thus were not tracked in these studies.
Fig. 1.
Putative PV+ interneurons (GFP+) are abundant and broadly distributed in the G42 mouse cortex (A). Imaging layer 5/6 under higher magnification (white box) reveals the density and morphology of these mature interneurons (C). Small, single-site transplantation of MGE progenitors from G42 mouse embryos results in a broad but sparse distribution of GFP+ cells (B). Shown is a section ∼300 μm anterior to the injection site. Imaging at higher magnification reveals mature neuronal morphology similar (D) to the cells in their native environment. Scale bar in A represents 500 μm for A and B and 200 μm for C and D.
Whole cell electrophysiology.
In vitro experiments were performed at 7, 14, 21, or 35 DAT in the experimental (transplanted) group or in P7, P14, P21, or P35 GFP+ G42-CD1 control (nontransplanted) mice. Data from 35 transplanted and 28 control mice were analyzed. Mice were deeply anesthetized and euthanized. Brains were immersed in ice-cold, oxygenated high-sucrose cutting solution containing (in mM) 150 sucrose, 50 NaCl, 25 NaHCO3, 10 dextrose, 7 MgSO4, 2.5 KCl, 1 NaH2PO4, and 0.5 CaCl2. Coronal sections (300 μm thick) of somatosensory cortex were made with a vibratome. Slices were incubated for 30 min at 35°C and thereafter at room temperature in artificial cerebrospinal fluid (ACSF) containing (in mM) 124 NaCl, 26 NaHCO3, 10 dextrose, 3 KCl, 2 CaCl2, 2 MgSO4, and 1.25 NaH2PO4.
For recording, slices were placed in a recording chamber mounted on a differential interference contrast/epifluorescent microscope. Glass recording pipettes (3–5 MΩ) were filled with the appropriate internal solution. For excitatory postsynaptic current (EPSC) and current-clamp recordings in interneurons, internal solution contained (in mM) 140 K-gluconate, 10 HEPES, 5 EGTA, 2 Mg-ATP, 1 NaCl, 1 MgCl2, 1 CaCl2, and 0.2 Na-GTP. For inhibitory postsynaptic current (IPSC) recordings in pyramidal neurons, internal solution contained (in mM) 140 CsCl, 11 EGTA, 10 HEPES, 2 Mg-ATP, 1 MgCl2, and 0.5 Na-GTP. Data were collected using pClamp v9 (Molecular Devices) and analyzed using pClamp v9 and MiniAnalysis v6 (Synaptosoft).
Immunohistochemistry.
For anatomic studies, experimental mice at 35 DAT or control mice at P35 were deeply anesthetized and perfused with ice-cold phosphate-buffered saline (PBS), followed by 4% paraformaldehyde in PBS. Brains were sections on a vibratome into 50-μm coronal slices. Sections were then stained with a primary antibody to GFP (chick; Aves Labs), followed by a secondary fluorescent antibody (Alexa 488 goat anti-chick; Molecular Probes). Images were obtained on an Eclipse Ni microscope using NIS-Elements software (Nikon).
Analyses and statistics.
Statistical analyses were performed using SigmaPlot v12. Unless otherwise stated, analyses consisted of two-way ANOVAs (age × group) followed by Holm-Sidak post hoc tests. Statistical significance was achieved when P < 0.05.
RESULTS
We focused our experiments primarily in somatosensory cortex, where GABA-expressing PV+ fast-spiking interneurons provide large inhibitory synaptic inputs (Rossignol 2011). Acute cortical slices were prepared from GFP+ G42 mice (Fig. 1, A and C) or CD1 mice transplanted with GFP+ progenitor cells (Fig. 1, B and D). Patch-clamp recordings were made from visually identified GFP+ interneurons in layer 5/6.
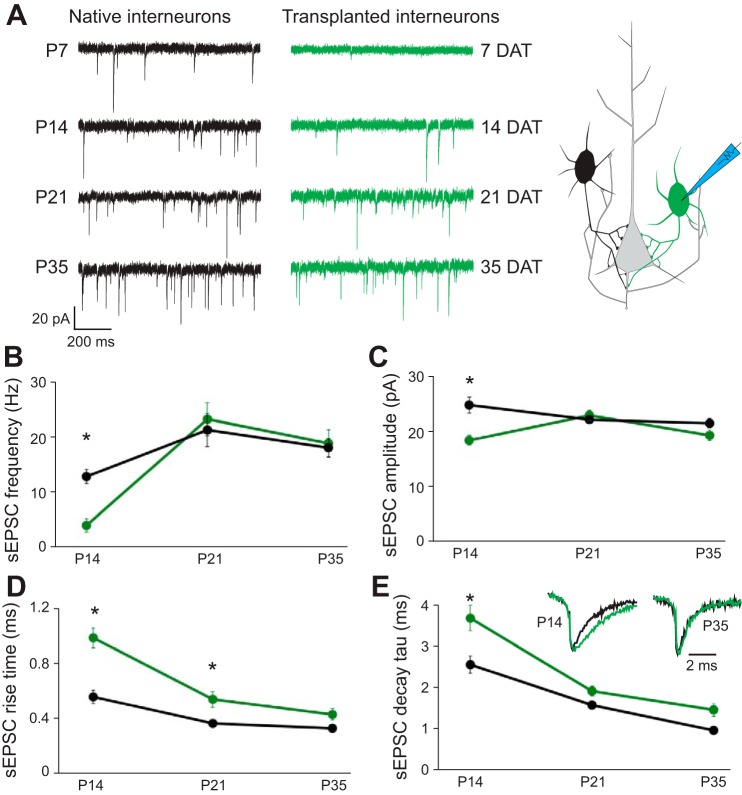
We performed voltage-clamp recordings to evaluate excitatory synaptic input to endogenous or transplanted PV+ interneurons (Fig. 2A). At 7 DAT, MGE-derived PV+ (MGE-PV) interneurons exhibited spontaneous EPSCs (sEPSCs; n = 11) at a very low frequency (∼0.15 Hz) that did not provide a large enough event sample size for quantitative analysis. At 14 DAT, sEPSCs onto MGE-PV interneurons (n = 17) were observed at a frequency approximately threefold smaller than that measured for endogenous PV+ (e-PV) interneurons (Fig. 2, A and B; n = 14; P = 0.002). EPSC amplitude (Fig. 2C; P = 0.006), rise time (Fig. 2D; P < 0.001), and decay constant (τ; Fig. 2E; P = 0.037) were also significantly different at 14 DAT compared with controls, consistent with properties exhibited by immature interneurons at early developmental stages (Okaty et al. 2009).
Fig. 2.
Transplanted MGE cells receive excitatory synapses with subtype-appropriate properties. Whole cell voltage-clamp recordings (A) of native (black) or transplanted (green) GFP+ cells reveal sEPSCs as early as P7 (7 DAT). sEPSC frequency (B) and amplitude (C) are significantly lower at earlier developmental time points but normalize by P21. Amplitude-normalized sEPSCs (inset in E) reveal significantly slower rise time (D) and decay τ (E) at earlier ages but full normalization by P35. *P < 0.05.
At 21 DAT, sEPSC frequency, amplitude and decay τ were comparable to those for P35 e-PV interneurons; rise time remained significantly smaller (P = 0.037). At 35 DAT, all sEPSC properties measured for MGE-PV interneurons were quantitatively comparable to those for P35 e-PV interneurons.
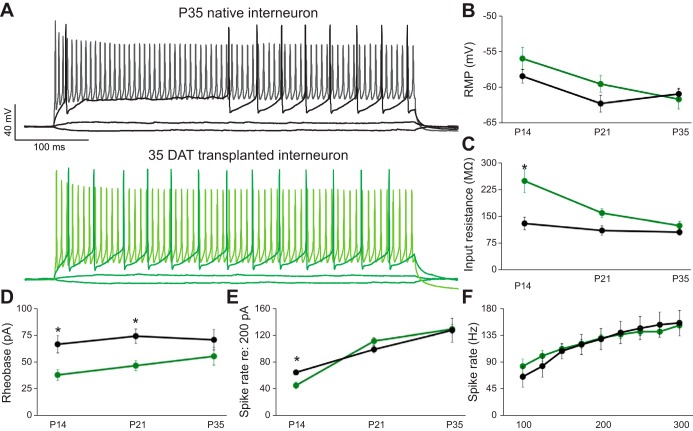
We performed current-clamp recordings to evaluate the intrinsic firing properties of endogenous or transplanted interneurons. Representative current-clamp recordings of e-PV and MGE-PV interneurons at P35 during 500-ms depolarizing or hyperpolarizing steps are shown for direct comparison in Fig. 3A. At 14 DAT, MGE-PV interneurons exhibited immature neuronal properties with significantly higher input resistance (Rin; Fig. 3C; P = 0.003) and lower rheobase (amplitude of a square current required to evoke spikes; Fig. 3D; P = 0.002) compared with P35 e-PV interneurons. Despite this lower threshold for initiating activity, spike rate evoked with a 200-pA current step was significantly lower in MGE-PV interneurons than in e-PV interneurons (Fig. 3E; P = 0.049). At 21 DAT, rheobase remained significantly lower (P = 0.006) for MGE-PV interneurons, but all other intrinsic properties were comparable to those for controls. At 35 DAT, MGE-PV interneurons exhibited Rin, rheobase, resting membrane potential (RMP), and spike rates across a broad range of current stimuli (Fig. 3F) indistinguishable from those of P35 e-PV interneurons (P > 0.05 for each measure).
Fig. 3.
Transplanted MGE cells develop subtype-appropriate intrinsic properties. Whole cell current-clamp recordings (A) of native (black) and transplanted (green) GFP+ cells at P35 reveal low input resistance to small current steps, fast action potentials with large afterhyperpolarizations to near-threshold current steps, and high-frequency firing to larger inputs (lighter traces). RMP did not show significant differences between groups (B). Input resistance (C), rheobase (D), and spike rate to a 200-pA current step (E) were all significantly different at early developmental time points but were equivalent by P35. Native and transplanted GFP+ cells exhibited equivalent high firing rates across a range of current inputs at P35 (F). *P < 0.05.
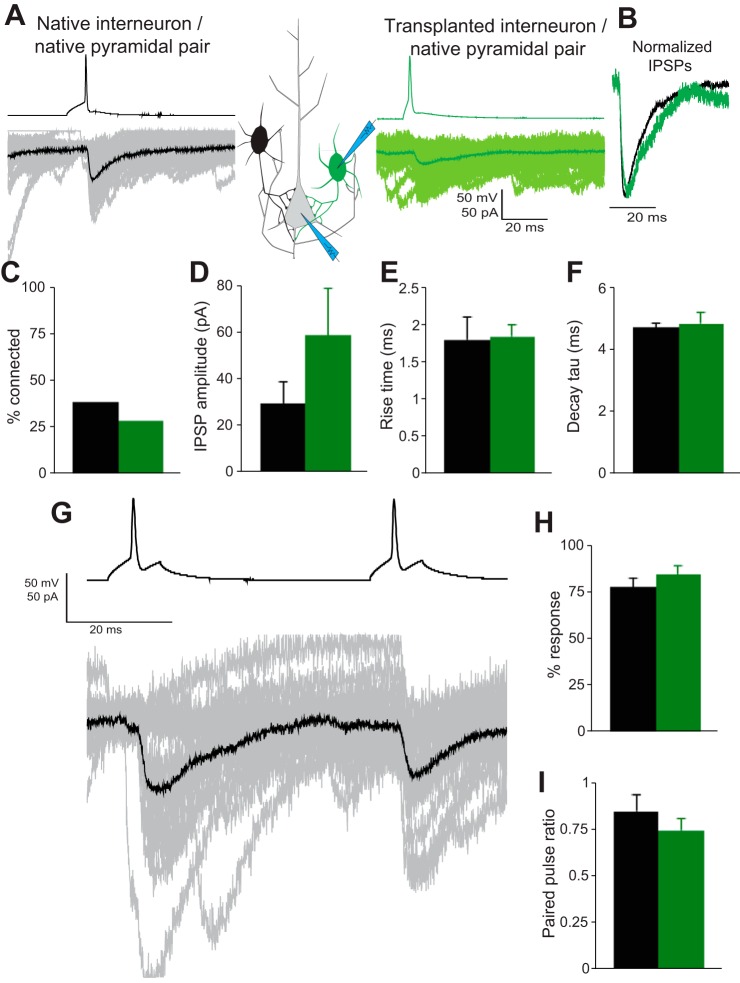
Finally, we performed dual whole cell patch recordings to test for functional synaptic connections between PV+ interneurons and layer 5/6 pyramidal neurons (PNs). PV+ interneurons were held in current clamp and stimulated with suprathreshold voltage steps to evoke single APs, whereas PNs were held in voltage clamp to record evoked IPSCs (Fig. 4A). We identified e-PV-to-PN pairs in 39% (7 of 18) of recordings compared with 29% (8 of 28) for transplanted MGE-PV-to-PN pairs (Fig. 4C). Statistical comparison (t-test) revealed no significant differences between the evoked IPSC amplitude (Fig. 4D; P = 0.223), rise time (Fig. 4E; P = 0.955), or decay τ (Fig. 4F; P = 0.763) of ePV-PN and MGE-PV-PN pairs. Interneuron subtypes exhibit subtype-specific probabilities of vesicular release from presynaptic terminals (Savanthrapadian et al. 2014). The failure rate of synapses was inferred by quantifying the number of measurable IPSCs evoked across 100 sweeps and showed no significant difference (Fig. 4H; P = 0.0346). Measurement of paired-pulse ratio revealed equal levels of synaptic depression (Fig. 4, G and I; P = 0.536) between groups in response to two stimuli with a 50-ms interstimulus interval.
Fig. 4.
Transplanted MGE cells project subtype-appropriate inhibitory synapses onto cortical pyramidal neurons. Paired recordings between GFP+ cells, held in current clamp, and pyramidal neurons, held in voltage clamp, reveal direct inhibitory connections (A). Connection probability was below 40% for both groups (C). Evoked IPSC amplitude was not significantly different (D). Normalized evoked IPSCs (B) revealed no significant difference in rise time (E), decay τ (F), or response probability (H) between groups. Multiple stimuli (50-ms interstimulus interval) revealed depressing synapses. Paired-pulse ratio (I) was not significantly different between groups.
DISCUSSION
Neural networks are highly complex, containing many neuronal subtypes each with a distinct role in the processing of information. The behavior of a neuron during network activity is, in large part, dictated by the physiological properties of its postsynaptic machinery, the intrinsic membrane properties that transform inputs into action potentials, and properties of the synapses it forms onto postsynaptic partners. In this study we tested the hypothesis that transplant-derived PV+ interneurons from the embryonic MGE integrate into host neural circuitry in a cell subtype-appropriate fashion. We found that after an accelerated developmental period, MGE-derived fast-spiking PV+ interneurons exhibited presynaptic, intrinsic, and postsynaptic properties that mirrored those of endogenous PV+ neurons within the cortical microcircuit.
Our data demonstrate how transplanted cells integrate into host networks to fulfill the distinct physiological role of their neuronal subtype. This subtype-specific functional integration, observed to be complete by 35 days after transplantation (DAT), contrasts with an earlier suggestion that MGE-derived interneurons may alter network excitability in a nonspecific fashion at times before these cells become mature, e.g., 7 to 14 DAT (De la Cruz et al. 2011; Hammad et al. 2015; Southwell et al. 2014). Our findings also suggest that emergence of subtype-specific physiological properties is determined early in development and, like interneuron precursor migration and subtype specification (Batista-Brito et al. 2009; Wichterle et al. 1999; Zhou et al, 2015), is cell autonomous. Transplanted MGE progenitors have been widely shown to generate neurons expressing markers consistent with an interneuron phenotype, e.g., GAD67, GABA, PV, Sst, and NPY (Alvarez-Dolado et al. 2006; Calcagnotto et al. 2010; De la Cruz et al. 2011; Hammad et al. 2015; Henderson et al. 2014; Howard et al. 2014; Hunt et al. 2014; Southwell et al. 2010; Wichterle et al. 1999). Using electrophysiological recording techniques, we now show that expression of these interneuron subtype-specific markers, PV in particular, is coincident with the functional development of a specific set of synaptic and intrinsic physiological properties. These findings are similar to experiments using transplanted human neural precursor cells, which exhibited electrophysiological properties appropriate to the expression of different genetic markers (Zhou et al. 2015).
MGE progenitor cell transplantation improved outcomes in animal models of epilepsy (Baraban et al. 2009; Calcagnotto et al. 2010; De la Cruz et al. 2011; Hammad et al. 2015; Henderson et al. 2014; Hunt et al. 2014), neuropathic pain (Bráz et al. 2012), Alzheimer's disease (Tong et al. 2014), and schizophrenia (Perez and Lodge 2013), all of which are associated with network hyperexcitability. Human neural progenitor cells, transplanted into mice, also show the ability to integrate as functional interneurons (Zhou et al. 2015) and suppress seizures (Cunningham et al. 2014). Although it has been suggested that modulation of inflammation (Southwell et al. 2014) may represent an underlying mechanism of action for these therapeutic effects, the congruence of evidence, including our present demonstration that MGE-derived PV+ neurons receive excitatory synaptic input and make inhibitory synaptic output onto principal cells, supports a conclusion that generation of new and fully functional inhibitory synapses in the host brain is a more plausible explanation. Inflammation in the host brain is minimal following MGE transplantation (Tong et al. 2014) and is normally associated with an increased propensity toward hyperexcitability or epileptogenesis (Vezzani et al. 2013). In regions of cortex containing transplanted MGE progenitors, analysis of inhibitory synaptic activity has consistently shown a 20–30% increase in activity (Alvarez-Dolado et al. 2006; Baraban et al. 2009), which, like antiepileptic drugs that enhance GABA-mediated synaptic inhibition, is a reasonable mechanism for the seizure suppressive effects observed with MGE progenitor cell transplantation.
Direct evidence for functional synaptic connections between transplanted MGE-derived interneurons and principal cells in host brain provides additional insight into the mechanism underlying these therapeutic activities. Earlier reports using loose-patch recordings to stimulate presynaptic neurons while simultaneously obtaining whole cell current-clamp recordings reported a very low connection probability (∼10%), weak excitatory inputs, and small mean inhibitory postsynaptic potential outputs in data from four successful transplant MGE-derived interneuron-to-pyramidal neuron recordings (Southwell et al. 2010, 2014). RMP and Rin properties were not monitored for these neurons, making it difficult to judge the functional maturity of these cells. Dual whole cell patch-clamp techniques allow for recording from interneurons with mature and stable RMP and Rin properties comparable to those of endogenous interneurons. We found inhibitory postsynaptic outputs with amplitude, kinetic properties, and connection probabilities (∼30%) similar to those measured for endogenous PV+ interneuron-to-PN pairs. A connection probability of 35% was recently reported for MGE-to-PN pairs in juvenile mice (P11 to P21) with the use of dual whole cell patch-clamp recordings (Wester and McBain 2016), also consistent with our findings. Excitatory inputs to MGE-derived PV+ interneurons, after a 2-wk delay, were found to be functionally comparable to those measured on age-matched endogenous PV+ interneurons. Importantly, the source of excitatory synaptic inputs onto MGE-PV neurons has yet to be determined. In contrast to previous reports, we measured strong inhibitory currents in synaptically connected MGE-PV-PN pairs even though this measure showed some expected variability. Thus the contrasting results could be due to relatively small sample sizes dictated by the low probability of successfully recording from synaptically connected pairs of neurons or to differences in experimental protocols. Inconsistencies between studies may also be due to the heterogeneous nature of cortical pyramidal neurons. It is also possible that variability in connectivity is due to competition between endogenous and transplanted interneurons for space on the dendritic tree or to the availability of postsynaptic receptor machinery necessary to make a new inhibitory synapse (PSDs, GABA receptor subunits). Both issues could be less meaningful in a disease condition-associated loss of inhibitory neurons, i.e., epilepsy, and do not change our overall conclusion that MGE-derived PV+ interneurons make mature functional synaptic connections with appropriate postsynaptic partners in the host brain.
GRANTS
This work was supported, in part, by National Institute of Neurological Disorders and Stroke Grant R37NS071785 (to S. C. Baraban) and a Dravet Syndrome Foundation Postdoctoral Research Fellowship (to M. A. Howard).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.A.H. and S.C.B. conception and design of research; M.A.H. performed experiments; M.A.H. analyzed data; M.A.H. and S.C.B. interpreted results of experiments; M.A.H. prepared figures; M.A.H. and S.C.B. drafted manuscript; M.A.H. and S.C.B. edited and revised manuscript; M.A.H. and S.C.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge John Rubenstein and Audrey Brumback for ongoing collaboration and helpful discussion on this and related projects.
REFERENCES
- Alvarez-Dolado M, Calcagnotto ME, Karkar KM, Southwell DG, Jones-Davis DM, Estrada RC, Rubenstein JL, Alvarez-Buylla A, Baraban SC. Cortical inhibition modified by embryonic neural precursors grafted into the postnatal brain. J Neurosci 26: 7380–7389, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science 278: 474–476, 1997. [DOI] [PubMed] [Google Scholar]
- Baraban SC, Southwell DG, Estrada RC, Jones DL, Sebe JY, Alfaro-Cervello C, García-Verdugo JM, Rubenstein JL, Alvarez-Buylla A. Reduction of seizures by transplantation of cortical GABAergic interneuron precursors into Kv1.1 mutant mice. Proc Natl Acad Sci USA 106: 15472–15477, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista-Brito R, Rossignol E, Hjerling-Leffler J, Denaxa M, Wegner M, Lefebvre V, Pachnis V, Fishell G. The cell-intrinsic requirement of Sox6 for cortical interneuron development. Neuron 63: 466–481, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bráz JM, Sharif-Naeini R, Vogt D, Kriegstein A, Alvarez-Buylla A, Rubenstein JL, Basbaum AI. Forebrain GABAergic neuron precursors integrate into adult spinal cord and reduce injury-induced neuropathic pain. Neuron 74: 663–675, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcagnotto ME, Ruiz LP, Blanco MM, Santos-Junior JG, Valente MF, Patti C, Frussa-Filho R, Santiago MF, Zipancic I, Alvarez-Dolado M, Mello LE, Longo BM. Effect of neuronal precursor cells derived from medial ganglionic eminence in an acute epileptic seizure model. Epilepsia 51, Suppl 3: 71–75, 2010. [DOI] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Higashiyama H, Knott GW, Kuhlman SJ, Welker E, Huang ZJ. Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. J Neurosci 24: 9598–9611, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham M, Cho JH, Leung A, Savvidis G, Ahn S, Moon M, Lee PK, Han JJ, Azimi N, Kim KS, Bolshakov VY, Chung S. hPSC-derived maturing GABAergic interneurons ameliorate seizures and abnormal behavior in epileptic mice. Cell Stem Cell 15: 559–573, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Cruz E, Zhao M, Guo L, Ma H, Anderson SA, Schwartz TH. Interneuron progenitors attenuate the power of acute focal ictal discharges. Neurotherapeutics 8: 763–773, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Cho RY, Lewis DA. Alterations in cortical network oscillations and parvalbumin neurons in schizophrenia. Biol Psychiatry 77: 1031–1040, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammad M, Schmidt SL, Zhang X, Bray R, Frohlich F, Ghashghaei HT. Transplantation of GABAergic interneurons into the neonatal primary visual cortex reduces absence seizures in Stargazer mice. Cereb Cortex 25: 2970–2979, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson KW, Gupta J, Tagliatela S, Litvina E, Zheng X, Van Zandt MA, Woods N, Grund E, Lin D, Royston S, Yanagawa Y, Aaron GB, Naegele JR. Long-term seizure suppression and optogenetic analyses of synaptic connectivity in epileptic mice with hippocampal grafts of GABAergic interneurons. J Neurosci 34: 13492–13504, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard MA, Rubenstein JL, Baraban SC. Bidirectional homeostatic plasticity induced by interneuron cell death and transplantation in vivo. Proc Natl Acad Sci USA 111: 492–497, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt RF, Girskis KM, Rubenstein JL, Alvarez-Buylla A, Baraban SC. GABA progenitors grafted into the adult epileptic brain control seizures and abnormal behavior. Nat Neurosci 16: 692–697, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavdas AA, Grigoriou M, Pachnis V, Parnavelas JG. The medial ganglionic eminence gives rise to a population of early neurons in the developing cerebral cortex. J Neurosci 19: 7881–7888, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Magueresse C, Monyer H. GABAergic interneurons shape the functional maturation of the cortex. Neuron 77: 388–405, 2013. [DOI] [PubMed] [Google Scholar]
- Okaty BW, Miller MN, Sugino K, Hempel CM, Nelson SB. Transcriptional and electrophysiological maturation of neocortical fast-spiking GABAergic interneurons. J Neurosci 29: 7040–7052, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez SM, Lodge DJ. Hippocampal interneuron transplants reverse aberrant dopamine system function and behavior in a rodent model of schizophrenia. Mol Psychiatry 18: 1193–1198, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell EM, Campbell DB, Stanwood GD, Davis C, Noebels JL, Levitt P. Genetic disruption of cortical interneuron development causes region- and GABA cell type-specific deficits, epilepsy, and behavioral dysfunction. J Neurosci 23: 622–631, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol E. Genetics and function of neocortical GABAergic interneurons in neurodevelopmental disorders. Neural Plast 2011: 649325, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savanthrapadian S, Meyer T, Elgueta C, Booker SA, Vida I, Bartos M. Synaptic properties of SOM- and CCK-expressing cells in dentate gyrus interneuron networks. J Neurosci 34: 8197–8209, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwell DG, Froemke RC, Alvarez-Buylla A, Stryker MP, Gandhi SP. Cortical plasticity induced by inhibitory neuron transplantation. Science 327: 1145–1148, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwell DG, Nicholas CR, Basbaum AI, Stryker MP, Kriegstein AR, Rubenstein JL, Alvarez-Buylla A. Interneurons from embryonic development to cell-based therapy. Science 344: 1240622, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong LM, Djukic B, Arnold C, Gillespie AK, Yoon SY, Wang MM, Zhang O, Knoferle J, Rubenstein JL, Alvarez-Buylla A, Huang Y. Inhibitory interneuron progenitor transplantation restores normal learning and memory in ApoE4 knock-in mice without or with Aβ accumulation. J Neurosci 34: 9506–9515, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyson JA, Anderson SA. GABAergic interneuron transplants to study development and treat disease. Trends Neurosci 37: 169–177, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verret L, Mann EO, Hang GB, Barth AM, Cobos I, Ho K, Devidze N, Masliah E, Kreitzer AC, Mody I, Mucke L, Palop JJ. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell 149: 708–721, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A, Friedman A, Dingledine RJ. The role of inflammation in epileptogenesis. Neuropharmacology 69: 16–24, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wester JC, McBain CJ. Interneurons differentially contribute to spontaneous network activity in the developing hippocampus dependent on their embryonic lineage. J Neurosci 36: 2646–2662, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichterle H, Garcia-Verdugo JM, Herrera DG, Alvarez-Buylla A. Young neurons from medial ganglionic eminence disperse in adult and embryonic brain. Nat Neurosci 2: 461–466, 1999. [DOI] [PubMed] [Google Scholar]
- Xu Q, Cobos I, De La Cruz E, Rubenstein JL, Anderson SA. Origins of cortical interneuron subtypes. J Neurosci 24: 2612–2622, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FW, Fortin JM, Chen HX, MartinezDiaz H, Chang LJ, Reynolds BA, Roper SN. Functional integration of human neural precursor cells in mouse cortex. PLoS One 10: e0120281, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]