Abstract
The savannahs of Asia remain locally unrecognized as distinctive ecosystems, and continue to be viewed as degraded forests or seasonally dry tropical forests. These colonial-era legacies are problematic, because they fail to recognize the unique diversity of Asian savannahs and the critical roles of fire and herbivory in maintaining ecosystem health and diversity. In this review, we show that: the palaeo-historical evidence suggests that the savannahs of Asia have existed for at least 1 million years, long before widespread landscape modification by humans; savannah regions across Asia have levels of C4 grass endemism and diversity that are consistent with area-based expectations for non-Asian savannahs; there are at least three distinct Asian savannah communities, namely deciduous broadleaf savannahs, deciduous fine-leafed and spiny savannahs and evergreen pine savannahs, with distinct functional ecologies consistent with fire- and herbivory-driven community assembly. Via an analysis of savannah climate domains on other continents, we map the potential extent of savannahs across Asia. We find that the climates of African savannahs provide the closest analogues for those of Asian deciduous savannahs, but that Asian pine savannahs occur in climates different to any of the savannahs in the southern continents. Finally, we review major threats to the persistence of savannahs in Asia, including the mismanagement of fire and herbivory, alien woody encroachment, afforestation policies and future climate uncertainty associated with the changing Asian monsoon. Research agendas that target these issues are urgently needed to manage and conserve these ecosystems.
This article is part of the themed issue ‘Tropical grassy biomes: linking ecology, human use and conservation’.
Keywords: Asian savannahs, diversity, fire, functional traits, herbivory, tropical dry forest
1. Savannahs in Asia: original or man-made?
There is a long-running debate about whether Asia has natural savannahs or whether Asia's savannahs are derived from forests as the result of long-term management by humans [1–3]. Both biogeography and history have contributed to widespread misperceptions about Asian savannahs. Most people visualize savannahs as vast open grassy landscapes with sparsely scattered trees. This iconic vegetation physiognomy is common in Africa where most savannahs occur in areas that receive less than 700 mm of rainfall per year [4]. In reality, the Earth's savannahs range from grasslands with scattered trees to densely tree-covered woodlands, typically along a gradient of increasing rainfall, with the defining characteristic being that the tree canopy is not closed and the understorey is grassy [4–7]. Tropical Asia is dominated by monsoonal climates with mean annual rainfall of more than 700 mm. Consequently, Asian savannahs support dense and tall woodlands, the physiognomies of which superficially resemble forests rather than the open grassy landscapes that occur widely across Africa.
The history of vegetation classification in the Asian tropics, which is closely tied to its socio-political history, has contributed to a forest-centric legacy in the nomenclature and understanding of Asian vegetation. Formal classifications of the vegetation of South and Southeast Asia originated in the colonial era [8,9], when colonial foresters trained in European forestry traditions were tasked with describing vegetation, generally from the perspective of timber and other extractive uses [2]. As a consequence, most vegetation types with some degree of tree cover in them were described as forests [10,11]. Since then, savannahs with their open tree canopies have been viewed as degraded forests resulting from human use and management [10,12]. This forest-centric perception was further reinforced by the savannahs of Asia occurring in lowland areas, where savannahs have been used and managed by humans for thousands of years [13,14]. Finally, in many of the regions where Asia's savannahs occur, forests and savannahs occur as a mosaic of alternate states within a landscape [5,6,15]. Together, the above factors have culminated in an entrenched ideology of Asian savannahs as ‘degraded forests’ [10–12,16–18].
Tropical savannahs can be defined as mixed tree–grass systems, where the herbaceous layer is usually dominated by C4 grasses that use the C4 photosynthetic pathway to fix carbon, while trees use the ancestral C3 pathway, although there are exceptions such as parts of the South American cerrado, where the herbaceous layer is dominated by C3 grasses [1,2,4,7]. Several lines of evidence suggest that savannahs were present in Asia before human arrival and were likely more extensive under past climates. These include fossil evidence for C4 grasses and mammalian herbivores, climates similar to that of other savannahs, functional diversity that reflects selection under fire or mammalian herbivory, high diversity of C4 grasses and the presence of endemic species with life histories adapted to savannah environments. We review the evidence for each of these in §2 and describe the major savannah formations of tropical and sub-tropical Asia in §§3–5. Subsequently, in §6, we explore the potential climate domain and geographical extent of savannahs in Asia by using the climate domains of savannahs in Africa, Australia and South America to predict their distribution. Finally, in §7, we provide an overview of the threats facing savannahs in Asia.
2. The antiquity of savannahs in Asia
Fossil and molecular evidence presented below suggest that savannahs across Asia, and the savannah-adapted species associated with them, mostly existed before 1 Ma, placing their existence prior to human modifications of the landscape, which began with the widespread use of fire by modern humans (Homo sapiens) at about 0.12 Ma [19], and before Homo erectus is thought to have used fire for cooking at 0.77 Ma [20,21].
C4 grassy vegetation became established in different parts of Asia at quite different times [22–24]. The history of Asian savannahs is therefore best understood by considering different regions separately. The three regions we consider here are (electronic supplementary material, figure S1): South Asia, equivalent to the Indian Subcontinent; East Asia, composed of Southeast and South-central China and Hainan Island; and Southeast Asia, composed of the continental region of Indochina and oceanic region of Malesia. These regions are separated by the Tibetan plateau, the Andaman Sea, the forest belt along the border between India and Myanmar, and montane forests along the southern border of China.
In South Asia, evidence from Himalayan Siwalik sediments suggests that C4 vegetation and C4 diets were established by about 16–14 Ma [22], but C4 grasses became the dominant vegetation type in the Late Miocene (9.3–6.5 Ma) as indicated by the evidence from palynofloras [25], and from δ13C values in tooth enamel and fossil soils [26–28]. Values of δ13C in carbonate rocks from Kudankulum, south India, suggest that C4 vegetation had extended across all South Asia by the Late Miocene [29]. Late Pleistocene values of δ13C in soil organic matter from the Gangetic plain suggest that the ratio of C3 to C4 plants has increased and decreased in response to glacial cycles, but has remained predominantly C4 throughout that period [30].
Within East Asia, savannahs are presently only recognized in the deep dry valleys of Yunnan and Sichuan provinces in southwest China, but C4 grassy vegetation also occurs in northern China (Chinese Academy of Sciences [31]). Historically, savannahs may have been more widely distributed across East Asia. Fossil evidence suggests that C4 diets first appear in China about 18 Ma, although extensive C4 vegetation, based on palaeosol carbonate δ13C is only recorded from 9 to 7 Ma [22]. The age of savannahs appears to be related both to the global aridification beginning in the Eocene, which created drier environments in northeast China, and the uplift of the Tibetan plateau, which either created or intensified the monsoonal climate in southwestern China at a later date. In northern and eastern China, the switch to C4 diets occurred in the Mid to Late Miocene [22], when gazelles also appeared in the fossil record [32] and herbaceous pollen was widely distributed across northern and eastern China [33]. By contrast, evidence collected on δ13C in fossil teeth and soil carbonate nodules [34], leaf and pollen records from dry valleys in Yunnan [35], and molecular data of an endemic savannah tree species [36], all suggest that the transition to savannah in southwest China took place in the Late Pliocene–Early Pleistocene (4.2–1.2 Ma).
The present extent of savannah in Southeast Asia is probably far smaller than that during past glacial maxima [23,37]. Today savannah physiognomies in Southeast Asia are found in three main areas: Indochina, east Java and the Lesser Sunda Islands northeast of Australia, and the northwest Philippines (Luzon and Mindoro) and scattered parts of Wallacea [38]. These areas are all separated by forests and sea, and today grassland-dependent ruminant species, including bovids (Bos javanicus, Bubalus mindorensis) and several cervids (species in the genera Panolia, Rusa, Hyelaphus) are restricted to these isolated savannah patches and islands across Malesia [39]. These modern disjunct distributions suggest that extant savannah communities were connected during past glaciations when sea levels were low and most areas of Sundaland and parts of Wallacea were connected by land or separated by narrow water straits [23,24,37]. Pollen and megafauna fossil records suggest that Southeast Asia was humid throughout the Miocene and drying began at the end of the Pliocene [23,24,40,41]. Extant savannah fauna and flora likely developed from the Late Pliocene to the present as Sundaland was connected for multiple extended periods during the Pliocene and Pleistocene. Fossil and pollen evidence suggest that by the Middle Pleistocene (less than 0.78 Ma) savannahs may have existed from Myanmar through to Java [23,41]. Recent molecular phylogenies estimate that grass-dependent cervids and bovines diversified in Asia after the start of the Pliocene, and that the species which are today isolated on islands across Southeast Asia diverged during the Pleistocene [42,43], possibly more than 1 Ma.
3. Asian savannahs: formations and environmental drivers
Worldwide understanding of savannahs suggests that they may be driven by water limitation or disturbances such as fire or herbivory, leading to distinctive savannah communities that each contain unique species and functional diversity [44–49]. Based on descriptions of extant vegetation across Asia, we have identified three major savannah types, where grasses form the dominant component of the herbaceous layer under open tree canopies, and which are associated with different tree lineages (table 1; electronic supplementary material, figure S1). Below we briefly describe each savannah type and review data on plant functional traits that suggest whether they are mediated by drought, fire or herbivory.
Table 1.
Dominant savannah formations in Asia. Location, composition and evidence for functional traits related to tolerance of fire, herbivory or drought. Cells with ‘no data’ indicate areas where information is lacking about Asian savannah types.
| formations | deciduous broadleaf savannah |
fine-leafed and spiny savannah | pine savannah | ||
|---|---|---|---|---|---|
| dipterocarp savannah | mixed savannaha | eucalypt savannaha | |||
| locations in Asiab | South, Southeast | South, Southeast, East | Southeast (Lesser Sunda Islands) | South, Southeast, East | South, Southeast, East? |
| dominant tree clades | Dipterocarpaceae | Anacardiaceae, Combretaceae, Lamiaceae, Lythraceae, Fabaceae (Deteriae, Caesalpinioideae) | Myrtaceae | Fabaceae (Mimosoideae), Rhamnaceae, Rubiaceae, Capparaceae, Burseraceae |
Pinaceae, Fagaceae |
| dominant grass clades | Andropogoneae | Andropogoneae | Andropogoneae | Andropogoneae, Chloridoideae | Andropogoneae |
| diverse non-Poaceae herb clades | no data | Fabaceae, Asteraceae, Euphorbiaceae | no data | Fabaceae, Malvaceae, Euphorbiaceae | Cyperaceae, Fabaceae, Xyridaceae, Eriocaulaceae |
| functional traits for fire | thickened bark sub-cutaneous meristems below-ground storage |
thickened bark subcutaneous meristems |
no data | no data | thickened bark subcutaneous meristems grass-stage juveniles below-ground storage underground bud banks |
| functional traits for herbivory | spiny plants along river drainage lines | no data | no data | spiny plants abundant mammal-dispersed fruits |
no data |
| functional traits for drought | deciduous | deciduous | deciduous | small leaves, deciduous | no data |
| references | [50–52] | [50,53–55], K. W. Tomlinson 2014, unpublished data | [38,53,56–59] | [50,54,60–62], W. Eiadthong 2014, unpublished data | |
aDensity of tree cover increases with rainfall.
bImages of savannahs from these regions can be viewed in figure 2a,b.
(a). Deciduous broadleaf savannahs
Tropical deciduous broadleaf savannahs range from tall deciduous dipterocarp savannahs (dominated by six Shorea and Dipterocarpus species [50]), to teak savannahs (dominated by Tectona species) and mixed deciduous savannahs (numerous genera including Anogeissus, Lannea, Hardwickia, Lagerstroemia, Pterocarpus, Tectona, Terminalia, Vitex). These occur across all three regions (East, Southeast and South Asia) [3,50,53,63] where 700 mm < MAP < 2100 mm [53,54,64,65]. A fourth formation dominated by Eucalyptus alba is restricted to the Lesser Sunda Island group to the northeast of Australia [56].
Several lines of evidence suggest that the deciduous broadleaf savannahs are maintained by fire. In these communities, composition is mostly dominated by the fire-adapted C4 lineage Andropogoneae (Heteropogon, Themeda, Cymbopogon, Dichanthium, Bothriochloa) [50,53,55,57,58]. In eastern Indochina, the grass layer of these savannahs is dominated by the herbaceous bamboo genus Vietnamosasa [66], a C3 grass [67], which resprouts and flowers following fire. Deciduous broadleaf savannahs can support frequent fire, although annual fire is probably only due to human management of these landscapes [68]. Ground fires are most common [66,68], although crown fires may occur where taller, upright bamboos can carry fire up to the canopy [66]. The characteristic tree species show functional traits typical of adaptation to fire including thick bark [66], sub-cutaneous meristems and tuberous roots [69]. Woody species rapidly resprout from subterranean stems following fire (KW Tomlinson 2015, personal observation). Saplings are able to tolerate annual low-intensity fires but they may be destroyed by high-intensity fires [66], and it has been suggested that deciduous dipterocarp savannahs convert to semi-evergreen forests where fire has been suppressed (Ashton [3] citing Sarayudh Bunyavejchewin). However, we found no evidence that this has been tested experimentally.
(b). Fine-leafed and spiny savannahs
In western and central South Asia, and much smaller areas of Southeast Asia (Central valley of Myanmar, east Java and the Lesser Sunda Islands) and in the dry interior valleys of China, fine-leafed and spiny savannahs occur in areas that have rainfall 400 mm < MAP < 1000 mm [65]. These communities are usually dominated by deciduous tree species of lower stature and smaller leaf size than found in the deciduous broadleaf savannahs, and numerous species have spines (locally dominant genera include Boswellia, Capparis, Acacia, Dichrostachys, Ziziphus and Catunaregum) [51,53,54,56,70]. Two environmental drivers seem most important for the structure of fine-leafed and spiny savannahs. Firstly, growing-season water stress and aridity select for smaller leaves and lower statured plants, consistent with observations from other low-rainfall savannahs [47]. In addition, the herbaceous community shows increased representation of grasses from the lineage Chloridoideae, but these rarely become dominant [51,59] as they do in arid parts of Africa, Australia and America [71,72]. For the most part, dominant genera are still from the lineage Andropogoneae. Possibly this is because MAP rarely falls lower than 500 mm in Asia (small areas in South Asia), whereas large areas of African and Australian savannahs are found where MAP < 500 mm [65]. Secondly, high soil fertility in these areas [73] increases the quality and palatability of plants for mammalian herbivores, such that herbivores may be the major disturbance agents in these savannahs rather than fire. Numerous species in these savannahs possess spines or have bushy forms known to reduce the rates of herbivory by large mammalian herbivores [74]. It is possible that these areas possess the greatest diversity of large mammals in Asia in much the same way as has been observed in Africa [75,76].
(c). Pine savannahs
Pine savannahs have a patchy distribution across the three regions in Asia, but Pinus species (P. kesiya, P. merkusii, P. roxburghii, P. yunannensis) are shared across these patches [3,50,54], which suggests that they were once more widely spread and connected [3,24]. They are usually found at higher altitude than broadleaf deciduous savannahs [54,60], reflecting a tolerance for cooler conditions. Asian pine savannahs appear to be maintained by fire [61]. Where fire is rare or suppressed, pine savannahs are often invaded by forest species (notably Quercus) [77] and convert to closed-canopy forest over time. The pine species are found over a huge rainfall range of 900 mm < MAP < 3200 mm ([65]; www.gbif.org, accessed 27 March 2016) and are often associated with low-nutrient soils, similar to pine savannahs in North America [78,79]. This physiognomic similarity may indicate that Asian pine savannahs persist where growth rates are slow due to soil nutrient limitation and fire occurs regularly [79]. The herbaceous layer is dominated by fire-adapted Andropogoneae grasses (Cymbopogon, Eulalia, Imperata, Themeda) [61]. Juveniles of some Pinus species develop a grassy stage, where they produce multiple lateral meristems with long photosynthetic needles and keep their apical buds close to the ground. Lateral meristems are embedded in the stem cortex, allowing them to survive fires and increase photosynthetic surface [61]. In this way, juveniles store carbohydrate reserves in their roots that can support subsequent stem elevation above fire heights [46,80]. Adult pine trees have thick bark that protects them from low-intensity fires, which increases in thickness as a response to increased fire frequency [61]. Asian savannah pine species can survive high-intensity fires, sloughing bark where it has been badly damaged by fire and reconnecting cambium to heal the exposed scar [61]. Nevertheless at high fire frequencies, adult pine trees become sparse [60].
The full extent of pine savannahs in Asia may be dramatically under-described in East Asia: the hills of southwest China are draped in Pinus yunnanensis and P. kesiya ‘forest’, where fire has been suppressed as a result of government policy [81,82]. The species are closely related [83] and recent evidence suggests that fire may have been an intricate part of these systems: P. yunnanensis in Yunnan develops grassy stage juveniles where it has been burnt, climate models predict that the region should be fire-prone [81], and a study on regeneration in P. yunnanensis savannahs subjected to wildfire indicate that more than 90% of native trees and shrubs recovered after fires, many resprouting from underground bud banks and that P. yunannensis is itself serotinous [62].
4. Endemism in Asian savannahs
Endemic plants and animals with life-histories characteristic of savannahs are found across the three savannah regions of Asia. Here, we provide some examples for different life forms as available from the literature. Where possible we link these to the three savannah types identified above.
Endemism of C4 grasses in the Asian regions (electronic supplementary material, figure S2), as estimated using the GrassBase database [84] combined with a database of C3/C4 pathways for grass taxa [67], indicates substantial diversity of endemic C4 taxa (electronic supplementary material, figure S2; details in the electronic supplementary material, table S1). Total C4 endemism for each region as a function of total grass diversity in each region is similar to estimates for other regions with large savannahs around the world (electronic supplementary material, figure S2a and table S1), except for East Asia, where the level of C4 endemism is much lower. In all regions, endemic diversity of Andropogoneae far exceeds Chloridoideae (electronic supplementary material, table S1), possibly reflecting the far greater extent of humid than semi-arid savannahs in Asia. Presently, we are not able to link these data to the three identified savannah types.
A recent analysis of the floristics of dry tropical regions across the world [85] suggests that some of the woody floras of savannahs in Asia have assembled by local evolution of lineages from other biomes within Asia. These include the six fire-adapted deciduous dipterocarp species that characterize dry dipterocarp savannahs [50,52], fire-adapted pine species that distinguish Asian pine savannahs [61,77] and Tectona [86]. There are likely substantially more species in the deciduous broadleaf savannahs (e.g. [51]) which possess adaptations to fire. However, presently there is insufficient data to confirm that these species disappear under closed forest conditions, as there are no long-term fire-exclusion experiments in Asia. Some species in the fine-leafed and spiny savannahs may be restricted to Asia (e.g. Acacia leucophloea) but many characteristic species extend beyond Asia (e.g. Dichrostachys cinerea, Ziziphus mauritiana, Acacia nilotica).
Several ruminant mammals are endemic to savannahs in Asia (distribution maps from IUCN Red List of threatened species http://maps.iucnredlist.org). Several Antilopinae (Antilope cervicapra, Gazella bennettii), Bovinae (Boselaphus tragocamelus) and Cervidae (Rusa timorensis) are associated with fine-leafed and spiny savannahs in South and Southeast Asia. Several Bovidae (Bos javanica, Tetracerus quadricornus) and cervidae (Panolia eldii) in South and Southeast Asia are associated with deciduous broadleaf savannahs. Additionally, at least one species uses both fine-leafed and spiny savannahs and deciduous broadleaf savannahs (Axis axis). In the Philippines (Southeast Asia), Cervidae (Rusa alfredi) and Bovinae (Bubalis mindorensis) are grazers [87] that can be found in C4 Imperata and Sorghum grasslands.
Several bird species that are typically grassland-associated are also endemic across Asia. These include buttonquails (Turnix spp.) with endemics in the Philippines, associated with pine savannahs (T. worcesteri, T. ocellatus), and one species in the Lesser Sunda Islands, associated with lowland fine-leafed and spiny savannahs (T. everetti); two bustard species associated with open savannahs with panicoid grasses in South and Southeast Asia (Houbaropsis bengalensis, Sypheotides indicus); three species of francolins (Francolinus francolins, F. pictus and F. pondicerianus) and two courser species (Cursorius coromandelius, Rhinoptilus bitorquatus) associated with fine-leafed and spiny savannahs in South Asia [88]. Two iconic species of South and Southeast Asia, the Indian and Green peafowls (Pavo spp.) prefer deciduous broadleaf savannahs to forests [89].
5. Herb species richness in Asian savannahs
Herb diversity is poorly documented throughout Asian savannahs and is in need of systematic research. Nevertheless, evidence at multiple scales suggests that Asian savannahs house significant herbaceous diversity. In a deciduous broadleaf savannah of South Asia, Sankaran [90] recorded 278 species within grassy communities across a 1000 km2 nature reserve. In a fine-leafed and spiny savannah in South Asia, Singh et al. [91] report that Poaceae was the most diverse group in the understorey with 110 species. In a pine savannah in Southeast Asia, Eiadthong (Wichan Eiadthong 2015, unpublished data, Kasertsart University) recorded 105 herbaceous species in a single 50 × 50 m plot.
At larger scales, species richness of C4 grasses in the Asian regions as estimated using the GrassBase database combined with a database of C3/C4 pathways for grass taxa [67,84], indicates that total C4 diversity in each Asian region as a function of land surface is comparable to values in other major savannah and grassland regions (electronic supplementary material, figure S2b and table S1). In a similar vein to endemism, diversity of Andropogoneae species is substantially greater than for Chloridoideae.
6. Defining the potential climatic envelope of Asian savannahs
Vegetation mapping of the savannahs of Asia is sparse and inconsistent [2]. As a first step towards identifying the geographical extent of Asian savannahs, we mapped their potential climatic envelope within Asia, based on the climate envelopes occupied by African, South American and Australian savannahs. At present, there is insufficient data from Asian savannahs to effectively define their climatic domains based on field observations. Savannahs on different continents have different floristic compositions with different associations with rainfall, fire and herbivory [7,92,93]. Hence, while globally savannahs are structurally similar, their geographical distributions with respect to climate differ [7], and we should expect Asian savannahs to be no different. Consequently, we mapped the potential extent of Asian savannahs based on climatic domains of African, Australian and South American savannahs separately to gauge the most probable geographical distribution of Asian savannahs.
We first delimited the extent of savannah versus non-savannah habitats in Africa, Australia and South America based on previous work (electronic supplementary material, figure S3a and see appendix I for full details of the methods) [4,94]. We then used stochastic gradient boosting [94–97] to statistically assess the distribution of savannahs on each continent based on climate, elevation and edaphic parameters. The predictor variables in the models included mean annual temperature, annual temperature range, mean temperature of the driest quarter, mean annual precipitation (MAP), precipitation of the driest month, precipitation seasonality (PS), potential evapotranspiration, soil N content (soil N), soil clay content (% clay) and elevation. We then used these models to predict the potential distribution of savannahs in Asia based on the climatic envelopes occupied by savannahs on each of these different continents.
Our models recreated the observed distribution of savannahs in Africa with a high degree of accuracy (92.2% of savannah pixels correctly classified) and to a lesser extent, those of South America (72.5% correctly classified) and Australia (68.2% correctly classified; see the electronic supplementary material, figure S3a,b). There are several potential reasons for the observed differences in model performance across continents. First, baseline vegetation maps used to build the models could have differed in their underlying accuracy, with savannah distributions more accurately depicted in vegetation maps of Africa than Australia or South America. Second, environmental and edaphic parameters not considered in our models (e.g. seasonal flooding, soil drainage, acidity, availability of exchangeable bases, aluminium concentrations) might be important determinants of savannah distributions in South America and Australia [7,92,93,98,99]. Finally, savannahs in South America and Australia extend into much wetter regions than in Africa, with some South American savannahs receiving as much as 750 mm more rainfall annually than Africa's wettest savannahs [92]. In these wetter sites, fires are well recognized to play a critical role in extending savannah distributions by maintaining the savannah state under climatic conditions that would otherwise support forests [5,6].
We subsequently used the models developed for each of these continents to predict the potential distribution of savannahs in Asia. Our results suggest that Asia supports larger areas with climates that are analogous to those occupied by African savannahs than either Australian or South American savannahs (figure 1). The African model identified large sections of Asia with very high (more than 0.9; approx. 1.1 × 106 km2) or high (0.75–0.9; approx. 0.9 × 106 km2) probabilities of supporting savannahs, including the central Indian plateau, parts of western India and the drier tracts of Southeast Asia in Myanmar and Thailand (figure 1a). By contrast, the predicted extent of savannahs in Asia that are analogous to Australian and South American savannahs is much lower. Based on the Australian model, 0.3 × 106 km2 and 0.6 × 106 km2 of the Asian continent were identified as potentially supporting savannahs with very high (more than 0.9) or high (0.75–0.9) probabilities (figure 1b), while in the case of the South American model these were 0.1 × 106 km2 and 0.2 × 106 km2, respectively (figure 1c).
Figure 1.
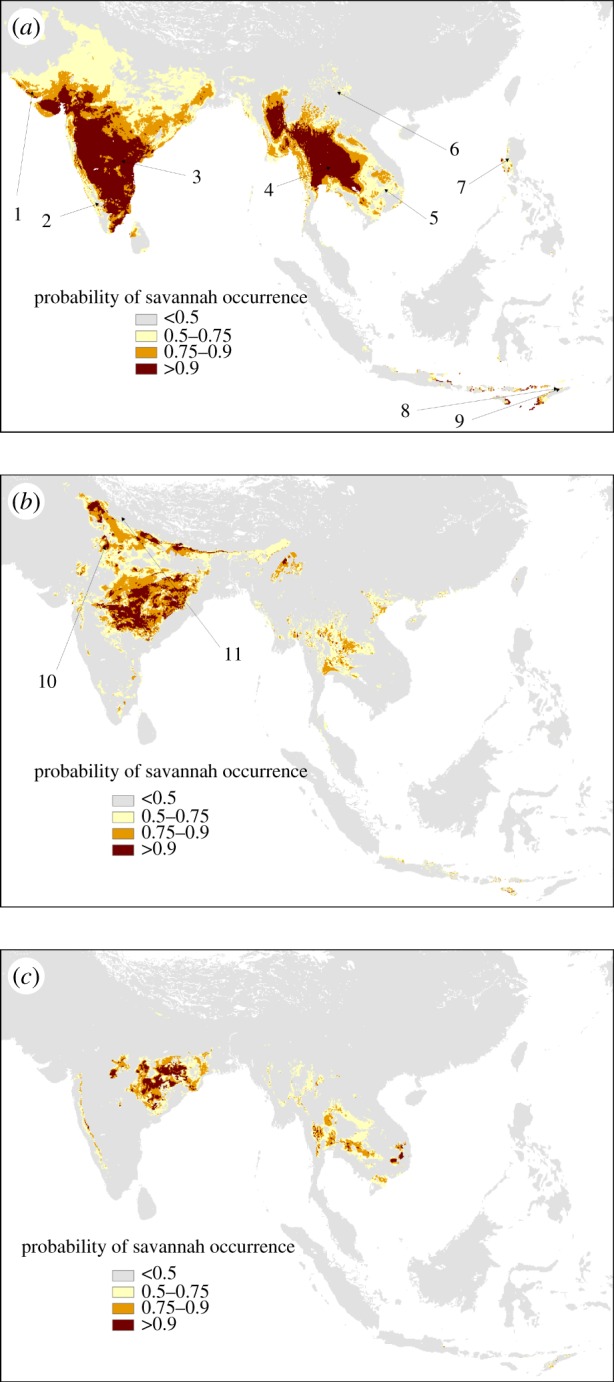
Predicted distributions of savannahs in Asia based on the climate envelope for (a) Africa, (b) Australia and (c) South America. The results suggest that Asia supports larger areas with climates that are similar to those occupied by African savannahs than either Australian or South American savannahs. The numbers shown are known field locations of different Asian savannahs. Images corresponding to these numbers are shown in figure 2.
Interestingly, the predicted occurrence of Asian savannahs based on the climate envelopes of Africa, Australia or South America capture different savannah types in Asia. Areas identified with a high probability of supporting savannahs based on the African model, but not by the Australian or South American models, include the fine-leaved spiny savannahs of western India and the southern Deccan plateau (see [10,100], images in figure 2). By contrast, the areas identified by Australian and South American models correspond largely to the deciduous broadleaf savannahs of the central Indian landscape (see [10,100], figures 1b,c and 2 for images). The Australian model additionally identifies the Terrai grassland and savannah habitats [101] of the Himalayan foothills (figure 1b). Only the African model weakly captures the East Asian savannah formations in Yunnan, China, and the savannahs in Luzon, the Philippines (figure 1a and images in figure 2). Surprisingly, all three models only weakly capture the location of savannahs in the Lesser Sunda Islands, but make different predictions about their locations. It is intriguing that while some of these savannahs are dominated by Australian tree taxa, others are dominated by African and Asian tree taxa [3,56]. Importantly, none of the models were able to predict the extensive pine savannahs in the Himalayas, China, Sumatra and the Philippines [3], suggesting that these savannahs may occupy a unique climate space that is not found in Africa, Australia or South America. Possibly this is because they occupy higher elevations and cooler climates than any of the southern continent savannahs and are analogous to pine savannahs in North America [79], which are also underlain by C4 grasses [102]. Thus, as is the case for the southern continents, the climatic space of Asian savannahs is different from other continents [7].
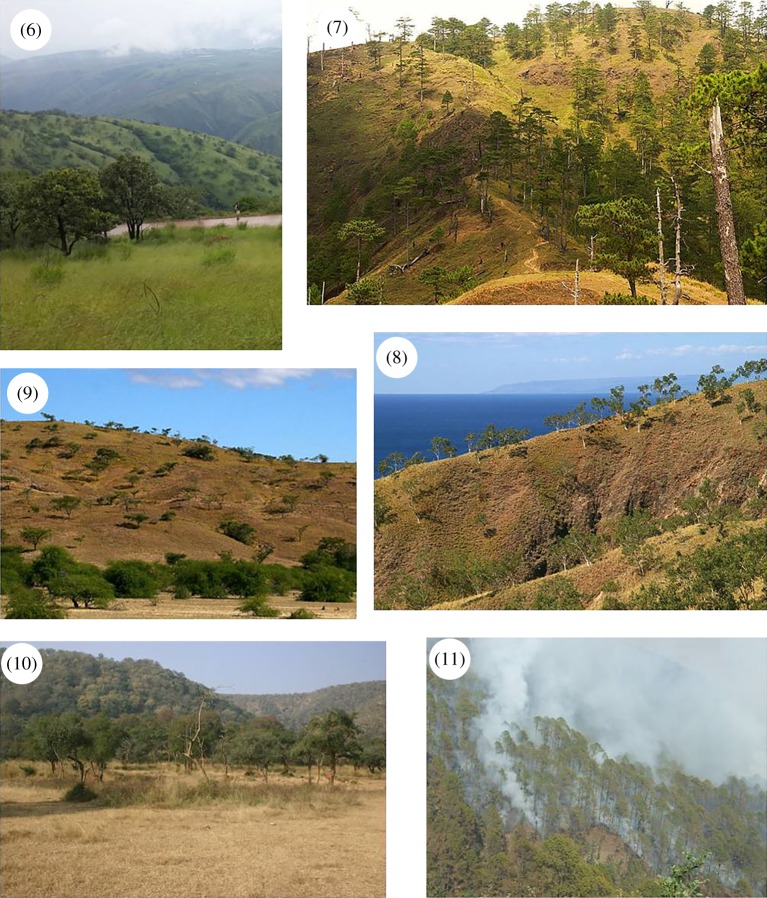
Figure 2.
Images of different Asian savannahs with numbers on the images corresponding to the numbered geographical locations shown in figure 1. (1) Fine-leafed and spiny Acacia savannah in Gujarat, western India. (2) Broadleaf Anogeissus–Terminalia savannah in Mudumalai, southern India. (3) Broadleaf Anogeissus savannah in Nagarjuna-Sagar in south-eastern India. (4) Broadleaf Dipterocarp savannah in Pa Hin Ngam, Thailand and (5) Broadleaf Dipterocarp savannah underlain by the deciduous bamboo Vietnamosasa spp. in Mondulkiri, Cambodia. (6) Mixed broadleaf savannah in Yuanjiang Valley savannah, Yunnan, China. (7) Pine savannah (Pinus kesiya) on Mt Ugo, The Philippines. (8) Eucalypt savannah (Eucalyptus alba) on Mount Curi, East Timor, The Lesser Sunda islands. (9) Fine-leafed and spiny Acacia savannah on Mount Manatuto, East Timor, The Lesser Sunda islands. (10) Fine-leafed and spiny savannah in Sariska Tiger Reserve, central India and (11) pine savannah (Pinus roxburghii) in the Himalayan foothills in Dehradun, north India. Photo credits: Jayashree Ratnam, Edmund February, Mahesh Sankaran, Komsant Inroung (http://lifexdiscovery.blogspot.in), Kyle Tomlinson, Don Franklin, Anne Jimenez (Wikimedia Commons).
Although our models provide us with a first approximation of the potential distribution and extent of savannahs in Asia, they are conspicuous in the lack of fire and herbivory as predictors of savannah distribution. Inclusion of such top–down drivers in future models will undoubtedly provide us with a more refined understanding of savannah distributions in Asia. Given that the vast majority of savannah habitats in Asia have already been converted or otherwise modified by humans [100,103], there is an urgent need for identifying and mapping Asian savannahs, both potential and extant, not only for their effective management but also for a more nuanced understanding of tropical savannahs globally.
7. Uncertain futures for savannahs in Asia
The lack of recognition of Asian savannahs as unique ecosystems distinct from forests, and the common misperception of savannahs as degraded forests pose significant conservation and management challenges. These include inappropriate fire and herbivore management resulting from a lack of understanding of the functional roles of these disturbances in these ecosystems, land-use conversions to agriculture and tree plantations, severe invasions by exotic woody species and uncertain trajectories under changing climatic regimes.
Beginning in the colonial period, official policy on protected area management in South Asia was to strictly suppress fire and livestock grazing [11,86]. For example, the Indian Forest Act of 1927 (http://envfor.nic.in/legis/forest/forest4.html) officially deemed the willful setting of fire in protected areas as a punishable crime. Many such policies remain official to this day, contrary to scientific consensus that fire and herbivory are key ecological processes that maintain ecosystem health and diversity in savannahs [79,104,105]. In practice, the implementation of such policies varies widely across Asia. In China, official government policies of strict fire prevention in conservation areas and public lands have been implemented, at least since the 1980s [81,82]. By contrast, departments charged with the stewardship of protected areas in other South and Southeast Asian countries often have limited resources, and fire suppression can be erratic [86].
In the dipterocarp savannahs of Thailand, fire suppression resulted in litter build-up and exceptionally intense fires in years when they did occur, with unusually high tree mortalities [11]. In the central and northern Indian mixed deciduous savannahs, it was widely recognized by the early 1900s that both teak (Tectona grandis) and sal (Shorea robusta) seedlings were not recruiting in areas with sustained fire suppression [86]. At the other extreme, Saha & Howe [106] found that annual, low-intensity fires in a deciduous savannah in central India resulted in a very restricted set of tree species being able to regenerate, and predicted large declines in tree diversity in these communities in the coming century. Thus, neither fire exclusion nor very frequent burning is desirable. Similarly, policy-driven exclusion of large bodied grazing cattle from a southern Indian deciduous broadleaf savannah released tall-grass species of low-nutrient quality from their control, allowing them to dominate the understorey, in turn suppressing populations of small-bodied wild herbivores for which these tall grasses are unpalatable [107]. In sharp contrast to Africa, where the role of herbivores in driving community and ecosystem dynamics has been extensively studied, current knowledge of the functional roles of herbivores in Asian savannahs is woefully inadequate [108]. Both long-term herbivore and fire-exclusion studies are almost non-existent in Asia. Likewise, studies documenting vegetation responses using experimental burns, which have been invaluable for understanding the role of fires in savannahs and grasslands elsewhere, are rare in Asia. Unfortunately, they are likely to remain so because of government policies of fire suppression in countries like India and China [81,82,86].
As is true for savannahs elsewhere, Asian savannahs are heavily threatened by land-use changes including conversion to agriculture and tree plantations. Of particular concern, government driven afforestation initiatives in the context of emerging programmes for carbon sequestration are a major threat to mixed tree–grass biomes globally [109–111]. For example, the Green India mission (http://www.envfor.nic.in/major-initiatives/national-mission-green-india-gim) aims ‘to increase and improve tree cover on 10 million hectares of forest and non-forest lands’ in the subcontinent. A key challenge in this context will be to distinguish derived savannahs via forest degradation (and therefore appropriate for reforestation) from ancient savannahs that should be conserved for their unique biodiversity, particularly in their herbaceous layers and the herbivore communities that they support [1,15,111,112]. This will require identification of indicator tree, grass and herbaceous species, or combinations of these, that are diagnostic of derived versus ancient savannahs across these regions [111,112].
Another major threat to Asia's savannahs arises from invasions by exotic plant species that have transformed the physiognomies of these landscapes. Drier tracts of fine-leafed spiny savannahs in both South Asia and Southeast Asia have been heavily invaded by Prosopis juliflora which transforms open tree–grass landscapes into woody thickets, often with extensive bare ground [113–116]. In the more mesic tracts of deciduous broadleaf savannahs, dense invasions of the woody shrub Lantana camara have replaced the C4 grass understorey [117–119], with potential consequences for fire and nutrient dynamics, and herbivore and predator communities of these systems. The causes for these invasions include tree seeding for fuelwood, overgrazing and suppression of native fire regimes [117–119]. Today, these widespread invasions appear irreversible in many South Asian savannahs and may well represent fundamental state shifts in these ecosystems. Research that elucidates how these invasions have transformed the functional ecology of these ecosystems, including the balance of woody and herbaceous components, responses to fire and herbivory, carbon and water dynamics, and how these may respond under anticipated climatic changes in the future is urgently needed.
Analyses of long-term precipitation records for Asia show that the South Asian summer monsoon is undergoing a directional shift, with a trend towards more intense rain events and longer dry periods between rain events during the monsoon season [120,121]. Shifts in patterns of water availability during the monsoon, along with predicted changes in total rainfall, seasonality and spatial variability of rainfall [122–124] will likely have large impacts on the structure and dynamics of Asian savannahs over the coming decades. A recent analysis for South Asia finds that for an ensemble of projected climate scenarios, the savannahs of the Indian subcontinent will likely show large losses in their geographical extent, as the drier areas they now occupy are replaced by higher rainfall regimes [125]. Likewise, climate models predict increasing rainfall over East Asia (IPCC 2013 report, http://www.ipcc.ch/report/ar5/wg1/), with the potential to shift these savannahs towards densely wooded states from where they may transition to forest ecosystems. Future research that considers the interactive effects of rainfall and temperature changes with changes in fire and herbivore regimes and human use will be critical for forecasting the dynamics of Asian systems. Further, experimental studies that explore changes in recruitment and growth responses of dominant trees of the different savannah types to simulated changes in temperature, precipitation and nutrient regimes will provide critical insights into the potential trajectories of these savannahs over the coming century.
Supplementary Material
Acknowledgements
We thank Huang Guohualing for assisting with data collection for species compositions and traits for Asian savannas and Caroline Lehmann for savannah distribution maps for South America and Australia. We thank three anonymous reviewers, Toby Pennington and Caroline Lehmann for feedback that greatly improved our manuscript.
Authors' contributions
J.R. and K.W.T. are equal first authors on this manuscript. D.N.R. and M.S. performed the analysis and modelling of climatic envelopes of Asian savannahs. J.R., K.W.T. and M.S. wrote the manuscript, with inputs from D.N.R.
Competing interests
The authors declare no competing interests.
Funding
K.W.T. acknowledges financial support from the National Science Foundation of China (grant no. 31470449) and the Chinese Academy of Sciences (Fellowship for Young International Scientists). J.R. and M.S. acknowledge financial support from the Department of Science and Technology of India (grant no. SERB/SR/SO/PS/78/2012). D.N.R. and M.S. are supported by the Department of Biotechnology of India (grant no. BT/01/NE/PS/NCBS/09 to M.S.).
References
- 1.Ratnam J, Bond WJ, Fensham RJ, Hoffmann WA, Archibald S, Lehmann CER, Anderson MT, Higgins SI, Sankaran M. 2011. When is a ‘forest’ a savanna, and why does it matter? Glob. Ecol. Biogeogr. 20, 653–660. ( 10.1111/j.1466-8238.2010.00634.x) [DOI] [Google Scholar]
- 2.Sankaran M, Ratnam J. 2013. African and Asian savannas. In Encyclopedia of biodiversity, vol. 1, 2nd edn (ed Levin SA.), pp. 58–74. New York, NY: Elsevier. [Google Scholar]
- 3.Ashton P. 2014. On the forests of tropical Asia: lest the memory fade. London, UK: Kew Publishing. [Google Scholar]
- 4.Sankaran M, et al. 2005. Determinants of woody cover in African savannas. Nature 438, 846–849. ( 10.1038/nature04070) [DOI] [PubMed] [Google Scholar]
- 5.Hirota M, Holmgren M, Van Nes EH, Scheffer M. 2011. Global resilience of tropical forest and savanna to critical transitions. Science 334, 232–235. ( 10.1126/science.1210657) [DOI] [PubMed] [Google Scholar]
- 6.Staver AC, Archibald S, Levin SA. 2011. The global extent and determinants of savanna and forest as alternative biome states. Science 334, 230–232. ( 10.1126/science.1210465) [DOI] [PubMed] [Google Scholar]
- 7.Lehmann CER, et al. 2014. Savanna vegetation-fire-climate relationships differ among continents. Science 343, 548–552. ( 10.1126/science.1247355) [DOI] [PubMed] [Google Scholar]
- 8.Kurz S. 1877. Forest flora of British Burma, vols I and II. Calcutta: Office of the Superintendent of Government Printing. [Google Scholar]
- 9.Champion HG. 1936. A preliminary survey of the forest types of India and Burma. Ind. For. Rec. 1, 1–286. [Google Scholar]
- 10.Champion HG, Seth SK. 1968. A revised survey of the forest types of India. Delhi: Government of India Press. [Google Scholar]
- 11.Stott P. 1990. Stability and stress in the savanna forests of mainland South-east Asia. J. Biogeogr. 17, 373–383. ( 10.2307/2845366) [DOI] [Google Scholar]
- 12.Puri GS, Gupta RK, Meher-Homini VM, Puri S. 1989. Forest ecology, plant form, diversity, communities and succession, vol. 2, 2nd edn New Delhi: Oxford and IBH Publishing. [Google Scholar]
- 13.Stott P. 1988. The forest as phoenix: towards a biogeography of fire in mainland South East Asia. Geogr. J. 154, 337–350. ( 10.2307/634607) [DOI] [Google Scholar]
- 14.McShea WJ, Davies SJ. 2011. Seasonally dry forests of tropical Asia: an ecosystem adapted to seasonal drought, frequent fire and human activity. In The ecology and conservation of seasonally dry forests in Asia (eds McShea WJ, Davies SJ, Bhumpakphan N), pp. 1–8. Washington, DC: Smithsonian Institution Scholarly Press. [Google Scholar]
- 15.Bond WJ, Parr CL. 2010. Beyond the forest edge: ecology, diversity and conservation of the grassy biomes. Biol. Conserv. 143, 2395–2404. ( 10.1016/j.biocon.2009.12.012) [DOI] [Google Scholar]
- 16.Gadgil M, Meher-Homji VM. 1985. Landuse and productive potential of Indian savannas. In Ecology and management of the world’s savannas (eds Tothill JC, Mott JJ), pp. 107–113. Canberra, Australia: Australian Academy of Sciences. [Google Scholar]
- 17.Boulbet J. 1982. Evolution des paysages vegetaux en Thailande du nord-est. Paris: Ecole Francaise d'Extreme-Orient. [Google Scholar]
- 18.Yadava PS. 1990. Savannas of north-east India. J. Biogeogr. 17, 385–394. ( 10.2307/2845367) [DOI] [Google Scholar]
- 19.James R. 1989. Hominid use of fire in the lower and middle Pleistocene. A review of the evidence. Curr. Anthropol. 30, 1–26. ( 10.1086/203705) [DOI] [Google Scholar]
- 20.Shen G, Gao X, Gao B, Granger DE. 2009. Age of Zhoukoudian Homo erectus determined with 26Al/10Be burial dating. Nature 458, 198–200. ( 10.1038/nature07741) [DOI] [PubMed] [Google Scholar]
- 21.Zhong M, Shi C, Gao X, Wu X, Chen F, Zhang S, Zhang X, Olsen J. 2014. On the possible use of fire by Homo erectus at Zhoukoudian, China. Chinese Sci. Bull. 59, 335–343. ( 10.1007/s11434-013-0061-0) [DOI] [Google Scholar]
- 22.Edwards EJ, et al. 2010. The origins of C4 grasslands: integrating evolutionary and ecosystem science. Science 328, 587–591. ( 10.1126/science.1177216) [DOI] [PubMed] [Google Scholar]
- 23.Louys J, Meijaard E. 2010. Palaeoecology of Southeast Asian megafauna-bearing sites from the Pleistocene and a review of environmental changes in the region. J. Biogeogr. 37, 1432–1449. ( 10.1111/j.1365-2699.2010.02297.x) [DOI] [Google Scholar]
- 24.Morley RJ. 2012. A review of the Cenozoic paleoclimate history of Southeast Asia. In Biotic evolution and environmental change in Southeast Asia (eds Gower DJ, Johnson KG, Richardson JE, Rosen BR, Ruber L, Williams S), pp. 79–114. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 25.Hoorn C, Ohja T, Quade J. 2000. Palynological evidence for vegetation development and climatic change in the sub-Himalayan zone (Neogene, Central Nepal). Palaeogeogr. Palaeoclimatol. Palaeoecol. 163, 133–161. ( 10.1016/S0031-0182(00)00149-8) [DOI] [Google Scholar]
- 26.Behrensmeyer AK, et al. 2007. The structure and rate of late Miocene expansion of C4 plants: evidence from lateral variation in stable isotopes in paleosols of the Siwalik Group, northern Pakistan. Geol. Soc. Am. Bull. 119, 1486–1505. ( 10.1130/B26064.1) [DOI] [Google Scholar]
- 27.Badgley C, Barry JC, Morgan ME, Nelson SV, Behrensmeyer AK, Cerling TE, Pilbeam D. 2008. Ecological changes in Miocene mammalian record show impact of prolonged climatic forcing. Proc. Natl Acad. Sci. USA 105, 12 145–12 149. ( 10.1073/pnas.0805592105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanyal P, Sarkar A, Bhattacharya SK, Kumar R, Ghosh SK, Agrawal S. 2010. Intensification of monsoon, microclimate and asynchronous C4 appearance: isotopic evidence from the Indian Siwalik sediments. Palaeogeogr. Palaeoclimatol. Palaeoecol. 296, 165–173. ( 10.1016/j.palaeo.2010.07.003) [DOI] [Google Scholar]
- 29.Armstrong-Altrin JS, Lee Y, Verma SP, Worden RH. 2009. Carbon, oxygen, and strontium isotope geochemistry of carbonate rocks of the upper Miocene Kudankulam Formation, southern India: implications for paleoenvironment and diagenesis. Chemie der Erde - Geochemistry 69, 45–60. ( 10.1016/j.chemer.2008.09.002) [DOI] [Google Scholar]
- 30.Agrawal S, Sanyal P, Sarkar A, Jaiswal M, Dutta K. 2012. Variability of Indian monsoonal rainfall over the past 100 ka and its implication for C3–C4 vegetational change. Quat. Res. 77, 159–170. ( 10.1016/j.yqres.2011.09.003) [DOI] [Google Scholar]
- 31.CAS. 2001. Vegetation atlas of China. Beijing, China: Science Press. [Google Scholar]
- 32.Qiu Z, Qiu Z. 1995. Chronological sequence and subdivision of Chinese Neogene mammalian faunas. Palaeogeogr. Palaeoclimatol. Palaeoecol. 116, 41–70. ( 10.1016/0031-0182(94)00095-P) [DOI] [Google Scholar]
- 33.Jiang H, Ding Z. 2009. Spatial and temporal characteristics of Neogene palynoflora in China and its implication for the spread of steppe vegetation. J. Arid Environ. 73, 765–772. ( 10.1016/j.jaridenv.2009.03.011) [DOI] [Google Scholar]
- 34.Biasatti D, Wang Y, Gao F, Xu Y, Flynn L. 2012. Paleoecologies and paleoclimates of late Cenozoic mammals from Southwest China: evidence from stable carbon and oxygen isotopes. J. Asian Earth Sci. 44, 48–61. ( 10.1016/j.jseaes.2011.04.013) [DOI] [Google Scholar]
- 35.Yao YF, Bruch AA, Cheng YM, Mosbrugger V, Wang YF, Li CS. 2012. Monsoon versus uplift in southwestern China-Late Pliocene climate in Yuanmou Basin, Yunnan. PLoS ONE 7, 1–8. ( 10.1371/journal.pone.0037760) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang TC, Comes HP, Sun H. 2011. Chloroplast phylogeography of Terminalia franchetii (Combretaceae) from the eastern Sino-Himalayan region and its correlation with historical river capture events. Mol. Phylogenet. Evol. 60, 1–12. ( 10.1016/j.ympev.2011.04.009) [DOI] [PubMed] [Google Scholar]
- 37.Bird MI, Taylor D, Hunt C. 2005. Palaeoenvironments of insular Southeast Asia during the Last Glacial Period: a savanna corridor in Sundaland? Q. Sci. Rev. 24, 2228–2242. ( 10.1016/j.quascirev.2005.04.004) [DOI] [Google Scholar]
- 38.van Steenis CGGJ. 1979. Plant-geography of east Malesia. Bot. J. Linn. Soc. 79, 97–178. ( 10.1111/j.1095-8339.1979.tb01511.x) [DOI] [Google Scholar]
- 39.Groves C, Grubb P. 2011. Ungulate taxonomy. Baltimore, MD: Johns Hopkins University Press. [Google Scholar]
- 40.Kershaw P, van der Kaars S, Moss P, Opdyke B, Guichard F, Rule S, Turney C. 2006. Environmental change and the arrival of people in the Australian region. Before Farming 1, 1–24. ( 10.3828/bfarm.2006.1.2) [DOI] [Google Scholar]
- 41.Suraprasit K, Chaimanee Y, Bocherens H, Chavasseau O, Jaeger J-J. 2014. Systematics and phylogeny of middle Miocene Cervidae (Mammalia) from Mae Moh Basin (Thailand) and a paleoenvironmental estimate using enamel isotropy of sympatric herbivore species. 1. J. Vertebr. Paleontol. 34, 179–194. ( 10.1080/02724634.2013.789038) [DOI] [Google Scholar]
- 42.Gilbert C, Ropiquet A, Hassanin A. 2006. Mitochondrial and nuclear phylogenies of Cervidae (Mammalia, Ruminantia): systematics, morphology, and biogeography. Mol. Phylogenet. Evol. 40, 101–117. ( 10.1016/j.ympev.2006.02.017) [DOI] [PubMed] [Google Scholar]
- 43.Bibi F. 2013. A multi-calibrated mitochondrial phylogeny of extant Bovidae (Artiodactyla, Ruminantia) and the importance of the fossil record to systematics. BMC Evol. Biol. 13, 166 ( 10.1186/1471-2148-13-166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoffmann WA, Franco AC. 2003. Comparative growth analysis of tropical forest and savanna woody plants using phylogenetically independent contrasts. J. Ecol. 91, 475–484. ( 10.1046/j.1365-2745.2003.00777.x) [DOI] [Google Scholar]
- 45.Staver AC, Bond WJ, Cramer MD, Wakeling JL. 2012. Top-down determinants of niche structure and adaptation among African acacias. Ecol. Lett. 15, 673–679. ( 10.1111/j.1461-0248.2012.01784.x) [DOI] [PubMed] [Google Scholar]
- 46.Tomlinson KW, et al. 2012. Biomass partitioning and root morphology of savanna trees across a water gradient. J. Ecol. 100, 1113–1121. ( 10.1111/j.1365-2745.2012.01975.x) [DOI] [Google Scholar]
- 47.Tomlinson KW, Poorter L, Sterck FJ, Borghetti F, Ward D, de Bie S, van Langevelde F. 2013. Leaf adaptations of evergreen and deciduous trees of semi-arid and humid savannas on three continents. J. Ecol. 101, 430–440. ( 10.1111/1365-2745.12056) [DOI] [Google Scholar]
- 48.Scheiter S, Langan L, Higgins SI. 2013. Next-generation dynamic global vegetation models: learning from community ecology. New Phytol. 198, 957–969. ( 10.1111/nph.12210) [DOI] [PubMed] [Google Scholar]
- 49.Dantas VDL, Pausas JG. 2013. The lanky and the corky: fire-escape strategies in savanna woody species. J. Ecol. 101, 1265–1272. ( 10.1111/1365-2745.12118) [DOI] [Google Scholar]
- 50.Bunyavejchewin S, Baker PJ, Davies SJ. 2011. Seasonally dry tropical forests in Continental Southeast Asia: structure, composition, and dynamics. In The ecology and conservation of seasonally dry forests in Asia (eds McShea WJ, Davies SJ, Bhumpakphan N), pp. 9–35. Washington, DC: Smithsonian Institution Scholarly Press. [Google Scholar]
- 51.Oo WP, Koike F. 2015. Dry forest community types and their predicted distribution based on a habitat model for the central dry zone of Myanmar. For. Ecol. Manage. 358, 108–121. ( 10.1016/j.foreco.2015.09.006) [DOI] [Google Scholar]
- 52.Rundel PW. 1999. Conservation priorities in Indochina - WWF Desk Study Forest habitats and flora in Lao PDR, Cambodia and Vietnam. Hanoi, Vietnam: World Wide Fund for Nature. [Google Scholar]
- 53.Suresh HS, Dattaraja HS, Mondal N, Sukumar R. 2011. Seasonally dry tropical forests in Southern India. An analysis of floristic composition, structure, and dynamics in Mudumalai Wildlife Sanctuary. In The ecology and conservation of seasonally dry forests in Asia (eds WJ McShea, SJ Davies, N Bhumpakphan), pp. 37–58. Washington, DC: Smithsonian Institution Scholarly Press. [Google Scholar]
- 54.Jin Z-Z, Ou X-K. 2000. Yuanjiang, Nujiang, Jinshajiang, Lancangjiang: vegetation of dry-hot valley. Kunming, China: Yunnan University Press. [Google Scholar]
- 55.Soreng RJ, Peterson PM, Romaschenko K, Davidse G, Zuloaga FO, Judziewicz EJ, Filgueiras TS, Davis JI, Morrone O. 2015. A worldwide phylogenetic classification of the Poaceae (Gramineae). J. Syst. Evol. 53, 117–137. ( 10.1111/jse.12150) [DOI] [Google Scholar]
- 56.Monk KA, De Fretes Y, Reksodiharjo-Lilly G. 1997. The ecology of Nusa Tenggara and Maluku. Hong Kong, China: Periplus Editions. [Google Scholar]
- 57.Jin Z-Z. 2002. Floristic features of dry-hot and dry-warm valleys, Yunnan and Sichuan. Kunming, China: Yunnan Science and Technology Press. [Google Scholar]
- 58.Sagar R, Pandey A, Singh JS. 2012. Composition, species diversity, and biomass of the herbaceous community in dry tropical forest of northern India in relation to soil moisture and light intensity. Environmentalist 32, 485–493. ( 10.1007/s10669-012-9414-5) [DOI] [Google Scholar]
- 59.Metzner JK. 1977. Man and environment in Eastern Timor. Canberra, Australia: Australian National University. [Google Scholar]
- 60.Wanthongchai KA, Tarusadamrongdet VC, Chinnawong KC. 2013. Fuel properties and fire behaviour characteristics of prescribed fire in pine-dominated forests at Nam Nao National Park, Thailand. Int. J. Wildl. Fire 22, 615–624. ( 10.1071/WF11183) [DOI] [Google Scholar]
- 61.Goldammer JG, Peñafiel SR. 1990. Fire in the pine-grassland biomes of tropical and subtropical Asia. In Fire in the tropical biota. Ecosystem processes and global challenges (ed. Goldammer JG.), pp. 45–62. Berlin, Germany: Springer. [Google Scholar]
- 62.Su W-H, Shi Z, Zhou R, Zhao Y-J, Zhang G-F. 2015. The role of fire in the Central Yunnan Plateau ecosystem, southwestern China. For. Ecol. Manage. 356, 22–30. ( 10.1016/j.foreco.2015.05.015) [DOI] [Google Scholar]
- 63.Sagar R, Singh JS. 2005. Structure, diversity, and regeneration of tropical dry deciduous forest of northern India. Biodivers. Conserv. 14, 935–959. ( 10.1007/s10531-004-0671-6) [DOI] [Google Scholar]
- 64.Wohlfart C, Wegmann M, Leimgruber P. 2014. Mapping threatened dry deciduous dipterocarp forest in South-east Asia for conservation management. Trop. Conserv. Sci. 7, 597–613. [Google Scholar]
- 65.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. 2005. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978. ( 10.1002/joc.1276) [DOI] [Google Scholar]
- 66.Stott P. 1986. The spatial pattern of dry season fires in the savanna forests of Thailand. J. Biogeogr. 13, 345–358. ( 10.2307/2845018) [DOI] [Google Scholar]
- 67.Osborne CP, Salomaa A, Kluyver TA, Visser V, Kellogg E, Morrone O, Vorontsova MS, Clayton WD, Simpson DA. 2014. A global database of C4 photosynthesis in grasses. New Phytol. 204, 441–446. ( 10.1111/nph.12942) [DOI] [PubMed] [Google Scholar]
- 68.Wanthongchai K, Goldammer JG, Bauhus J. 2011. Effects of fire frequency on prescribed fire behaviour and soil temperatures in dry dipterocarp forests. Int. J. Wildl. Fire 20, 35–45. ( 10.1071/WF08098) [DOI] [Google Scholar]
- 69.Eiadthong W. 2009. Endemic and rare plants in dry deciduous dipterocarp forest in Thailand. In Tropical forestry change in a changing world. Vol. 5: Dry forest ecology and conservation, pp. 133–142. Bangkok, Thailand: Kasetsart University Faculty of Forestry. [Google Scholar]
- 70.Anitha K, Joseph S, Chandran RJ, Ramasamy EV, Prasad SN. 2010. Tree species diversity and community composition in a human-dominated tropical forest of Western Ghats biodiversity hotspot, India. Ecol. Complex. 7, 217–224. ( 10.1016/j.ecocom.2010.02.005) [DOI] [Google Scholar]
- 71.Taub DR. 2000. Climate and the U.S. distribution of C4 grass subfamilies and decarboxylation variants of C4 photosynthesis. Am. J. Bot. 87, 1211–1215. ( 10.2307/2656659) [DOI] [PubMed] [Google Scholar]
- 72.Wand SJE, Midgley GF, Stock WD. 2001. Growth responses to elevated CO2 in NAdp-ME, NAD-ME and PCK C4 grasses and a C3 grass from South Africa. Aust. J. Bot. 28, 13–25. ( 10.1071/pp99104) [DOI] [Google Scholar]
- 73.FAO/IIASA/ISRIC/ISSCAS/JRC. 2012. Harmonized World Soil Database (version 1.2).
- 74.Cooper SM, Owen-Smith N. 1986. Effects of plant spinescence on large mammalian herbivores. Oecologia 68, 446–455. ( 10.1007/BF01036753) [DOI] [PubMed] [Google Scholar]
- 75.Olff H, Ritchie ME, Prins HHT. 2002. Global environmental controls of diversity in large herbivores. Nature 415, 901–904. ( 10.1038/415901a) [DOI] [PubMed] [Google Scholar]
- 76.Hempson GP, Archibald S, Bond WJ. 2015. A continent-wide assessment of the form and intensity of large mammal herbivory in Africa. Science 350, 1056–1061. ( 10.1126/science.aac7978) [DOI] [PubMed] [Google Scholar]
- 77.Kiianmaa S. 2005. Natural regeneration and ecological succession in Pinus kesiya watershed plantations in northern Thailand: implications for plantation management. MSc thesis, Univeristy of Helsinki.
- 78.Keddy PA, Smith L, Campbell DR, Clark M, Montz G. 2006. Patterns of herbaceous plant diversity in southeastern Louisiana pine savannas. Appl. Veg. Sci. 9, 17–26. ( 10.1111/j.1654-109X.2006.tb00652.x) [DOI] [Google Scholar]
- 79.Veldman JW, Brudvig LA, Damschen EI, Orrock JL, Mattingly WB, Walker JL. 2014. Fire frequency, agricultural history and the multivariate control of pine savanna understorey plant diversity. J. Veg. Sci. 25, 1438–1449. ( 10.1111/jvs.12195) [DOI] [Google Scholar]
- 80.Wigley BJ, Cramer MD, Bond WJ. 2008. Sapling survival in a frequently burnt savanna: mobilisation of carbon reserves in Acacia karroo. Plant Ecol. 203, 1–11. ( 10.1007/s11258-008-9495-x) [DOI] [Google Scholar]
- 81.Krawchuk MA, Moritz MA. 2009. Fire regimes of China: inference from statistical comparison with the United States. Glob. Ecol. Biogeogr. 18, 626–639. ( 10.1111/j.1466-8238.2009.00472.x) [DOI] [Google Scholar]
- 82.Lu A, Tian H, Liu M, Liu J, Melillo JM. 2006. Spatial and temporal patterns of carbon emissions from forest fires in China from 1950 to 2000. J. Geophys. Res. 111, D05313 ( 10.1029/2005JD006198) [DOI] [Google Scholar]
- 83.Eckert AJ, Hall BD. 2006. Phylogeny, historical biogeography, and patterns of diversification for Pinus (Pinaceae): phylogenetic tests of fossil-based hypotheses. Mol. Phylogenet. Evol. 40, 166–182. ( 10.1016/j.ympev.2006.03.009) [DOI] [PubMed] [Google Scholar]
- 84.Clayton WD, Vorontsova MS, Harman KT. 2016. World grass species: synonymy. See http://www.kew.org/data/grasses-syn.html. (accessed 26 February 2016). [Google Scholar]
- 85.Dexter KG, et al. 2015. Floristics and biogeography of vegetation in seasonally dry tropical regions. Int. For. Rev. 17, 10–32. ( 10.1505/146554815815834859) [DOI] [Google Scholar]
- 86.Wanthongchai K, Goldammer JG. 2011. Fire management in South and Southeast Asia's seasonally dry forests: colonial approaches, current problems and perspectives. In The ecology and conservation of seasonally dry forests in Asia (eds McShea WJ, Davies SJ, Bhumpakphan N), pp. 97–114. Washington, DC: Smithsonian Institution Scholarly Press. [Google Scholar]
- 87.Nowak RM. 1999. Walker‘s mammals of the world Baltimore, MD: Johns Hopkins University Press. [Google Scholar]
- 88.Birdlife & NatureServe 2015. Bird species distribution maps of the world. Cambridge, UK: BirdLife International and NatureServe. [Google Scholar]
- 89.Brickle NW. 2002. Habitat use, predicted distribution and conservation of green peafowl (Pavo muticus) in Dak Lak province, Vietnam. Biol. Conserv. 105, 189–197. ( 10.1016/S0006-3207(01)00182-3) [DOI] [Google Scholar]
- 90.Sankaran M. 2009. Diversity patterns in savanna grassland communities: implications for conservation strategies in a biodiversity hotspot. Biodivers. Conserv. 18, 1099–1115. ( 10.1007/s10531-008-9519-9) [DOI] [Google Scholar]
- 91.Singh K, Shukla A, Singh J. 2010. Floristic diversity and taxonomic profile of Achanakmar-Amarkantak Biosphere Reserve, Central India. J. Bombay Nat. Hist. Soc. 107, 135–145. [Google Scholar]
- 92.Lehmann CER, Archibald SA, Hoffmann WA, Bond WJ. 2011. Deciphering the distribution of the savanna biome. New Phytol. 191, 197–209. ( 10.1111/j.1469-8137.2011.03689.x) [DOI] [PubMed] [Google Scholar]
- 93.Murphy BP, Bowman DMJS. 2012. What controls the distribution of tropical forest and savanna? Ecol. Lett. 15, 748–758. ( 10.1111/j.1461-0248.2012.01771.x) [DOI] [PubMed] [Google Scholar]
- 94.Friedman J, Hastie T, Tibshirani R. 2000. Additive logistic regression: a statistical view of boosting. Ann. Stat. 28, 337–407. ( 10.1214/aos/1016218223) [DOI] [Google Scholar]
- 95.Friedman JH. 2001. Greedy function approximation: a gradient boosting machine. Ann. Stat. 29, 1189–1232. ( 10.1214/aos/1013203451) [DOI] [Google Scholar]
- 96.Friedman JH. 2002. Stochastic gradient boosting. Comput. Stat. Data Anal. 38, 367–378. ( 10.1016/S0167-9473(01)00065-2) [DOI] [Google Scholar]
- 97.Elith J, Leathwick JR, Hastie T. 2008. A working guide to boosted regression trees. J. Anim. Ecol. 77, 802–813. ( 10.1111/j.1365-2656.2008.01390.x) [DOI] [PubMed] [Google Scholar]
- 98.Haridasan M. 1982. Aluminium accumulation by some cerrado native species of central Brazil. Plant Soil 65, 265–273. ( 10.1007/BF02374657) [DOI] [Google Scholar]
- 99.Hoffmann WA, Geiger EL, Gotsch SG, Rossatto DR, Silva LCR, Lau OL, Haridasan M, Franco AC. 2012. Ecological thresholds at the savanna-forest boundary: how plant traits, resources and fire govern the distribution of tropical biomes. Ecol. Lett. 15, 759–768. ( 10.1111/j.1461-0248.2012.01789.x) [DOI] [PubMed] [Google Scholar]
- 100.ICFRE. 2013. Forest types of India: revisited. Dehradun, India: Indian Council of Forestry Research & Eduction. [Google Scholar]
- 101.Olson DM, et al. 2001. Terrestrial ecoregions of the world: a new map of life on earth. BioScience 51, 933–938. ( 10.1641/0006-3568(2001)051%5B0933:TEOTWA%5D2.0.CO;2) [DOI] [Google Scholar]
- 102.Noss RF, Platt WJ, Sorrie BA, Weakley AS, Means DB, Costanza J, Peet RK. 2015. How global biodiversity hotspots may go unrecognized: lessons from the North American Coastal Plain. Divers. Distrib. 21, 236–244. ( 10.1111/ddi.12278) [DOI] [Google Scholar]
- 103.Blasco F, Bellan MF, Aizpuru M. 1996. A vegetation map of tropical continental Asia at 1:5 million. J. Veg. Sci. 7, 623–634. ( 10.2307/3236374) [DOI] [Google Scholar]
- 104.Bond WJ. 2008. What limits trees in C4 grasslands and savannas? Annu. Rev. Ecol. Evol. Syst. 39, 641–659. ( 10.1146/annurev.ecolsys.39.110707.173411) [DOI] [Google Scholar]
- 105.Clarke PJ, Lawes MJ, Midgley JJ, Lamont BB, Ojeda F, Burrows GE, Enright NJ, Knox KJE. 2012. Resprouting as a key functional trait: how buds, protection and resources drive persistence after fire. New Phytol. 197, 19–35. ( 10.1111/nph.12001) [DOI] [PubMed] [Google Scholar]
- 106.Saha S, Howe HF. 2003. Species composition and fire in a dry deciduous forest. Ecology 84, 3118–3123. ( 10.1890/02-3051) [DOI] [Google Scholar]
- 107.Sankaran M. 2005. Fire, grazing and the dynamics of tall-grass savannas in the Kalakad-Mundanthurai Tiger Reserve, South India. Conserv. Soc. 3, 4. [Google Scholar]
- 108.Sankaran M, Ahrestani FS. 2016. Large herbivores of South and Southeast Asia: synthesis and future directions. In The ecology of large herbivores in South & South East Asia (eds Ahrestani FS, Sankaran M), pp. 237–250. Berlin, Germany: Springer. [Google Scholar]
- 109.Parr CL, Lehmann CER, Bond WJ, Hoffmann WA, Andersen AN. 2014. Tropical grassy biomes: misunderstood, neglected, and under threat. Trends Ecol. Evol. 29, 205–213. ( 10.1016/j.tree.2014.02.004) [DOI] [PubMed] [Google Scholar]
- 110.Veldman JW, et al. 2015. Where tree planting and forest expansion are bad for biodiversity and ecosystem services. Bioscience 65, 1011–1018. ( 10.1093/biosci/biv118) [DOI] [Google Scholar]
- 111.Bond WJ. 2016. Ancient grasslands at risk. Science 351, 120–122. ( 10.1126/science.aad5132) [DOI] [PubMed] [Google Scholar]
- 112.Veldman JW, et al. 2015. Toward an old-growth concept for grasslands, savannas, and woodlands. Front. Ecol. Environ. 13, 154–162. ( 10.1890/140270) [DOI] [Google Scholar]
- 113.Kaur R, Gonzales WL, Llambi LD, Soriano PJ, Callaway RM, Rout ME, Gallaher TJ. 2012. Community impacts of Prosopis juliflora invasion: biogeographic and congeneric comparisons. PLoS ONE 7, e44966 ( 10.1371/journal.pone.0044966) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pandey CN, Pandey R, Bhatt JR. 2012. Woody, alien and invasive Prosopis juliflora (Swartz) D.C.: management dilemmas and regulatory issues in Gujarat. In Invasive alien plants: an ecological appraisal for the Indian subcontinent (ed. Bhatt JR.), pp. 299–304. Wallingford, UK: CABI-International. [Google Scholar]
- 115.Hiremath AJ, Sundaram B. 2013. Invasive plant species in Indian protected areas: conserving biodiversity in cultural landscapes. In Plant invasions in protected areas (eds Foxcroft LC, Pyšek P, Richardson DM, Genovesi P), pp. 241–266. Berlin, Germany: Springer. [Google Scholar]
- 116.Aung T, Koike F. 2015. Identification of invasion status using a habitat invasibility assessment model: the case of Prosopis species in the dry zone of Myanmar. J. Arid Environ. 120, 87–94. ( 10.1016/j.jaridenv.2015.04.016) [DOI] [Google Scholar]
- 117.Kannan R, Shackleton CM, Shaanker RU. 2013. Reconstructing the history of introduction and spread of the invasive species, Lantana, at three spatial scales in India. Biol. Invasions 15, 1287–1302. ( 10.1007/s10530-012-0365-z) [DOI] [Google Scholar]
- 118.Hiremath AJ, Sundaram B. 2005. The fire-lantana cycle hypothesis in Indian forests. Conserv. Soc. 3, 26. [Google Scholar]
- 119.Pasiecznik NM, Felker P. 2001. The ‘Prosopis juliflora’-‘Prosopis pallida’ complex: a monograph. Coventry, UK: HDRA. [Google Scholar]
- 120.Goswami BN, Venugopal V, Sengupta D, Madhusoodanan MS, Xavier K. 2006. Increasing trend of extreme rain events over India in a warming environment. Science 314, 1442–1445. ( 10.1126/science.1132027) [DOI] [PubMed] [Google Scholar]
- 121.Singh D, Tsiang M, Rajaratnam B, Diffenbaugh NS. 2014. Observed changes in extreme wet and dry spells during the South Asian summer monsoon season. Nat. Clim. Chang. 4, 456–461. ( 10.1038/nclimate2208) [DOI] [Google Scholar]
- 122.Ghosh S, Das D, Kao S, Ganguly AR. 2011. Lack of uniform trends but increasing spatial variability in observed Indian rainfall extremes. Nat. Clim. Change 2, 86–91. ( 10.1038/nclimate1327) [DOI] [Google Scholar]
- 123.Yang S, Ding Z, Li Y, Wang X, Jiang W, Huang X. 2015. Warming-induced northwestward migration of the East Asian monsoon rain belt from the Last Glacial Maximum to the mid-Holocene. Proc. Natl Acad. Sci. USA 112, 13 178–13 183. ( 10.1073/pnas.1504688112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Singh D. 2016. South Asian monsoon: tug of war on rainfall changes. Nat. Clim. Change 6, 20–22. ( 10.1038/nclimate2901) [DOI] [Google Scholar]
- 125.Rasquinha DN, Sankaran M. 2016 Modelling biome shifts in the Indian subcontinent under scenarios of future climate change. Curr. Sci. 111, 147–156. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.