There have been numerous studies demonstrating a paradoxical proexcitatory role for BK potassium channels in some neurons. This study provides the first demonstration of a BK-ryanodine receptor functional coupling that underlies this unusual proexcitatory effect in dentate gyrus granule neurons. Functional coupling occurs during a single action potential that enhances the fast afterhyperpolarization amplitude and reduces the interspike interval. Given their broad expression, these findings may be relevant to many neurons of the central nervous system.
Keywords: large-conductance calcium- and voltage-activated potassium channels, action potentials, dentate gyrus, fast afterhyperpolarization
Abstract
BK channels are large-conductance calcium- and voltage-activated potassium channels with diverse properties. Knockout of the accessory BK β4-subunit in hippocampus dentate gyrus granule neurons causes BK channels to change properties from slow-gated type II channels to fast-gated type I channels that sharpen the action potential, increase the fast afterhyperpolarization (fAHP) amplitude, and increase spike frequency. Here we studied the calcium channels that contribute to fast-gated BK channel activation and increased excitability of β4 knockout neurons. By using pharmacological blockers during current-clamp recording, we find that BK channel activation during the fAHP is dependent on ryanodine receptor activation. In contrast, L-type calcium channel blocker (nifedipine) affects the BK channel-dependent repolarization phase of the action potential but has no effect on the fAHP. Reducing BK channel activation during the repolarization phase with nifedipine, or during the fAHP with ryanodine, indicated that it is the BK-mediated increase of the fAHP that confers proexcitatory effects. The proexcitatory role of the fAHP was corroborated using dynamic current clamp. Increase or decrease of the fAHP amplitude during spiking revealed an inverse relationship between fAHP amplitude and interspike interval. Finally, we show that the seizure-prone ryanodine receptor gain-of-function (R2474S) knockin mice have an unaltered repolarization phase but larger fAHP and increased AP frequency compared with their control littermates. In summary, these results indicate that an important role of the β4-subunit is to reduce ryanodine receptor-BK channel functional coupling during the fAHP component of the action potential, thereby decreasing excitability of dentate gyrus neurons.
NEW & NOTEWORTHY
There have been numerous studies demonstrating a paradoxical proexcitatory role for BK potassium channels in some neurons. This study provides the first demonstration of a BK-ryanodine receptor functional coupling that underlies this unusual proexcitatory effect in dentate gyrus granule neurons. Functional coupling occurs during a single action potential that enhances the fast afterhyperpolarization amplitude and reduces the interspike interval. Given their broad expression, these findings may be relevant to many neurons of the central nervous system.
the pore-forming subunit of large-conductance calcium- and voltage-activated potassium channels (BK channels) contributes to a diverse potassium channel current that is encoded by a single gene (KCNMA1). In large part, diversity is mediated by families of accessory β- and γ-subunits that regulate BK channel biophysical properties and pharmacology (recent reviews, Sun et al. 2012; Yan and Aldrich 2012). BK channels have a long-established role in repolarizing action potentials (APs) and contributing to the fast afterhyperpolarization (fAHP) in many neurons (Sah 1996; Sah and Faber 2002). Their very large conductance (∼250 pS) suggests that these channels must be carefully regulated since small changes in probability of opening can cause large changes in excitability. Studies indicate that some accessory subunits are key regulators of BK activation during APs. For example, fast inactivation that is likely mediated by BK β2- or β3-subunits (Wallner et al. 1999; Xia et al. 1999) restricts BK channel activity to early but not later APs of a train. This results in so-called frequency-dependent spike broadening that is seen in CA1 (Shao et al. 1999) and lateral amygdala neurons (Faber and Sah 2003). Neuronal β4-subunits also modulate BK channel function by slowing channel opening and closing, which reduces BK channel contribution to AP repolarization in dentate gyrus (DG) granule neurons (Brenner et al. 2005) and CA3 neurons (Deng et al. 2013), but may sustain BK channel activity during interspike times of cerebellar Purkinje neurons (Benton et al. 2013).
Although BK channels can open in response to depolarization and calcium independently (Cui et al. 1997), the combination appears to be required for opening at physiological voltage ranges (Berkefeld and Fakler 2008; Cox 2014; Wang et al. 2009). The fact that BK channels bind calcium at low affinity (Cox et al. 1997) suggests that BK channels require a colocalized (high-concentration) calcium source to achieve sufficient opening for AP repolarization (Berkefeld and Fakler 2008; Cox 2014; Wang et al. 2009). Thus, there is likely to be interplay between colocalized calcium channels and accessory subunits that determines BK contribution to regulation of AP shape. Consistent with this, biochemical studies indicate that BK channels are physically coupled to a number of voltage-dependent calcium channels, including N-type, P/Q-type and L-type calcium channels (Berkefeld et al. 2006; Loane et al. 2007). Functional studies indicate a more promiscuous but cell-specific association of BK channels with various calcium channels. For example, BK is activated during the AP by P/Q-type channels in striatal neurons (Goldberg and Wilson 2005), N-type channels in CA1 hippocampal neurons (Loane et al. 2007), P/Q- and L-type channels in adrenal chromaffin cells (Prakryia and Lingle 1999, Marcantoni, et al. 2010), T type in cerebellar Purkinje neurons and medial vestibular nucleus neurons (Smith et al. 2002; Womack and Khodakhah 2004), and ryanodine receptors (RyRs) in sympathetic neurons (Akita and Kuba 2000).
In some studies, BK channels have been shown to increase, rather than decrease, AP frequency (Brenner et al. 2005; Gu et al. 2007; Jaffe et al. 2011; Lovell and McCobb 2001; Martinez-Espinosa et al. 2014; Montgomery et al. 2012; Shruti et al. 2008). Given that BK channels are activated by specific calcium sources, this suggests that some calcium channels' effects on excitability may be partly mediated by activation of proexcitatory BK channels. Studies in DG granule neurons of the hippocampus have shown that BK channels become proexcitatory due to a gain-of-function phenotype following knockout (KO) of the BK β4-subunit (Brenner et al. 2005). The β4-subunit dramatically slows BK channel activation to timescales that theoretically reduce BK channel activation during the AP (Jaffe et al. 2011). This is evidenced by lack of effect of BK channel blocker paxilline in wild-type (WT) DG neurons (Brenner et al. 2005). Without β4, BK gain-of-function is revealed as sharpened APs that correlate with an increase in AP frequency and temporal lobe spontaneous seizures (Brenner et al. 2005). However, the calcium sources that activate BK channels in DG neurons have not been identified. Here we found that β4 reduces functional coupling to two distinct calcium sources that otherwise activate β4 KO BK channels during the repolarization phase and fAHP. By blocking calcium channels that activate BK during different components of the AP, and by directly modulating AP shape using dynamic clamp, we determine which aspect of BK channel effect on AP waveform serves to decrease or increase AP frequency.
MATERIALS AND METHODS
Isolation of brain slices.
All mice were humanely treated, and procedures were reviewed and approved by the University of Texas Health Science Center at San Antonio Institutional Animal Care and Use Committee. Brain slices were prepared from 3- to 5-wk-old animals. β4 KO mice were generated as described previously (Brenner et al. 2005). The RyRs R2474S knockin mice were previously reported (Lehnart et al. 2008). Animals used for this study were inbred for at least five generations to C57BL/6J and compared with control WT C57BL/6J mice. In contrast to the original mixed 129svj/C57BL/6J background strain, the inbred strain β4 KO C57BL/6J does not exhibit spontaneous seizures. Therefore, observed changes more likely represent direct effects of β4 KO rather than secondary effects of seizures. Animals were fully anesthetized by isoflurane (Butler Animal Health Supply, Dublin, OH) before their death by decapitation. The whole brain was extracted from the skull after decapitation and placed in ice-cold cutting solution [containing (in mM) 2 KCl, 2 MgSO4, 1.25 NaH2PO4, 1 CaCl2, 2 MgCl2, 26 NaHCO3, 10 d-dextrose, 0.4 vitamin C, and 206 sucrose]. The brain was than attached to a cutting platform and sliced (while constantly bubbled with 95% O2-5% CO2 mixture) to 400-μM-thick sagittal sections no later than 3–4 min after the brain extraction. The slicing was performed on a Leica VT1200 vibratome (Leica Microsystems, Bannockburn, IL) in ice-cold cutting solution. The brain slices were recovered in 30°C extracellular solution for 1 h and then were kept at room temperature for at least another 1 h before first measurements. The extracellular solution contained (in mM) 124 NaCl, 2 KCl, 2 MgSO4, 1.25 NaH2PO4, 2 CaCl2, 26 NaHCO3, 10 d-dextrose, and 0.4 mM vitamin C.
Whole cell current-clamp recordings.
The above extracellular solution was also used for the patch-clamp experiments in the whole cell configuration. The recordings were conducted at room temperature with an internal solution of (in mM) 120 potassium gluconate, 20 KCl, 2 MgCl2, 10 HEPES, 2 ATP, 0.25 GTP, and 0.1 EGTA (pH = 7.4); free calcium content was calculated to be ∼50 nM using MaxC software (Patton, Stanford University). When applicable, paxilline was added to the extracellular (bath) solution in 1:2,000 dilutions from its stock solution (10 mM in DMSO; Sigma, St. Louis, MO). The brain slice was perfused with paxilline for at least 5 min before first measurements. When applicable, ryanodine (20 μM) and thapsigargin (1 μM) were applied through the pipette. Calcium channel blockers were applied in the extracellular solution, including L-type blocker (10 μM nifedipine), T-type CaV blocker (50 μM flunarizine hydrochloride), N-type blocker (3 μM ω-conotoxin GVIA), and R-type blocker (200 nM SNX-482).
Granule cells of the hippocampus were identified at × 60 magnification using a water-submersible objective and DIC/infrared optics on a BX51WI Olympus microscope. Recordings were conducted at 25°C. Cells were analyzed only if they maintained a resting membrane potential of at least −75 mV and if they had an input resistance of 300 MΩ or larger. Recordings of voltage were done with bridge balance compensation via the amplifier (HEKA, EPC10). If slow capacitance changed by >20% during recordings, the cell was excluded from further analysis. A hardware filter of 3 kHz was used for data collection. Current-clamp recordings were performed starting with a holding current to maintain −80 mV, and spikes were generated by rectangular current injections (1 s duration) at 15-pA increments (from 0 to 300 pA). APs were measured 3 min after break in into the whole cell. Similarly, controls were also measured 3 min after the break in.
Measurement of APs.
Characterization of AP waveform was performed on the first spike of a train (of 2 or more spikes) triggered by minimal current stimulation. Frequency was measured as the interspike interval between the first and second spike of a train. AxoGraph software (Kagi, Berkeley, CA) was used to characterize individual AP waveform properties. AP 10% width was the width of AP at 10% of the peak amplitude relative to threshold voltage. The amplitude of fAHP was measured from the threshold voltage to the most negative overshoot after repolarization. The slope of the rise to threshold was obtained by a linear fit from completion of the fAHP to the threshold of the subsequent AP. The input resistance was regularly monitored by using a current-clamp injection of −20 pA from an −80-mV holding current. Interspike intervals (ISIs) were measured as the time between first evoked AP peak to the second AP. All data are represented as means ± SE. The Student's t-test for unpaired samples was used for statistical analysis. Asterisk represents P < 0.05. Data are presented using Igor Pro (WaveMetrics, Portland, OR) and statistically analyzed using Kaleidagraph (Synergy Software).
Dynamic current-clamp recording.
Whole cell brain slice recordings of DG neurons were used to evoke APs. Membrane potential (Vm) was monitored in current-clamp mode (bridge mode) and acquired via the patch amplifier (EPC10, HEKA) under control of a Windows PC. Vm is passed to a Linux PC running RTXI (real-time experiment interface) dynamic clamp software (http://rtxi.org). Neurons were stimulated over a range of currents (15–300 pA) at 30-s intervals from a holding potential of −80 mV. The dynamic clamp computes at each time step a state variable (n) based on the following voltage-dependent rate constants: αn(V) = 0.02 × (V − 25)/{1 − exp[−(V − 25)/9]} and β(V) = −0.002 × (V − 25)/{1 − exp[(V − 25)/9]}. K+ current (IK) is then calculated based on Vm, measured by the bridge circuit, where Ik = ḡkn4(Vm − EK) assuming EK = −90 mV and given a selected value for the maximum conductance (ḡK).
Using a voltage window (Graham and Schramm 2009), we limited the activation of the modeled K+ current to the fAHP component of the AP waveform. The calculated current was added to or subtracted from the injected current, and the sum is then passed to the current-clamp amplifier where the total current injected in the cell is adjusted in real time. Membrane potential acquisition, updating of the state variable, and calculation of the output current were accomplished at a rate of 10 kHz.
RESULTS
The β4-subunit modulates AP shape and frequency near threshold potentials.
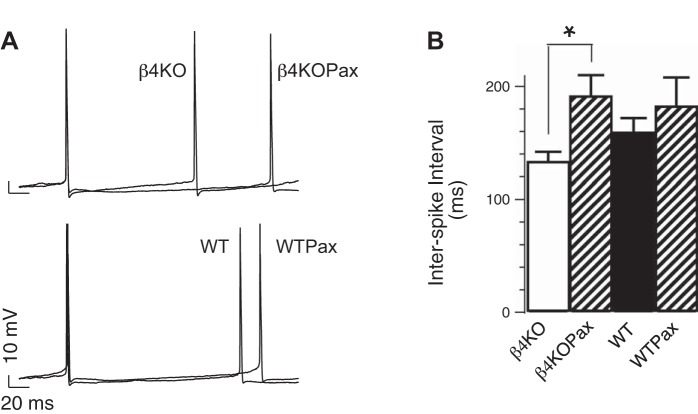
Recordings were made of DG neurons from freshly prepared hippocampal slices in whole cell current-clamp mode. APs were elicited with 1-s square-wave near-threshold current injection sufficient to obtain a minimum of two APs. In β4−/− neurons, APs were elicited with an average 40 ± 3- and 39 ± 4-pA current injection in the absence and presence of BK-channel blocker paxilline (5 μM), respectively. However, paxilline significantly increased the ISI (133 ± 9 vs. 191 ± 19 ms with paxilline, P < 0.05). Significant effect of paxilline was not observed in WT neurons (159 ± 13 vs. 182 ± 26 ms with paxilline, P > 0.05; Fig. 1). Together, these findings suggest that β4 normally inhibits a proexcitatory effect of BK channels in DG neurons near the AP threshold. This is consistent with previous studies revealing a proexcitatory effect of β4 KO at high current injections (Brenner et al. 2005).
Fig. 1.
Effect of large-conductance calcium- and voltage-activated potassium (BK) channel blockers on dentate gyrus (DG) action potential (AP) firing. A: representative APs recorded from knockout (KO) β4−/− or wild-type (WT) DG neurons at threshold current injection. Traces represent APs in the absence or presence of 5 μM paxilline (Pax). X and Y scales represent 20 ms and 10 mV, respectively. B: averaged interspike interval (ISI; in ms, β4KO: 133 ± 9, n = 23, β4KOPax: 191 ± 19, n = 8; WT: 159 ± 13, n = 22, WTPax: 182 ± 26, n = 8). β4−/− and β4−/−Pax are significantly different (P = 0.006). Difference between WT and WTPax was not significant (P = 0.4). *P < 0.05.
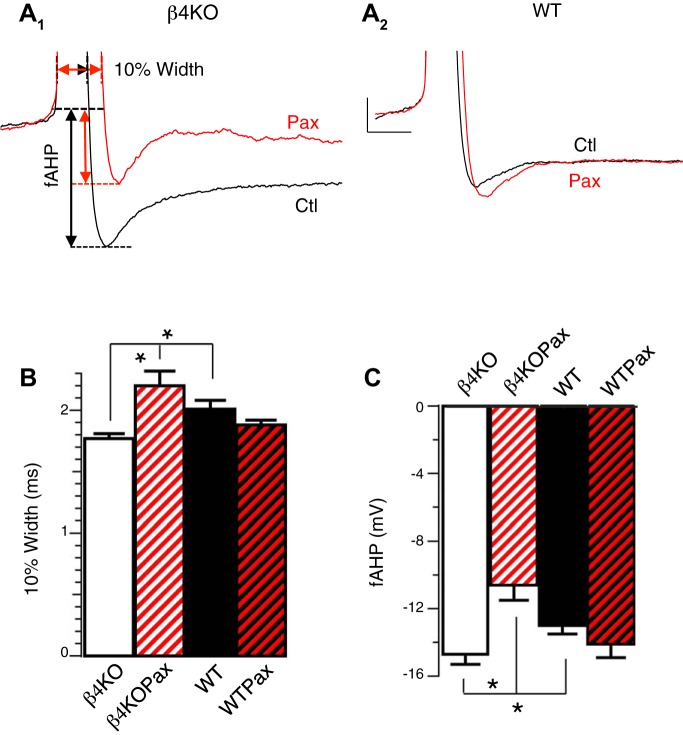
In previous studies, β4 was shown to reduce BK channel contribution to AP repolarization at relatively large depolarizing current injections (Brenner et al. 2005). Here, we examined effects on the fAHP and at near-threshold current injections. We found that the fAHP size was significantly larger in β4−/− neurons (Fig. 2A, summarized in Fig. 2C). Consistent with previous studies, β4−/− 10% AP width was significantly narrower than WT (Fig. 2A, summarized in Fig. 2B). Furthermore, only in β4−/− neurons did paxilline significantly broaden the 10% width (1.77 ± 0.04 vs. 2.20 ± 0.12, P < 0.05; Fig. 2, A1 and B) and decrease fAHP size (−14.7 ± 0.6 vs. −10.6 ± 0.9, P < 0.05; Fig. 2, A1 and 2C). In contrast, blocking BK channels did not significantly alter AP shape in WT neurons (Fig. 2, A2, B, and C). Therefore, these results suggest that β4 inhibits BK channel activation not only during repolarization but also the fAHP phase of the AP.
Fig. 2.
β4 Inhibits BK channel function during AP repolarization and fast afterhyperpolarization (fAHP). A: representative APs recorded from β4KO (A1) and WT (A2) DG neurons at threshold current injection in the absence (black) or presence of 5 μM Pax (red). The AP was cut off at the peak to focus on the 10% width and fAHP that were measured as indicated from arrows in A1. B: Pax significantly reduced the 10% AP width in β4KO but not in WT DG. Averaged 10% widths in ms: β4KO 1.77 ± 0.04 (n = 24); β4KOPax 2.20 ± 0.12 (n = 8); WT 2.02 ± 0.06 (n = 29); WTPax 1.88 ± 0.04 (n = 9). WT and β4KO are significantly different (P = 0.004). β4KO and β4KOPax are significantly different (P < 0.001). Difference between WT and WTPax did not reach significance (P = 0.3). C: Pax significantly reduced fAHP amplitude in β4KO but not in WT DG. Averaged fAHP amplitudes in mV: β4KO −14.7 ± 0.6 (n = 24); β4KOPax −10.6 ± 0.9 (n = 8); WT −13.0 ± 0.52 (n = 29); WTPax −14.1 ± 0.8 (n = 9). WT and β4KO are significantly different (P = 0.04). β4KO and β4KOPax are significantly different (P = 0.001). Difference between WT and WTPax was not significant (P = 0.3). *P < 0.05.
β4 KO BK channels are functionally coupled to L-type CaV channels during the repolarization phase, and RyRs during the fAHP.
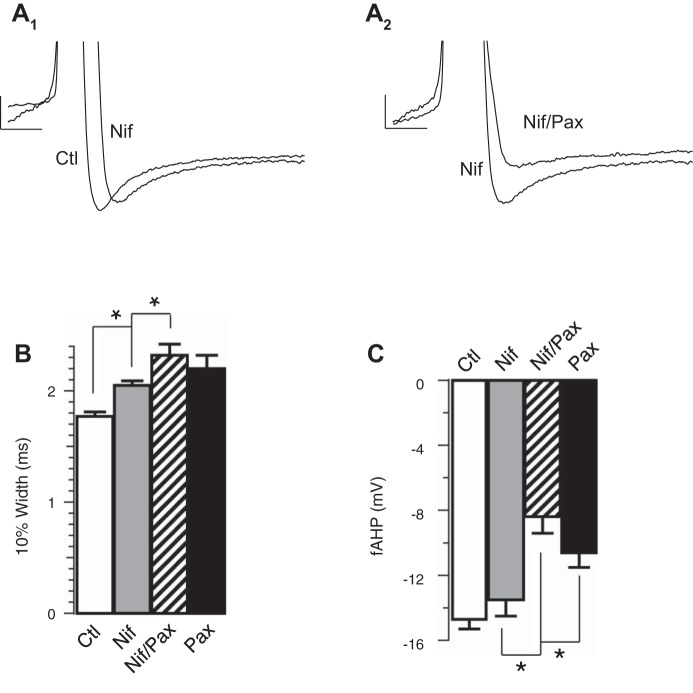
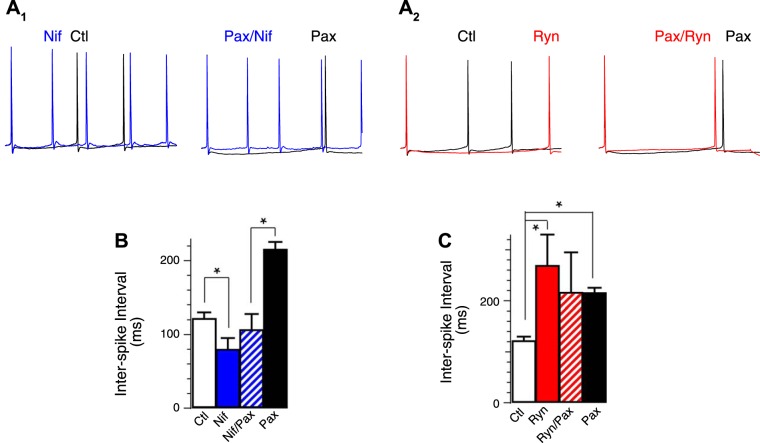
We investigated the calcium source that activates BK channels and thereby contributes to increased excitability in β4−/− neurons. To identify calcium channels that are functionally coupled to BK channel activation, we recorded granule cells with different calcium channel blockers to determine their effects on BK-mediated repolarization and fAHP. We identified two major calcium sources affecting BK channels during the AP of β4 KO neurons. We observed that L-type CaV blocker (10 μM nifedipine) significantly slowed the repolarization by an average of 0.28 ms compared with the effect of paxilline that slowed by an average of 0.43 ms (control 1.77 ± 0.04, nifedipine 2.05 ± 0.04, paxilline 2.20 ± 0.12; Fig. 3A1, summarized Fig. 3B). The effect of nifedipine on the AP width was significant but incomplete compared with BK channel blocker paxilline. This suggests that an additional calcium source may partially contribute to BK channel activation during the repolarization phase. The finding that the AP were not significantly broadened further than paxilline with combined treatment of paxilline and nifedipine (paxilline 2.20 ± 0.12, paxilline/nifedipine: 2.32 ± 0.10, Fig. 3B) suggests that L-type calcium channels mediate their effects through BK channels. Surprisingly, nifedipine had no significant effect on the fAHP amplitude, depolarizing the fAHP by an average of 1.2 mV compared with paxilline, which depolarized the fAHP by 4.1 mV [P = 0.28 (Fig. 3A1, summarized in Fig. 3C), control −14.7 ± 0.6, nifedipine −13.5 ± 1.0, paxilline −10.6 ± 0.9].
Fig. 3.
Ca2+ influx through L-type calcium channels affects the repolarization phase. A1: representative threshold APs in the absence or presence of 10 μM nifedipine (Nif) in β4KO neurons. A2: representative threshold APs in the absence or presence of 5 μM Pax in addition to 10 μM Nif. B: Nif increased the 10% width of threshold AP in β4KO neurons. Averaged 10% width in ms: control 1.77 ± 0.04 (n = 24); Pax 2.20 ± 0.12 (n = 8); Nif 2.05 ± 0.04 (n = 11); Nif/Pax 2.32 ± 0.10 (n = 15). Nif and control are significantly different (P ≈ 0.0005). Nif/Pax and Nif are significantly different (P ≈ 0.04). Nif/Pax and Pax are not significantly different (P ≈ 0.46). C: Nif did not reduce the fAHP amplitudes of threshold AP in β4KO neurons. Averaged fAHP amplitudes in mV: control −14.7 ± 0.6 (n = 24); Pax −10.6 ± 0.9 (n = 8); Nif −13.5 ± 1.0 (n = 11); Pax/Nif −8.4 ± 1.0 (n = 15). Nif and control are not significantly different (P ≈ 0.28). Pax/Nif and Pax are not significantly different (P ≈ 0.19). Nif/Pax and Nif are significantly different (P ≈ 0.003). *P < 0.05.
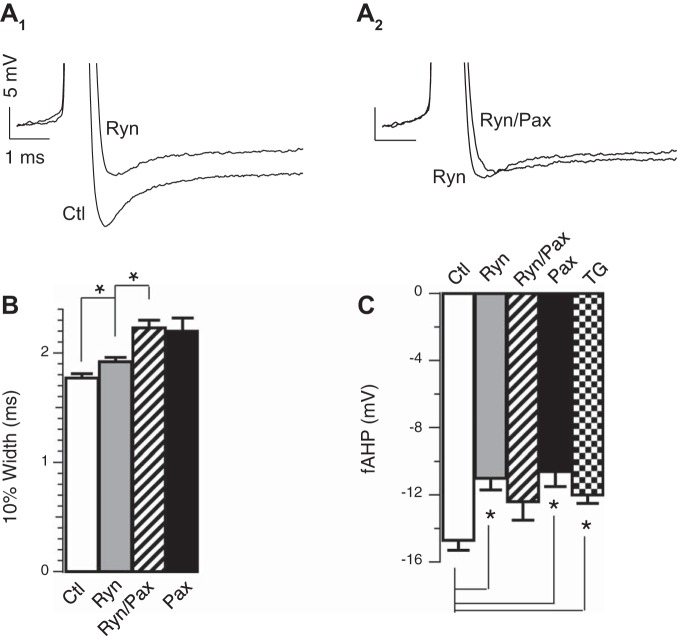
In contrast to nifedipine, RyR blocker (20 μM ryanodine) had a nearly identical effect on the fAHP amplitude as BK blocker paxilline (control −14.7 ± 0.6 mV, paxilline −10.6 ± 0.9 mV, ryanodine −11.0 ± 0.7 mV; Fig. 4A1, summarized in Fig. 4C) but only weakly affected the 10% width (control 1.77 ± 0.04 ms, paxiline 2.20 ± 0.12 ms, ryanodine 1.92 ± 0.04 ms; Fig. 4A1, summarized Fig. 4B). The effect of ryanodine was nonadditive with BK channel blocker paxilline (11.0 ± 0.7 ms ryanodine, −12.4 ± 1.1 ryanodine/paxilline; Fig. 4, A1, A2, and C), suggesting that BK channel activation during the fAHP is exclusively through RyRs. We further confirmed the role of calcium-induced calcium release (RyR) on the fAHP by passively depleting calcium stores with SERCA blocker thapsigargin (1 μM; Fig. 4C), which also reduced the fAHP from β4 KO DG granule neurons (control −14.7 ± 0.6 mV, thapsigargin −12.0 ± 0.5 mV, P < 0.05). While RyRs appear to contribute in large part to BK channel activation during the fAHP, we also observed that T-type CaV blocker (50 μM flunarizine hydrochloride) had a moderate but significant effect on the fAHP (Δ10% width = 0 ms, P = 0.95, ΔfAHP = −1.5 mV, P = 0.01, paired t-test). This may suggest that T-type CaV channels may serve as a calcium influx pathway that partly evokes calcium-induced calcium release.
Fig. 4.
Ca2+ release through ryanodine receptors (RyR) activates BK during the fAHP. A1: representative threshold APs in the absence or presence of 20 μM ryanodine (Ryn) in β4KO neurons. A2: representative threshold APs in the absence or presence of 5 μM Pax in addition to 20 μM Ryn. B: averaged 10% width in ms: control 1.77 ± 0.04 (n = 24); Pax 2.20 ± 0.12 (n = 8); Ryn 1.92 ± 0.04 (n = 13); Ryn/Pax 2.23 ± 0.07 (n = 9); Ryn and control are significantly different (P ≈ 0.03). Ryn/Pax and Pax are not significantly different (P = 0.82). C: Ryn significantly decreased fAHP amplitudes. Averaged fAHP amplitudes in mV: control −14.7 ± 0.6 (n = 24); Pax −10.6 ± 0.9 (n = 8); Ryn −11.0 ± 0.7 (n = 13); Ryn/Pax −12.4 ± 1.1 (n = 9); thapsigargin (TG) −12.0 ± 0.5 (n = 6). Ryn and control are significantly different (P = 0.0005). Ryn/Pax and Ryn are not significantly different (P = 0.30). Ryn/Pax and Pax are not significantly different (P ≈ 0.24). TG (1 μM) and control are significantly different (P ≈ 0.03). TG and Ryn are not significantly different (P ≈ 0.41). *P < 0.05.
We screened with other calcium channel blockers, including N-type (3 μM ω-conotoxin GVIA, Δ10% width = 0.06 ms, P = 0.48, ΔfAHP = −0.3 mV, P = 0.6) and R-type (200 nM SNX-482, Δ10% width = −0.06 ms, P = 0.12, ΔfAHP = −0.3 mV, P = 0.32) blockers that did not significantly affect the AP waveform. In summary, we find that BK channels lacking β4 are functionally coupled to distinct calcium sources during different components of the AP waveform. L-type calcium channels contribute to repolarization, whereas RyR channels maintain or activate an additional pool of BK channels during the fAHP.
RyR-dependent fAHP generation increases firing rate.
Given that selective calcium channel blockers affect BK channel activation during different components of the AP waveform, this provided the unique opportunity to determine whether the sharpening of the AP or increase of the fAHP amplitude underlies the BK-mediated increase of AP frequency. We observed that L-type channels blockers, which mainly affect repolarization, actually decreased ISI (Fig. 5A1, summarized in Fig. 5B). In contrast, blocking the RyRs, which affect the BK-mediated fAHP, increased ISIs in a similar manner as BK channel block with paxilline (Fig. 5A2, summarized n Fig. 5C). Paxilline occluded any further effect of ryanodine (Fig. 5, A2 and C). In conclusion, pharmacological block of BK calcium sources indicates that proexcitatory effects of β4−/− BK channels are mediated through the increased fAHP.
Fig. 5.
Nif increased excitability and ryanodine reduced excitability of β4KO neurons. A1 and A2: representative APs in β4KO neurons. The first APs of each trace were aligned. A1: Nif reduced ISI between the 1st and 2nd AP in β4KO neurons. A2: Ryn increased ISI between the 1st and 2nd AP in β4KO neurons. B: averaged ISI in ms, control 121 ± 9 (n = 12); Pax 215 ± 11 (n = 4); Nif 79 ± 16 (n = 8); Nif/Pax 106 ± 22 (n = 5). Nif and control are significantly different (P ≈ 0.02). Nif/Pax is significantly different from Pax (P ≈ 0.005). C: averaged ISI in ms, control 121 ± 9, (n = 12); Pax 215 ± 11 (n = 4); Ryn 268 ± 62 (n = 4); Ryn/Pax 216 ± 80 (n = 5). Pax and control are significantly different (P < 0.0001). Ryn and control are significantly different (P ≈ 0.001). Ryn/Pax is not significantly different from Pax (P ≈ 0.99) or Ryn (P ≈ 0.63). *P < 0.05.
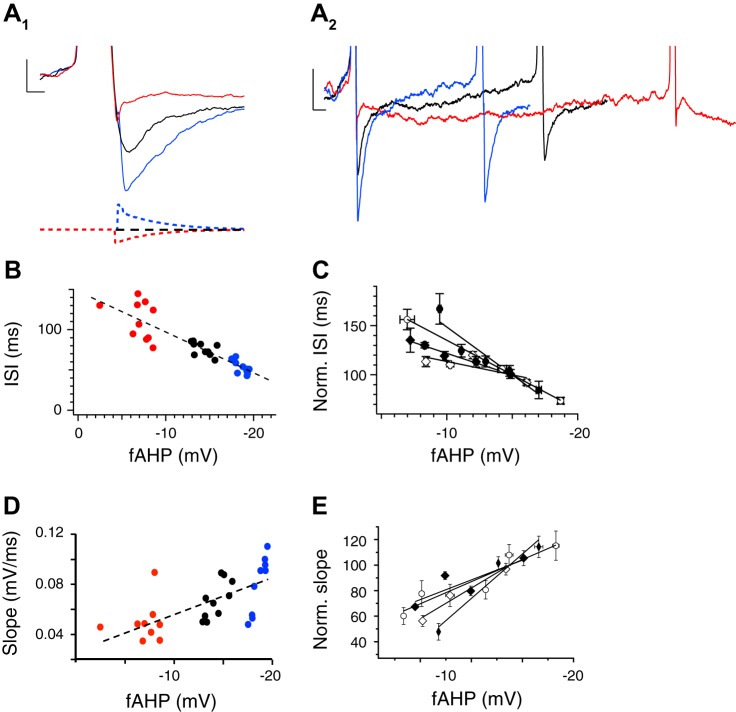
To directly test the contribution of the fAHP on excitability, we used the dynamic clamp method to modify the hyperpolarization phase of the AP in β4−/− DG neurons and measure its effects on the neuron's firing response. Injection of outward and inward current provided by the dynamic clamp (Fig. 6A1, traces on bottom) amplified or reduced fAHP amplitude relative to control (Fig. 6A1, traces on top). We observed that a larger fAHP reduced ISIs relative to control (Fig. 6A2). In contrast, reduction of the fAHP extended ISIs relative to control. Figure 6B shows an example response of a single neuron, and Fig. 6C shows the response of several neurons where the fAHP amplitude was systematically altered. There was a clear negative correlation between fAHP amplitude and ISI (R2 = −0.83 ± 0.09). In addition to changes in ISI, we also observed a steeper rise to threshold approaching the subsequent spike for large fAHPs compared with small fAHPs (Fig. 6A2). The positive relationship between fAHP amplitude and slope is shown for a representative neuron in Fig. 6D and summarized for several neurons in Fig. 6E (R2 = +0.88 ± 0.05). These results indicate that fAHP amplitude alters AP frequency and may underlie BK excitatory effects.
Fig. 6.
fAHP amplitude and AP frequency are correlated in β4KO neurons. A1: AP evoked by constant current injection (black trace) is compared with dynamic clamp that increases (blue) and reduces (red) fAHP amplitude. A2: expanded time scale from A1 shows effects of fAHP amplitude on ISIs. B: summary data showing an inverse correlation between fAHP amplitude and ISI for each neuron tested. C: ISI-fAHP relationship for multiple neurons (different symbols for each neuron) normalized to the ISI at the −15 mV fAHP (n = 4). D: summary data showing a correlation between fAHP amplitude and slope (mV/ms) between the fAHP and subsequent spike. The slope is measured from completion of the fAHP of the first spike to threshold of the second spike. E: relative slope-fAHP relationship for multiple neurons (different symbols for each neuron) normalized to the slope at the −15 mV fAHP (n = 4).
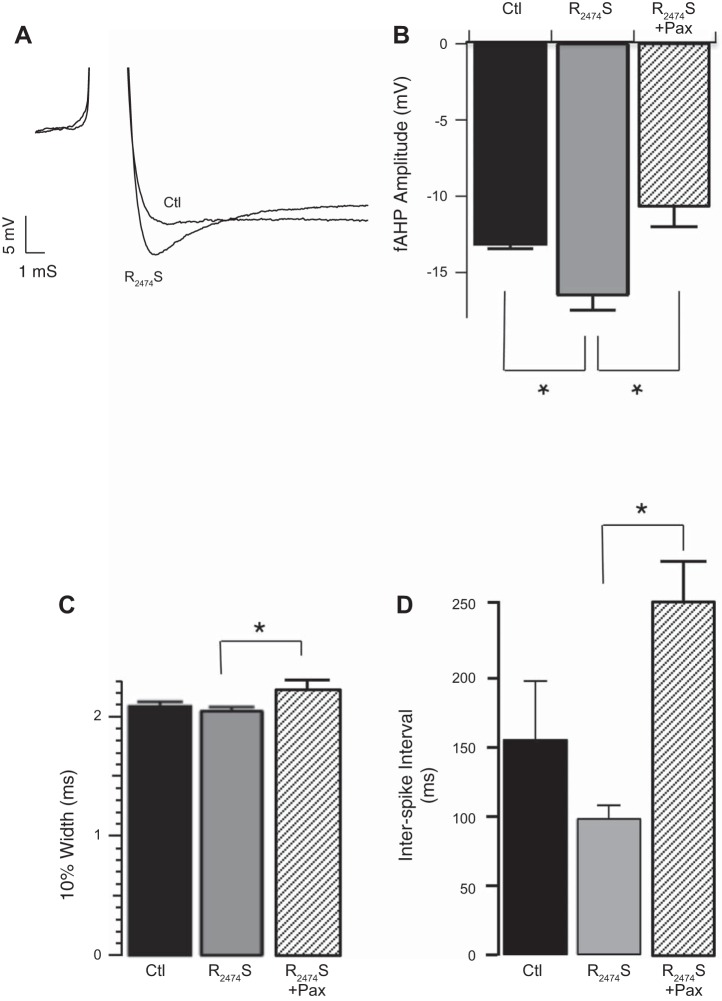
Studies have shown that ryanodine receptor 2 subtype (RyR2) mutations cause epileptic seizures in humans (LaPage et al. 2012; Nagrani et al. 2011) and also in a knockin mouse model containing the mutation R2474S (Lehnart et al. 2008). The R2474S RyR2 mutation perturbs binding of calstabin (also known as FKBP12.6), resulting in channels that open in response to relatively low cytosolic calcium concentrations (Wehrens et al. 2003). Calcium imaging of brain slices from the R2474S mouse indicates that increased calcium release events are particularly evident in the DG (Lehnart et al. 2008), suggesting that RyR2 subtype are positioned to activate BK channels in DG neurons. We therefore investigated if the RyR gain-of-function could contribute to increased fAHP amplitudes and AP frequency in DG neurons. Figure 7A (summarized in Fig. 7B) shows that mice carrying the RyR2 R2474S mutation indeed have larger fAHP amplitudes compared with their WT littermates (fAHP control −13.1 ± 0.3 mV, R2474S −16.5 ± 1.0 mV, P < 0.05). The effect of the gain-of-function was specific to the fAHP since AP width was not affected (10% width control 2.0 ± 0.05 ms, R2474S 2.0 ± 0.03 ms; Fig. 7C). The R2474S mutation tended to reduce the ISI, but this did not reach statistical significance due to variability in the sibling control mice (control 155 ± 43; R2474S: 98 ± 10; P = 0.2 for control vs. R2474S; Fig. 7D). However, paxilline reduced the fAHP amplitude (P = 0.002; Fig. 7B) and significantly increased the ISI (P = 0.0001; Fig. 7D) of R2474S mutant mice, suggesting that the effects of the R2474S mutation are mediated through modulation of BK channels.
Fig. 7.
RyR2 gain-of-function mutation R2474S causes an increased fAHP amplitude. A: representative fAHP from R2474S and from control WT sibling dentate gyrus (DG) granule neuron. B and C: fAHP amplitude (control −13.1 ± 0.3; R2474S −16.5 ± 1.0; R2474S + Pax 10.6 ± 1.4, P = 0.004 for control vs. R2474S, P = 0.002 for R2474S vs. R2474S + Pax, n = 8, 9, and 6 respectively, B) and AP 10% width (control 2.0 ± 0.05; R2474S 2.0 ± 0.03; R2474S + Pax 2.2 ± 0.07, P = 0.92 for control vs. R2474S, P = 0.02 for R2474S vs. R2474S + Pax, n = 9, 13 and 6, respectively, C) at threshold current injections. D: ISI at threshold current injections (control 155 ± 43; R2474S 98 ± 10; R2474S + Pax 252 ± 29, P = 0.2 for control vs. R2474S, P = 0.0001 for R2474S vs. R2474S + Pax, n = 8, 8 and 6, respectively). Gray bars are R2474S mice, stippled bars are R2474S + Pax, and black bars are control littermates. *P < 0.05.
DISCUSSION
β4 KO mice reveal BK functional coupling to distinct calcium sources during different phases of the AP.
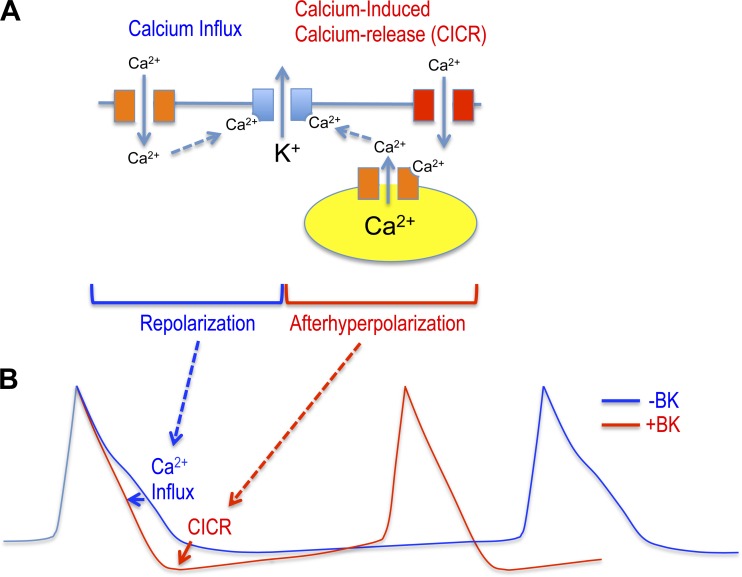
Our studies indicate that the BK channel in DG neurons may be coupled to distinct calcium sources during the AP (summarized in Fig. 8). During the repolarization phase, BK channels are activated by L-type calcium channels, whereas BK channels are functionally coupled to RyRs during the fAHP (Fig. 8A). The proexcitatory effect of the β4 KO is mediated by functional coupling via calcium-induced calcium release to increase the fAHP that decreases ISIs (Fig. 8B). While physical and functional coupling of BK to L-type channels in neurons has been described (Berkefeld et al. 2006; Davies et al. 1996; Sun et al. 2003), reports of coupling to RyRs are more prevalent in smooth muscle (Bolton and Imaizumi 1996; Jaggar et al. 1998) than the central nervous system. Nevertheless, others have observed BK-dependent spontaneous transient outward currents in DG neurons resembling those of smooth muscle cells (Shirasaki et al. 2001). Ryanodine effects during the fAHP, but not the repolarizing phase, suggest calcium release is either somehow temporally isolated from L-type calcium influx or that there are distinct pools of BK channels, one activated by L-type CaV channels and the other by RyRs. Supporting the latter hypothesis are electron microscopy studies showing that there are indeed distinct BK channels associated with submembrane cisternae in DG granule neurons (Kaufmann et al. 2010). Submembrane cisternae are positions of the endoplasmic reticulum containing RyRs that come into close apposition to the plasma membrane (Berridge 1998) and may provide a local source of calcium that activates BK channels. The finding that BK channels are activated in DG neurons, only in the absence of β4, suggests that this accessory subunit has an important role in preventing proexcitatory effects of the fAHP.
Fig. 8.
BK proexcitatory effects are mediated by RyR BK channel coupling that increases the fAHP. A: fast-gated BK potassium channels (lacking β4, blue) are activated during the repolarization phase by calcium influx originating from L-type voltage-dependent calcium channels (orange). Different voltage-dependent calcium channels (including T type, red) specifically contribute to calcium-induced calcium release through RyRs (orange) to activate BK channels and increase the fAHP amplitude. B: fAHP, mediated by RyR-BK channel coupling, causes a shorter ISI to confer the BK channel's proexcitatory effect (red trace). Block of BK channels, block of RyRs, or slow-gating conferred by β4 reduces the fAHP amplitude and increases the ISI (blue trace).
Previous studies of β4 KO mice in DG neurons were conducted under high current injections (Brenner et al. 2005), whereas studies here used threshold current injections to more closely approach physiological conditions. Both studies demonstrate a gain-of-function and proexcitatory effect of BK channels when the β4-subunit is not expressed. Under high current clamp, we observed that the β4 KO supported a tonic firing and reduced spike-frequency adaptation (Brenner et al. 2005), perhaps similar to the firing patterns that support a seizure state. This suggests that β4 protects the hippocampus from high synchronous discharges that otherwise contribute to temporal lobe seizures. Here, under threshold current injections, the β4 KO demonstrated a reduced ISI or increased probability of two spikes over a time domain of 100–200 ms. In awake rats, granule cells fire APs rarely but undergo periods of depolarization that involve multiple APs more so than single spikes, with an average of 3.3 APs per burst (Pernia-Andrade and Jonas 2014). Thus one can speculate that the BK/β4 channels, by regulating the ISI, affect the temporal coding of information by DG granule neurons.
Proexcitatory effects of BK channels are mediated by RyR activation of BK channels during the fAHP.
Pharmacological studies and computational modeling also revealed proexcitatory effects of BK channels in CA1 neurons (Gu et al. 2007). However, the computational model did not distinguish whether the sharper width or larger fAHP contributed to increased excitability (Gu et al. 2007). In this study of DG neurons, specific effect of ryanodine on the fAHP allowed us to ascribe BK proexcitatory effects to this component, and not to BK effect on AP repolarization. This was further corroborated using dynamic current clamp to specifically perturb the fAHP and observe an effect on AP frequency. These findings also explain the paradoxical effect that, while nifedipine and BK β4 deletion increase AP frequency, the former broadens the AP while the latter narrows it. We find that it is mainly the reduction of fAHP and not AP broadening that underlies the BK β4 deletion proexcitatory effect. However, there is also the possibility that nifedipine increases frequency by blocking an L-type calcium current that otherwise feeds calcium-activated channels other than BK, such as SK or chloride channels.
As yet, it is not clearly understood how a larger fAHP decreases the ISI. Because BK regulation of AP shape has effects that occur long after conclusion of the spike, models generally incorporate secondary effects of the AP shape on KCNQ, HCN, or SK currents that are sustained during ISIs (Jaffe et al. 2011, Ly et al. 2011). Models also consider the possibility that BK-mediated sharpening of APs is proexcitatory by removing sodium channel inactivation (Gu et al. 2007). Previous studies suggest that β4 KO neurons increase excitability due to reduced recruitment of SK-type calcium-activated potassium channels (Brenner et al. 2005). If this is the case, then we might surmise that the larger fAHP reduces activation of a calcium source that otherwise feeds SK channel activation. As a result, the various sources of calcium appear to have opposing effects on membrane excitability depending on the properties of BK channels. In general, in β4 KO neurons, they enhance excitability through type I BK channels (i.e., decreased spike width, larger fAHP), whereas, when more strongly coupled to SK channels (with the expression of type II BK channels), they reduce excitability.
BK channels may be plasma membrane effectors of RyR calcium dysregulation.
While RyR2 is most notable for its role in mediating excitation-contraction coupling in the heart and in arrhythmia pathologies (Betzenhauser and Marks 2010), reports are emerging of RyR2 mutations also causing seizures in humans (LaPage et al. 2012; Nagrani et al. 2011) and in a knockin mouse model (Lehnart et al. 2008). RyRs are regulated by phosphorylation and are susceptible to hyperphosphorylation that contributes to stress-induced cognitive dysfunction (Liu et al. 2012). In addition, RyRs have a role in long-term potentiation in DG neurons in response to strong high-frequency stimulation (Wu et al. 2008). Thus, while RyRs likely have many effects on neuronal function, how these proteins communicate to the membrane to affect membrane excitability is poorly understood. These studies showing a functional coupling between RyRs and BK channels suggest that BK channels can serve as a membrane effector of RyRs and could potentially contribute to endoplasmic reticulum calcium dysregulation.
GRANTS
We acknowledge the Xiangya Medical Student Research Program support to Ling Ling. This material is based upon work supported by the National Science Foundation under Grant No. 1456862 and National Institute of Neurological Disorders and Stroke Grant NS-52574 to R. Brenner.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.W., D.B.J., and R.B. conception and design of research; B.W., V.B., L.L., and H.-H.C. performed experiments; B.W. and V.B. analyzed data; B.W. and D.B.J. interpreted results of experiments; B.W. prepared figures; B.W., D.B.J., and R.B. edited and revised manuscript; B.W., D.B.J., and R.B. approved final version of manuscript; R.B. drafted manuscript.
ACKNOWLEDGMENTS
We thank Andrew R. Marks, Columbia University, for the kind gift of the RyR2474S mice.
REFERENCES
- Akita T, Kuba K. Functional triads consisting of ryanodine receptors, Ca(2+) channels, and Ca(2+)-activated K(+) channels in bullfrog sympathetic neurons. Plastic modulation of action potential. J Gen Physiol 116: 697–720, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton MD, Lewis AH, Bant JS, Raman IM. Iberiotoxin-sensitive and -insensitive BK currents in Purkinje neuron somata. J Neurophysiol 109: 2528–2541, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkefeld H, Fakler B. Repolarizing responses of BKCa-Cav complexes are distinctly shaped by their Cav subunits. J Neurosci 28: 8238–8245, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkefeld H, Sailer CA, Bildl W, Rohde V, Thumfart JO, Eble S, Klugbauer N, Reisinger E, Bischofberger J, Oliver D, Knaus HG, Schulte U, Fakler B. BKCa-Cav channel complexes mediate rapid and localized Ca2+-activated K+ signaling. Science 314: 615–620, 2006. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Neuronal calcium signaling. Neuron 21: 13–26, 1998. [DOI] [PubMed] [Google Scholar]
- Betzenhauser MJ, Marks AR. Ryanodine receptor channelopathies. Pflugers Arch 460: 467–480, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton TB, Imaizumi Y. Spontaneous transient outward currents in smooth muscle cells. Cell Calcium 20: 141–152, 1996. [DOI] [PubMed] [Google Scholar]
- Brenner R, Chen QH, Vilaythong A, Toney GM, Noebels JL, Aldrich RW. BK channel beta4 subunit reduces dentate gyrus excitability and protects against temporal lobe seizures. Nat Neurosci 8: 1752–1759, 2005. [DOI] [PubMed] [Google Scholar]
- Cox DH, Cui J, Aldrich RW. Allosteric gating of a large conductance Ca-activated K+ channel. J Gen Physiol 110: 257–281, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DH. Modeling a Ca2+ channel/BKCa at the single complex level. Biophys J 107: 2797–2814, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Cox DH, Aldrich RW. Intrinsic voltage dependence and Ca2+ regulation of mslo large conductance Ca-activated K+ channels. J Gen Physiol 109: 647–673, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PJ, Ireland DR, McLachlan EM. Sources of Ca2+ for different Ca(2+)-activated K+ conductances in neurones of the rat superior cervical ganglion. J Physiol Lond 495: 353–366, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng PY, Rotman Z, Blundon JA, Cho Y, Cui J, Cavalli V, Zakharenko SS, Klyachko VA. FMRP regulates neurotransmitter release and synaptic information transmission by modulating action potential duration via BK channels. Neuron 77: 696–711, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber ES, Sah P. Ca2+-activated K+ (BK) channel inactivation contributes to spike broadening during repetitive firing in the rat lateral amygdala. J Physiol 552: 483–497, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JA, Wilson CJ. Control of spontaneous firing patterns by the selective coupling of calcium currents to calcium-activated potassium currents in striatal cholinergic interneurons. J Neurosci 25: 10230–10238, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham LJ, Schramm A. In vivo dynamic-clamp manipulation of extrinsic and intrinsic conductances: functional roles of shunting inhibition and ibk in rat and cat cortex. In: Dynamic-Clamp: From Principles to Applications, edited by Destexhe A, Bal T. New York: Springer, 2009, p. xiv. [Google Scholar]
- Gu N, Vervaeke K, Storm JF. BK potassium channels facilitate high-frequency firing and cause early spike frequency adaptation in rat CA1 hippocampal pyramidal cells. J Physiol 580: 859–882, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe DB, Wang B, Brenner R. Shaping of action potentials by type I and type II large-conductance Ca(2)+-activated K+ channels. Neuroscience 192: 205–218, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaggar JH, Wellman GC, Heppner TJ, Porter VA, Perez GJ, Gollasch M, Kleppisch T, Rubart M, Stevenson AS, Lederer WJ, Knot HJ, Bonev AD, Nelson MT. Ca2+ channels, ryanodine receptors and Ca(2+)-activated K+ channels: a functional unit for regulating arterial tone. Acta Physiol Scand 164: 577–587, 1998. [DOI] [PubMed] [Google Scholar]
- Kaufmann WA, Kasugai Y, Ferraguti F, Storm JF. Two distinct pools of large-conductance calcium-activated potassium channels in the somatic plasma membrane of central principal neurons. Neuroscience 169: 974–986, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPage MJ, Russell MW, Bradley DJ, Dick M 2nd. Novel ryanodine receptor 2 mutation associated with a severe phenotype of catecholaminergic polymorphic ventricular tachycardia. J Pediatr 161: 362–364, 2012. [DOI] [PubMed] [Google Scholar]
- Lehnart SE, Mongillo M, Bellinger A, Lindegger N, Chen BX, Hsueh W, Reiken S, Wronska A, Drew LJ, Ward CW, Lederer WJ, Kass RS, Morley G, Marks AR. Leaky Ca2+ release channel/ryanodine receptor 2 causes seizures and sudden cardiac death in mice. J Clin Invest 118: 2230–2245, 2008.18483626 [Google Scholar]
- Liu X, Betzenhauser MJ, Reiken S, Meli AC, Xie W, Chen BX, Arancio O, Marks AR. Role of leaky neuronal ryanodine receptors in stress-induced cognitive dysfunction. Cell 150: 1055–1067, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loane DJ, Lima PA, Marrion NV. Co-assembly of N-type Ca2+ and BK channels underlies functional coupling in rat brain. J Cell Sci 120: 985–995, 2007. [DOI] [PubMed] [Google Scholar]
- Lovell PV, McCobb DP. Pituitary control of BK potassium channel function and intrinsic firing properties of adrenal chromaffin cells. J Neurosci 21: 3429–3442, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly C, Melman T, Barth AL, Ermentrout GB. Phase-resetting curve determines how BK currents affect neuronal firing. J Comp Neurosci 30: 211–223, 2011. [DOI] [PubMed] [Google Scholar]
- Marcantoni A, Vandael DHF, Mahapatra S, Carabelli V, Sinnegger-Brauns MJ, Striessnig J, Carbone Loss of Cav1 E. 3 . Channels reveals the critical role of L-type and BK channel coupling in pacemaking mouse adrenal chromaffin cells. J Neurosci 30: 491–504, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Espinosa PL, Yang C, Gonzalez-Perez V, Xia XM, Lingle CJ. Knockout of the BK beta2 subunit abolishes inactivation of BK currents in mouse adrenal chromaffin cells and results in slow-wave burst activity. J Gen Physiol 144: 275–295, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery JM, Meredith AL. Genetic activation of BK currents in vivo generates bi-directional effects on neuronal excitability. Proc Natl Acad Sci USA 109: 18997–9002, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagrani T, Siyamwala M, Vahid G, Bekheit S. Ryanodine calcium channel: a novel channelopathy for seizures. Neurologist 17: 91–94, 2011. [DOI] [PubMed] [Google Scholar]
- Pernía-Andrade AJ, Jonas P. Theta-gamma-modulated synaptic currents in hippocampal granule cells in vivo define a mechanism for network oscillations. Neuron 81: 140–152, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakriya M, Lingle CJ. BK channel activation by brief depolarizations requires Ca2+ influx through L- and Q-type Ca2+ channels in rat chromaffin cells. J Neurophysiol 81: 2267–2278, 1999. [DOI] [PubMed] [Google Scholar]
- Sah P. Ca(2+)-activated K+ currents in neurones: types, physiological roles and modulation. Trends Neurosci 19: 150–154, 1996. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ES. Channels underlying neuronal calcium-activated potassium currents. Prog Neurobiol 66: 345–353, 2002. [DOI] [PubMed] [Google Scholar]
- Shao LR, Halvorsrud R, Borg-Graham L, Storm JF. The role of BK-type Ca2+-dependent K+ channels in spike broadening during repetitive firing in rat hippocampal pyramidal cells. J Physiol 521: 135–146, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasaki T, Houtani T, Sugimoto T, Matsuda H. Spontaneous transient outward currents: modulation by nociceptin in murine dentate gyrus granule cells. Brain Res 917: 191–205, 2001. [DOI] [PubMed] [Google Scholar]
- Shruti S, Clem RL, Barth AL. A seizure-induced gain-of-function in BK channels is associated with elevated firing activity in neocortical pyramidal neurons. Neurobiol Dis 30: 323–330, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MR, Nelson AB, Du Lac S. Regulation of firing response gain by calcium-dependent mechanisms in vestibular nucleus neurons. J Neurophysiol 87: 2031–2042, 2002. [DOI] [PubMed] [Google Scholar]
- Sun X, Gu XQ, Haddad GG. Calcium influx via L- and N-type calcium channels activates a transient large-conductance Ca2+-activated K+ current in mouse neocortical pyramidal neurons. J Neurosci 23: 3639–3648, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Zaydman MA, Cui J. Regulation of voltage-activated k(+) channel gating by transmembrane beta subunits. Front Pharmacol 3: 63, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner M, Meera P, Toro L. Molecular basis of fast inactivation in voltage and Ca2+-activated K+ channels: a transmembrane beta-subunit homolog. Proc Natl Acad Sci USA 96: 4137–4142, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Rothberg BS, Brenner R. Mechanism of increased BK channel activation from a channel mutation that causes epilepsy. J Gen Physiol 133: 283–294, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrens XH, Lehnart SE, Huang F, Vest JA, Reiken SR, Mohler PJ, Sun J, Guatimosim S, Song LS, Rosemblit N, D'Armiento JM, Napolitano C, Memmi M, Priori SG, Lederer WJ, Marks AR. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell 113: 829–840, 2003. [DOI] [PubMed] [Google Scholar]
- Womack MD, Khodakhah K. Dendritic control of spontaneous bursting in cerebellar Purkinje cells. J Neurosci 24: 3511–3521, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Harney S, Rowan MJ, Anwyl R. Involvement of group I mGluRs in LTP induced by strong high frequency stimulation in the dentate gyrus in vitro. Neurosci Lett 436: 235–239, 2008. [DOI] [PubMed] [Google Scholar]
- Xia XM, Ding JP, Lingle CJ. Molecular basis for the inactivation of Ca2+- and voltage-dependent BK channels in adrenal chromaffin cells and rat insulinoma tumor cells. J Neurosci 19: 5255–5264, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Aldrich RW. BK potassium channel modulation by leucine-rich repeat-containing proteins. Proc Natl Acad Sci USA 109: 7917–7922, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]