Our study is the first to report that Fyn kinase is critical for the development of tactile allodynia in the state of prediabetes. Furthermore, the increased expression/function of NR2B subunit-containing N-methyl-d-aspartate receptors in the spinal cord may contribute to the progression of neuropathy in type 2 diabetes.
Keywords: Fyn kinase, NMDA, prediabetes, neuropathic pain, spinal cord
Abstract
Diabetic neuropathy is a common complication of diabetes. This study evaluated the role of Fyn kinase and N-methyl-d-aspartate receptors (NMDARs) in the spinal cord in diabetic neuropathy using an animal model of high-fat diet-induced prediabetes. We found that prediabetic wild-type mice exhibited tactile allodynia and thermal hypoalgesia after a 16-wk high-fat diet, relative to normal diet-fed wild-type mice. Furthermore, prediabetic wild-type mice exhibited increased tactile allodynia and thermal hypoalgesia at 24 wk relative to 16 wk. Such phenomena were correlated with increased expression and activation of NR2B subunit of NMDARs, as well as Fyn-NR2B interaction in the spinal cord. Fyn−/− mice developed prediabetes after 16-wk high-fat diet treatment and exhibited thermal hypoalgesia, without showing tactile allodynia or altered expression and activation of NR2B subunit, relative to normal diet-fed Fyn−/− mice. Finally, intrathecal administrations of Ro 25-6981 (selective NR2B subunit-containing NMDAR antagonist) dose-dependently alleviated tactile allodynia, but not thermal hypoalgesia, at 16 and 24 wk in prediabetic wild-type mice. Our results suggested that Fyn-mediated NR2B signaling plays a critical role in regulation of prediabetic neuropathy and that the increased expression/function of NR2B subunit-containing NMDARs may contribute to the progression of neuropathy in type 2 diabetes.
NEW & NOTEWORTHY
Our study is the first to report that Fyn kinase is critical for the development of tactile allodynia in the state of prediabetes. Furthermore, the increased expression/function of NR2B subunit-containing N-methyl-d-aspartate receptors in the spinal cord may contribute to the progression of neuropathy in type 2 diabetes.
one of the most common complications of diabetes mellitus is diabetic neuropathy, which affects nerve fibers of the peripheral nervous system. Diabetic neuropathy occurs in more than 50% of adult diabetic patients (Boulton et al. 2005). Patients with diabetic neuropathy often experience hyperalgesia or allodynia, which are accompanied by excessive sensitivity to nociceptive stimuli, or may perceive normal stimuli as painful stimuli (Maritim et al. 2003; Obrosova 2009; Yasuda et al. 2003). Despite extensive studies to determine the neurobiological mechanisms underlying diabetic neuropathy, it remains a challenge for the development of successful therapy for diabetic neuropathy (Habib and Brannagan 2010).
Previous studies have illustrated that the spinal cord dorsal horn is critical in the perception of nociceptive signals in diabetic neuropathy (Chen and Pan 2003). It has been proposed that central sensitization can occur in the dorsal horn of the spinal cord after inflammation or nerve injury, which may result in increased primary afferents, neuroplasticity in the spinal cord, and hyperactivity of dorsal horn neurons in the spinal cord in diabetic neuropathic pain (Burchiel et al. 1985; Chen and Levine 2001; Chen and Pan 2002; Khan et al. 2002). One of the putative mechanisms underlying the hyperactivity of dorsal horn neurons in the spinal cord may involve N-methyl-d-aspartate receptors (NMDARs). In support of this, administration of NMDA induces a greater increase in calcium influx in spinal lamina II neurons in nerve-ligated rats relative to control rats (Isaev et al. 2000). Consistent with this, NMDAR antagonism remarkably reduces evoked responses of dorsal horn neurons in spinal nerve-ligated rats (Suzuki et al. 2001). Such an effect might involve NR2B subunit-containing NMDARs, since extensive evidence has shown that spinal NR2B subunit-containing NMDARs play a critical role in nociceptive processing and pathological pain (Meller and Gebhart 1993; Scholz and Woolf 2002).
Most animal studies of diabetic neuropathy have been carried out in diabetic rodents prepared by injections of streptozotocin (STZ), a pancreatic β-cell cytotoxin. These rodents develop a syndrome that resembles type 1 diabetes. With this model of diabetes, it has been shown that STZ-induced diabetic rats exhibit mechanical allodynia and hyperalgesia (Bujalska 2008; Ulugol et al. 2004). In addition, type 1 diabetic neuropathy is associated with increased glutamate release from primary afferent terminals in the spinal cord (Li et al. 2010; Wang et al. 2007). Furthermore, the tactile allodynia in STZ-induced diabetic rats is associated with increased expression of NR2B subunits of NMDARs in the spinal cord (Bai et al. 2014). However, it remains unclear whether NMDARs are involved in diabetic neuropathy in type 2 diabetic animals. Given that type 2 diabetes accounts for ∼90–95% of all diagnosed cases of diabetes in adult humans (Campbell et al. 2009; Mohamadi and Cooke 2010) and animal studies have demonstrated diabetic neuropathy in obesity-associated prediabetic animals (Oltman et al. 2008; Otto et al. 2011; Romanovsky et al. 2008), it will be important to examine the role of NMDARs in prediabetic neuropathy.
NMDA receptor function is critically regulated by Src-family tyrosine kinases (Wang and Salter 1994; Yu et al. 1997). In the spinal cord, NR2A and NR2B are the predominant subunits. NR2B subunits are highly expressed in the superficial dorsal horn of the spinal cord (Boyce et al. 1999; Momiyama 2000). Importantly, both NR2A and NR2B subunits can be tyrosine phosphorylated in the central nervous system (Moon et al. 1994). A previous study has demonstrated that NR2B-containing NMDARs in the spinal cord are responsible for the maintenance of chemically induced neuropathic pain and that NR2B phosphorylation at Tyr1472 by Fyn kinase is essential for the maintenance of neuropathic pain (Abe et al. 2005). However, few studies have been conducted to examine the role of Fyn kinase in prediabetic neuropathy. Therefore, the present study was designed to evaluate the role of Fyn kinase and NMDARs in the spinal cord in prediabetic neuropathy with an animal model of high-fat diet-induced prediabetes.
MATERIALS AND METHODS
Animals.
Fyn wild-type C57BL6/SV129 mice and Fyn-knockout (Fyn−/−) mice (C57BL6/SV129 background) were obtained from Jackson Laboratory (Bar Harbor, ME). Wild-type and knockout mice were littermates bred in house from heterozygous parents. Only male mice were used in the experiments. Mice were housed in groups of four in a temperature- and humidity-controlled vivarium in the Animal Center of Shandong Provincial Hospital Affiliated to Shandong University under a 12:12-h light-dark cycle and were given food and water ad libitum. All animal experiments were approved by the Institutional Animal Care and Use Committee of Shandong Provincial Hospital Affiliated to Shandong University. The housing and treatment of the rats followed the guidelines of the Guide for the Care and Use of Laboratory Rats (Institute of Laboratory Animal Resources, 2011).
High-fat diet-induced prediabetes.
Male wild-type or Fyn−/− mice, weighing 23–25 g at the age of 8 wk, were fed with normal mouse chow (PMI Nutrition International, Brentwood, MO) or high-fat diet (D12330 formula, 58kcal% fat with corn starch, Research Diets, New Brunswick, NJ) and had ad libitum access to water for 16 wk or 24 wk. After 16 wk on either normal mouse chow or high-fat diet, mice were then randomly assigned to separate groups for subsequent biochemical experiments or behavioral experiments, based on body weight, blood glucose concentration, and diet type. Some mice were used for spinal cord tissue collection after euthanization at 16 wk or 24 wk on normal diet or high-fat diet treatment. Separate groups of mice were behaviorally tested for tactile responses to flexible von Frey filaments (first day) and for thermal algesia by paw withdrawal test (second day) at 16 wk or 24 wk.
Blood glucose and weight monitoring.
Weight and blood glucose measurements (glucose diagnostic reagents; Sigma, St. Louis, MO) were collected after the first week of high-fat diet treatment and at 16 and 24 wk. Mice were fasted for 3 h prior to collection of blood collection from the tail. While most of the studies used a benchmark of blood glucose between 11.1 and 16.6 mmol/l (200–300 mg/dl) as the diabetic condition, the prediabetic mice usually had blood glucose ranging around 8.0–9.4 mmol/l after a 3-h fast in our study, which is slightly higher than mice with normal diet (7.0–7.8 mmol/l) but is significantly lower than the level of the diabetic condition. This range of blood glucose (8.0–9.4 mmol/l) was defined as the prediabetic condition in the present study and was consistent with some of the previous studies on high-fat diet-induced prediabetes and obesity (Obrosova et al. 2007; Watcho et al. 2010; Winzell and Ahren 2004).
Intrathecal administration.
Intrathecal administrations of vehicle (i.e., 1% DMSO in phosphate-buffered saline) or Ro 25-6981, a selective NR2B subunit-containing NMDAR antagonist, were conducted through the intervertebral space in unanesthetized mice between L5 and L6 of the spinal cord, as described previously (Hylden and Wilcox 1980). In brief, a volume of 5 μl was administered intrathecally with a 26-gauge stainless steel needle (Harvard Apparatus, Shanghai, China) connected to a 100-μl Hamilton microsyringe, the animal being lightly restrained to maintain the position of the needle during the injection. A slight flick of the tail was used as a sign of the puncture of the dura. Five minutes after the injections, mice behavioral tests were conducted. The doses of Ro 25-6981 were selected based on the literature (Gabra et al. 2007).
Behavioral tests.
All behavioral testing was conducted at 16 wk or 24 wk on normal diet or high-fat diet treatment in wild-type mice (n = 40) or Fyn−/− mice (n = 40). Each mouse received two behavioral tests. Prior to behavioral testing, mice were acclimated to the behavioral apparatus and equipment for a minimum of 2 days. On test days, mice were placed in the behavioral apparatus and allowed to acclimate to the environment for 30 min. To test mouse tactile response, a von Frey assay was conducted. Mice were placed in a clear plastic cage on top of a wire mesh grid that allowed access to their hind paws for the duration of the analysis. Mechanical withdrawal thresholds with von Frey monofilaments were measured to track the progression of neuropathy and evaluate the effects of acute intrathecal administration of Ro 25-6981 on neuropathy, using the up-down method to determine 50% withdrawal thresholds (Carter and Shieh 2010; Kruger 2001). The next day after Von Frey assay, a hot plate test was also performed to track progression of thermal sensitivity and measure the effects of acute intrathecal administration of Ro 25-6981 on thermal sensitivity. The mice were placed on a hot plate maintained at 55°C, and the latency for licking of the front or hind paws was monitored with a video camera and recorded on videotape. Mechanical withdrawal thresholds and latency time were analyzed in a blinded manner by two examiners.
Western blot analysis.
To examine the role of Fyn-mediated NMDAR signaling cascade in spinal cord of mice with prediabetic neuropathy, the spinal cord lumbar enlargement of wild-type mice (n = 40) or Fyn−/− mice (n = 40) was removed under deep anesthesia (pentobarbital, 60 mg/kg ip) and spinal cord tissues were collected from each mouse at 16 wk or 24 wk in wild-type or Fyn−/− mice and were then separately homogenized with a glass Dounce homogenizer in ice-cold lysis buffer containing 10 mM HEPES, 1% SDS, 11% sucrose, and 1× protease and phosphatase inhibitor cocktails (Sigma-Aldrich, St. Louis, MO). The homogenates were centrifuged (20 min at 50,000 rpm) and stored at −80°C. Tissue samples were then boiled in a dry heat block at 100°C for 10 min. The protein concentration of samples was determined with the BioRad DC Protein Assay kit. Proteins were separated on 7.5% Tris·HCl polyacrylamide gel and transferred to a polyvinylidene difluoride membrane (Millipore, Billerica, MA) for 1 h at 100 V. Membranes were then blocked in 5% milk for 1 h and incubated with rabbit anti-phospho-NMDA NR2B polyclonal antibody (no. M2442, pTyr1472, 1:1,000; Sigma-Aldrich, Shanghai, China) or rabbit anti-Fyn polyclonal antibody (no. HPA023887, 1:1,000; Sigma-Aldrich, Shanghai, China) overnight (16–20 h) at 4°C. Membranes were incubated in donkey anti-rabbit polyclonal secondary antibody (1:10,000, GE Healthcare, Piscataway, NJ) conjugated to horseradish peroxidase (HRP) for 1 h, followed by development with an enhanced chemiluminescence system (Pierce Biotech, Rockford, IL). Membranes were washed with stripping buffer (62.5 mM Tris·HCl at pH 6.7, 2% SDS, 100 mM beta-mercaptoethanol) to permit reprobing with antibodies to total NR2B (no. M265, 1:1,000; Sigma-Aldrich, Shanghai, China) and actin-HRP (i.e., loading control; 1:50,000, Santa Cruz Biotechnology, Shanghai, China). pNR2B, total NR2B, and actin protein levels were quantified by densitometry with National Institutes of Health ImageJ software. The discussion of NR2B activation below refers to normalized pNR2B/total NR2B levels.
Coimmunoprecipitation and immunoblotting.
To study physical interaction between Fyn kinase and NR2B, coimmunoprecipitation assays were conducted by immunoprecipitation of the spinal cord samples with mouse monoclonal anti-Fyn antibody followed by probe of the blots with the antibody against NR2B in separate groups of wild-type mice (n = 40). Specifically, spinal cord samples of each mouse were lysed in ice-cold NP-40 buffer (1% IGEPAL, 150 mM NaCl, 10 mM sodium phosphate, 2 mM EDTA, pH = 7.2). The total lysate (80–100 μg of protein) was incubated with mouse monoclonal anti-Fyn antibody (no. ab1881, 1:1,000; Abcam, Shanghai, China) at 4°C for 1 h, followed by precipitation with protein G-Sepharose beads (50 μl) for 30 min at 4°C. The immunocomplexes were then washed and resuspended in sample buffer. Samples were boiled for 5 min, subjected to SDS-PAGE, and immunoblotted with anti-NR2B antibodies (no. M265, 1:1,000; Sigma-Aldrich, Shanghai, China). HRP-conjugated secondary antibody (1:10,000; GE Healthcare) was used. Immunoreactive bands were detected and protein levels were quantified with the methods described above.
Statistical analysis.
Data are expressed as means ± SE. Data were analyzed with one-way or mixed-factorial analyses of variance (ANOVAs), where appropriate. Significant ANOVA main and interaction effects were further investigated with Tukey post hoc tests, when appropriate. α was set at 0.05.
RESULTS
Body weights, blood glucose levels, and food intake.
After high-fat diet treatment, body weight, blood glucose level, and food intake were altered similarly in wild-type and Fyn−/− mice (Table 1). With high-fat diet treatment, body weight gains in prediabetic groups increased over time. Specifically, after the first week of high-fat diet treatment, both wild-type and Fyn−/− mice exhibited slightly increased gains of body weight and food intake, but not blood glucose, compared with mice with normal diet. At 16 or 24 wk of high-fat diet treatment, mice exhibited higher body weight gains relative to normal diet control mice (Table 1). In addition, food intake (1 day) in high-fat diet-fed groups was significantly increased compared with normal diet control mice (Table 1). Furthermore, at 16 or 24 wk of high-fat diet treatment, the levels of blood glucose in all prediabetic mice were enhanced relative to normal diet control mice (Table 1).
Table 1.
Body weight, 1-day food intake, and blood glucose concentration
| Normal Diet Group |
High-Fat Diet Group |
||||
|---|---|---|---|---|---|
| Weeks of Treatment | Wild type | Fyn−/− | Wild type | Fyn−/− | |
| 1 | Body wt, g | 24.1 ± 0.3 | 24.0 ± 0.5 | 28.8 ± 0.6* | 28.5 ± 0.4* |
| Food intake, g | 3.1 ± 0.6 | 3.2 ± 0.5 | 4.6 ± 0.4* | 4.5 ± 0.3* | |
| Blood glucose, mmol/l | 7.4 ± 0.4 | 7.4 ± 0.3 | 7.5 ± 0.3 | 7.5 ± 0.6 | |
| 16 | Body wt, g | 36.3 ± 0.6 | 36.0 ± 0.9 | 56.1 ± 1.2* | 55.5 ± 1.8* |
| Food intake, g | 3.1 ± 0.4 | 3.3 ± 0.6 | 6.4 ± 0.4* | 6.8 ± 0.3* | |
| Blood glucose, mmol/l | 7.4 ± 0.5 | 7.6 ± 0.6 | 8.9 ± 0.5* | 8.6 ± 0.3* | |
| 24 | Body wt, g | 37.1 ± 2.1 | 37.7 ± 2.5 | 57.4 ± 2.1* | 56.7 ± 1.7* |
| Food intake, g | 3.7 ± 0.4 | 3.3 ± 0.2 | 6.9 ± 0.2* | 7.1 ± 0.2* | |
| Blood glucose, mmol/l | 7.4 ± 0.6 | 7.4 ± 0.5 | 9.0 ± 0.3* | 8.9 ± 0.6* | |
Data are expressed as means ± SE; n = 10–33 per group.
Significant difference relative to normal diet group (P < 0.05).
High-fat diet treatment results in tactile allodynia and thermal hypoalgesia in wild-type mice.
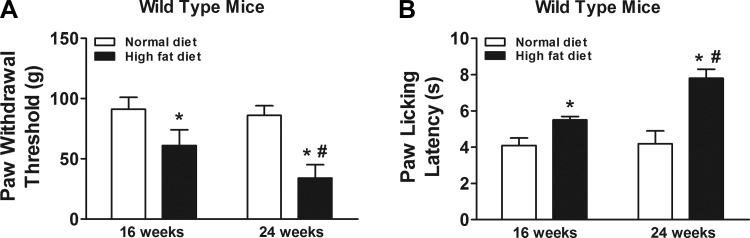
Prediabetic mice exhibited tactile allodynia and thermal hypoalgesia after high-fat diet treatment at 16 and 24 wk compared with normal diet control mice (Fig. 1). Additionally, tactile allodynia and thermal hypoalgesia became more severe in mice at 24 wk of high-fat diet treatment compared with 16 wk of high-fat diet treatment (Fig. 1).
Fig. 1.
Effects of high-fat diet treatment on prediabetic neuropathy in wild-type mice. Prediabetic mice exhibited tactile allodynia (A) and thermal hypoalgesia (B) (n = 10/group). Mice were fed with normal diet or high-fat diet for 16 wk and were behaviorally tested for tactile responses to flexible von Frey filaments (first day) and for thermal algesia by paw withdrawal test (second day) at 16 wk or 24 wk. *Significant difference relative to normal diet control (P < 0.05); #significant difference relative to 16 wk (P < 0.05).
High-fat diet treatment results in alterations of Fyn-mediated NR2B activation in spinal cord in wild-type mice.
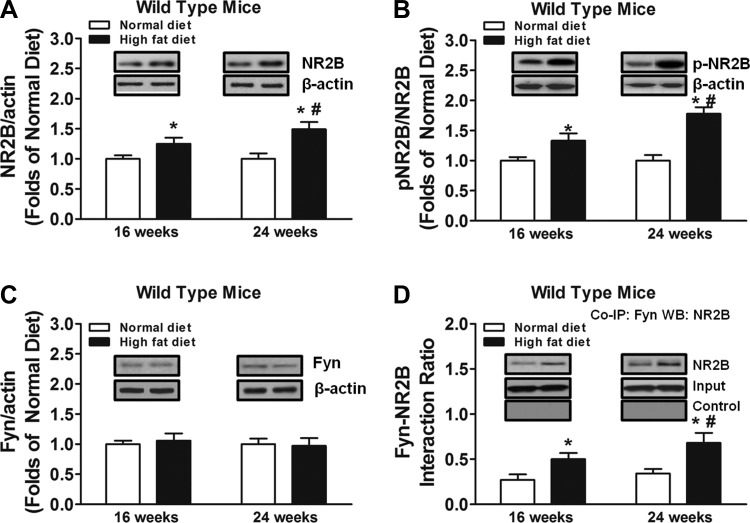
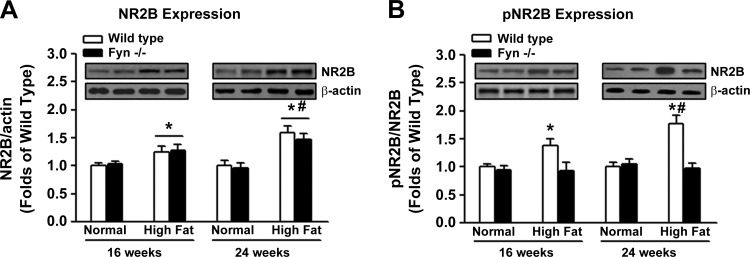
We found that high-fat diet treatment increased NR2B subunit expression in the spinal cord of wild-type mice at 16 and 24 wk compared with normal diet control mice (Fig. 2A). Furthermore, NR2B subunit expression in the spinal cord was enhanced at 24 wk of high-fat diet treatment compared with 16 wk (Fig. 2A). Similar patterns of changes in NR2B phosphorylation at Tyr1472 were also observed (Fig. 2B), resulting in increased NR2B activation (i.e., p-NR2B-to-NR2B ratio) over time in wild-type mice treated with high-fat diet (Fig. 2B). In contrast, high-fat diet treatment failed to alter the expression of Fyn kinase in the spinal cord of wild-type mice compared with normal diet treatment (Fig. 2C). However, high-fat diet treatment promoted the interaction of Fyn kinase and NR2B subunit in the spinal cord of wild-type mice compared with mice fed with normal diet (Fig. 2D). This interaction was enhanced at 24 wk of high-fat diet treatment compared with 16 wk (Fig. 2D).
Fig. 2.
Effects of high-fat diet treatment on Fyn-mediated NR2B activation (n = 10/group). To explore the putative mechanisms underlying the effects of high-fat diet treatment on prediabetic neuropathy, spinal cord tissues were collected from each mouse at 16 wk or 24 wk in wild-type mice. Western blot analysis was performed on total NR2B levels (A), pNR2B-to-NR2B ratio (B), and Fyn kinase levels (C). D: to study physical interaction between Fyn kinase and NR2B, coimmunoprecipitation (Co-IP) assays were conducted by immunoprecipitation of the spinal cord samples with mouse monoclonal anti-Fyn antibody, followed by probing the blots with the antibody against NR2B (WB). *Significant difference relative to normal diet control (P < 0.05); #significant difference relative to 16 wk (P < 0.05).
High-fat diet treatment results in thermal hypoalgesia but not tactile allodynia in Fyn−/− mice.
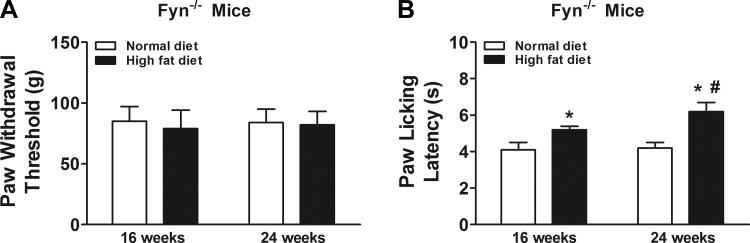
We found that prediabetic Fyn−/− mice exhibited thermal hypoalgesia (Fig. 3B) but not tactile allodynia (Fig. 3A) after high-fat diet treatment for 16 and 24 wk compared with normal diet Fyn−/− mice. Additionally, thermal hypoalgesia became more pronounced in mice at 24 wk of high-fat diet treatment compared with 16 wk of high-fat diet treatment (Fig. 3B).
Fig. 3.
Effects of high-fat diet treatment on prediabetic neuropathy in Fyn−/− mice (n = 10/group). Prediabetic Fyn−/− mice did not exhibit tactile allodynia (A) but showed thermal hypoalgesia (B). Mice were fed with normal diet or high-fat diet for 16 wk and were behaviorally tested for tactile responses to flexible von Frey filaments (first day) and for thermal algesia by paw withdrawal test (second day) at 16 wk or 24 wk. *Significant difference relative to normal diet control (P < 0.05); #significant difference relative to 16 wk (P < 0.05).
High-fat diet treatment enhances NR2B subunit expression in spinal cord in Fyn−/− mice.
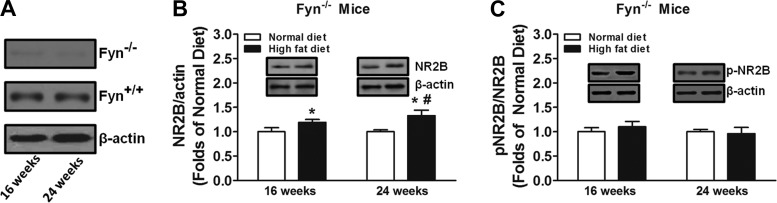
We first verified the levels of Fyn kinase expression in the spinal cord in wild-type and Fyn−/− mice (Fig. 4A). We found that high-fat diet treatment increased NR2B subunit expression in the spinal cord of Fyn−/− mice at 16 and 24 wk compared with normal diet control mice (Fig. 4B). Similar to wild-type mice, NR2B subunit expression in the spinal cord was enhanced at 24 wk of high-fat diet treatment compared with 16 wk in Fyn−/− mice (Fig. 4B). However, high-fat diet treatment did not alter NR2B phosphorylation at Tyr1472 in the spinal cord of Fyn−/− mice over time compared with normal diet control mice (Fig. 4C).
Fig. 4.
Effects of high-fat diet treatment on expression and activation of NR2B in prediabetic Fyn−/− mice (n = 10/group). Western blot analysis was performed on Fyn kinase levels (A), total NR2B levels (B), and pNR2B-to-NR2B ratio (C). *Significant difference relative to normal diet control (P < 0.05); #significant difference relative to 16 wk (P < 0.05).
When we compared the effects of high-fat diet treatment on NR2B expression or activation in the spinal cord of wild-type mice and Fyn−/− mice, we found that high-fat diet increased the expression of NR2B subunit in the spinal cord of both wild-type mice and Fyn−/− mice compared with normal diet treatment (Fig. 5A). Furthermore, such an enhancement of NR2B subunit expression was increased after 24 wk of high-fat diet treatment, compared with 16 wk, in the spinal cord of both wild-type mice and Fyn−/− mice (Fig. 5A). We also found that high-fat diet increased the activation of NR2B subunit (i.e., pNR2B-to-NR2B ratio) in the spinal cord of wild-type mice, but not in Fyn−/− mice, compared with normal diet treatment (Fig. 5B). Furthermore, such an enhancement of NR2B subunit activation in the spinal cord of wild-type mice was increased after 24 wk of high-fat diet treatment compared with 16 wk (Fig. 5B).
Fig. 5.
Comparison of effects of high-fat diet treatment on expression and activation of NR2B subunit in the spinal cord of wild-type and Fyn−/− mice. Western blot analysis was performed on total NR2B levels (A) and pNR2B-to-NR2B ratio (B). *Significant difference relative to normal diet control (P < 0.05); #significant difference relative to 16 wk (P < 0.05).
Intrathecal administration of Ro 25-6981 alleviates high-fat diet-induced tactile allodynia but not thermal hypoalgesia in Fyn−/− mice.
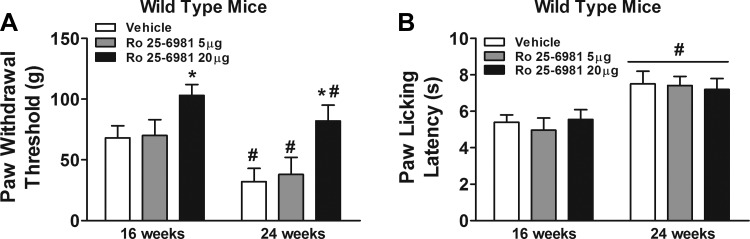
Replicating the findings in wild-type mice, mice receiving intrathecal injections of vehicle exhibited increased tactile allodynia and thermal hypoalgesia following high-fat diet treatment at 16 wk compared with 24 wk (Fig. 6). However, intrathecal injections of 20 μg of Ro 25-6981, but not 5 μg of Ro 25-6981, increased paw withdrawal threshold in wild-type mice relative to vehicle (Fig. 6A). In contrast, intrathecal injections of either dose of Ro 25-6981 did not alter paw licking latency in wild-type mice relative to vehicle (Fig. 6B).
Fig. 6.
Effects of intrathecal administration of Ro 25-6981 on prediabetic neuropathy in wild-type mice. Intrathecal administrations of Ro 25-6981 were conducted through the intervertebral space in unanesthetized mice between L5 and L6 of the spinal cord. Mice were behaviorally tested for tactile responses to flexible von Frey filaments (first day) (A) and for thermal algesia by paw withdrawal test (second day) (B) at 16 wk or 24 wk. *Significant difference relative to vehicle (P < 0.05); #significant difference relative to 16 wk (P < 0.05).
DISCUSSION
Our study is the first to examine the role of Fyn kinase and NR2B-containing NMDARs in prediabetic neuropathy. We found that prediabetic wild-type mice exhibited tactile allodynia and thermal hypoalgesia after 16-wk high-fat diet treatment relative to normal diet-fed wild-type mice. Furthermore, prediabetic wild-type mice exhibited increased tactile allodynia and thermal hypoalgesia at 24 wk relative to 16 wk. These findings are consistent with previous studies (Watcho et al. 2010). Importantly, this phenomenon was correlated with increased expression and Tyr1472 phosphorylation of NR2B subunit of NMDARs as well as Fyn-NR2B interaction in the spinal cord. The Fyn−/− mice developed prediabetes after 16-wk high-fat diet treatment and exhibited thermal hypoalgesia, without showing tactile allodynia or altered expression or Tyr1472 phosphorylation of NR2B subunit, relative to normal diet-fed Fyn−/− mice. Finally, intrathecal administration of Ro 25-6981 dose-dependently alleviated tactile allodynia, but not thermal hypoalgesia, at 16 and 24 wk in prediabetic wild-type mice. Our results suggested that Fyn-mediated NR2B signaling plays a critical role in regulation of prediabetic neuropathy and that the increased expression/function of NR2B-containing NMDARs may contribute to the progression of neuropathy in type 2 diabetes.
While the present study revealed the contributions of Fyn kinase to diabetic neuropathy, others have reported that Fyn kinase is critical for various types of neuropathic pain. For example, nerve injury-induced neuropathic pain disappears in mice lacking Fyn kinase (Abe et al. 2005). Additionally, Fyn kinase inhibition in the spinal cord results in reduction of inflammatory pain induced by intradermal injection of complete Freund's adjuvant (Liu et al. 2014; Yang et al. 2011). Furthermore, expression of constitutively active Fyn mutant alone in the spinal cord of intact mice is sufficient to elicit persistent mechanical allodynia and thermal hyperalgesia (Liu et al. 2014). Adding to this literature, our study is the first to show that Fyn kinase activity in the spinal cord is critical for the development of tactile allodynia, but not thermal hypoalgesia, in mice with prediabetes.
While increased activity of spinal cord NMDARs is involved in nociception induced by nerve ligation and peripheral nerve injury (Roh et al. 2008), our study has also demonstrated that upregulation of spinal cord NMDAR-mediated functions is necessary for the progression of neuropathy in an animal with high-fat diet-induced prediabetes. While various mechanisms may contribute to the alteration of expression and/or function of NMDARs during the course of neuropathy, our study indicated that Fyn kinase might be involved in regulating the functions of NMDARs in neuropathy associated with prediabetes. Specifically, we found that Tyr1472 phosphorylation of NR2B subunit in the spinal cord is enhanced in wild-type mice but not in Fyn−/− mice after high-fat diet treatment. Furthermore, high-fat diet treatment resulted in increased interaction of Fyn kinase and NR2B subunit. Given that Tyr1472 phosphorylation of NR2B subunit is critical for NR2B subunit surface expression and function (VanDongen 2009), these results suggested that Fyn kinase in the spinal cord is necessary for activation of NR2B-containing NMDARs. Consistent with previous studies, it has been shown that spinal dorsal horn neurons exhibit abnormal hyperactivity and increased responses to innocuous stimuli in rats with diabetic neuropathy (Chen and Pan 2002). Thus the increased activation of NR2B-containing NMDAR expression likely contributes to the hyperactivity of spinal cord dorsal horn neurons in prediabetic neuropathy and reflects the progression of neuropathy associated with type 2 diabetes.
On the basis of these findings, it is reasonable to hypothesize that upregulation of NR2B subunit activation in the spinal cord may potentiate the development of prediabetic neuropathy in wild-type mice. While to our knowledge no study has been conducted to examine the effects of upregulation of NR2B subunit expression in the spinal cord on the development of neuropathy in general, our study showed that Fyn−/− mice with increased NR2B expression in the spinal cord did not develop tactile allodynia, suggesting that increased NR2B expression in the spinal cord is not critical for the development of tactile allodynia associated with prediabetes. However, previous studies have shown that spinal NR2B expression or activation is also increased in animal models of spinal cord injury-induced neuropathic pain (Geng et al. 2010; Kim et al. 2012b; Qu et al. 2009). While we are still not clear whether upregulation of NR2B subunit expression in the supraspinal regions is involved in modulation of pain responses associated with prediabetic neuropathy per se, it seems possible that NR2B subunit expression in the spinal cord might be differently involved in various types of neuropathic pain.
In the present study, we also found that high-fat diet treatment resulted in enhanced NR2B subunit expression in the spinal cord of wild-type and Fyn−/− mice. This effect thus seems to be independent of Fyn kinase activity, suggesting that other mechanisms are likely involved in regulating spinal cord NR2B subunit expression in prediabetic neuropathy. In fact, it has been demonstrated that STZ-induced diabetic neuropathy is associated with enhanced expression of NR2B subunit in the spinal cord (Bai et al. 2014). Phosphorylated CREB is essential to maintain the basal level of NMDARs (Lau et al. 2004), and phosphorylated CREB increases expression of NMDARs (Kim et al. 2012a) in cortical neurons. While the level of phosphorylated CREB in the spinal cord in mice with high-fat diet induced prediabetes remains unclear, several studies have demonstrated that phosphorylated CREB level is significantly increased in the spinal cord in STZ-induced diabetic neuropathy (Bai et al. 2014; Dang et al. 2014; Francis et al. 2009). Therefore, future studies will be important to examine the effects of high-fat diet on CREB phosphorylation in the spinal cord.
Interestingly, our study revealed that high-fat diet treatment increases the interaction of Fyn kinase and NR2B subunit but does not alter the expression of Fyn kinase per se. While it is not clear as to the mechanisms that are involved in promoting the interaction of Fyn kinase and NR2B subunit in the present study, it has been reported that protein kinase A (PKA) can regulate Fyn kinase activity. Specifically, stimulation of spinal PKA can enhance the enzymatic activity of Fyn kinase (Yang et al. 2011). Such an effect may promote the phosphorylation of NR2B subunit. To support this hypothesis, it has been shown that PKA activation in the hippocampus disrupts the association of inhibitory scaffolding protein RACK1 (receptor for activated C-kinase 1) with Fyn and NR2B, thus permitting Fyn-dependent tyrosine phosphorylation of NR2B subunit and enhancing NMDAR-mediated synaptic currents (Yaka et al. 2003). Therefore, it will be necessary to determine whether PKA-mediated Fyn/RACK1 interaction is critical for the development of prediabetic neuropathy.
One of the interesting findings in our study was that the function of Fyn kinase and NR2B-containing NMDARs is not required for the development of thermal hypoalgesia in high-fat diet-induced prediabetes. While few studies have focused on hypoalgesia associated with obesity and prediabetes, studies using a STZ-induced type 1 diabetes model have demonstrated that T-type calcium channels are critical for thermal hypoalgesia. Specifically, it has been shown that thermal hyperalgesia in rats with short-term (2–4 wk) STZ-induced diabetes is mediated by upregulation of T-type Ca2+ current in dorsal root ganglion (DRG) neurons. However, in longer-term diabetes (6–8 wk) thermal hyperalgesia is changed to hypoalgesia, which is accompanied by downregulation of T-type Ca2+ current in DRG neurons (Duzhyy et al. 2015; Khomula et al. 2013). Although no studies so far have investigated the effects of high-fat diet treatment on the expression/function of T-type Ca2+ channels in the spinal cord of prediabetic animals, it has been shown that inhibition of T-type calcium channels inhibits high-fat diet-induced weight gain in mice (Uebele et al. 2009).
In summary, our study has confirmed that high-fat diet treatment results in Fyn-mediated NR2B activation in the spinal cord and that NR2B-containing NMDARs in the spinal cord play a critical role in regulation of tactile allodynia associated with high-fat diet-induced prediabetes. Importantly, the increased Fyn-mediated NR2B activation might contribute to the progression of type 2 diabetic neuropathy. Given that prediabetes increases the risk to develop type 2 diabetes and various evidence has suggested that prediabetes can be reversed by regular exercise and healthy diet, the dynamic alterations in Fyn-mediated NR2B activation in the spinal cord during the course of type 2 diabetes should be further elucidated. Furthermore, phosphorylation of NR2A by Fyn kinase can also potentiate NMDAR function (Chen and Leonard 1996; Kohr and Seeburg 1996). While our study focused on Fyn kinase and NR2B subunit, other studies have shown that NR2A subunit is critically involved in neuropathic pain induced by chronic compression of the DRG (Yang et al. 2014). Thus it will be important to examine the role of spinal cord NR2A subunit in the development of prediabetic neuropathy in the future. Such a line of research on the interaction of Fyn kinase and NMDA receptor subunit will help the development of effective pharmacotherapies for prediabetic neuropathy as well as complications associated with type 2 diabetes in general.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.S. and M.Z. conception and design of research; M.S. and P.W. performed experiments; M.S. and P.W. analyzed data; M.S. and P.W. interpreted results of experiments; M.S. and P.W. prepared figures; M.S. and M.Z. drafted manuscript; M.S., P.W., and M.Z. approved final version of manuscript; M.Z. edited and revised manuscript.
REFERENCES
- Abe T, Matsumura S, Katano T, Mabuchi T, Takagi K, Xu L, Yamamoto A, Hattori K, Yagi T, Watanabe M, Nakazawa T, Yamamoto T, Mishina M, Nakai Y, Ito S. Fyn kinase-mediated phosphorylation of NMDA receptor NR2B subunit at Tyr1472 is essential for maintenance of neuropathic pain. Eur J Neurosci 22: 1445–1454, 2005. [DOI] [PubMed] [Google Scholar]
- Bai HP, Liu P, Wu YM, Guo WY, Guo YX, Wang XL. Activation of spinal GABAB receptors normalizes N-methyl-d-aspartate receptor in diabetic neuropathy. J Neurol Sci 341: 68–72, 2014. [DOI] [PubMed] [Google Scholar]
- Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, Malik RA, Maser RE, Sosenko JM, Ziegler D. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care 28: 956–962, 2005. [DOI] [PubMed] [Google Scholar]
- Boyce S, Wyatt A, Webb JK, O'Donnell R, Mason G, Rigby M, Sirinathsinghji D, Hill RG, Rupniak NM. Selective NMDA NR2B antagonists induce antinociception without motor dysfunction: correlation with restricted localisation of NR2B subunit in dorsal horn. Neuropharmacology 38: 611–623, 1999. [DOI] [PubMed] [Google Scholar]
- Bujalska M. Effect of cannabinoid receptor agonists on streptozotocin-induced hyperalgesia in diabetic neuropathy. Pharmacology 82: 193–200, 2008. [DOI] [PubMed] [Google Scholar]
- Burchiel KJ, Russell LC, Lee RP, Sima AA. Spontaneous activity of primary afferent neurons in diabetic BB/Wistar rats. A possible mechanism of chronic diabetic neuropathic pain. Diabetes 34: 1210–1213, 1985. [DOI] [PubMed] [Google Scholar]
- Campbell RK, Neumiller JJ, White J, Sisson E, Kuhn C. Type 2 diabetes: epidemiology and treatment, pathophysiology, new therapeutics, and the evolving role of the pharmacist. J Am Pharm Assoc (2003) 49, Suppl 1: S2, 2009. [DOI] [PubMed] [Google Scholar]
- Carter M, Shieh JC. Guide to Research Techniques in Neuroscience. Amsterdam: Elsevier/Academic, 2010. [Google Scholar]
- Chen C, Leonard JP. Protein tyrosine kinase-mediated potentiation of currents from cloned NMDA receptors. J Neurochem 67: 194–200, 1996. [DOI] [PubMed] [Google Scholar]
- Chen SR, Pan HL. Hypersensitivity of spinothalamic tract neurons associated with diabetic neuropathic pain in rats. J Neurophysiol 87: 2726–2733, 2002. [DOI] [PubMed] [Google Scholar]
- Chen SR, Pan HL. Spinal GABAB receptors mediate antinociceptive actions of cholinergic agents in normal and diabetic rats. Brain Res 965: 67–74, 2003. [DOI] [PubMed] [Google Scholar]
- Chen X, Levine JD. Hyper-responsivity in a subset of C-fiber nociceptors in a model of painful diabetic neuropathy in the rat. Neuroscience 102: 185–192, 2001. [DOI] [PubMed] [Google Scholar]
- Dang JK, Wu Y, Cao H, Meng B, Huang CC, Chen G, Li J, Song XJ, Lian QQ. Establishment of a rat model of type II diabetic neuropathic pain. Pain Med 15: 637–646, 2014. [DOI] [PubMed] [Google Scholar]
- Duzhyy DE, Viatchenko-Karpinski VY, Khomula EV, Voitenko NV, Belan PV. Upregulation of T-type Ca2+ channels in long-term diabetes determines increased excitability of a specific type of capsaicin-insensitive DRG neurons. Mol Pain 11: 29, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis G, Martinez J, Liu W, Nguyen T, Ayer A, Fine J, Zochodne D, Hanson LR, Frey WH 2nd, Toth C. Intranasal insulin ameliorates experimental diabetic neuropathy. Diabetes 58: 934–945, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gabra BH, Kessler FK, Ritter JK, Dewey WL, Smith FL. Decrease in N-methyl-d-aspartic acid receptor-NR2B subunit levels by intrathecal short-hairpin RNA blocks group I metabotropic glutamate receptor-mediated hyperalgesia. J Pharmacol Exp Ther 322: 186–194, 2007. [DOI] [PubMed] [Google Scholar]
- Geng SJ, Liao FF, Dang WH, Ding X, Liu XD, Cai J, Han JS, Wan Y, Xing GG. Contribution of the spinal cord BDNF to the development of neuropathic pain by activation of the NR2B-containing NMDA receptors in rats with spinal nerve ligation. Exp Neurol 222: 256–266, 2010. [DOI] [PubMed] [Google Scholar]
- Habib AA, Brannagan TH 3rd. Therapeutic strategies for diabetic neuropathy. Curr Neurol Neurosci Rep 10: 92–100, 2010. [DOI] [PubMed] [Google Scholar]
- Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol 67: 313–316, 1980. [DOI] [PubMed] [Google Scholar]
- Isaev D, Gerber G, Park SK, Chung JM, Randik M. Facilitation of NMDA-induced currents and Ca2+ transients in the rat substantia gelatinosa neurons after ligation of L5-L6 spinal nerves. Neuroreport 11: 4055–4061, 2000. [DOI] [PubMed] [Google Scholar]
- Khan GM, Chen SR, Pan HL. Role of primary afferent nerves in allodynia caused by diabetic neuropathy in rats. Neuroscience 114: 291–299, 2002. [DOI] [PubMed] [Google Scholar]
- Khomula EV, Viatchenko-Karpinski VY, Borisyuk AL, Duzhyy DE, Belan PV, Voitenko NV. Specific functioning of Cav3.2 T-type calcium and TRPV1 channels under different types of STZ-diabetic neuropathy. Biochim Biophys Acta 1832: 636–649, 2013. [DOI] [PubMed] [Google Scholar]
- Kim JH, Roberts DS, Hu Y, Lau GC, Brooks-Kayal AR, Farb DH, Russek SJ. Brain-derived neurotrophic factor uses CREB and Egr3 to regulate NMDA receptor levels in cortical neurons. J Neurochem 120: 210–219, 2012a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Cho HY, Ahn YJ, Kim J, Yoon YW. Effect of NMDA NR2B antagonist on neuropathic pain in two spinal cord injury models. Pain 153: 1022–1029, 2012b. [DOI] [PubMed] [Google Scholar]
- Kohr G, Seeburg PH. Subtype-specific regulation of recombinant NMDA receptor-channels by protein tyrosine kinases of the src family. J Physiol 492: 445–452, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger L. Methods in Pain Research. Boca Raton, FL: CRC, 2001. [Google Scholar]
- Lau GC, Saha S, Faris R, Russek SJ. Up-regulation of NMDAR1 subunit gene expression in cortical neurons via a PKA-dependent pathway. J Neurochem 88: 564–575, 2004. [DOI] [PubMed] [Google Scholar]
- Li JQ, Chen SR, Chen H, Cai YQ, Pan HL. Regulation of increased glutamatergic input to spinal dorsal horn neurons by mGluR5 in diabetic neuropathic pain. J Neurochem 112: 162–172, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YN, Yang X, Suo ZW, Xu YM, Hu XD. Fyn kinase-regulated NMDA receptor- and AMPA receptor-dependent pain sensitization in spinal dorsal horn of mice. Eur J Pain 18: 1120–1128, 2014. [DOI] [PubMed] [Google Scholar]
- Maritim AC, Sanders RA, Watkins JB 3rd. Diabetes, oxidative stress, antioxidants: a review. J Biochem Mol Toxicol 17: 24–38, 2003. [DOI] [PubMed] [Google Scholar]
- Meller ST, Gebhart GF. Nitric oxide (NO) and nociceptive processing in the spinal cord. Pain 52: 127–136, 1993. [DOI] [PubMed] [Google Scholar]
- Mohamadi A, Cooke DW. Type 2 diabetes mellitus in children and adolescents. Adolesc Med State Art Rev 21: 103–119, 2010. [PubMed] [Google Scholar]
- Momiyama A. Distinct synaptic and extrasynaptic NMDA receptors identified in dorsal horn neurones of the adult rat spinal cord. J Physiol 523: 621–628, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon IS, Apperson ML, Kennedy MB. The major tyrosine-phosphorylated protein in the postsynaptic density fraction is N-methyl-d-aspartate receptor subunit 2B. Proc Natl Acad Sci USA 91: 3954–3958, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrosova IG. Diabetes and the peripheral nerve. Biochim Biophys Acta 1792: 931–940, 2009. [DOI] [PubMed] [Google Scholar]
- Obrosova IG, Ilnytska O, Lyzogubov VV, Pavlov IA, Mashtalir N, Nadler JL, Drel VR. High-fat diet induced neuropathy of pre-diabetes and obesity: effects of “healthy” diet and aldose reductase inhibition. Diabetes 56: 2598–2608, 2007. [DOI] [PubMed] [Google Scholar]
- Oltman CL, Davidson EP, Coppey LJ, Kleinschmidt TL, Lund DD, Adebara ET, Yorek MA. Vascular and neural dysfunction in Zucker diabetic fatty rats: a difficult condition to reverse. Diabetes Obes Metab 10: 64–74, 2008. [DOI] [PubMed] [Google Scholar]
- Otto KJ, Wyse BD, Cabot PJ, Smith MT. Longitudinal study of painful diabetic neuropathy in the Zucker diabetic fatty rat model of type 2 diabetes: impaired basal G-protein activity appears to underpin marked morphine hyposensitivity at 6 months. Pain Med 12: 437–450, 2011. [DOI] [PubMed] [Google Scholar]
- Qu XX, Cai J, Li MJ, Chi YN, Liao FF, Liu FY, Wan Y, Han JS, Xing GG. Role of the spinal cord NR2B-containing NMDA receptors in the development of neuropathic pain. Exp Neurol 215: 298–307, 2009. [DOI] [PubMed] [Google Scholar]
- Roh DH, Kim HW, Yoon SY, Seo HS, Kwon YB, Han HJ, Beitz AJ, Lee JH. Depletion of capsaicin-sensitive afferents prevents lamina-dependent increases in spinal N-methyl-d-aspartate receptor subunit 1 expression and phosphorylation associated with thermal hyperalgesia in neuropathic rats. Eur J Pain 12: 552–563, 2008. [DOI] [PubMed] [Google Scholar]
- Romanovsky D, Walker JC, Dobretsov M. Pressure pain precedes development of type 2 disease in Zucker rat model of diabetes. Neurosci Lett 445: 220–223, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz J, Woolf CJ. Can we conquer pain? Nat Neurosci 5, Suppl: 1062–1067, 2002. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Matthews EA, Dickenson AH. Comparison of the effects of MK-801, ketamine and memantine on responses of spinal dorsal horn neurones in a rat model of mononeuropathy. Pain 91: 101–109, 2001. [DOI] [PubMed] [Google Scholar]
- Uebele VN, Gotter AL, Nuss CE, Kraus RL, Doran SM, Garson SL, Reiss DR, Li Y, Barrow JC, Reger TS, Yang ZQ, Ballard JE, Tang C, Metzger JM, Wang SP, Koblan KS, Renger JJ. Antagonism of T-type calcium channels inhibits high-fat diet-induced weight gain in mice. J Clin Invest 119: 1659–1667, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulugol A, Karadag HC, Ipci Y, Tamer M, Dokmeci I. The effect of WIN 55,212-2, a cannabinoid agonist, on tactile allodynia in diabetic rats. Neurosci Lett 371: 167–170, 2004. [DOI] [PubMed] [Google Scholar]
- VanDongen AM. Biology of the NMDA Receptor. Boca Raton, FL: CRC, 2009. [Google Scholar]
- Wang XL, Zhang HM, Chen SR, Pan HL. Altered synaptic input and GABAB receptor function in spinal superficial dorsal horn neurons in rats with diabetic neuropathy. J Physiol 579: 849–861, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YT, Salter MW. Regulation of NMDA receptors by tyrosine kinases and phosphatases. Nature 369: 233–235, 1994. [DOI] [PubMed] [Google Scholar]
- Watcho P, Stavniichuk R, Ribnicky DM, Raskin I, Obrosova IG. High-fat diet-induced neuropathy of prediabetes and obesity: effect of PMI-5011, an ethanolic extract of Artemisia dracunculus L. Mediators Inflamm 2010: 268547, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzell MS, Ahren B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes 53, Suppl 3: S215–S219, 2004. [DOI] [PubMed] [Google Scholar]
- Yaka R, He DY, Phamluong K, Ron D. Pituitary adenylate cyclase-activating polypeptide [PACAP(1-38)] enhances N-methyl-d-aspartate receptor function and brain-derived neurotrophic factor expression via RACK1. J Biol Chem 278: 9630–9638, 2003. [DOI] [PubMed] [Google Scholar]
- Yang HB, Yang X, Cao J, Li S, Liu YN, Suo ZW, Cui HB, Guo Z, Hu XD. cAMP-dependent protein kinase activated Fyn in spinal dorsal horn to regulate NMDA receptor function during inflammatory pain. J Neurochem 116: 93–104, 2011. [DOI] [PubMed] [Google Scholar]
- Yang L, Gu X, Zhang W, Zhang J, Ma Z. Cdk5 inhibitor roscovitine alleviates neuropathic pain in the dorsal root ganglia by downregulating N-methyl-d-aspartate receptor subunit 2A. Neurol Sci 35: 1365–1371, 2014. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Terada M, Maeda K, Kogawa S, Sanada M, Haneda M, Kashiwagi A, Kikkawa R. Diabetic neuropathy and nerve regeneration. Prog Neurobiol 69: 229–285, 2003. [DOI] [PubMed] [Google Scholar]
- Yu XM, Askalan R, Keil GJ 2nd, Salter MW. NMDA channel regulation by channel-associated protein tyrosine kinase Src. Science 275: 674–678, 1997. [DOI] [PubMed] [Google Scholar]