Abstract
Woody encroachment in ‘open’ biomes like grasslands and savannahs is occurring globally. Both local and global drivers, including elevated CO2, have been implicated in these increases. The relative importance of different processes is unresolved as there are few multi-site, multi-land-use evaluations of woody plant encroachment. We measured 70 years of woody cover changes over a 1020 km2 area covering four land uses (commercial ranching, conservation with elephants, conservation without elephants and communal rangelands) across a rainfall gradient in South African savannahs. Different directions of woody cover change would be expected for each different land use, unless a global factor is causing the increases. Woody cover change was measured between 1940 and 2010 using the aerial photo record. Detection of woody cover from each aerial photograph was automated using eCognitions' Object-based image analysis (OBIA). Woody cover doubled in all land uses across the rainfall gradient, except in conservation areas with elephants in low-rainfall savannahs. Woody cover in 2010 in low-rainfall savannahs frequently exceeded the maximum woody cover threshold predicted for African savannahs. The results indicate that a global factor, of which elevated CO2 is the likely candidate, may be driving encroachment. Elephants in low-rainfall savannahs prevent encroachment and localized megafaunal extinction is a probable additional cause of encroachment.
This article is part of the themed issue ‘Tropical grassy biomes: linking ecology, human use and conservation’.
Keywords: savannah, woody encroachment, woody thickening, land use, aerial photography, tree cover
1. Introduction
Increases in woody cover have been documented across the globe [1–3] with the largest increases occurring in the open ecosystems (grasslands [4], tundra [3], savannahs [1]). Woody encroachment in savannahs is most frequently attributed to changes in land management, particularly the alteration of fire and herbivory [1,5,6]. More recently, however, studies suggest that global factors, particularly elevated CO2 [7–9] and changes in rainfall regimes [10] can be the primary cause of encroachment. A clear overview of the different causes of encroachment is difficult to obtain as the scale and magnitude of the changes are not explicit, and conclusions of the causes of change are often based on localized studies, making it difficult to gain a regional perspective as to which factors are causing the change. To address this we have conducted a multi-site, multi-land-use evaluation of woody plant encroachment across South African savannahs.
Fire and herbivory are frequently altered in the management and utilization of savannah rangelands. Changes in either can influence trajectories of woody cover change over time. Sustained heavy livestock grazing reduces above- and below-ground grass biomass [11], benefitting tree growth and establishment by increasing tree rooting spaces and soil moisture availability [10]. However, where grazing lawns are created, tree seedling establishment is prevented. Fire suppression and the reduction in grass biomass which reduces fire frequency and intensity [12], increases the likelihood of sapling escape from the fire trap [6,13], increasing woody biomass over time [1,8]. On the other hand, browsers, ranging from goats to elephants can reduce tree seedling survival and growth rates [14–16]. In Africa, the recent widespread elimination of megafauna, e.g. elephants, and the overall reduction in the numbers of mammal browsers [17,18], can be considered as an alternative cause of wide-scale woody cover increases. This is, however, set against a backdrop of increasing human densities and use that exert heavy pressures on woody plants by harvesting [19–21] to the extent that widespread decreases in woody cover are expected for several areas [19,22].
Increasing atmospheric CO2 concentrations have been proposed to drive woody encroachment. Elevated CO2 can enhance plant water-use efficiencies (WUE) via reduced stomatal conductance [23,24] which can improve seedling survival and plant growth rates. This mechanism is likely most effective in water-limited savannahs as these changes are the equivalent of increasing water availability. Trees in high-rainfall savannahs (mean annual precipitation; MAP > 650 mm) are likely to benefit directly from increased CO2 through increases in maximum photosynthetic rates which allow plants to allocate the extra carbon gains to below-ground root reserves. These form a critical resource to enhance tree growth rates and resprouting following fires and damage [25–27]. These changes are the equivalent of reducing the fire frequency in an area, as high regrowth rates increase the probability of escaping the fire trap and recruiting between fire events [8,27]. In addition, increases in MAP and changes in rainfall intensity [28,29] in water-limited savannahs could increase woody cover as maximum woody cover is constrained by water availability [30].
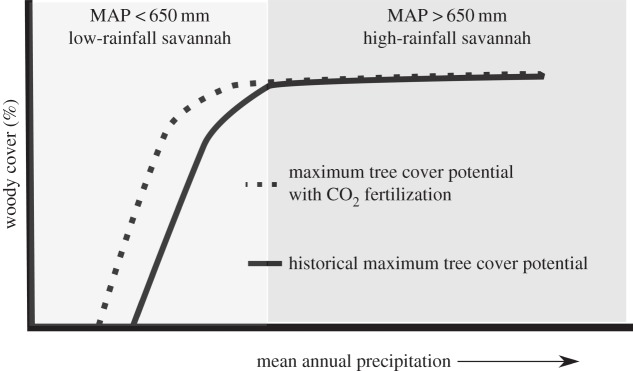
Key studies examining woody cover in African savannahs enable us to form a theoretical framework to conceptualize processes of woody cover change relative to land use, rainfall and trends of increasing CO2. In low-rainfall savannahs (MAP < 650 mm), maximum woody cover is limited by water availability so that the potential for woody encroachment is bounded (figure 1). Here, in the absence of increased rainfall, increases in woody cover beyond the maximum woody cover threshold savannahs should indicate that CO2 fertilization is occurring (figure 1). The elevated atmospheric CO2 should increase available soil moisture and, therefore, increase in the maximum woody cover threshold (definition per [30]) for a given rainfall regime. In high-rainfall savannahs, where there is sufficient water availability for canopy closure [8,30] fire, herbivory and human disturbance are the primary limits on woody cover. In these high-rainfall savannahs, the potential for greater woody cover change is high as the potential maximum woody cover is high (i.e. 80% cover (forest)) [30].
Figure 1.
Current defined limits of maximum tree cover (as defined by Sankaran et al. [30]) (solid black line) across a rainfall gradient. Maximum tree cover in low-rainfall savannahs (MAP < 650 mm) is constrained by water availability. CO2 fertilization is expected to increase the maximum tree cover, for the same amount of rainfall, as well as increase the probability of reaching maximum woody cover.
To investigate the relative importance of global versus local drivers in savannah woody cover change, we quantified the magnitude and direction of woody cover change over 1020 km2 of NE South Africa using aerial photographs across a multi-site, multi-land-use, regional-scale study. We measured woody cover change over 70 years across four different land-use types in both low- and high-rainfall savannahs. If land management is the primary determinant of woody cover change, we expect variable trajectories of woody cover change across land-use types and that the magnitude of change should vary relative to rainfall. However, if increased atmospheric CO2 concentrations drive changes in woody cover, the direction of change will be consistent across different land-use types across the rainfall gradient.
2. Material and methods
(a). Study sites
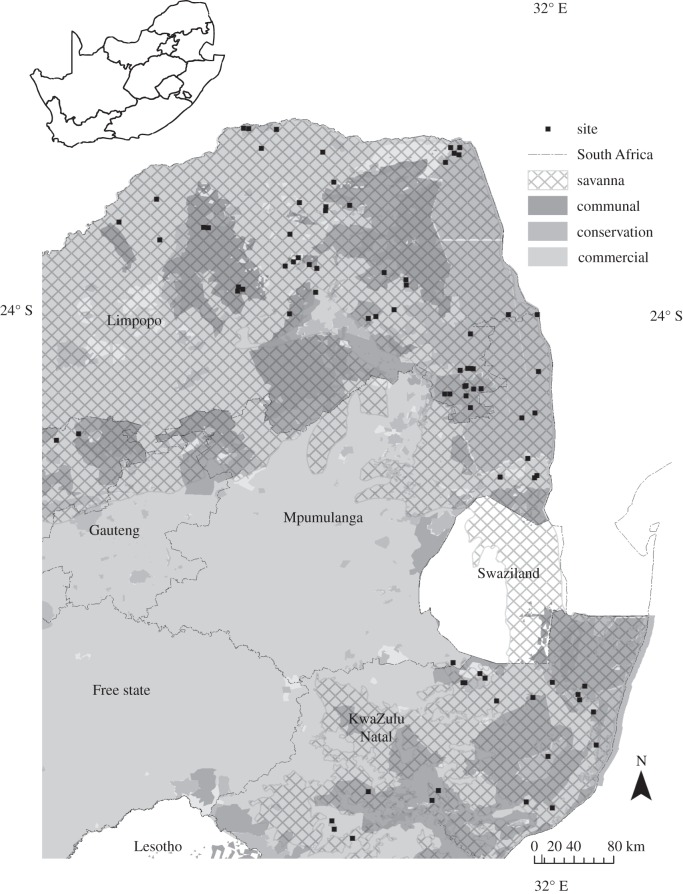
We restricted the selection of study sites to untransformed South African savannahs. The study region was located in northeast South Africa, occurring in Limpopo province, south and east Mpumulanga and the northern savannah regions of Kwazulu Natal (figure 2). Within Limpopo observed rainfall trends from 1960 to 2010 [31] demonstrate that there have been reductions in rainfall in the late wet season (January–March), and a decline in the number of rain days during the wet season (October–March) [31]. Significant increases in maximum temperatures, particularly, in the winter months have been recorded along with increases in minimum daily temperatures during the summer months. Northern KwaZulu Natal and southern Mpumulanga (figure 2) have experienced a consistent decrease in the number of rain days and the amount of rainfall in the late wet season. These changes have also been accompanied by increases in maximum daily temperatures and reductions in minimum daily temperatures [31].
Figure 2.
Site map, showing positions of 81 aerial photograph pairs in the savannah biome of South Africa. Diagonal lines indicate the limits of the savannah biome. The shades of grey indicate the different land uses. Conservation areas in this map are not separated out by elephant and non-elephant.
Using a GIS [32], we restricted the analysis within the savannah biome [33]. All transformed land was excluded from the analysis using a satellite derived landcover map of South Africa [34], where lands that had been converted or cleared were excluded. The non-transformed areas were divided into four land-use types occurring in natural land; conservation areas with elephants, conservation areas without elephants, commercial ranches and communal rangelands, each with distinct management characteristics. Each land use is expected to have different trajectories of woody cover change [35].
(1) Communal rangelands are home to subsistence farmers in rural villages, who run herds of goats (browsers) and cattle (grazers). This land use is characterized by a constant heavy wood harvesting pressure which is predicted to reduce woody cover (table 1) [19,22]. Within the study region a positive population growth rate from at least 1992–2004 occurred [21,37]. Although electrification of these areas has occurred, 90% of households still use fuelwood as a primary, cheaper energy source [37,38].
(2) Commercial rangelands are commercial cattle farming entities on natural land. The farming practice is concerned with maximizing livestock production resulting in a grazer (cattle) dominated system with moderate but constant stocking rates and a low fire frequency [6,39]. Here due to constant grazing and a policy of fire suppression, woody cover increases are predicted for commercial ranches (table 1).
(3) Conservation areas are the closest ‘control’ systems available; here, the natural processes of frequent fires and moderate herbivory (grazers and browser) are mostly maintained. A convenient distinction between conservation areas is the presence or absence of elephant, a difference that enables us to test the impact of this single agent on woody cover change (table 1).
As it is difficult to reconstruct the long-term historical land uses for all the areas, where possible we set the criteria that the present land use was the majority use for the past 20 years.
Table 1.
Land-use management characteristics, and their expected outcome on woody cover change in South Africa.
| land-use | mean no. years between fires | human population | grazer density (kg ha−1) | area sampled (km2) (site) in low-rainfall sites | area sampled (km2) (site) in high-rainfall sites |
|---|---|---|---|---|---|
| commercial | 4 | 722 759 | 36.04 | 221 (18) | 28 (5) |
| communal | 5 | 10.67 million | 49.07 | 171 (15) | 4.65 (11) |
| elephants | 2 | 20 952 | 30.48 | 15.9 (16) | 7.2 (5) |
| no elephants | 9 (5) | 6 (6) | |||
| reference | [12] | (www.census2011. ac.za) | Global Livestock Distributions 3 arc-min filled using [12,36] and data reported herein |
A database of conservation land was created using data from South African National Parks, the World database of conservation areas (www.wdpa.org), Vegmap [33] and De Beers Mining group. This represented both formal and informal conservation areas. The complete database of protected areas was joined and each area was assigned a classification of elephant present or absent. This classification was made from gathering local expert knowledge, site visits and the site specific websites. Communal areas and commercial ranches were selected from land tenure maps (used from [12]).
To ensure an equal representation of sites across the rainfall gradient, all potentially selectable areas were overlaid onto a long-term rainfall map [40]. To ensure an even spread of sites across the rainfall gradient, the savannah map was separated into rainfall areas: low rainfall (less than 650 mm MAP) and high rainfall (greater than 650 mm MAP) [30]. The division between the rainfall areas was based on the findings of Sankaran et al. [30], which separates savannahs into stable (water-limited) and unstable savannahs at 650 mm MAP. In the initial phase of site selection, 30 random points, a minimum of 4 km apart, were overlaid within each land use within each rainfall zone. The latitude and longitude of the potential sites were exported to Google Earth. The area around a point was visually inspected to determine if the area was largely untransformed (greater than 10%). Unsuitable sites, which were transformed, were discarded. The process was stopped when we found 10 suitable untransformed sites. The remaining suitable points were then each matched with the paired 2010 aerial photograph. Eighty-one aerial photo pairs were selected, covering an area of 1020 km2 in 1940 and an area of 1020 km2 in 2010 (table 1). Sites occurred across the rainfall range 302–1134 mm.
(b). Measuring woody cover change
The aerial photograph flight plans were digitized and geo-referenced using ARCMAP v. 10 [32] and the selected random points were overlaid onto the flight plans and the appropriate aerial photographs were selected. If a random point fell on an aerial photograph with a scale smaller than 1 : 25 000, it was excluded from the analysis.
The selected aerial photograph negatives were scanned at a high resolution of 600 dpi. Orthorectified colour aerial photographs from 2010 were used as a reference to quantify vegetation change since 1940. Using the 2010 orthorectified images and the flight plans for reference, the 1940 aerial photographs were geo-referenced using ERDAS Imagine 11 [41]. The georeferencing process used a minimum of five manual tie points in a third-order polynomial model. The orthorectification was accepted if the root-mean-square error (RMSE) < 4.0. The 1940 photographs were subject to image equalization and brightness adjustments to maximize woody canopy visibility.
Woody vegetation canopy cover was extracted from each photograph pair, using object-based image analysis (OBIA) (see [42,43] for similar applications). eCognition Developer [44] is a software package that implements OBIA for general image classification problems and was used here to automate woody cover detection from the aerial photographs, thus allowing a large area coverage. OBIA is particularly suited to the problem of tree detection since the classification process relies on aggregates of pixels (called objects) and the characteristics of these aggregates of pixels. Tree canopies tend to be more homogeneous compared to the surrounding vegetation, so are well suited to OBIA approaches.
Each image was segmented using a method called multi-resolution segmentation which divides the photograph into homogeneous aggregates of pixels, called objects. The size and shape of objects are determined by user-defined parameters of scale, shape and compactness. The images were segmented into objects at two difference levels, using values of 14 and 200 for the scale parameter. The shape parameter, which defines the shape when segmenting the image, was set at 0.1. The higher the value of shape the lower the influence of colour on the segmentation procedure. The compactness criteria were set at 0.5 where the higher the value the more compact the objects. These values were derived from published studies, and adapted to ensure that objects corresponded as closely as possible to tree canopies and tree clumps.
To aim for a high level of accuracy, 5% of the image (calculated as 5% of all objects) was manually classified into woody canopies and non-woody canopies; this was used as a training dataset to run a feature space optimization. For each of these selected objects (training data), 30 variables based on the colour, shape, texture and size of objects as well as their context and relationships between each other within and across scales, were calculated. The variables that resulted in the highest separation distance were used to automatically classify trees and ground layer for the entire extent of the image. After classification was complete the accuracy was assessed using the built in accuracy assessment of eCognition. An additional 100 woody and non-woody objects were manually selected (test data). These manual classifications were compared with the automated classifications using eCognition accuracy reports. If the accuracy was less than 90% then more training points were manually added and the images were re-classified. Accepted classifications were exported as rasters to Arcmap 10 [32]. Tall vegetation casts black shadows. The uniform black colour of the shadows, which differs from the varying hues in woody plants meant that shadows were not confused with woody cover during the training and classification process.
The accepted classified rasters were overlaid onto the aerial photographs and small transformations to the areas, e.g. roads and small fields were clipped and removed from both old and new images. The rasters were used to assess woody cover change from 1940. The per cent woody cover change was assessed using zonal statistics in Arcmap 10 [32]. Woody cover was assessed as the total number of woody pixels as a proportion of all the pixels in the image. Samples, totalling an area of 1020 km2, were analysed and assessed for woody cover change.
(c). Data analysis
All data were analysed using R v. 3.0 [45] with the packages ‘stats’ and ‘AICmodavg’ v. 2.0.3 for model selection and averaging. Data were normally distributed and remained untransformed. A repeated measures ANOVA was used to detect if there was any difference in woody cover across the different land uses over the rainfall gradient between 1940 and 2010. Paired t-tests were used to determine if woody cover increases were significant for each separate land use in both high- and low-rainfall savannahs.
To examine the correlates of woody cover change, we used an ANOVA in R (v. 3.1.1) [45]. Important environmental variables that also vary across this rainfall gradient were included as covariates. The covariates were assessed for high levels of correlation, before they were analysed. The following environmental correlates were considered: MAP; initial woody cover (IC); land use (LU), number of frost days (FD), mean annual temperature (MAT) and altitude. The candidate model set included all combinations of the aforementioned environmental variables. Candidate models were compared and ranked using Akaike's information criterion, corrected for small sample sizes (AICc). The model with the lowest AICc was considered to have the most support, and there were no other models with AICc values that differed by less than 2, so no model averaging was performed. We used the final parameter estimates, standard errors and confidence intervals to demonstrate the effect size of the different parameters.
To place our data in a regional context and allow comparison with past studies, we compared our data points to the equation developed by Sankaran et al. [30], which describes the upper bound of tree cover in low-rainfall areas by a 95th quantile piece-wise linear regression, with a 650 mm MAP breakpoint. As trees are typically absent below 101 mm MAP, the equation for the line quantifying the upper bound on woody cover occurs between 101 and 650 mm MAP (equation (2.1)). At higher rainfall sites maximum woody cover is bounded at 80% woody cover.
| 2.1 |
We plotted the 1940 tree cover against rainfall, restricting the analysis to areas with rainfall below 650 mm. We fitted a 95th quantile to our data and compared the linear output to equation (2.1) derived from Sankaran et al. [30]. The equation intercept of the 1940 data (y-intercept = 13.7), and the slope (0.138) closely match the fit to that of equation (2.1), thereby allowing us to relate changes in woody cover at our sites to that of previous observations.
3. Results
(a). Magnitude of total tree cover change
Woody cover increased both at low- and high-rainfall sites and across all land-use types from 1940 to 2010 with the exception of the conservation areas with elephants in low-rainfall areas (figure 3) (repeated measures ANOVA F3,145 = 857.92; p < 0.001). Paired t-tests confirmed, with one exception, that woody cover increases were significant (figure 3). In the low-rainfall commercial sites, woody cover increased from 27% to 42%, and from 20% to 46% in the high-rainfall sites. Communal rangelands experienced the greatest increase in cover which doubled (17.5–40%) in low-rainfall areas and more than doubled from 12% to 36% in high-rainfall areas. In low-rainfall conservation areas without elephants, woody cover increased from 30% to 42% and from 33% to 58% in the high-rainfall savannahs. By contrast, woody cover in the low-rainfall conservation area with elephants barely changed (33.7% to 34.2%). However, in high-rainfall savannahs when elephants were present, tree cover increased from 19.9% to 37%.
Figure 3.
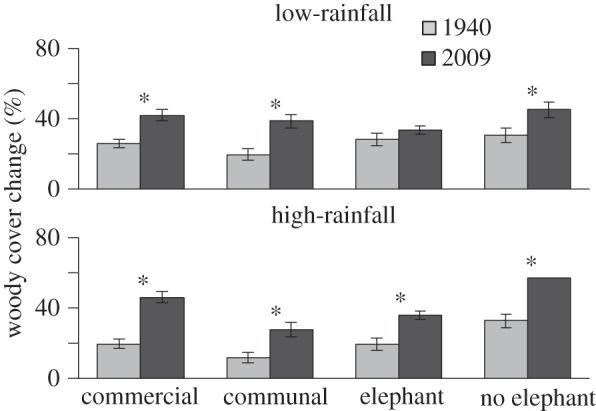
Woody cover increased significantly at low (MAP < 650 mm) and high (MAP > 650 mm) rainfall sites and across all land-use types from 1940 to 2010—the one exception being low-rainfall conservation areas with elephants which showed no significant change since 1940. Bars indicate standard errors, n = 81. Asterisks indicate significant differences as shown by paired t-tests.
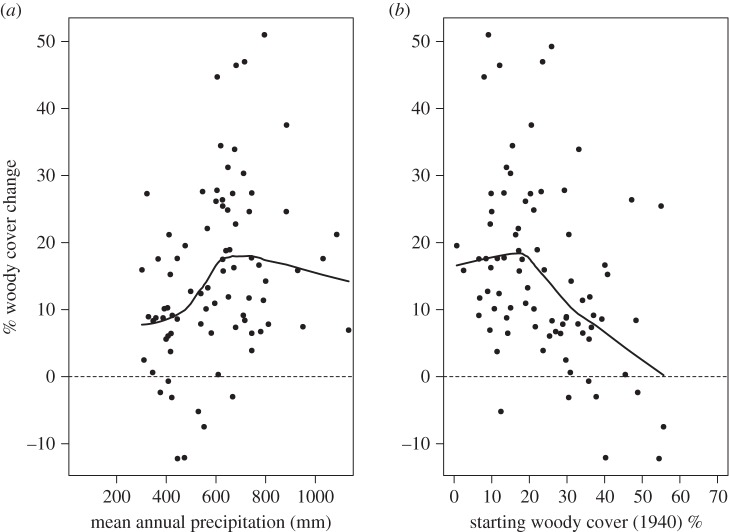
The annual rate of woody cover change was best explained by a model of land use, MAP, initial tree cover, MAT, the FD and an interaction of MAP and land use. This model explained 47.7% of the variation in rates of woody cover change (table 2). Increases in site MAP and temperature caused an increase in the rate of annual woody cover change (table 2). When MAP > 600 mm woody cover increases become larger (figure 3a). Starting woody cover also affected the vulnerability of the area to change, and the higher the starting woody cover (i.e. 1940 woody cover) the lower the potential for woody cover increase (table 2 and figure 4b). When IC exceeds 20%, the likelihood of a large increase in cover declines rapidly (figure 3b). As described above, land use interacted with rainfall.
Table 2.
Final parameter estimates (β), standard errors (s.e.) and confidence intervals of best model. Significant parameters, i.e. where confidence intervals do not overlap zero, are shown in bold. LU, land use; IC, initial cover in 1940; MAP, mean annual precipitation; FD, frost days; MAT, mean annual temperature.
| predictors | β | s.e. | lower CI | upper CI |
|---|---|---|---|---|
| (intercept) | −38.3379 | 22.4146 | −82.2697 | 5.593962 |
| LU (communal) | 27.00936 | 10.15735 | 7.10133 | 46.91739 |
| LU (cons. with elephant) | −1.14238 | 10.98495 | −22.6725 | 20.38773 |
| LU (cons) | 1.09788 | 16.73598 | −31.704 | 33.8998 |
| MAP | 0.04843 | 0.01379 | 0.021401 | 0.075461 |
| IC | −0.40649 | 0.09787 | −0.59831 | −0.21468 |
| FD | −0.09427 | 0.36059 | −0.80102 | 0.612483 |
| MAT | 2.09241 | 0.90514 | 0.318363 | 3.866457 |
| LU (communal):MAP | −0.05343 | 0.01665 | −0.08606 | −0.0208 |
| LU (cons with elephant):MAP | −0.02585 | 0.01874 | −0.06259 | 0.01088 |
| LU (cons):MAP | −0.01465 | 0.02449 | −0.06265 | 0.033346 |
Figure 4.
Scatterplot of mean annual precipitation (a) and initial (1940) cover (b) against total woody cover change (%). Lowess lines are fitted. When starting tree cover exceeds 20% the potential for woody cover increase declines. When MAP > 600 mm, tree cover changes are greatest.
A comparison of woody cover site pairs (1940 versus 2010) against the Sankaran et al. [30] relationship for maximum woody cover and MAP for African savannahs (Cover (%) = 0.14(MAP) − 14.2, between 101 mm and 674 mm) showed that 6% of the site pairs exceeded the maximum tree cover threshold in 1940 (figure 5). However, by 2010, 20% of the sites exceeded the maximum tree cover threshold calculated for African savannahs. In terms of area, 11% of the measured area exceeded the maximum woody cover threshold (figures 4 and 5).
Figure 5.
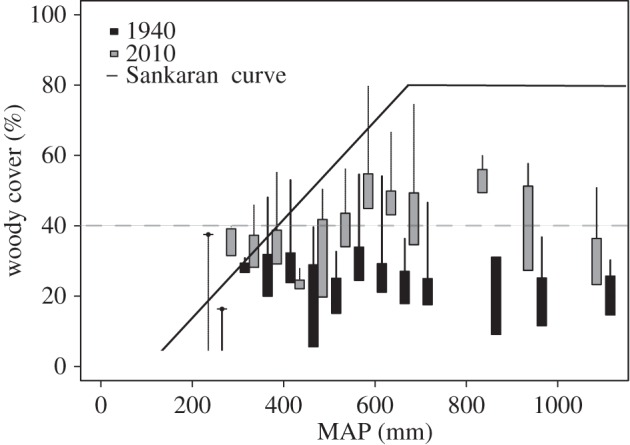
% Woody cover (in 50 mm bins) against MAP for 1940 (black) and 2010 (grey). The box represents the standard error and the lines represent the 0.99 quantile for the 50 mm classes. The solid black line represents the maximum woody cover versus map relationship for African savannahs and is represented by the equation: Cover(%) = 0.14(MAP) − 14.2 between 101 and 650 mm of MAP. Dashed lines represents the 40% woody cover threshold.
4. Discussion
Widespread increases in woody cover have occurred across South African savannahs since 1940, regardless of land use. The sole exception was in arid conservation areas containing elephants. In comparing our data with the woody cover thresholds in [30], in 2010, 20% of arid savannah sites exceeded the maximum tree cover threshold calculated for African savannahs. This is in contrast with 1940 where only 6% of arid savannah sites exceeded the same threshold.
(a). Local-scale contributors of woody cover change
Our results demonstrate that widespread woody cover increases have occurred across the rainfall gradient. The degree of change is contingent on MAP, MAT, the starting woody cover and land use. Sites with higher rainfall and warmer temperatures are more vulnerable to increases in woody cover. As water availability is a key constraint on plant growth rates, it is expected that high-rainfall areas will result in higher levels of encroachment [30]. Conversely, a high starting woody cover reduced subsequent encroachment rates; this is likely a result of density dependence [6] where further establishment of trees is hindered by competition for space, light and water. Local land use can be an important component of woody cover change [18,46]. However, our data indicate that the influence of local factors is overridden by regional or global processes given the consistency in the direction of change across very different land uses across the rainfall gradient. The largest increases in woody cover occurred in communal rangelands, where we predicted a decline in woody cover [19,22] due to high human population densities (table 1) and associated heavy utilization of wood for fuel and building [19]. While in many cases large trees suitable for building and fuel have disappeared, widespread increases of cover have occurred caused by increases in shrubs [47]. Frequently, shrubs are the dominant woody growth form responsible for encroachment [7,47]. One possible explanation is that shrub species most responsible for thickening spread clonally by root suckering, and root suckering may be enhanced by additional carbon gain from increasing atmospheric CO2 [7,8,48].
We predicted large woody cover increases in the commercial rangelands, which were observed in these data. In addition to a potential global driver, the management of commercial rangelands may facilitate encroachment as there is typically a high abundance of grazers (domestic cattle), long fire return intervals and a low number of browsers (goats), all of which have been shown to aid woody plant growth [12,39,49]. In Africa, conservation areas with elephants represent a unique ‘control’ for global change as they retain an indigenous megafauna, have low human impacts and experience regular fire. Yet where elephants are present in fenced areas, we predicted that woody cover would decline [50,51]. However, in conservation areas without elephants, the regular fires and rich grazer and browser communities would result in woody cover remaining stable over time. Instead, large increases in woody cover in conservation areas without elephants occurred across the rainfall gradient. Where elephants were present, woody cover still increased in high-rainfall savannahs, but not in low-rainfall savannahs where woody cover remained stable. This result points to the importance of this single species in shaping the structure of low-rainfall African savannahs. The widespread extinction of megaherbivores from across the landscape should, therefore, be considered an additional component of woody cover increase in the low-rainfall savannahs [18]. This finding also supports the hypothesis that Africa has much larger areas of arid savannahs than Australia and South America because of the persistence of megafauna, contrasting with Pleistocene extinctions on the other southern continents [52]. The impact of elephants can be disproportionately higher in low-rainfall savannahs as direct effects of herbivory are modulated by ecosystem productivity [53]. In low-rainfall areas productivity is low, and plant regrowth following heavy browsing is also low when compared with that of higher rainfall savannahs [53].
(b). The potential role of global factors in woody cover change
Increases in woody cover across the rainfall gradient and across different land uses indicate that a global process is overriding the impact of land use in causing encroachment. Regional changes in fire regime, specifically a trend of fire suppression, could cause encroachment [48]. However, although we have limited information about long-term trends in fires across Africa, there is no current evidence to indicate a change in fire trends over time. An analysis of approximately 60 years of fire data from the Kruger National Park and Hluhluwe-iMfolozi Park showed no clear trends [54] in fire patterns. As a proxy, we could use long-term trends in livestock densities for amount of burnt area and intensity. We would expect reductions in fire area and intensity in areas where livestock numbers have increased, as there would be less fuel available to burn [55]. However, long-term data on stocking densities in communal and commercial rangelands [1,56] show no increases in livestock densities despite large increases in human population sizes. Additionally, as expected, fire patterns differ between land uses. The total area burned is twice as high in protected areas than associated communal lands [57] due to high livestock numbers [55]. The differences across land-use categories in fire return time are less obvious, but fires are slightly more intense inside than outside protected areas on average [57]. The fire regimes observed are not conditions of fire suppression and the fire regimes, especially in conservation areas, are not conducive to encroachment and do not explain the patterns noted in this analysis. In these areas, we would expect decreases in woody cover, especially in mesic savannahs where fire intensity and return periods are the highest. Instead, despite regular and intense fire, high levels of encroachment were observed.
Our results are most consistent with existing predictions [25], models [58] and field studies [7,59] which indicate that a primary global influence of increasing CO2 concentrations is causing a regional increase in woody plant biomass and dominance. This result is further supported by the finding that woody cover in the low-rainfall sites increasingly exceeded the expected rainfall-limited maximum woody cover reported by Sankaran et al. [30]. No regional increase in rainfall has been recorded within the study area. Weather station records from 1960 to 2010 indicate that the amount of rainfall and number of rain days within these regions has declined [31]. Therefore, it is most likely that encroachment in low-rainfall savannahs is driven by elevated CO2, probably through increasing soil moisture, driven by improved WUEs [23]. Similarly, using this principle, Donohue et al. [9] demonstrated that elevated CO2 most likely accounted for an 11% increase in annual maximum greening in semi-arid areas, worldwide. The CO2 driven increases in high-rainfall savannahs, where fires are frequent, can best be explained by an increased probability of sapling escape from the fire trap [25,26,48] with higher growth rates and below-ground storage reserves [26,48]. Additionally, these results are important in highlighting the extent of encroachment in South Africa low-rainfall savannahs, as previously it has been suggested that encroachment was most prominent in African high-rainfall savannahs [7,48].
(c). Relevance of these results for other grassy ecosystems
Despite the fact that this was a regional study (due to data constraints all sites were located within South Africa), we sampled a rainfall range from 302 to 1134 mm (i.e. nearly the full range of the savannah biome in Africa [60]). This enabled us to explore interactions between rainfall, land-use and global-change drivers, and gives confidence that the results are generalizable across a range of grassy ecosystems. However, we did not sample the nutrient-poor Brachystegia (Miombo) woodlands which dominate in high-rainfall savannahs elsewhere in Africa. The relevance of these results to Brachystegia (Miombo) woodlands remains to be tested: the clonal growth form of the dominant species (Brachystegia sp. and Julbernardia sp.) imply that they could be responsive to CO2, but nutrient limitation in these systems might limit any response. Although the observed increase in woody cover is regional, the underlying mechanisms driving this increase change can change across rainfall gradients and in interaction with land use. For instance, it is likely that CO2 fertilization benefits plants through improving WUE in low-rainfall savannahs versus improving resprouting ability in high-rainfall savannahs [23,25]. Plants are also less likely to be responsive if they are nutrient or space limited [61]. Therefore, generalizing these results to other continents and other parts of Africa first requires an improved understanding of the processes limiting woody cover in these ecosystems.
(d). Consequences of widespread tree cover increases
Woody encroachment across the extent of African savannahs will alter economic activities and ecological processes. Ecosystem services, including water supply and nutrient cycling, will be altered and biodiversity changes will occur [62–64]. As increasing woody cover reduces grass cover, grazer stocking rates are expected to drop, tourism potential for conservation areas may decline [65] and the costs of woody plant clearing will increase [66]. On the other hand, this widespread regional change represents new opportunities that need to be explored, e.g. potential energy provision and commercialization of the goat industry. This study highlights the need for a review of existing land-management policies, and the formulation of novel management and adaption strategies to cope with these changes. As encroachment occurs relatively gradually, the extent of the changes up until now has been underestimated. An assessment of the ecological and economic costs/benefits of woody encroachment, at a national and continental level, should be made to bring attention to its impacts and the diverse ecological and socio-economic consequences.
Data accessibility
Woody cover change data: provisional http://dx.doi.org/10.5061/dryad.4544k.
Authors' contributions
N.S., B.F.N.E. and W.J.B. conceived the study. N.S. collected the data, analysed the data and wrote the paper. S.A. assisted with data analysis. B.F.N.E., W.J.B., S.A. provided comments on a draft of the manuscript. B.F.N.E. provided the facilities for the analysis.
Competing interests
We have no competing interests.
Funding
N.S. was funded by CSIR parliamentary grant on land-atmosphere feedbacks. B.F.N.E. was supported by the NRF-DST Applied Centre for Climate and Earth System Science 440 (www.access.ac.za).
References
- 1.O'Connor TG, Puttick JR, Hoffman MT. 2014. Bush encroachment in southern Africa: changes and causes. Afr. J. Range Forage Sci. 31, 67–88. ( 10.2989/10220119.2014.939996) [DOI] [Google Scholar]
- 2.Murphy BP, Lehmann CE, Russell-Smith J, Lawes MJ. 2014. Fire regimes and woody biomass dynamics in Australian savannas. J. Biogeogr. 41, 133–144. ( 10.1111/jbi.12204) [DOI] [Google Scholar]
- 3.Myers-Smith IH, et al. 2011. Shrub expansion in tundra ecosystems: dynamics, impacts and research priorities. Environ. Res. Lett. 6, 045509 ( 10.1088/1748-9326/6/4/045509) [DOI] [Google Scholar]
- 4.Van Auken OW. 2000. Shrub invasions of North American semiarid grasslands. Annu. Rev. Ecol. Syst. 31, 197–215. ( 10.1146/annurev.ecolsys.31.1.197) [DOI] [Google Scholar]
- 5.Archer S. 1995. Tree-grass dynamics in a Prosopis-thornscrub savanna parkland: reconstructing the past and predicting the future. Ecoscience 2, 83–99. [Google Scholar]
- 6.Roques KG, O'Connor TG, Watkinson AR. 2001. Dynamics of shrub encroachment in an African savanna: relative influences of fire, herbivory, rainfall and density dependence. J. Appl. Ecol. 38, 268–280. ( 10.1046/j.1365-2664.2001.00567.x) [DOI] [Google Scholar]
- 7.Buitenwerf R, Bond WJ, Stevens N, Trollope WSW. 2012. Increased tree densities in South African savannas: >50 years of data suggests CO2 as a driver. Glob. Change Biol. 18, 675–684. ( 10.1111/j.1365-2486.2011.02561.x) [DOI] [Google Scholar]
- 8.Bond WJ, Midgley GF. 2012. Carbon dioxide and the uneasy interactions of trees and savannah grasses. Phil. Trans. R. Soc. B 367, 601–612. ( 10.1098/rstb.2011.0182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donohue RJ, Roderick ML, McVicar TR, Farquhar GD. 2013. Impact of CO2 fertilization on maximum foliage cover across the globe's warm, arid environments. Geophys. Res. Lett. 40, 3031–3035. ( 10.1002/grl.50563) [DOI] [Google Scholar]
- 10.Skarpe C. 1990. Shrub layer dynamics under different herbivore densities in an arid savanna, Botswana. J. Appl. Ecol. 27, 873–885. ( 10.2307/2404383) [DOI] [Google Scholar]
- 11.Holland EA, Detling JK. 1990. Plant response to herbivory and belowground nitrogen cycling. Ecology 71, 1040–1049. ( 10.2307/1937372) [DOI] [Google Scholar]
- 12.Archibald S, Roy DP, Wilgen V, Brian W, Scholes RJ. 2009. What limits fire? An examination of drivers of burnt area in Southern Africa. Glob. Change Biol. 15, 613–630. ( 10.1111/j.1365-2486.2008.01754.x) [DOI] [Google Scholar]
- 13.Higgins SI, Bond WJ, Trollope WS. 2000. Fire, resprouting and variability: a recipe for grass–tree coexistence in savanna. J. Ecol. 88, 213–229. ( 10.1046/j.1365-2745.2000.00435.x) [DOI] [Google Scholar]
- 14.Staver AC, Bond WJ, Stock WD, Van Rensburg SJ, Waldram MS. 2009. Browsing and fire interact to suppress tree density in an African savanna. Ecol. Appl. 19, 1909–1919. ( 10.1890/08-1907.1) [DOI] [PubMed] [Google Scholar]
- 15.Guldemond R, AARDE R. 2008. A meta-analysis of the impact of African elephants on savanna vegetation. J. Wildl. Manage. 72, 892–899. ( 10.2193/2007-072) [DOI] [Google Scholar]
- 16.Hester AJ, Scogings PF, Trollope WS. 2006. Long-term impacts of goat browsing on bush-clump dynamics in a semi-arid subtropical savanna. Plant Ecol. 183, 277–290. ( 10.1007/s11258-005-9039-6) [DOI] [Google Scholar]
- 17.Owen-Smith RN. 1992. Megaherbivores: the influence of very large body size on ecology. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 18.Daskin JH, Stalmans M, Pringle RM. 2015. Ecological legacies of civil war: 35-year increase in savanna tree cover following wholesale large-mammal declines. J. Ecol. 104, 79–89. ( 10.1111/1365-2745.12483) [DOI] [Google Scholar]
- 19.Banks DI, Griffin NJ, Shackleton CM, Shackleton SE, Mavrandonis JM. 1996. Wood supply and demand around two rural settlements in a semi-arid savanna, South Africa. Biomass Bioenergy 11, 319–331. ( 10.1016/0961-9534(96)00031-1) [DOI] [Google Scholar]
- 20.Bucini G, Hanan NP. 2007. A continental-scale analysis of tree cover in African savannas. Glob. Ecol. Biogeogr. 16, 593–605. ( 10.1111/j.1466-8238.2007.00325.x) [DOI] [Google Scholar]
- 21.Garenne ML, Tollman SM, Collinson MA, Kahn K. 2007. Fertility trends and net reproduction in Agincourt, rural South Africa, 1992–2004. Scand. J. Public Health 35, 68–76. ( 10.1080/14034950701355650) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wessels KJ, Colgan MS, Erasmus BFN, Asner GP, Twine WC, Mathieu R, Van Aardt JAN, Fisher JT, Smit IP. 2013. Unsustainable fuelwood extraction from South African savannas. Environ. Res. Lett. 8, 014007 ( 10.1088/1748-9326/8/1/014007) [DOI] [Google Scholar]
- 23.Polley HW, Mayeux HS, Johnson HB, Tischler CR. 1997. Viewpoint: atmospheric CO2, soil water, and shrub/grass ratios on rangelands. J. Range Manage. 50, 278–284. ( 10.2307/4003730) [DOI] [Google Scholar]
- 24.Ainsworth EA, Long SP. 2005. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 165, 351–372. ( 10.1111/j.1469-8137.2004.01224.x) [DOI] [PubMed] [Google Scholar]
- 25.Bond WJ, Midgley GF. 2000. A proposed CO2-controlled mechanism of woody plant invasion in grasslands and savannas. Glob. Change Biol. 6, 865–869. ( 10.1046/j.1365-2486.2000.00365.x) [DOI] [Google Scholar]
- 26.Kgope BS, Bond WJ, Midgley GF. 2010. Growth responses of African savanna trees implicate atmospheric [CO2] as a driver of past and current changes in savanna tree cover. Austral Ecol. 35, 451–463. ( 10.1111/j.1442-9993.2009.02046.x) [DOI] [Google Scholar]
- 27.Hoffmann WA, Bazzaz FA, Chatterton NJ, Harrison PA, Jackson RB. 2000. Elevated CO2 enhances resprouting of a tropical savanna tree. Oecologia 123, 312–317. ( 10.1007/s004420051017) [DOI] [PubMed] [Google Scholar]
- 28.Kulmatiski A, Beard KH. 2013. Woody plant encroachment facilitated by increased precipitation intensity. Nat. Clim. Change 3, 833–837. ( 10.1038/nclimate1904) [DOI] [Google Scholar]
- 29.Good SP, Caylor KK. 2011. Climatological determinants of woody cover in Africa. Proc. Natl Acad. Sci. USA 108, 4902–4907. ( 10.1073/pnas.1013100108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sankaran M., et al. 2005. Determinants of woody cover in African savannas. Nature 438, 846–849. ( 10.1038/nature04070) [DOI] [PubMed] [Google Scholar]
- 31.MacKellar N, New M, Jack C. 2014. Observed and modelled trends in rainfall and temperature for South Africa: 1960–2010. S. Afr. J. Sci. 110, 1–13. ( 10.1590/sajs.2014/20130353) [DOI] [Google Scholar]
- 32.ESRI ArcGIS Desktop. 2011 ESRI ArcGIS Desktop, Release 10. Redlands, CA: Environ. Syst. Res. Inst.
- 33.Mucina L, Rutherford MC, Powrie Leslie W. 2006. The vegetation of South Africa, Lesotho and Swaziland. Silverton, South Africa: South African National Biodiversity Institute. [Google Scholar]
- 34.Fairbanks DHK, Thompson MW, Vink DE, Newby TS, Van den Berg HM, Everard DA. 2000. The South African land-cover characteristics database: a synopsis of the landscape S. Afr. J. Sci. 96, 69–82. [Google Scholar]
- 35.Scholes RJ. 2003. Convex relationships in ecosystems containing mixtures of trees and grass. Environ. Resour. Econ. 26, 559–574. ( 10.1023/B:EARE.0000007349.67564.b3) [DOI] [Google Scholar]
- 36.Fritz H, Duncan P. 1994. On the carrying capacity for large ungulates of African savanna ecosystems. Proc. R. Soc. Lond. B 256, 77–82. ( 10.1098/rspb.1994.0052) [DOI] [PubMed] [Google Scholar]
- 37.Hunter LM, Nawrotzki R, Leyk S, Maclaurin GJ, Twine W, Collinson M, Erasmus B. 2014. Rural outmigration, natural capital, and livelihoods in South Africa. Popul. Space Place 20, 402–420. ( 10.1002/psp.1776) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Twine W, Moshe D, Netshiluvhi T, Siphugu V. 2003. Consumption and direct-use values of savanna bio-resources used by rural households in Mametja, a semi-arid area of Limpopo province, South Africa: research letter. S. Afr. J. Sci. 99, 467. [Google Scholar]
- 39.Scholes RJ. 2009. Syndromes of dryland degradation in southern Africa. Afr. J. Range Forage Sci. 26, 113–125. [Google Scholar]
- 40.Schulze RE. 2008. South African atlas of climatology and agrohydrology. Pretoria, South Africa: Water Research Commission. [Google Scholar]
- 41.Leica Geosystems 2004. ERDAS imagine. Atlanta, GA: Leica Geosystems. [Google Scholar]
- 42.Levick SR, Rogers KH. 2011. Context-dependent vegetation dynamics in an African savanna. Landsc. Ecol. 26, 515–528. ( 10.1007/s10980-011-9578-2) [DOI] [Google Scholar]
- 43.Laliberte AS, Rango A, Havstad KM, Paris JF, Beck RF, McNeely R, Gonzalez AL. 2004. Object-oriented image analysis for mapping shrub encroachment from 1937 to 2003 in southern New Mexico. Remote Sens. Environ. 93, 198–210. ( 10.1016/j.rse.2004.07.011) [DOI] [Google Scholar]
- 44.Trimble Geospatial eCognition developer. Munich, Germany: Trimble.
- 45.R Core Team 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 46.Fynn RWS, O'connor TG. 2000. Effect of stocking rate and rainfall on rangeland dynamics and cattle performance in a semi-arid savanna, South Africa. J. Appl. Ecol. 37, 491–507. ( 10.1046/j.1365-2664.2000.00513.x) [DOI] [Google Scholar]
- 47.Mograbi PJ, Erasmus BF, Witkowski ETF, Asner GP, Wessels KJ, Mathieu R, Knapp DE, Martin RE, Main R. 2015. Biomass increases go under cover: woody vegetation dynamics in South African rangelands. PLoS ONE 10, e0127093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wakeling JL, Bond WJ. 2007. Disturbance and the frequency of root suckering in an invasive savanna shrub, Dichrostachys cinerea. Afr. J. Range Forage Sci. 24, 73–76. ( 10.2989/AJRFS.2007.24.2.3.157) [DOI] [Google Scholar]
- 49.Smit IP, Asner GP, Govender N, Kennedy-Bowdoin T, Knapp DE, Jacobson J. 2010. Effects of fire on woody vegetation structure in African savanna. Ecol. Appl. 20, 1865–1875. ( 10.1890/09-0929.1) [DOI] [PubMed] [Google Scholar]
- 50.Dublin HT. 1986. Decline of the Mara woodlands: the role of fire and elephants. PhD thesis, University of British Columbia. [Google Scholar]
- 51.Asner GP, Levick SR, Kennedy-Bowdoin T, Knapp DE, Emerson R, Jacobson J, Colgan MS, Martin RE. 2009. Large-scale impacts of herbivores on the structural diversity of African savannas. Proc. Natl Acad. Sci. USA 106, 4947–4952. ( 10.1073/pnas.0810637106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lehmann CE, et al. 2014. Savanna vegetation-fire-climate relationships differ among continents. Science 343, 548–552. ( 10.1126/science.1247355) [DOI] [PubMed] [Google Scholar]
- 53.Pringle RM, Young TP, Rubenstein DI, McCauley DJ. 2007. Herbivore-initiated interaction cascades and their modulation by productivity in an African savanna. Proc. Natl Acad. Sci. USA 104, 193–197. ( 10.1073/pnas.0609840104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Archibald S, Nickless A, Govender N, Scholes RJ, Lehsten V. 2010. Climate and the inter-annual variability of fire in southern Africa: a meta-analysis using long-term field data and satellite-derived burnt area data. Glob. Ecol. Biogeogr. 19, 794–809. ( 10.1111/j.1466-8238.2010.00568.x) [DOI] [Google Scholar]
- 55.Norton-Griffiths M. 1979. The influence of grazing, browsing, and fire on the vegetation dynamics of the Serengeti. In Serengeti: dynamics of an ecosystem (eds ARE Sinclair, M Norton-Griffiths), pp. 310–352 Chicago, IL: University of Chicago Press. [Google Scholar]
- 56.Vetter S, Bond WJ. 2012. Changing predictors of spatial and temporal variability in stocking rates in a severely degraded communal rangeland. Land Degrad. Dev. 23, 190–199. ( 10.1002/ldr.1076) [DOI] [Google Scholar]
- 57.Archibald S, Scholes RJ, Roy DP, Roberts G, Boschetti L. 2010. Southern African fire regimes as revealed by remote sensing. Int. J. Wildland Fire 19, 861–878. ( 10.1071/WF10008) [DOI] [Google Scholar]
- 58.Higgins SI, Scheiter S. 2012. Atmospheric CO2 forces abrupt vegetation shifts locally, but not globally. Nature 488, 209–212. ( 10.1038/nature11238) [DOI] [PubMed] [Google Scholar]
- 59.Wigley BJ, Bond WJ, Hoffman M. 2010. Thicket expansion in a South African savanna under divergent land use: local vs. global drivers? Glob. Change Biol. 16, 964–976. ( 10.1111/j.1365-2486.2009.02030.x) [DOI] [Google Scholar]
- 60.Lehmann CE, Sally A, Archibald, Hoffmann WA, Bond WJ. 2011. Deciphering the distribution of the savanna biome. New Phytol. 191, 197–209. ( 10.1111/j.1469-8137.2011.03689.x) [DOI] [PubMed] [Google Scholar]
- 61.Higgins SI, Keretetse M, February EC. 2015. Feedback of trees on nitrogen mineralization to restrict the advance of trees in C4 savannahs. Biol. Lett. 11, 20150572 ( 10.1098/rsbl.2015.0572) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blaum N, Rossmanith E, Popp A, Jeltsch F. 2007. Shrub encroachment affects mammalian carnivore abundance and species richness in semiarid rangelands. Acta Oecol. 31, 86–92. ( 10.1016/j.actao.2006.10.004) [DOI] [Google Scholar]
- 63.Sirami C, Seymour C, Midgley G, Barnard P. 2009. The impact of shrub encroachment on savanna bird diversity from local to regional scale. Divers. Distrib. 15, 948–957. ( 10.1111/j.1472-4642.2009.00612.x) [DOI] [Google Scholar]
- 64.Ratajczak Z, Nippert JB, Collins SL. 2012. Woody encroachment decreases diversity across North American grasslands and savannas. Ecology 93, 697–703. ( 10.1890/11-1199.1) [DOI] [PubMed] [Google Scholar]
- 65.Gray EF, Bond WJ. 2013. Will woody plant encroachment impact the visitor experience and economy of conservation areas? Koedoe 55, 9 ( 10.4102/koedoe.v55i1.1106) [DOI] [Google Scholar]
- 66.Grossman D, Gandar MV. 1989. Land transformation in South African savanna regions. South Afr. Geogr. J. 71, 38–45. ( 10.1080/03736245.1989.9713503) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Woody cover change data: provisional http://dx.doi.org/10.5061/dryad.4544k.