Abstract
Deforestation as a result of burning and land conversion in the tropics and subtropics has been widely studied and active restoration of forests has been widely promoted. Besides other benefits, reforestation can sequester carbon thereby reducing CO2 emissions to the atmosphere. However, before grasslands are targeted for ‘reforestation', it is necessary to distinguish whether they are ancient natural grasslands or secondary vegetation colonizing deforested areas. Here we report the results of a study comparing primary grasslands in South Africa with 4–40 year old secondary grasslands recovering from afforestation with Pinus species. Primary grasslands had significantly higher plant species richness overall, especially of forb species. Ground cover of primary grasslands was more evenly distributed among species than secondary grasslands which tended to mono-dominance. Forbs with underground storage organs (USOs) were common in primary grasslands but conspicuously absent in the recovering systems. Comparison of secondary grasslands of different ages (up to 40 years) showed negligible recovery of the original species composition. Three key features distinguish old growth primary from secondary grasslands: total and forb species numbers, evenness of species contributions to cover and the presence of USOs. Old growth grasslands also differed in their fire response, showing significant post-burn resprouting and fire-stimulated flowering in contrast to secondary grasslands. Though similar contrasting attributes of ancient and secondary grasslands have been reported in South America, more studies are needed to explore their generality in other geographical regions.
This article is part of the themed issue ‘Tropical grassy biomes: linking ecology, human use and conservation’.
Keywords: grassland biodiversity and function, forest restoration, underground storage organs, grassland succession
1. Introduction
Deforestation in the tropics has long been a concern and has led to international and national initiatives to slow the pace of forest destruction and to restore forest cover where possible [1,2]. Reforestation methods have been explored and successfully applied, especially in the neotropics, bringing multiple benefits [3,4]. Recently, the motivation for reforestation has changed from local benefits to global benefits in the form of carbon sequestration as mitigation for global warming [5,6]. The proposed global benefits have prompted the development of ambitious forest restoration plans at regional [7] and, recently, global scales [8]. Remote sensing by satellites has opened up new methods for global scale analyses of above-ground woody biomass allowing the identification of areas with anomalously low biomass for a given region. These are areas with the potential for large gains in woody biomass since environmental conditions can support forests, but the vegetation currently consists of open ecosystems such as grasslands and savannahs [7,8]. Very large areas of the world have been mapped as suitable for forest restoration by the World Resources Institute [8,9] with the African continent having the most potential of all continents.
It has long been assumed that higher rainfall tropical grasslands and savannahs are the products of deforestation, especially as a result of anthropogenic burning [10]. However, there is a growing consensus that these open grassy ecosystems are alternative biome states to forests [11–15]. Each biome is maintained by positive feedbacks created by the plants characteristic of the biome. Fire is the main feedback maintaining the grassy layer where the climate could support forests. Fossil evidence supports the antiquity of Africa's grassy biomes. Savannahs first spread in Africa at least 7 Ma [16,17]. They support a rich fauna and flora endemic to the grassland habitat. Growth forms adapted to savannah fire regimes are also millions of years old [18–20].
For the conservation of ancient grassy ecosystems, it would be very useful to have simple, rapid methods for distinguishing primary from secondary grasslands. Recently, attempts have been made to identify the characteristics of primary grasslands or, borrowing a term from North America, ‘old growth’ grasslands [21]. If general attributes of primary grasslands could be identified, then forest restoration projects could be targeted to secondary grasslands. Here we present a case study in South African C4 grassy ecosystems. We explore attributes of ‘old growth’ grasslands and contrast these with secondary grasslands recovering after afforestation with conifer plantations.
Our study was located in the mesic subtropical grasslands of South Africa where plantation forestry is a common form of land use. There are two major grassy vegetation types in the country: the grassland biome, where trees are restricted to small forest patches in fire-protected sites, and the savannah biome, where trees and shrubs co-occur with the grasses [22]. Mesic savannahs, as for mesic grasslands, also support patches of closed forests. For convenience, we refer to both grasslands and savannahs as ‘grasslands’ since our focus was primarily on plant species in the grassy herbaceous layer. As elsewhere in the world, the grasslands were interpreted as secondarily derived from forests by felling and burning. In the South African context, this was thought to have occurred after the arrival of Iron Age farmers in the past two millennia [23,24]. This narrative was first challenged by Meadows & Linder [25], who noted the continuous presence of grassland pollen throughout the Holocene, thousands of years before the arrival of farmers, in several southern African montane grasslands. Subsequent palynological and isotope studies confirmed that the grasslands are ancient and pre-date large-scale human settlement [26]. Yet the general occurrence of old, widespread grasslands in the region does not preclude the existence of secondary grasslands produced by anthropogenic deforestation (or cultivation and abandonment of crop lands). Forest–grassland mosaics are common and may represent natural patterns or landscapes produced by local deforestation. Aerial photography can be used to trace landscape history as far back as the mid-twentieth century. Isotope analyses of soil carbon can reveal the antiquity of the different systems over longer timescales [27,28] but are technically demanding for large-scale studies. Thus, biological markers of old growth versus secondary grasslands could contribute usefully to identifying areas suitable for reforestation as distinct from old growth grasslands where afforestation should be avoided [21].
South African grassy biomes have been well researched from a rangeland management perspective because they are important for livestock and game farming [29]. They have also been well studied from a hydrological perspective because they form the main vegetation cover in the mountain catchments that supply water to the major urban and industrial areas of the country. Paired catchment experiments demonstrated that conversion of the grasslands to plantation forests causes a large reduction in stream flow as a result of an increase in evapotranspiration of approximately 400 mm yr–1 from the grassland baseline of 700–900 mm [30]. Thus, in this water-scarce country, stringent regulations restrict afforestation of grasslands because of competing downstream water demand [31]. Studies on the biodiversity of the grassy biomes have been comparatively neglected, especially relative to forests, in common with most forest–grassland mosaics in the tropics ([15,32] but see e.g. [33]). However, the grassland biome is known to be rich in plant species with a high level of endemism [25,34,35]. Many of the endemics occur in montane grasslands with high rainfall and exposed to periodic frost, and have affinities with the Cape flora of the winter rainfall regions of South Africa. Mesic savannah grasslands occur at lower elevation with warmer temperatures. Mesic and arid savannah floras are quite distinct with divergent dominant families of forbs and of characteristic growth forms [36]. Mesic savannahs, for example, are characterized by long-lived perennial forbs with large underground storage organs (USOs) with few, if any, annuals. By contrast, arid savannahs lack this growth form but, instead, have a rich annual flora [36]. The distinction has not been recognized in flora accounts of savannahs.
We studied both montane grasslands and low elevation savannah grasslands to determine whether there were common differences between old growth and secondary grasslands. The secondary grasslands, in all cases, were derived from primary grasslands converted to conifer plantations, then cleared and left to return to a grassland state by natural succession. If, as assumed by advocates of large-scale ‘reforestation’, mesic grasslands are secondary products of deforestation, then we would expect little difference between species composition in primary (natural) grasslands and those recovering after afforestation. We were able to study different ages of post-clearing recovery (up to 40 years) and could therefore identify trajectories of change in grassland composition [37]. No active restoration efforts were applied after removal of the plantations at any of the study sites. All grasslands, both primary and secondary, were subject to frequent management fires (3–10 fires per decade in the dry season). We assessed differences in a number of vegetation attributes in plot samples of natural versus secondary grasslands. If species changes were significant, we wished to determine the most distinct and consistent indicators of ‘old growth’ grasslands in the South African context. Armed with such information, forest restoration could be directed to secondary grasslands while old growth grasslands could be targeted for conservation and other land uses that conserve their biodiversity.
2. Study areas
We carried out this study in the mesic grasslands of eastern South Africa. We compared primary and secondary grasslands at three study sites. Two were situated in montane grasslands; Makobulaan Nature Reserve (MNR; 25°12'30″ S, 30°33'40″ E) and Buffelskloof Nature Reserve (BKNR; 25°18'15″ S, 30°31'12″ E) both classified as Lydenburg Montane Grassland [22]. The terrain is mountainous and large areas have been afforested with pine plantations since the 1940s to 1960s. All sites have had one rotation. Mean annual precipitation (MAP) is 860 mm with frequent mists during most months of the year. January mean maximum temperature is 23°C and mean minimum 13°C, and for July, 18°C and 4°C, respectively. The region experiences periodic frost. Soils are mostly derived from shale and quartzite with occasional dolerite intrusions. The third site was in coastal grasslands on the eastern shores of Lake St Lucia (ESI; 28°11'26″ S, 32°28'45″ E) forming part of a grassland–forest mosaic classified as the Indian Ocean Coastal Belt biome [22]. MAP is approximately 1200 mm declining inland. Most days have high humidity. Mean January maximum temperature is 29°C, mean minimum 25°C and for July, 24°C and 12°C, respectively. There is no frost. The sandy soils are highly leached and nutrient poor. All study areas had a mosaic of natural grasslands, pine plantations and secondary grasslands formed after removal of a plantation. Pine species used for these plantations were Pinus patula and P. elliottii. Natural grasslands are defined here as grasslands with no history of afforestation or ploughing according to forestry or conservation records, or from examination of historical aerial photographs.
(a). Plot selection
Plots were spread out through each of the sample areas in natural and secondary grasslands. These are referred to as ‘vegetation treatments'. Plots in the two treatments were selected to be similar in geologies, post-burn age (recently burnt, less than 1 year, or not), and were within close proximity (less than 1 km). Where possible, age (time since plantation clear-felling) treatments were included. Plots were randomly selected within a treatment using a spun stick.
MNR had 32 plots, 16 for each vegetation treatment with 164 species recorded. All plots were burnt annually. Plots ranged between 2000 and 2080 m.a.s.l. Recovering plot ages ranged from 10 to 40 years since clear-felling. BKNR had 24 plots, 12 for each vegetation treatment with 174 species recorded. All plots were burnt every second year. Plots ranged from 1600 and 1750 m.a.s.l. Secondary grassland plots were 20 years old. Intact and recently clear-felled and burnt plantations were also sampled. ESI had 64 plots, 32 per vegetation treatment with 305 species recorded. Post-burn age varied within the study area. Fire return intervals varied from 1 to 3 years. Plots ranged between 30 and 90 m.a.s.l. The time since clear-felling ranged from 4 to 18 years.
3. Material and methods
Plot sampling occurred during two summer growth seasons from late September until late February. We used a nested quadrat method [37]. Plots were nested 1 × 1 m, 1 × 2 m, 2 × 2 m all nested within a circular quadrat of 5 m radius (total plot area = 78.5 m2). To determine species in the understory of plantations, and in recently clear-felled plots, transects of 78 × 1 m were sampled. Secondary grassland recovery age was determined from records provided by reserve managers.
(a). Plant survey
Plant species were recorded in the initial 1 × 1 m plot. Additional species were added with successive quadrat sizes giving a cumulative total. The first individual of each plant species was excavated for further species identification and documentation of plant functional traits, specifically below-ground root structure. Within the initial 1 m2 plot, the total plant ground cover was assessed and the top five most abundant species were identified and their individual percentage cover estimated. Species were noted as resprouters if they had any USOs in the form of a thickened underground structure (roots, tubers, bulbs, rhizomes, etc.; electronic supplementary material, figure S1) [36,38].
(b). Underground biomass survey
At MNR and ESI, we estimated underground forb biomass. Within the plots, an area of 50 × 50 cm was excavated to a depth of 25 cm. Below-ground biomass of non-grassy plants was removed. Root structures were separated from soil and grass roots using a 2 mm sieve. The wet weight was measured immediately after excavation. Samples were dried in a drying oven at 70°C for 96 h to obtain dry weight.
(c). Data analysis
To gauge the impact of afforestation on grasslands, we used species richness as a major response variable. Species composition may be strongly impacted by tree planting but requires different metrics and analyses. We include an analysis of species turnover within plots which indicates heterogeneity from plot to plot for a study area within each treatment. To help generalize the results, we also considered major growth forms, graminoids (grasses, sedges), forbs (non-grassy herbaceous species), woody plants (trees and shrubs) and geoxylic suffrutices. Geoxylic suffrutices are forb-like plants but with leaves emerging from an underground woody branch system. They were described as ‘underground trees' by White [39] and are often common in high rainfall, frequently burnt African savannahs [20]. Finally, plant cover was used as a variable to measure responses to treatments and to post-burn age.
(d). Species richness
GLMs were used to investigate the contribution of study area, vegetation treatment and post-burn age (recently burnt, less than 1 year or unburnt, more than 1 year since fire) and interactions between plots for all plants and for forb species in particular. Species turnover was compared between vegetation treatments using a similar method to [40], measuring intra-plot richness and accumulation by regressing the average cumulative plant and forb richness against quadrat size for each nested plot.
(e). Vegetative cover
GLMs were used to analyse plant cover. The effect of treatment (secondary or natural) and post-burn age were used in the analyses. Total vegetative cover of plots and the total cover of the five species with the highest cover per plot were used to explore treatment effects on the evenness of the distribution of cover among species.
(f). Community composition
The distribution of plant species for natural and secondary treatments for each sample area was broken down into those that only occurred in natural plots, secondary plots or those that were common to both. Plant species were placed into four growth forms: forbs, graminoids, woody species and geoxylic suffrutices for each study area. Woody species included trees and shrubs.
(g). Biomass
Dried below-ground non-graminoid root biomass was compared using a T-test between natural and secondary treatments for both MNR and ESI study sites separately. Average plot biomass was calculated as dry mass in kilogram per square metre.
(h). Succession
Both the ESI and MNR gave us the rare opportunity to investigate grassland succession during recovery. ESI had four different ages of time since recovery of up to 17 years, and MNR also had four different post-felling ages with the oldest being 40 years before this study. At MNR, we estimated initial composition as a fifth point by sampling the plantation understorey. With these datasets, we were able to determine whether or not recovering afforested areas showed a successional trajectory for plant and forb species richness. The means for all plant and forb species recorded within succession plots were regressed against age since clearance.
(i). Statistical analyses
Statistical analyses were performed using the Statistica v. 11 software package (StatSoft: Tulsa, OK, USA) with some analyses being done in R studio.
4. Results
(a). Species richness
Total plant and forb species richness in natural grasslands was two to four times greater than secondary grasslands in the three study areas (figure 1: total plants χ2 = 39.22, p < 0.0001 and table 2: forbs (F), χ2 = 28.58, p < 0.0001). The average richness of natural grasslands was lower in the coastal study area (ESI) compared with the inland montane grasslands (MNR and BKNR). Secondary grasslands had similar total plant and forb species richness across all three study areas. Post-burn counts of total and forb species richness in natural grasslands was greater than unburnt plots while secondary plots showed no such increase (figure 2: total species: F-value 17.90, p < 0.0001 and forbs: F-value 13.66, p < 0.0005). As regards heterogeneity, intra-plot species accumulation rates for natural vegetation treatments were far greater for average total plant species for all study areas (table 1).
Figure 1.
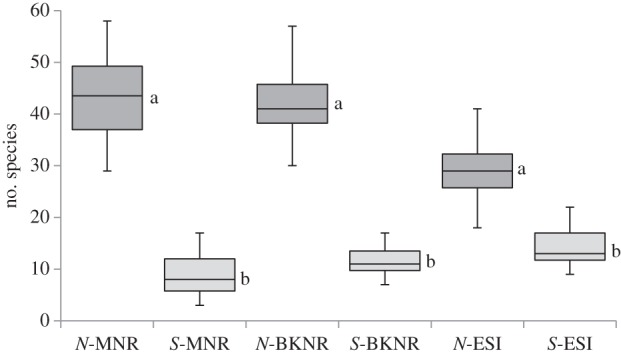
Plant species richness per nested plot (78.5 m2) for natural (N) and secondary (S) grasslands for each study area. Montane sites: MNR (n = 16 per treatment), BKNR (n = 12 per treatment). Coastal site: ESI (n = 32 per treatment). For all box-whisker plots, whiskers show maximum and minimum values, boxes show medians and quartiles.
Table 2.
Growth form composition in natural versus secondary grasslands for each study area. Values are mean number of species in each category, N, with standard error (s.e.) in parentheses. The percentage contribution is also shown. The distribution of growth forms is significantly different in natural versus secondary grasslands in MNR and ESI study areas but not BKNR. (χ2-test, d.f. = 3, MNR: χ2 = 25.8, p < 0.005, BKNR: χ2 = 4.7, p > 0.05, ESI: χ2 = 12.05, p < 0.01).
| growth form | natural |
secondary |
||
|---|---|---|---|---|
| N (s.e.) | (%) | N (s.e.) | (%) | |
| MNR | ||||
| forb | 18.5 (1.07) | 63.5 | 6.4 (0.29) | 54.6 |
| woody | 2.4 (0.20) | 8.4 | 0.2 (0.07) | 1.4 |
| geoxylic | 1.7 (0.23) | 5.8 | 0.1 (0.05) | 0.7 |
| graminoid | 6.5 (0.43) | 22.4 | 5.1 (0.39) | 43.3 |
| BKNR | ||||
| forb | 30.2 (2.08) | 69.5 | 5.4 (0.66) | 61.4 |
| woody | 0.3 (0.23) | 0.6 | 1.1 (0.18) | 12.1 |
| geoxylic | 0.4 (0.08) | 0.9 | 0.1 (0.05) | 0.7 |
| graminoid | 12.6 (0.92) | 29.1 | 2.3 (0.13) | 25.7 |
| ESI | ||||
| forb | 18.5 (1.07) | 63.5 | 6.0 (0.45) | 42.6 |
| woody | 2.4 (0.20) | 8.4 | 2.7 (0.27) | 19.3 |
| geoxylic | 1.7 (0.23) | 5.8 | 0.0 (0.00) | 0 |
| graminoid | 6.5 (0.43) | 22.4 | 5.4 (0.35) | 38.1 |
Figure 2.
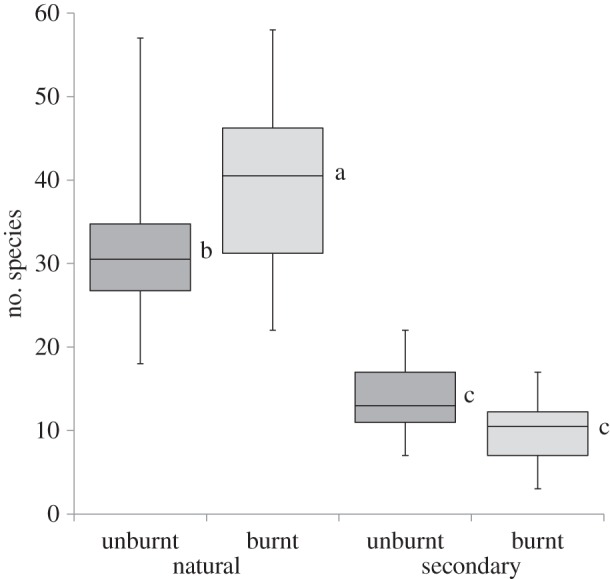
Plant species richness per nested plot in unburnt versus burnt natural and secondary grasslands. n plots: natural unburnt = secondary unburnt = 36; natural burnt = secondary burnt = 24. Plots were distributed throughout the study area.
Table 1.
Mean species richness (standard error) for nested plots of increasing area from 1 to 78 m2 for both natural (N) and secondary (S) treatments for each study area. Regressions of species on log area were, for MNR: (N: Slope = 0.25, y int. = 24.4, R2 = 0.87, p = 0.07. S: Slope = 0.07, y int. = 3.1, R2 = 0.88, p < 0.05), BKNR (N: Slope = 0.24, y int. = 23.0, R2 = 0.89, p = 0.06. S: Slope = 0.10, y int. = 4.0, R2 = 0.97, p < 0.05), ESI (N: Slope = 0.14, y int. = 18.4, R2 = 0.79, p = 0.11. S: Slope = 0.08, y int. = 8.1, R2 = 0.89, p = 0.06).
| plot size (m2) | MNR |
BKNR |
ESI |
|||
|---|---|---|---|---|---|---|
| N | S | N | S | N | S | |
| 1 | 20.2 (0.67) | 2.7 (0.43) | 19.2 (1.04) | 3.2 (0.46) | 15.2 (0.61) | 7.0 (0.55) |
| 2 | 24.8 (0.48) | 3.2 (0.16) | 24.0 (0.78) | 4.5 (0.26) | 18.9 (0.35) | 8.1 (0.21) |
| 4 | 29.9 (0.51) | 3.9 (0.25) | 27.7 (0.57) | 5.2 (0.19) | 22.3 (0.42) | 9.8 (0.25) |
| 78 | 43.4 (1.34) | 8.8 (0.88) | 41.6 (1.74) | 11.8 (0.87) | 29.1 (0.67) | 14.1 (0.42) |
(b). Understorey in pine stands
Species richness in natural plots was at least four times greater than in the plantation understorey or recently harvested pine stands plots, both of which had comparably low richness (figure 3; F-value 178.11, p < 0.0001). Abundance was also very low in both standing (5.4 ± 2.4 plants per plot) and recently cleared plantations (9 ± 2.6 plants per plot). No species were shared between the plantation understorey and the natural grasslands.
Figure 3.
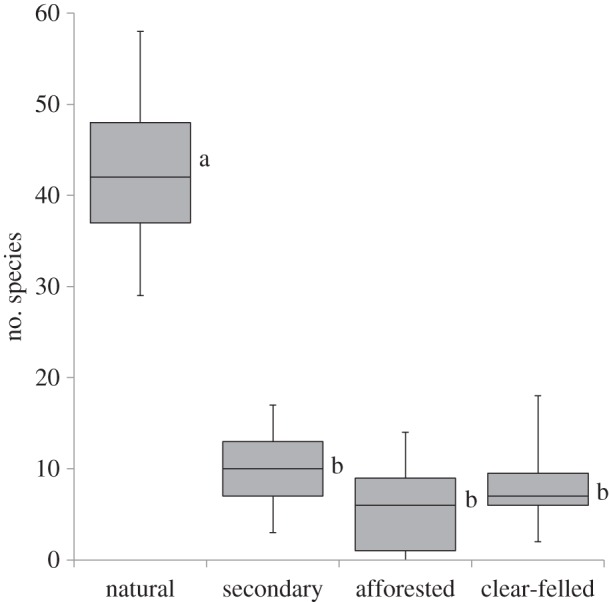
Average number of species found under afforested and recently clear-felled, burnt plots compared to natural and secondary treatment plots for the MNR and BKNR study areas.
(c). Plant ground and dominant species cover
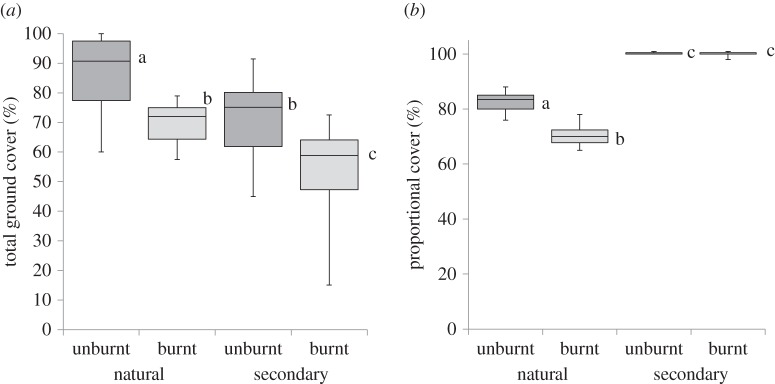
Plant cover was similar in unburnt natural and secondary grasslands but diverged after burning with lower total cover (figure 4a; F-value 27.80, p < 0.0001), and cover of the top five species, in natural versus secondary grasslands (figure 4b). There was no difference between cover of the dominant species in burnt and unburnt secondary grasslands (figure 4b; F-value 67.20, p < 0.0001). The cover of the top five dominant species in all natural vegetation plots was less compared with secondary vegetation plots (figure 4b; F-value, 1017.94, p < 0.0001). Rank abundance curves differed strikingly in natural versus secondary grasslands, with the former showing a more even distribution of cover among species, while secondary grasslands tended towards single species dominance (figure 5). The species composition of the five dominant species (measured as cover) was strikingly different in natural versus secondary grasslands and these differences were maintained for decades after clear-felling (electronic supplementary material, table S1). Themeda triandra, a member of the Andropogoneae, was the most abundant grass in the montane natural grasslands and common in the coastal grasslands but absent from secondary grasslands. Eragrostis curvula (Chloridoideae) was the dominant grass in montane secondary grasslands and retained this dominance in 40 year old stands.
Figure 4.
Plant cover (a) and the proportion of cover (b) taken up by the five top-ranked species in natural versus secondary grasslands in burnt versus unburnt treatments.
Figure 5.
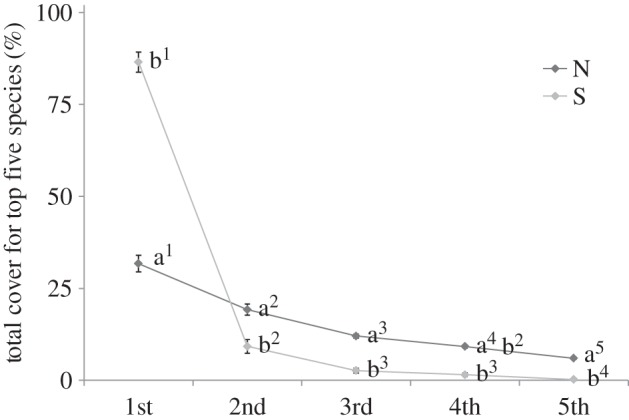
Average total percentage cover for each of the five top-ranked species per plot for each vegetative treatment: N, natural; S, secondary. Numbers on the x-axis indicate species rank, 1st = species with greatest cover, 2nd = next greatest, etc.
(d). Community composition
Most forb species were restricted to natural treatments in each of the study areas. As a percentage of all forb species, 69% were recorded only in natural grasslands at MNR, 77% at BKNR and 65% in ESI. Forb species restricted to secondary grasslands accounted for only 8% of all forb species at MNR, 13% at BKNR and 17% at ESI. Only a small proportion of forbs were shared between primary and secondary grasslands (MNR 23%, BKNR 9%, ESI 18%). In terms of growth form composition, forbs made up the majority of plant species in plots, with graminoids being the second most abundant (table 2). The species numbers in the different growth form types changed dramatically between vegetation treatments. In secondary grassland plots, species numbers in different plant functional types were far less than primary grasslands with the exception of woody plants (table 2). Geoxylic suffrutices, common in the coastal grasslands of ESI, all but disappeared in secondary grasslands and those that remained may have been remnants that survived afforestation. Changes in the proportional contribution of growth forms varied among sites (table 2). Forbs had proportionally fewer species and woody plants proportionally more in secondary grasslands. Graminoid species were proportionally more common in the two montane sites but not the coastal site. Change in the number of species in each growth form was a more consistent indicator than proportional changes among growth forms in distinguishing primary from secondary grasslands in our dataset (table 2).
(e). Below-ground biomass
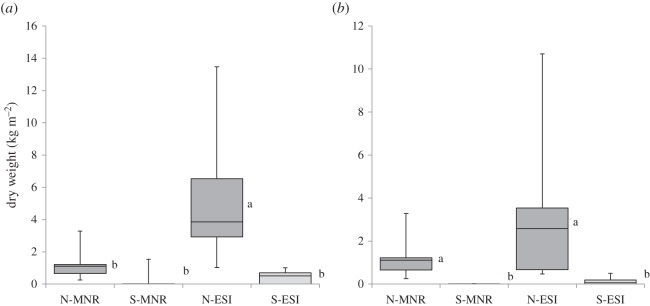
In the natural grasslands, below-ground biomass of non-graminoid species averaged 5.33 kg m−2 for ESI and 1.41 kg m−2 for MNR. These values were far greater than below-ground biomass of secondary grasslands (0.41 and 0.26 kg m−2, respectively; figure 6a; T-value −3.54, p < 0.005 and T-value −2.39, p < 0.05). Forb root biomass showed a similar pattern with natural dry mass in ESI (3.17 kg m−2) and MNR (1.30 kg m−2) being significantly greater than the secondary plot averages (0.12 and 0.01 kg m−2, respectively; figure 6b; T-value −2.57, p < 0.05 and T-value −2.75, p < 0.05). The non-graminoid biomass of natural grasslands at MNR was made up almost entirely of forbs while a large proportion (40%) of ESI biomass was made up of geoxylic suffrutices. For the secondary grasslands, forbs made up only 3% of the root biomass at MNR with the rest being rhizomes of bracken fern. At ESI, the proportion of forb root biomass dropped from 60% in old growth grasslands to 30% of the below-ground biomass in secondary grasslands with the remainder being made up mostly of the roots of the shrub Helichrysum kraussii and also alien woody species such as guava (Psidium guajava) and Chromolaena odorata.
Figure 6.
Average below-ground biomass of non-graminoid plant species (dry mass in kg m−2) for two study areas, MNR and the ESI. (a) Non-graminoid root biomass (includes woody species, i.e. geoxylic suffrutices) and (b) forb only root biomass for natural (N) and secondary (S) treatments.
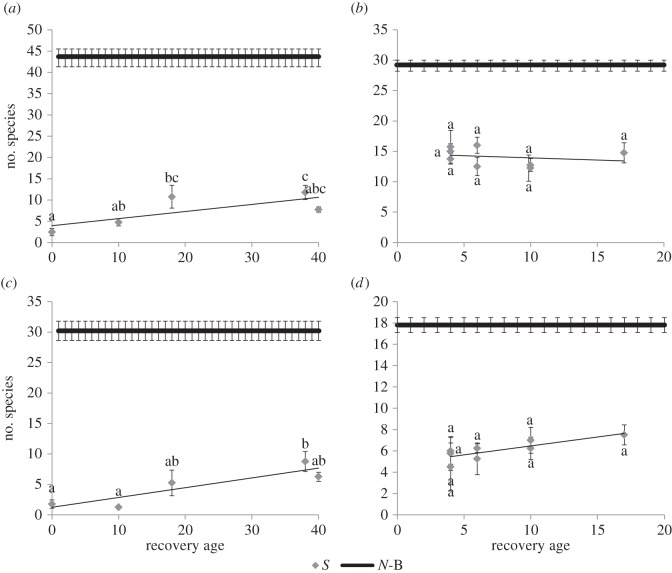
(f). Succession
There was no successional trend of increasing plant and forb species richness at ESI but a slight increase from pine understorey (t0) to mid-successional stages at MNR (figure 7a–d, Tukey post-hoc tests). No turnover in species composition was seen between younger and older secondary MNR plots, with the same species being dominant across ages. Some turnover in species richness was seen in the older secondary ESI plots but these were only additional pioneer or weedy species not found in natural plots.
Figure 7.
Change in species richness with increasing time since deforestation for MNR (a,c—40 years) and ESI study areas (b,d—20 years). Panels (a,b) show total species richness with time and (c,d) show forb species richness with time: (a) R2 = 0.43, p < 0.0005, (b) R2 = 0.00, p = 0.88, (c) R2 = 0.50, p < 0.0001, (d) R2 = 0.04, p = 0.30). The solid line (N-B) indicates the average number of species for natural treatments and the grey diamonds (S) indicate averages for secondary treatments.
5. Discussion
Natural grasslands were diverse in species, dominated by a huge variety of forb species but also rich in several other plant elements, including geoxylic suffrutices. The montane grasslands had even higher species richness per metre squared than their coastal counterpart. Coastal grasslands, however, sharing a landscape mosaic with coastal forests, had a much higher diversity of geoxylic suffrutices. Forb richness in natural grasslands was seemingly increased in burnt areas, possibly as a result of fire-stimulated growth and flowering of forb species. This post-burn growth flush is promoted by decreased ground cover in post fire environments as above-ground competition is reduced [41]. Post-burn flowering of forbs, before grasses suppress them, is a common feature of high rainfall South African grasslands [42].
(a). What is the impact of afforestation on ‘old growth’ grasslands?
The composition of secondary grasslands differed fundamentally from natural grasslands, a result that was repeated in all three study sites. Coastal secondary grasslands and montane secondary grasslands supported far more homogeneous communities that are species poor with little intra-plot species turnover. Secondary grasslands were dominated by a few widespread invasive species and communities tended to be monospecific. Burnt secondary grasslands had a barren appearance. The plants within them had no fire-stimulated flowering response. Unlike natural grasslands, there was no change in proportional plant cover, or a seeming increase in post-burn forb richness, as there were no forbs with USOs to take advantage of the reduced grass competition. Grasses therefore remained the visually dominant plant life form.
Secondary grasslands were not only missing a whole suite of natural grassland species, but families and plant functional types had been lost. Most notable was the loss of resprouting forbs. Natural grasslands contained up to 31 Mg ha−1 of below-ground forb root mass compared with less than 2 Mg ha−1 of below-ground biomass in secondary grasslands. Resprouting forbs had been replaced by weedy or woody elements, members of the Asteraceae and Fabaceae being the most frequent. The large quantity of underground forb biomass probably contributes significantly to the carbon cycle, trapping carbon in the soil within perennial USOs. When these forbs are removed, all this carbon is released back into the atmosphere while secondary grasslands lack the forbs with large USOs and may therefore be slow to sequester carbon or contribute little to carbon sequestration and storage in general. In this study, we only sampled below-ground biomass of the non-graminoid plant species. However, grass root biomass, which appeared to be far lower in secondary grassland, also contributes to carbon stocks in natural grasslands [43].
There was little evidence of any successional trend even after 40 years of passive recovery. There appears to be a biological limitation in respect of natural forb ability to easily colonize secondary grasslands, also noted in [37]. Traits that promote persistence come at the cost of those that promote recruitment [44]. This trade-off makes grassland forbs poor colonizers and probably contributes to the very slow successional trend towards a natural grassland state. Seed and dispersal limitations as key factors limiting restoration have been noted in many other instances of restoration, particularly in grassland systems [45–48].
(b). Distinguishing characteristics of ‘old growth’ grasslands
This research indicated several possible criteria that could be used to identify old growth grasslands and distinguish them from secondary grasslands. There was a clear difference in all study areas in the diversity and cover of plant species. Old growth grasslands had (i) greater evenness in the cover of dominant grasses combined with (ii) a high alpha diversity of forb species and (iii) a high underground biomass of resprouting forbs characterized by large USOs. These criteria are particularly obvious in the first growing season after a burn. The prominence of fire-stimulated flowering in the forb component in old growth grasslands is also a clear distinction, with negligible post-burn flowering in secondary grasslands.
A few more characteristic features of old growth grasslands could be added to this list, but were not uniform across study sites. These include a high abundance of geoxylic suffrutices in the lowland grasslands. Although this growth form occurs in some upland grasslands exposed to frost, it is far more common in grasslands that do not experience frost but where there are other constraints on plant growth such as nutrient poor soils or seasonal waterlogging [20]. In South Africa, the presence of ‘rooigras’ (Themeda triandra), an important natural grassland grass species, was also an indicator of old growth grasslands. The species has a reddish appearance in the dry season in common with other members of the Andropogoneae. This contrasts with the pale grey colour of chloridoid grasses, such as Eragrostis curvula, which are characteristic of secondary grasslands. If this pattern proves more general, it is a potential indicator of species-rich old growth versus secondary grasslands visible from satellite imagery. Similar differences in grass species composition, potentially detectable by remote sensing, have also been reported for old growth versus secondary grasslands in South America [49].
6. Conclusion
As the threat of climate change and the increasing pressure on vegetated systems from human-related activities increases, restoration has become a top global priority, especially in forested areas [3,4]. Our work adds to case studies elsewhere [21] in suggesting that unchecked ‘reforestation’ of ‘degraded’ lands, which are in fact ‘old growth’ grasslands, poses a serious threat to the biodiversity of grassy systems. Our results show that identification of natural grassy systems is technically possible. However, further regional studies are needed to help distinguish old growth from secondary grasslands elsewhere when making decisions as to where to conserve ancient grasslands. To emphasize this point, we note, for example, that underground trees (geoxylic suffrutices), though common in southern African savannahs and Brazilian Cerrado, are apparently absent from the savannahs of West Africa and Australia [39]. Thus, different regions may have different indicators of old growth grasslands.
The ecosystem services provided by grasslands need to be carefully evaluated relative to proposed reforestation schemes to ensure that local needs are not displaced by global geoengineering [50]. In South Africa, grasslands are the preferred vegetative cover in the major mountain catchments of the country based on the results of long-term experiments showing the negative effects of afforestation on streamflow [31]. Furthermore, the net effect of large-scale afforestation is a relatively small reduction in atmospheric carbon [6] and has seldom been evaluated relative to changes in solar energy absorbed by the dark surface of forests versus the light, reflective surfaces of grasslands [51,52]. Reforestation of deforested land is clearly beneficial in many circumstances. However, the global scale of recent ‘reforestation’ proposals needs careful evaluation of the net costs and benefits, including the assumed major benefit of mitigating global warming [50]. Old growth grasslands are of high intrinsic conservation value and merit much more attention as the other major tropical biome.
Supplementary Material
Acknowledgements
We would like to thank the staff of Mpumalanga Parks Board, EKZN Wildlife and iSimangaliso Wetland Authority for helping facilitate access to study areas, in particular, Mervyn Lotter from MBP, Dirk Rousouw, the EKZN head ranger for the Eastern Shores and Nerosha Govender and Bronwyn James of IWA. I would also like to thank Makobulaan Nature Reserve and John and Sandy Burrows of Buffelskloof Nature Reserve for their help and interest in allowing this project to happen, and Rob Skelton, Cathy Pineo and Alex Zaloumis for the field assistance.
Data accessibility
All data are available through contacting the primary author Nicholas Zaloumis in the form of Excel spreadsheets. Statistica and MS Excel where used in the analysis of the data.
Authors' contributions
Both authors contributed equally to this study.
Competing interests
We have no competing interests.
Funding
Funding for this study was provided by Andrew W Mellon foundation to W.J.B. and Andrew W Mellon foundation, the SANBI Grasslands Project and the Postgraduates Funding Office of the University of Cape Town to N.P.Z.
References
- 1.DeFries RS, Houghton RA, Hansen MC, Field CB, Skole D, Townshend J. 2002. Carbon emissions from tropical deforestation and regrowth based on satellite observations for the 1980s and 1990s. Proc. Natl Acad. Sci. USA 99, 14 256–14 261. ( 10.1073/pnas.182560099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harris NL, Brown S, Hagen SC, Saatchi SS, Petrova S, Salas W, Hansen MC, Potapov PV, Lotsch A. 2012. Baseline map of carbon emissions from deforestation in tropical regions. Science 336, 1573–1576. ( 10.1126/science.1217962) [DOI] [PubMed] [Google Scholar]
- 3.Chazdon RL. 2008. Beyond deforestation: restoring forests and ecosystem services on degraded lands. Science 320, 1458–1460. ( 10.1126/science.1155365) [DOI] [PubMed] [Google Scholar]
- 4.Suding KN. 2011. Toward an era of restoration in ecology: successes, failures, and opportunities ahead. Annu. Rev. Ecol. Evol. Syst. 42, 465–487. ( 10.1146/annurev-ecolsys-102710-145115) [DOI] [Google Scholar]
- 5.Niles JO, Brown S, Pretty J, Ball AS, Fay J. 2002. Potential carbon mitigation and income in developing countries from changes in use and management of agricultural and forest lands. Phil. Trans. R. Soc. Lond. A 360, 1621–1639. ( 10.1098/rsta.2002.1023) [DOI] [PubMed] [Google Scholar]
- 6.Canadell JG, Raupach MR. 2008. Managing forests for climate change mitigation. Science 320, 1456–1457. ( 10.1126/science.1155458) [DOI] [PubMed] [Google Scholar]
- 7.Greve M, Reyers B, Lykke AM, Svenning JC. 2013. Spatial optimization of carbon-stocking projects across Africa integrating stocking potential with co-benefits and feasibility. Nat. Commun. 4, 2975 ( 10.1038/ncomms3975) [DOI] [PubMed] [Google Scholar]
- 8.World Resources Institute 2014. Atlas of forest and landscape restoration opportunities. Washington, DC: World Resources Institute; (www.wri.org/resources/maps/Atlas-forest-and-landscape-restoration-opportunities) (accessed 1 June 2015). [Google Scholar]
- 9.Laestadius L, Maginnis S, Minnemeyer S, Potapov P, Saint-Laurent C, Sizer N. 2011. Mapping opportunities for forest landscape restoration. Unasylva 238, 47–48. [Google Scholar]
- 10.Aubreville A. 1947. La mort des forêts de l'Afrique tropicale. Unasylva 1, 5–11. [Google Scholar]
- 11.Staver AC, Archibald S, Levin SA. 2011. The global extent and determinants of savanna and forest as alternative biome states. Science 334, 230–232. ( 10.1126/science.1210465) [DOI] [PubMed] [Google Scholar]
- 12.Staver AC, Archibald S, Levin S. 2011. Tree cover in sub-Saharan Africa: rainfall and fire constrain forest and savanna as alternative stable states. Ecology 92, 1063–1072. ( 10.1890/10-1684.1) [DOI] [PubMed] [Google Scholar]
- 13.Hirota M, Holmgren M, Van Nes EH, Scheffer M. 2011. Global resilience of tropical forest and savanna to critical transitions. Science 334, 232–235. ( 10.1126/science.1210657) [DOI] [PubMed] [Google Scholar]
- 14.Lehmann CE, Archibald SA, Hoffmann WA, Bond WJ. 2011. Deciphering the distribution of the savanna biome. New Phytol. 191, 197–209. ( 10.1111/j.1469-8137.2011.03689.x) [DOI] [PubMed] [Google Scholar]
- 15.Parr CL, Lehmann CE, Bond WJ, Hoffmann WA, Andersen AN. 2014. Tropical grassy biomes: misunderstood, neglected, and under threat. Trends Ecol. Evol. 29, 205–213. ( 10.1016/j.tree.2014.02.004) [DOI] [PubMed] [Google Scholar]
- 16.Cerling TE, Harris JM, MacFadden BJ, Leakey MG, Quade J, Eisenmann V, Ehleringer JR. 1997. Global vegetation change through the Miocene/Pliocene boundary. Nature 389, 153–158. ( 10.1038/38229) [DOI] [Google Scholar]
- 17.Ségalen L, Lee-Thorp JA, Cerling T. 2007. Timing of C4 grass expansion across sub-Saharan Africa. J. Hum. Evol. 53, 549–559. ( 10.1016/j.jhevol.2006.12.010) [DOI] [PubMed] [Google Scholar]
- 18.Simon MF, Grether R, de Queiroz LP, Skema C, Pennington RT, Hughes CE. 2009. Recent assembly of the Cerrado, a neotropical plant diversity hotspot, by in situ evolution of adaptations to fire. Proc. Natl Acad. Sci. USA 106, 20 359–20 364. ( 10.1073/pnas.0903410106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simon MF, Pennington T. 2012. Evidence for adaptation to fire regimes in the tropical savannas of the Brazilian Cerrado. Int. J. Plant Sci. 173, 711–723. ( 10.1086/665973) [DOI] [Google Scholar]
- 20.Maurin O, Davies TJ, Burrows JE, Daru BH, Yessoufou K, Muasya AM, van der Bank M, Bond WJ. 2014. Savanna fire and the origins of the ‘underground forests’ of Africa. New Phytol. 204, 201–214. ( 10.1111/nph.12936) [DOI] [PubMed] [Google Scholar]
- 21.Veldman JW, et al. 2015. Toward an old-growth concept for grasslands, savannas, and woodlands. Front. Ecol. Environ. 13, 154–162. ( 10.1890/140270) [DOI] [Google Scholar]
- 22.Mucina L, Rutherford MC. 2006. The vegetation of South Africa, Lesotho and Swaziland. Strelitzia, 19. Pretoria, South Africa: SANBI. [Google Scholar]
- 23.Acocks JPH. 1953. Veld types of South Africa. Mem. Bot. Surv. SA 28, 1–192. [Google Scholar]
- 24.Feely JM. 1980. Did iron-age man have a role in the history of Zululand wilderness landscapes. S. Afr. J. Sci. 76, 150–152. [Google Scholar]
- 25.Meadows ME, Linder HP. 1993. A palaeoecological perspective on the origin of Afromontane grasslands. J. Biogeogr. 20, 345–355. ( 10.2307/2845584) [DOI] [Google Scholar]
- 26.Scott L. 2002. Grassland development under glacial and interglacial conditions in southern Africa: review of pollen, phytolith and isotope evidence. Palaeogeogr. Palaeoclimatol. Palaeoecol. 177, 47–57. ( 10.1016/S0031-0182(01)00351-0) [DOI] [Google Scholar]
- 27.West AG, Bond WJ, Midgley JJ. 2000. Soil carbon isotopes reveal ancient grassland under forest. S. Afr. J. Sci. 96, 253. [Google Scholar]
- 28.Gillson L. 2015. Evidence of a tipping point in a southern African savanna? Ecol. Complexity 21, 78–86. ( 10.1016/j.ecocom.2014.12.005) [DOI] [Google Scholar]
- 29.Tainton NM. 1999. Veld management in South Africa. Pietermaritzburg, South Africa: University of Natal Press. [Google Scholar]
- 30.Bosch JM, Hewlett JD. 1982. A review of catchment experiments to determine the effect of vegetation changes on water yield and evapotranspiration. J. Hydrol. 55, 3–23. ( 10.1016/0022-1694(82)90117-2) [DOI] [Google Scholar]
- 31.Dye P, Versfeld D. 2007. Managing the hydrological impacts of South African plantation forests: an overview. For. Ecol. Manag. 251, 121–128. ( 10.1016/j.foreco.2007.06.013) [DOI] [Google Scholar]
- 32.Bond WJ, Parr CL. 2010. Beyond the forest edge: ecology, diversity and conservation of the grassy biomes. Biol. Conserv. 143, 2395–2404. ( 10.1016/j.biocon.2009.12.012) [DOI] [Google Scholar]
- 33.O'Connor TG. 2005. Influence of land use on plant community composition and diversity in Highland Sourveld grassland in the southern Drakensberg, South Africa. J. Appl. Ecol. 42, 975–988. ( 10.1111/j.1365-2664.2005.01065.x) [DOI] [Google Scholar]
- 34.Cowling RM, Hilton-Taylor C. 1997. Phytogeogaphy, flora and endemism. In Vegetation of Southern Africa (eds Cowling RM, Richardson DM, Pierce SM), pp. 43–61. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 35.Carbutt C, Edwards TJ. 2003. The flora of the Drakensberg alpine centre. Edinb. J. Bot. 60, 581–607. ( 10.1017/S0960428603000428) [DOI] [Google Scholar]
- 36.Uys R. 2006. Patterns of plant diversity and their management across South African rangelands. Dissertation, University of Cape Town.
- 37.Zaloumis NP, Bond WJ. 2011. Grassland restoration after afforestation: no direction home? Austral Ecol. 36, 357–366. ( 10.1111/j.1442-9993.2010.02158.x) [DOI] [Google Scholar]
- 38.Overbeck GE, Pfadenhauer J. 2007. Adaptive strategies in burned subtropical grassland in southern Brazil. Flora-Morphol. Distrib. Funct. Ecol. Plants 202, 27–49. ( 10.1016/j.flora.2005.11.004) [DOI] [Google Scholar]
- 39.White F. 1976. The underground forests of Africa: a preliminary review. Gardens’ Bull. Singap. 24, 57–71. [Google Scholar]
- 40.Schwilk DW, Keeley JE, Bond WJ. 1997. The intermediate disturbance hypothesis does not explain fire and diversity pattern in fynbos. Plant Ecol. 132, 77–84. ( 10.1023/A:1009755320731) [DOI] [Google Scholar]
- 41.Uys RG, Bond WJ, Everson TM. 2004. The effect of different fire regimes on plant diversity in southern African grasslands. Biol. Conserv. 118, 489–499. ( 10.1016/j.biocon.2003.09.024) [DOI] [Google Scholar]
- 42.Lamont BB, Downes KS. 2011. Fire-stimulated flowering among resprouters and geophytes in Australia and South Africa. Plant Ecol. 212, 2111–2125. ( 10.1007/s11258-011-9987-y) [DOI] [Google Scholar]
- 43.Mills AJ, O'Connor TG, Donaldson JS, Fey MV, Skowno AL, Sigwela AM, Lechmere-Oertel RG, Bosenberg JD. 2005. Ecosystem carbon storage under different land uses in three semi-arid shrublands and a mesic grassland in South Africa. S. Afr. J. Plant Soil 22, 183–190. ( 10.1080/02571862.2005.10634705) [DOI] [Google Scholar]
- 44.Bond WJ, Midgley JJ. 2001. Ecology of sprouting in woody plants: the persistence niche. Trends Ecol. Evol. 16, 45–51. ( 10.1016/S0169-5347(00)02033-4) [DOI] [PubMed] [Google Scholar]
- 45.Pywell RF, Bullock JM, Hopkins A. 2002. Restoration of species-rich grassland on arable land: assessing the limiting processes using a multi-site experiment. J. Appl. Ecol. 39, 294–309. ( 10.1046/j.1365-2664.2002.00718.x) [DOI] [Google Scholar]
- 46.Buisson E, Dutoit T. 2004. Colonisation by native species of abandoned farmland adjacent to a remnant patch of Mediterranean steppe. Plant Ecol. 174, 371–384. [Google Scholar]
- 47.Prober SM, Thiele KR. 2005. Restoring Australia's temperate grasslands and grassy woodlands: integrating function and diversity. Ecol. Man. Rest. 6, 16–27. ( 10.1111/j.1442-8903.2005.00215.x) [DOI] [Google Scholar]
- 48.Kardol P, Wal AVD, Bezemer TM, Boer WD, Duyts H, Holtkamp R, Putten WH. 2008. Restoration of species-rich grasslands on ex-arable land: seed addition outweighs soil fertility reduction. Biol. Conserv. 141, 2208–2217. ( 10.1016/j.biocon.2008.06.011) [DOI] [Google Scholar]
- 49.Veldman JW, Putz FE. 2011. Grass-dominated vegetation, not species-diverse natural savanna, replaces degraded tropical forests on the southern edge of the Amazon Basin. Biol. Conserv. 144, 1419–1429. ( 10.1016/j.biocon.2011.01.011) [DOI] [Google Scholar]
- 50.Veldman JW, et al. 2015. Where tree planting and forest expansion are bad for biodiversity and ecosystem services. BioScience 65, 1011–1018. ( 10.1093/biosci/biv118) [DOI] [Google Scholar]
- 51.Jackson RB, et al. 2008. Protecting climate with forests. Environ. Res. Lett. 3, 044006 ( 10.1088/1748-9326/3/4/044006) [DOI] [Google Scholar]
- 52.Naick M, Abiodun BJ. In press Potential impacts of forestation on future climate change in Southern Africa. Int. J. Climatol. ( 10.1002/joc.4652) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available through contacting the primary author Nicholas Zaloumis in the form of Excel spreadsheets. Statistica and MS Excel where used in the analysis of the data.